Introduction
Human parvovirus B19 (B19) is a small,
non-enveloped, single-stranded DNA virus in the Erythrovirus
genus of the Parvoviridae family (1). The left side of the B19 genome
encodes nonstructural protein 1 (NS1), whereas the right side
encodes two capsid proteins: VP1 and VP2, which are identical
disregarding the 227 amino acids at the amino-terminal end of the
VP1 protein, termed the VP1 unique region (VP1u) (2). The B19 virus, which was initially
discovered in 1975 (1), is
associated with various clinical manifestations, including rash,
thrombocytopenia, leukopenia, fetal wastage, hypocomplementemia,
autoimmune hemolytic anemia, arthritis, vasculitis and systemic
lupus erythematosus (3–5).
B19 can be transmitted by blood and blood-derived
products, and can also be transmitted vertically from mother to
fetus (6). The P blood group
antigen (P-antigen) was first discovered in 1927, while identifying
novel human blood group antigens by immunizing rabbits with human
erythrocytes (7). Globoside, an
alternative name for the P-antigen, is the major cellular receptor
for B19 infection, and is a neutral glycosphingolipid that is
predominantly found on erythroid cells and their progenitors
(8,9). As well as globoside, α5β1 integrin
and Ku80 autoantigen have been identified as co-receptors of B19
infection. The expression levels of α5β1 integrin and P-antigen are
believed to be associated with restricted cellular lineage for B19
entry (10). In addition,
induction of Ku80 proteins on cell surfaces is involved in the
pathologic immunity associated with B19 infection (11).
B19 induces cytotoxic effects in infected cells via
the promotion of cell cycle arrest and apoptosis (12). Although women exposed to B19 in
pregnancy are usually asymptomatic, ~3% of infected women exhibit
severe clinical symptoms, including hydrops fetalis and fetal
mortality (13,14). Furthermore, various prospective
studies have reported that the risks of developing hydrops fetalis
and of fetal mortality during pregnancy are significantly higher in
patients with B19 infection, as compared with in controls (15–17).
B19 infection, alongside the induction of antibodies against B19
viral proteins, during pregnancy has been associated with various
disorders, particularly hydrops fetalis and fetal mortality
(18,19); however, the effects of antibodies
against B19 viral proteins during pregnancy remain unclear. The
present study used human BeWo trophoblasts to investigate the
effects of anti-B19 antibodies, and aimed to provide a possible
explanation for the transmission of the B19 virus during
pregnancy.
Materials and methods
Ethics
Animal experiments were approved by the
Institutional Animal Care and Use Committee at Chung Shan Medical
University (Taichung, Taiwan; IACUC approval no. 1130). Animal
welfare and experimental procedures were performed according to the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (8th edition).
Rabbit anti-B19-VP1u/VP2/NS1
immunoglobulin G (IgG) antibodies
Preparations of B19-VP1u, B19-VP2 and B19-NS1
recombinant proteins were performed as described in our previous
study (20). Yields of the
purified recombinant B19-NS1, B19-VP1u and B19-VP2 proteins were
5.7, 4.1 and 8.7 mg/l, and their purities approximated 97.3, 98.1
and 96.8%, respectively. New Zealand White rabbits (age, 6 months;
weight, 2–3 kg; Lu-Ho Rabbit Farm, Chunghua, Taiwan) were
maintained under a 12 h light-dark cycle, and ambient temperature
was maintained at 24–26°C. The rabbits had ad libitum access
to water and standard laboratory chow. Control rabbit IgG
antibodies and IgG antibodies against the various B19 viral
proteins were prepared by subcutaneously immunizing two female New
Zealand White rabbits per group in the neck region with Freund's
complete adjuvant (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany; control group), or with 0.5 mg purified recombinant
B19-VP1u (VP1u group), B19-VP2 (VP2 group) or B19-NS1 (NS1 group)
in Freund's complete adjuvant. After the first immunization, the
various groups were immunized at 2-week intervals with Freund's
incomplete adjuvant, or with 0.25 mg B19-VP1u, B19-VP2 or B19-NS1
recombinant proteins in Freund's incomplete adjuvant. The
experimental period lasted for 8 weeks. The rabbits were
anesthetized with chloral hydrate (1 ml/25 g) prior to
CO2 asphyxiation. Blood samples were obtained from the
rabbits for antibody extraction. The rabbit IgG antibodies were
subsequently purified by protein G agarose (Roche Life Science,
Indianapolis, IN, USA) chromatography. Each eluent was concentrated
using a Centricon-P10 column (Amicon; EMD Millipore, Billerica, MA,
USA) and filtered using a 0.22 µm microporous membrane (EMD
Millipore) (21). Endotoxin tests
were conducted using the Limulus Amebocyte Lysate Endochrome Assay
(Charles River Laboratories, Inc., Charleston, SC, USA). The
endotoxin levels were found to be below the detection limit [0.25
endotoxin unit (EU) ml-1] for all IgG preparations at the
concentrations used in the present study.
Cell culture and treatment
The BeWo choriocarcinoma cell line (no. BCRC-60073),
which is the most extensively used cellular model of placental
trophoblasts, was purchased from the Bioresource Collection and
Research Center (Food Industry Research and Development Institute,
Hsinchu, Taiwan). The cells were maintained in 75 cm2 Falcon flasks
(BD Biosciences, Erembodegem, Belgium) under standard culture
conditions at 37°C in an atmosphere containing 5% CO2.
The culture medium comprised 85% F-12 nutrient mixture (Ham)
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml
streptomycin and 15% heat-inactivated fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc.). For treatments, BeWo trophoblast
cells were grown to confluence and were incubated with 100 or 200
µg/ml purified rabbit IgG antibodies for 24 h as described
previously (21).
Flow cytometric analysis
Cells were seeded in culture plates at 1×106
cells/100 mm2 for flow cytometry. The cell monolayers
were cultured in serum-free F12 medium for 24 h at 37°C in a 5% CO2
incubator. Cells were then incubated for 24 h with the following
antibodies: i) Affinity-purified IgG (100 or 200 µg/ml) from
control rabbits that were immunized with adjuvant; ii)
affinity-purified rabbit anti-B19-VP1u, anti-B19-VP2 or
anti-B19-NS1 IgG (100 or 200 µg/ml), or iii) medium alone. After
incubation, the cells were harvested, washed with PBS, and were
fixed with 70% alcohol for 12–16 h at 4°C. The cells were then
washed with PBS and transferred to 12×75-mm tubes. Human leukocyte
antigen (HLA)-ABC (cat. no. 555553), β1 integrin [cluster of
differentiation (CD)29 (cat. no. 556049); BD Biosciences, San
Diego, CA, USA] or human HLA-G (cat. no. 335906) (BioLegend, Inc.,
San Diego, CA, USA) were detected by adding 20 µl
phycoerythrin-conjugated mouse anti-human monoclonal antibodies.
Globoside was detected by incubating the cells with rabbit
polyclonal anti-globoside GL4 (cat. no. ab23949; Abcam, Cambridge,
UK), followed by rhodamine-conjugated secondary antibody (cat. no.
AP132R; Chemicon; EMD Millipore). The stained cells were analyzed
using a FASCSCalibur analyzer (BD Biosciences, Bedford, MA, USA).
In addition, the cells (~2×106) were fixed in 75%
alcohol for 12–16 h at 4°C. Subsequently, the cells were treated
with RNase (1 mg/ml) at 25°C for 30 min, and were stained with
propidium iodide (10 mg/ml) for 30 min prior to cell cycle analysis
with a flow cytometer. For each sample, 10,000-gated events were
collected, and mean fluorescence intensity was analyzed. For each
molecule, all staining procedures were performed in a single batch
to avoid inter-batch variation.
ELISA
Analysis of caspase-3 activity was performed in
triplicate using the caspase-3, Active Form, ELISA pair kit (BD
Biosciences, San Diego, CA, USA) according to the manufacturer's
protocol.
Immunoblotting
Immunoblotting was performed as described previously
(22). Briefly, protein samples
were denatured for 5 min in boiling water containing sample buffer
(0.0625 M Tris-HCl buffer, pH 6.8; 2.3% SDS, 5% 2-mercaptoethanol,
10% glycerol). Samples were separated on a 12.5% acrylamide gel
(100–150 V; 1.5 h) and were electrophoretically transferred to
nitrocellulose membranes (Amersham; GE Healthcare, Piscataway, NJ,
UK). The membranes were then incubated in PBS containing 5% nonfat
dry milk for 30 min at room temperature, in order to saturate
irrelevant protein binding sites. Antibodies against Fas-associated
death domain protein (FADD; cat. no. sc-6035), activated caspase-8
(cat. no. sc-166320), activated caspase-3 (cat. no. sc-7148),
B-cell lymphoma 2-associated X protein (Bax; cat. no. sc-7480),
cytochrome c (cat. no. sc-13156), apoptotic peptidase
activating factor 1 (Apaf-1; cat. no. sc-8339), activated caspase-9
(cat. no. sc-7885) (1:500; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and β-actin (1:5,000; MAB1501, Chemicon; EMD Millipore,
Temecula, CA, USA) were diluted in PBS with 2.5% bovine serum
albumin. Membranes were incubated with the antibodies for 1.5 h
with gentle agitation at room temperature. After washing twice with
PBS-0.05% Tween, horseradish peroxidase (HRP)-conjugated secondary
antibody (cat. nos. sc-2004 or sc-2005; Santa Cruz Biotechnology,
Inc.) was added to the blots, which were incubated for a further 1
h. Immobilion Western HRP Chemiluminescent Substrate (EMD
Millipore) was then used to detect the antigen-antibody complexes.
The blots were scanned and semi-quantified by densitometry
(Alpha-Imager 2200; ProteinSimple, San Jose, CA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) by
one-way analysis of variance followed by Tukey multiple-comparisons
test. Data are presented as the mean ± standard error of the mean,
and results were verified in at least three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of rabbit anti-B19 IgG on the
expression levels of HLA-ABC and HLA-G
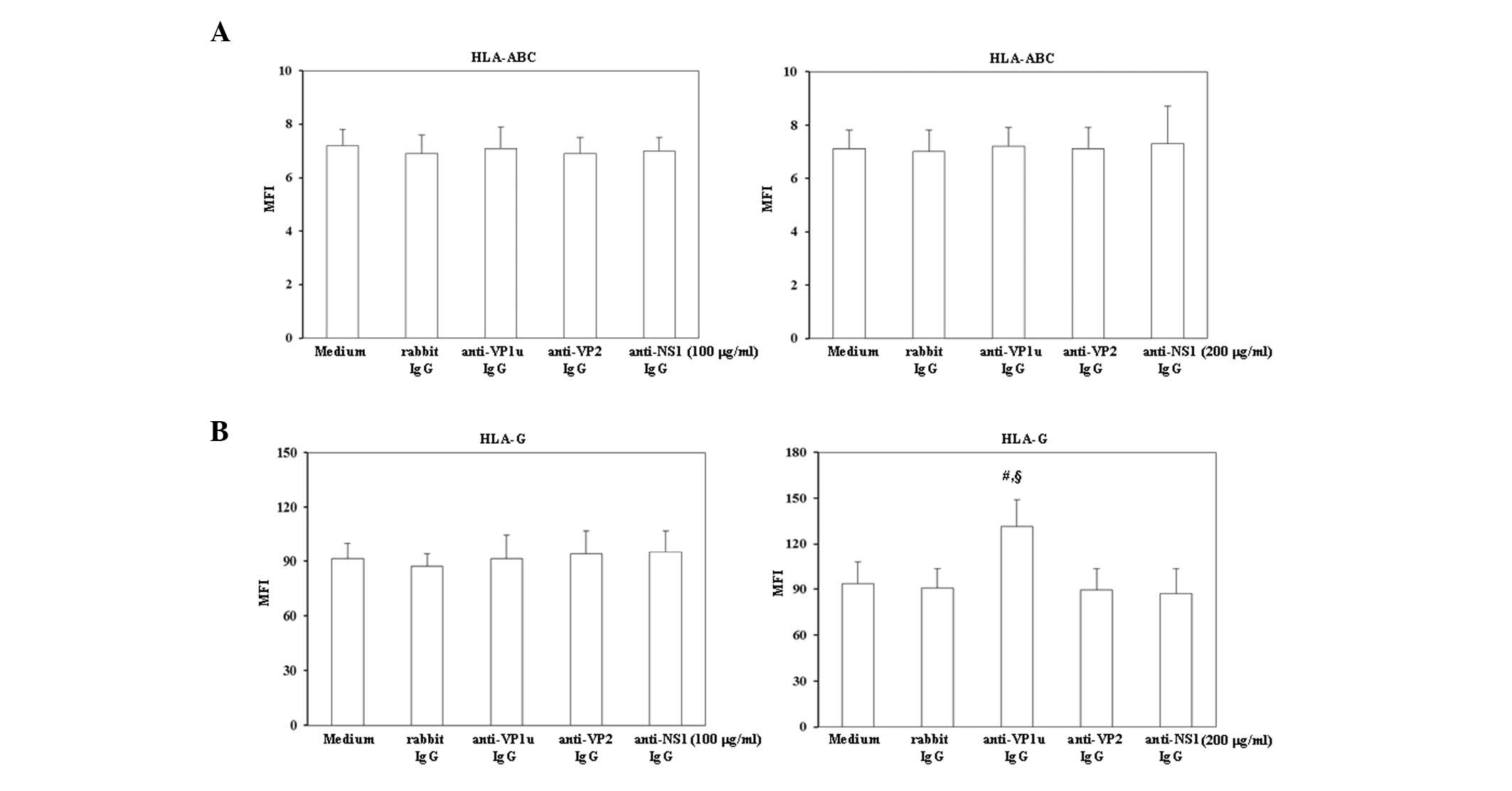
To investigate whether trophoblasts were activated
by antibodies against B19 viral proteins, the expression levels of
HLA-ABC and HLA-G were analyzed by flow cytometry (Fig. 1). As shown in Fig. 1A, treatment with 100 or 200 µg/ml
control rabbit IgG, rabbit anti-B19-VP1u, anti-B19-VP2 and
anti-B19-NS1 IgG did not significantly increase HLA-ABC expression
on BeWo trophoblasts. Notably, HLA-G expression was significantly
increased on BeWo trophoblasts treated with 200 µg/ml rabbit
anti-B19-VP1u IgG (Fig. 1B).
Conversely, no significant variation in HLA-G expression was
detected on BeWo trophoblasts treated with 200 µg/ml rabbit
anti-B19-VP2 and anti-B19-NS1 IgG compared with those treated with
control rabbit IgG.
Effects of rabbit anti-B19 IgG on the
expression levels of globoside and CD29
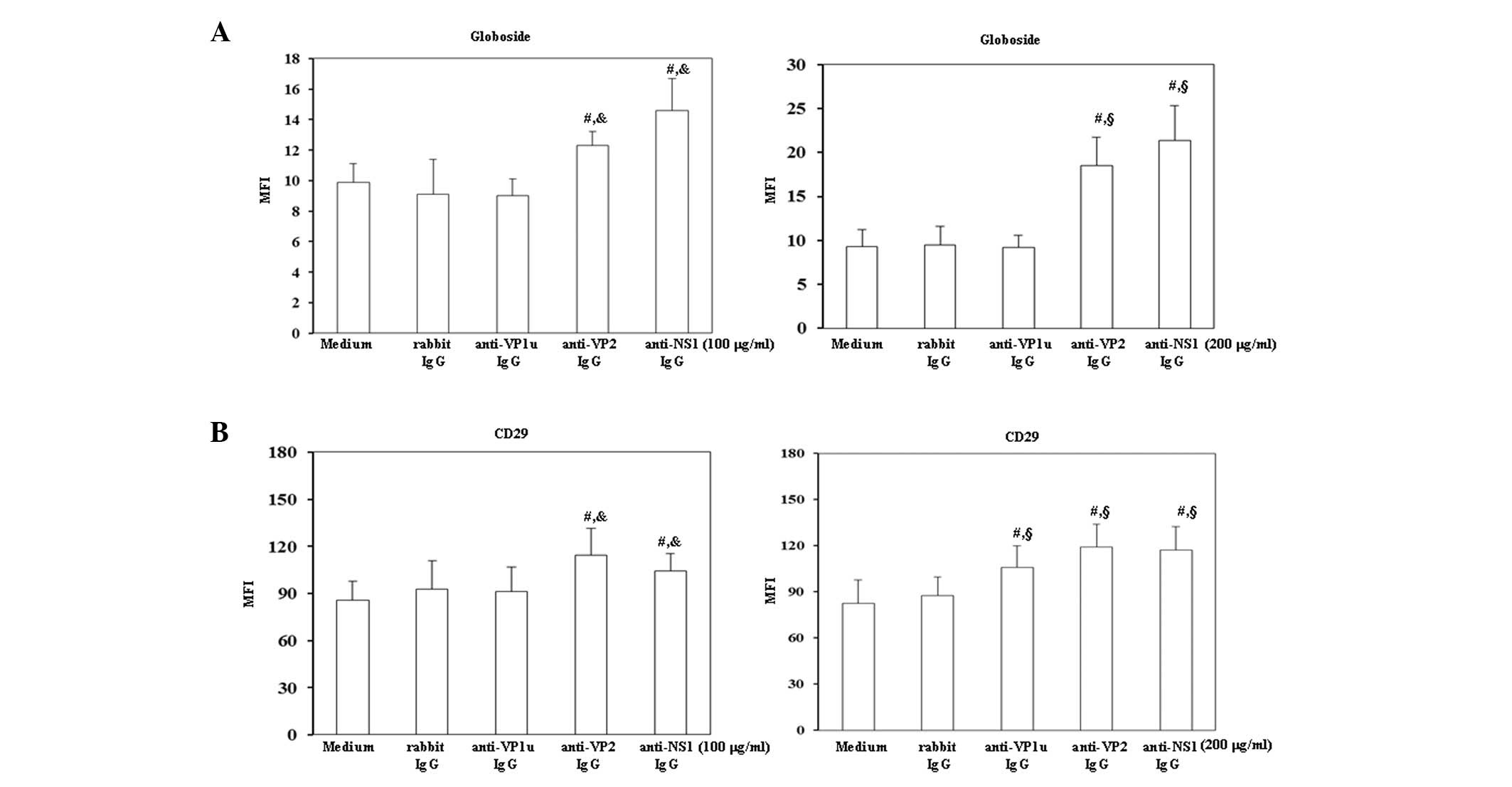
To investigate the effects of antibodies against B19
viral proteins on B19 receptors, the expression levels of
globoside, i.e., P-antigen, and CD29, also known as β1 integrin,
were detected on BeWo trophoblasts. As shown in Fig. 2A globoside expression was
significantly increased on BeWo trophoblasts treated with 100 or
200 µg/ml rabbit anti-B19-VP2 and anti-B19-NS1 IgG compared with
those treated with control rabbit IgG. Furthermore, the expression
levels of CD29 were significantly increased on BeWo trophoblast
cells treated with 200 µg/ml rabbit anti-B19-VP1u IgG, and with 100
or 200 µg/ml rabbit anti-B19-VP2 and anti-B19-NS1 IgG compared with
those treated with control rabbit IgG.
Effects of rabbit anti-B19 IgG on the
induction of apoptosis
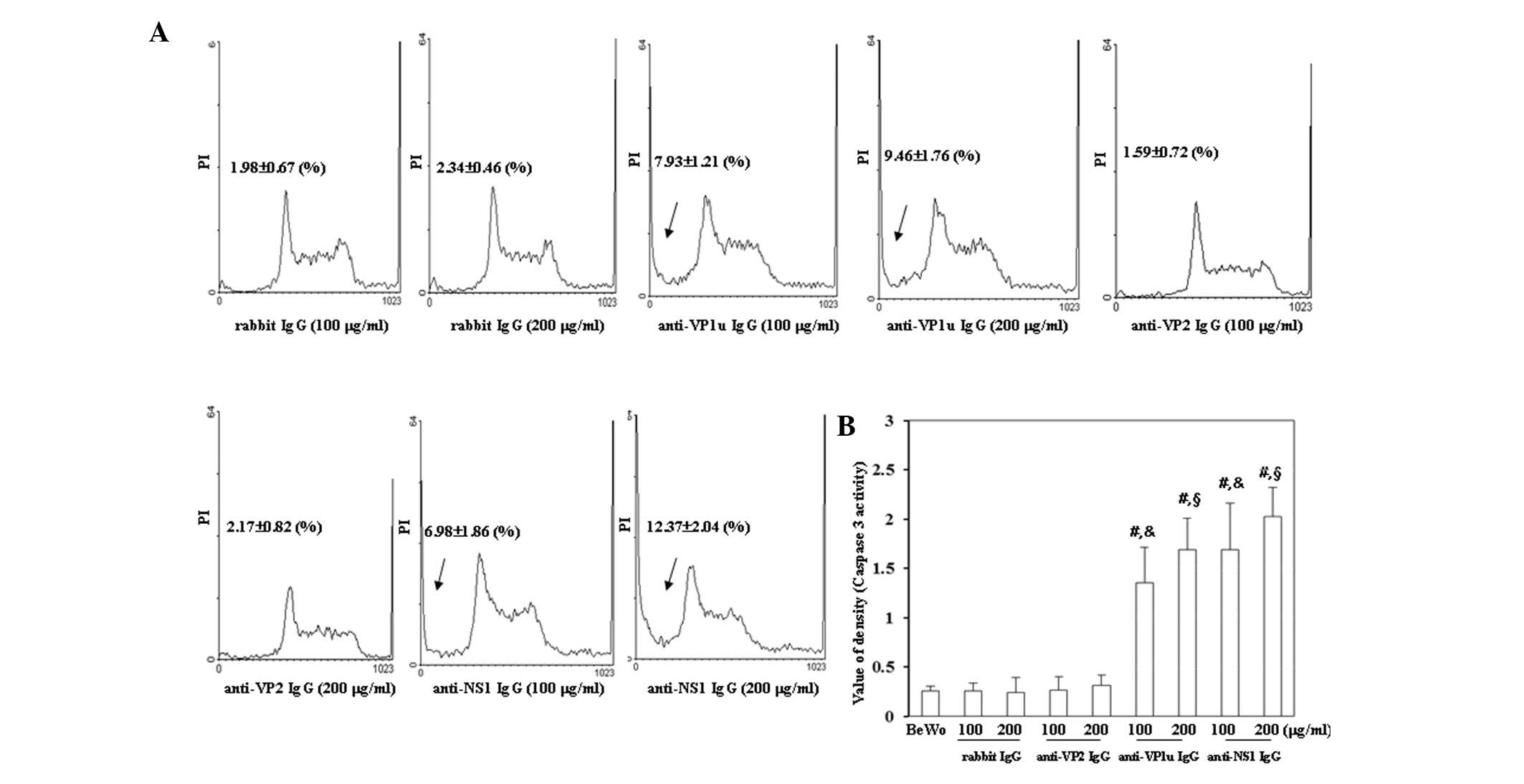
To investigate whether antibodies against B19 viral
proteins were able to induce apoptosis of BeWo trophoblasts, flow
cytometry and a caspase-3 activity assay were performed. As shown
in Fig. 3A, the number of cells in
sub-G1 phase was significantly increased in BeWo
trophoblasts treated with 100 or 200 µg/ml anti-B19-VP1u and
anti-B19-NS1 IgG compared with those treated with control rabbit
IgG. Conversely, the sub-G1 population was not
significantly different between BeWo trophoblasts treated with
control rabbit IgG and anti-B19-VP2 IgG. In addition, caspase-3
activity was significantly increased in BeWo trophoblasts treated
with 100 or 200 µg/ml anti-B19-VP1u and anti-B19-NS1 IgG compared
with those treated with control rabbit IgG (Fig. 3B).
Effects of rabbit anti-B19 IgG on the
expression levels of apoptotic molecules
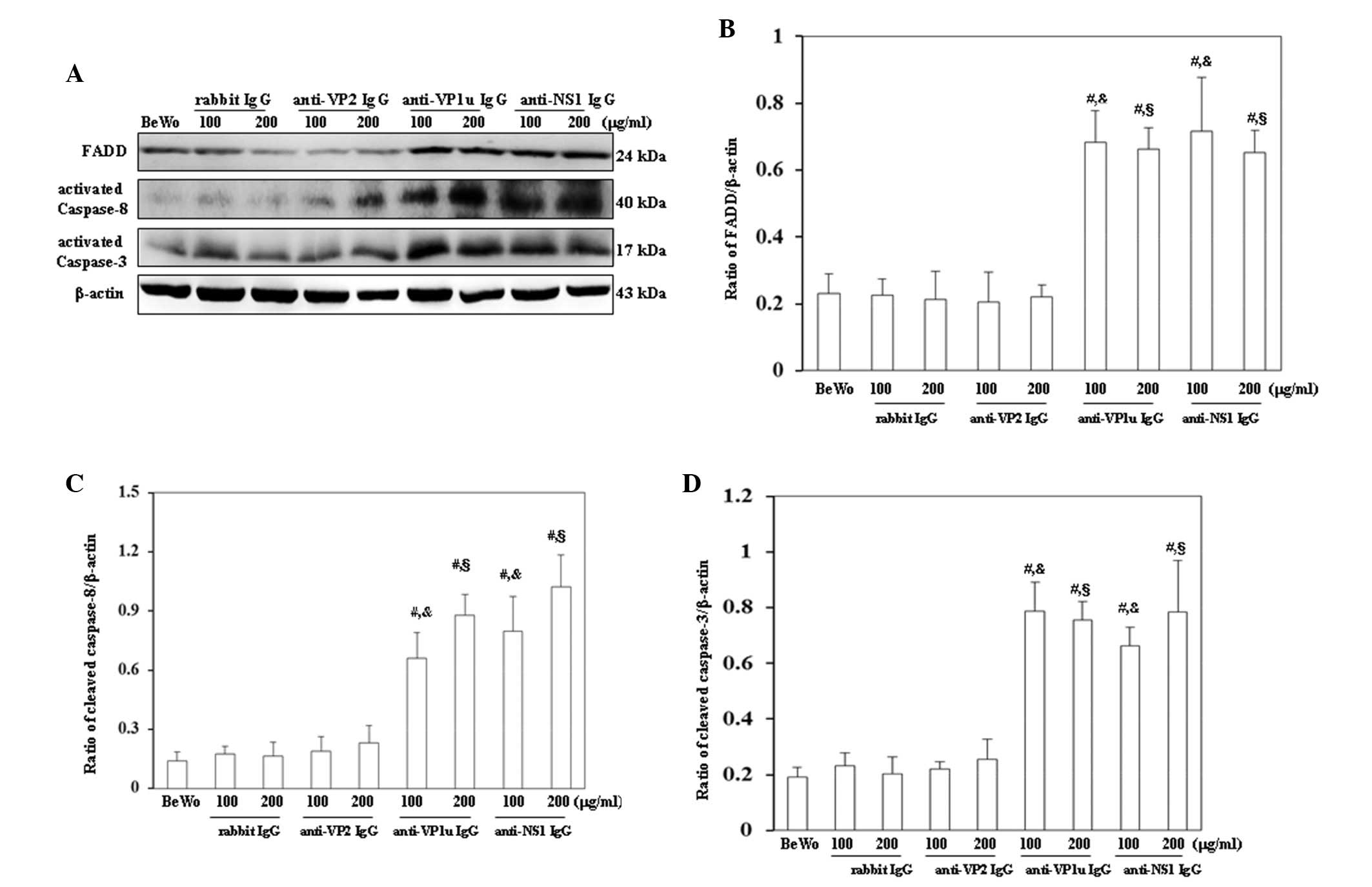
For further confirmation that apoptosis was induced
by anti-B19 antibodies, the expression levels of molecules
associated with the extrinsic and intrinsic apoptotic pathways were
examined. As shown in Fig. 4A,
FADD, activated caspase-8 and activated caspase-3 were
significantly increased in BeWo trophoblasts treated with 100 or
200 µg/ml anti-B19-VP1u and anti-B19-NS1 IgG compared with those
treated with control rabbit IgG. Conversely, FADD, activated
caspase-8 and activated caspase-3 expression levels were not
significantly different between BeWo trophoblasts treated with
control rabbit IgG and anti-B19-VP2 IgG (Fig. 4A). The results of western blotting
were semi-quantified, as presented in Fig. 4B-D. Furthermore, Bax, cytochrome
c, Apaf-1 and activated caspase-9 were significantly
increased in BeWo trophoblasts treated with 100 or 200 µg/ml
anti-B19-VP1u and anti-B19-NS1 IgG compared with those treated with
control rabbit IgG (Fig. 5A).
Conversely, no significant difference in Bax, cytochrome c,
Apaf-1 and activated caspase-9 expression was detected between BeWo
trophoblasts treated with control rabbit IgG and anti-B19-VP2 IgG
(Fig. 5A). The results of western
blotting were semi-quantified, as presented in Fig. 5B-E.
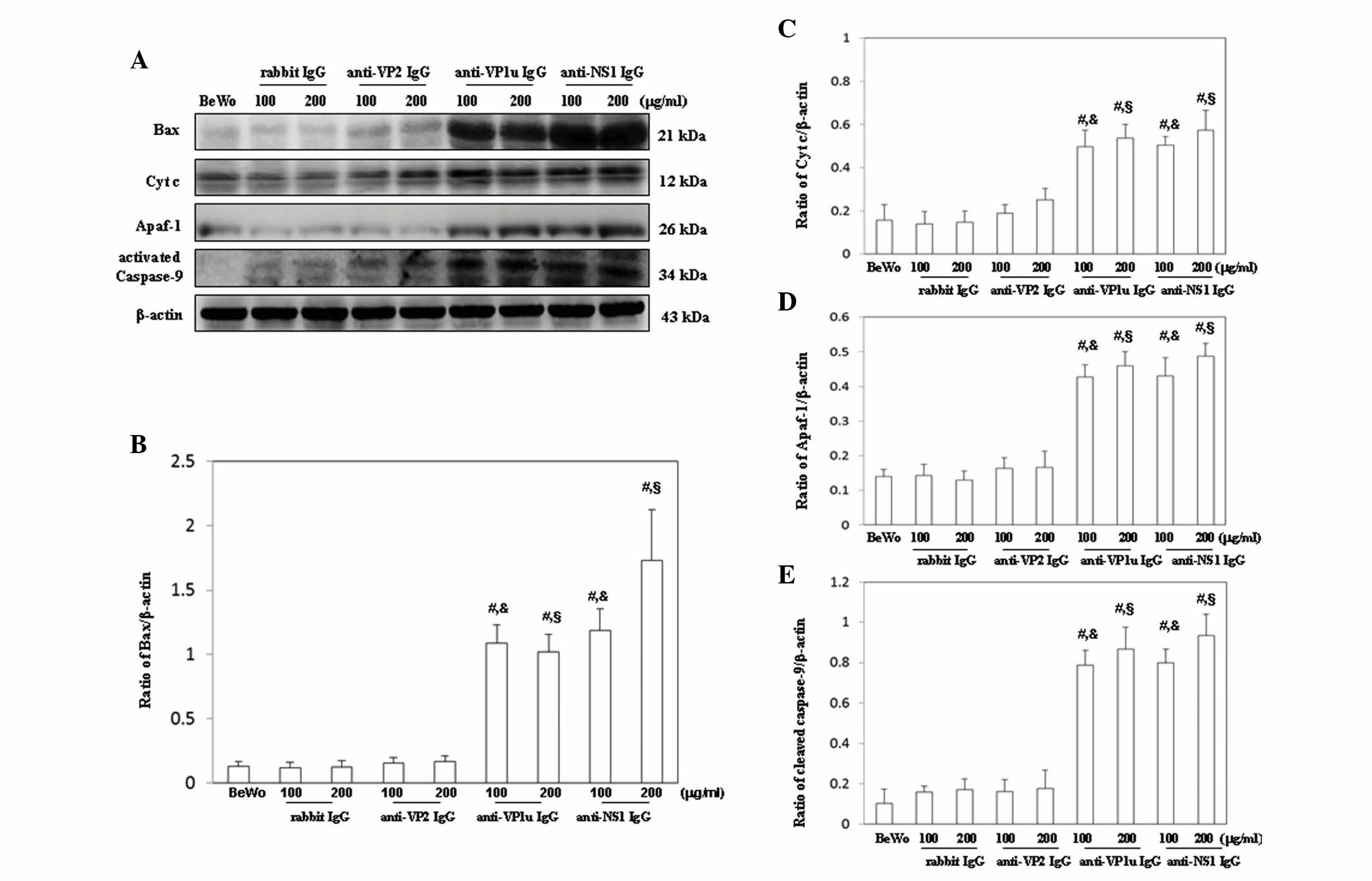 | Figure 5.Detection of (A) Bax, cytochrome
c, Apaf-1 and activated caspase-9 expression in BeWo
trophoblasts treated with normal rabbit IgG, anti-B19-VP1u,
anti-B19-VP2 and anti-B19-NS1 IgG for 24 h. Signal intensity of (B)
Bax, (C) cytochrome c, (D) Apaf-1 and (E) activated
caspase-9 was quantified using AlphaImager 2200. Equal loading was
assessed with the anti-β-actin antibody. Three independent
experiments were performed. #P<0.05 vs. BeWo cells;
&P<0.05 vs. 100 µg/ml rabbit IgG group;
§P<0.05 vs. 200 µg/ml rabbit IgG group. IgG,
immunoglobulin G; B19, human parvovirus B19; VP1u, VP1 unique
region; NS1, nonstructural protein 1; Bax, B-cell lymphoma
2-associated X protein; Cyt c, cytochrome c; Apaf-1,
apoptotic peptidase activating factor 1; BeWo group. BeWo cells
treated with medium only. |
Discussion
Fetal trophoblasts, maternal leukocytes, stromal
cells and endothelial cells in the maternal-fetal interface
comprise the decidua, and orchestrate placental vascularization and
tissue remodeling (23,24). Notably, fetal trophoblasts serve
crucial roles in modulating maternal immune responses, and educate
leukocytes, endometrial stromal cells and endothelial cells to
prepare a receptive decidual microenvironment that is required for
a successful pregnancy (25,26).
When the placental interface is forming with the maternal systemic
circulation, fetal trophoblasts begin to express HLA-G (23). HLA-G, which is a non-classical HLA
class I molecule, was initially discovered on fetal trophoblasts in
1986 (27). HLA-G is predominantly
expressed on fetal trophoblasts and is endowed with
immune-regulatory functions, which are known to suppress immune
responses and induce tolerance during pregnancy (24). Increased HLA-G expression has also
been associated with several immunological diseases, including
multiple sclerosis, asthma, allergic rhinitis and hepatitis C
virus-induced liver fibrosis (28,29).
These previous studies suggested that HLA-G has roles in normal
physiological and pathological processes. Notably, the present
study demonstrated that anti-B19-VP1u IgG significantly increased
HLA-G expression on BeWo trophoblasts. Although HLA-ABC expression
is associated with B19 transmission and replication in fetal
erythroid progenitor cells (30),
HLA-ABC expression was not significantly altered among BeWo
trophoblasts treated with anti-B19-VP1u, anti-B19-VP2 and
anti-B19-NS1 IgG. These findings indicated that anti-B19-VP1u,
anti-B19-VP2 and anti-B19-NS1 IgG have different effects on HLA-G
expression, but not on HLA-ABC expression, on BeWo trophoblasts.
Therefore, it may be hypothesized that anti-B19-VP1u, anti-B19-VP2
and anti-B19-NS1 IgG have pathological roles in disrupting the
maternal-fetal interface via altering the expression of HLA-G.
However, further studies are required to verify the precise
mechanism.
In addition to erythroid lineage cells, globoside is
known to be expressed on human placental trophoblasts (31). Globoside, namely P-antigen, and its
co-receptor, α5β1 integrin, are known to be major receptors for B19
infection. The P-antigen is required for primary B19 attachment to
permissive cells via the VP2 capsid protein, and subsequent
high-affinity conformation by α5β1 integrin is needed to trigger
the internalization step and enhance the probability of productive
infections (8,10,32).
Absence of P-antigen or α5β1 integrin is associated with loss of
cellular attachment, which may occur independently of viral
infection (33). These previous
findings suggested that increased expression of P-antigen or its
co-receptors may facilitate pathological processes after B19
infection. Notably, the present study demonstrated that antibodies
against B19-VP2 and B19-NS1 were able to induce the expression of
globoside and CD29, whereas anti-B19-VP1u only induced the
expression of CD29. These findings indicated that various
antibodies may have different pathological roles against B19 viral
proteins, and may facilitate the transmission of B19 across the
maternal-fetal interface via the activation of B19 receptors.
The placenta is a barrier that separates the fetal
and maternal blood and lymphatic systems. Within the placenta,
fetal trophoblasts serve crucial roles in evading recognition by
the maternal immune system and in defending against microorganisms
(34). Trophoblasts in the
placenta differentiate into villous and extravillous trophoblasts,
which provide epithelial cover of the placental villous tree in
direct contact with maternal blood, and invade maternal uterine
tissues resulting in direct contact with the maternal stromal and
immune cells, respectively (35).
Apoptosis of either type of trophoblast may cause severe
conditions, including pre-eclampsia and intrauterine growth
restriction. Abnormal apoptotic regulation of villous and/or
extravillous trophoblasts may also result in altered trophoblast
invasion and/or shedding into the maternal circulation. Notably, an
association between B19-induced fetal mortality and increased
apoptosis of placental villous trophoblasts has been reported
(36). Damage due to apoptotic
death of the placental trophoblast layer can compromise the
integrity of the protective barrier and have a role in pathogenesis
(36). Previous studies have
reported that B19 viral proteins induce apoptosis in vitro
and in vivo (37,38); however, little is currently known
regarding the effects of antibodies against B19 viral proteins on
placental trophoblasts during pregnancy. Notably, the present study
detected a significantly increased sub-G1 population;
caspase-3 activity; and expression of proteins associated with
intrinsic and extrinsic apoptotic signaling in BeWo trophoblasts
following treatment with anti-B19-VP1u and anti-B19-NS1 IgG.
Therefore, significantly increased apoptosis of BeWo trophoblasts
caused by anti-B19-VP1u and anti-B19-NS1 IgG may provide a rational
explanation for the transmission of B19 into the fetus.
Induction of anti-B19 antibodies due to B19
infection is associated with numerous severe disorders during
pregnancy; however, information regarding the effects of these
antibodies on trophoblasts is limited. The present study is the
first, to the best of our knowledge, to reveal the effects of
anti-B19-VP1u, anti-B19-NS1 and anti-B19-VP2 IgG on HLA-G,
globoside and CD29 expression, and the induction of intrinsic and
extrinsic apoptotic cascades in BeWo trophoblasts. The results of
the present study provide a possible explanation for the role of
anti-B19 antibodies in disrupting the fetal-maternal barrier via
modulating HLA-G, globoside and CD29 expression, and inducing
apoptosis of trophoblast cells.
Acknowledgements
The present study was supported in part by grants
from the National Science Council (nos. NSC 98-2314-B-040-008-MY3
and NSC 101-2314-B-040-008), the Department of Health (no. North
98030), and the Chung Shan Medical University Hospital, Taiwan
R.O.C. (no. CSH-2014-C-013). The funders had no role in study
design, data collection and analysis, decision to publish, or
preparation of the manuscript. The authors would also like to thank
Mr. Ted Knoy (Writing Center, Hsinchu, Taiwan, R.O.C.) for his
editorial assistance. Analyses using the FASCSCalibur, luminescent
image analyzer and digital imaging analyzer were performed at the
Instrument Center of Chung Shan Medical University, which is
supported by the National Science Council, Ministry of Education
and Chung Shan Medical University.
References
|
1
|
Cossart YE, Field AM, Cant B and Widdows
D: Parvovirus-like particles in human sera. Lancet. 1:72–73. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson S, Momoeda M, Kawase M, Kajigaya
S and Young NS: Peptides derived from the unique region of B19
parvovirus minor capsid protein elicit neutralizing antibodies in
rabbits. Virology. 206:626–632. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finkel TH, Török TJ, Ferguson PJ, Durigon
EL, Zaki SR, Leung DY, Harbeck RJ, Gelfand EW, Saulsbury FT,
Hollister JR, et al: Chronic parvovirus B19 infection and systemic
necrotizing vasculitis: Opportunistic infection or etiological
agent? Lancet. 343:1255–1258. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nesher G, Osborn TG and Moore TL:
Parvovirus infection mimicking systemic lupus erythematosus. Semin
Arthritis Rheum. 24:297–303. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogo LD, Mokhtari-Azad T, Kabir MH and
Rezaei F: Human parvovirus B19: A review. Acta Virol. 58:199–213.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enders M, Weidner A, Zoellner I, Searle K
and Enders G: Fetal morbidity and mortality after acute human
parvovirus B19 infection in pregnancy: Prospective evaluation of
1018 cases. Prenat Diagn. 24:513–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Landsteiner K and Levine P: On a specific
substance of the cholera vibrio. J Exp Med. 46:213–221. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown KE, Anderson SM and Young NS:
Erythrocyte P Antigen: Cellular receptor for B19 parvovirus.
Science. 262:114–117. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown KE and Young NS: Parvovirus B19
infection and hematopoiesis. Blood Rev. 9:176–182. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weigel-Kelley KA, Yoder MC and Srivastava
A: α5β1 integrin as a cellular coreceptor for human parvovirus B19:
Requirement of functional activation of b1 integrin for viral
entry. Blood. 102:3927–3933. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Munakata Y, Saito-Ito T, Kumura-Ishii K,
Huang J, Kodera T, Ishii T, Hirabayashi Y, Koyanagi Y and Sasaki T:
Ku80 autoantigen as a cellular coreceptor for human parvovirus B19
infection. Blood. 106:3449–3456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chisaka H, Morita E, Yaegashi N and
Sugamura K: Parvovirus B19 and the pathogenesis of anaemia. Rev Med
Virol. 13:347–359. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown T, Anand A, Ritchie LD, Clewley JP
and Reid TM: Intrauterine parvovirus infection associated with
hydrops fetalis. Lancet. 2:1033–1034. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Staroselsky A, Klieger-Grossmann C,
Garcia-Bournissen F and Koren G: Exposure to fifth disease in
pregnancy. Can Fam Physician. 55:1195–1198. 2009.PubMed/NCBI
|
|
15
|
Rodis JF, Quinn DL, Gary GW Jr, Anderson
LJ, Rosengren S, Cartter ML, Campbell WA and Vintzileos AM:
Management and outcomes of pregnancies complicated by human B19
parvovirus infection: A prospective study. Am J Obstet Gynecol.
163:1168–1171. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gratacós E, Torres PJ, Vidal J, Antolín E,
Costa J, Jiménez de Anta MT, Cararach V, Alonso PL and Fortuny A:
The incidence of human parvovirus B19 infection during pregnancy
and its impact on perinatal outcome. J Infect Dis. 171:1360–1363.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koch WC, Harger JH, Barnstein B and Adler
SP: Serologic and virologic evidence for frequent intrauterine
transmission of human parvovirus B19 with a primary maternal
infection during pregnancy. Pediatr Infect Dis J. 17:489–494. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwarz TF, Roggendorf M and Simader R:
Association of non-immunologically-induced hydrops fetalis with
Parvovirus B19 infection. Geburtshilfe Frauenheilkd. 47:572–573.
1987.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Searle K, Schalasta G and Enders G:
Development of antibodies to the nonstructural protein NS1 of
parvovirus B19 during acute symptomatic and subclinical infection
in pregnancy: Implications for pathogenesis doubtful. J Med Virol.
56:192–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai CC, Chiu CC, Hsu JD, Hsu HS, Tzang BS
and Hsu TC: Human parvovirus B19 NS1 protein aggravates liver
injury in NZB/W F1 mice. PLoS One. 8:e597242013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tzang BS, Tsai CC, Chiu CC, Shi JY and Hsu
TC: Up-regulation of adhesion molecule expression and induction of
TNF-alpha on vascular endothelial cells by antibody against human
parvovirus B19 VP1 unique region protein. Clin Chim Acta.
395:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiu CC, Shi YF, Yang JJ, Hsiao YC, Tzang
BS and Hsu TC: Effects of human parvovirus B19 and bocavirus VP1
unique region on tight junction of human airway epithelial A549
cells. PLoS One. 9:e1079702014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loke YW, King A and Burrows TD: Decidua in
human implantation. Hum Reprod 10 (Suppl 2). 14–21. 1995.
View Article : Google Scholar
|
|
24
|
Moffett A and Loke C: Immunology of
placentation in eutherian mammals. Nat Rev Immunol. 6:584–594.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szekeres-Bartho J: Immunological
relationship between the mother and the fetus. Int Rev Immunol.
21:471–495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gregori S, Amodio G, Quattrone F and
Panina-Bordignon P: HLA-G orchestrates the early interaction of
human trophoblasts with the maternal niche. Front Immunol.
6:1282015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ellis SA, Sargent IL, Redman CW and
McMichael AJ: Evidence for a novel HLA antigen found on human
extravillous trophoblast and a choriocarcinoma cell line.
Immunology. 59:595–601. 1986.PubMed/NCBI
|
|
28
|
Wiendl H, Feger U, Mittelbronn M, Jack C,
Schreiner B, Stadelmann C, Antel J, Brueck W, Meyermann R, Bar-Or
A, et al: Expression of the immune-tolerogenic major
histocompatibility molecule HLA-G in multiple sclerosis:
Implications for CNS immunity. Brain. 128:2689–2704. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
White SR: Human leucocyte antigen-G:
Expression and function in airway allergic disease. Clin Exp
Allergy. 42:208–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morey AL and Fleming KA: Immunophenotyping
of fetal haemopoietic cells permissive for human parvovirus B19
replication in vitro. Br J Haematol. 82:302–309. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jordan JA and DeLoia JA: Globoside
expression within the human placenta. Placenta. 20:103–108. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wegner CC and Jordan JA: Human parvovirus
B19 VP2 empty capsids bind to human villous trophoblast cells in
vitro via the globoside receptor. Infect Dis Obstet Gynecol.
12:69–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dalton SL, Marcantoni EE and Assoian RK:
Cell attachment controls fibronectin and alpha 5 beta 1 integrin
levels in fibroblasts. Implications for anchorage-dependent
and-independent growth. J Biol Chem. 267:8186–8191. 1992.PubMed/NCBI
|
|
34
|
Weetman AP: The immunology of pregnancy.
Thyroid. 9:643–646. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huppertz B, Kadyrov M and Kingdom JC:
Apoptosis and its role in the trophoblast. Am J Obstet Gynecol.
195:29–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jordan JA and Butchko AR: Apoptotic
activity in villous trophoblast cells during B19 infection
correlates with clinical outcome: Assessment by the caspase-related
M30 Cytodeath antibody. Placenta. 23:547–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moffatt S, Yaegashi N, Tada K, Tanaka N
and Sugamura K: Human parvovirus B19 nonstructural (NS1) protein
induces apoptosis in erythroid lineage cells. J Virol.
72:3018–3028. 1998.PubMed/NCBI
|
|
38
|
Yaegashi N, Niinuma T, Chisaka H, Uehara
S, Moffatt S, Tada K, Iwabuchi M, Matsunaga Y, Nakayama M, Yutani
C, et al: Parvovirus B19 infection induces apoptosis of erythroid
cells in vitro and in vivo. J Infect. 39:68–76. 1999. View Article : Google Scholar : PubMed/NCBI
|