Introduction
Colorectal cancer (CRC) is the third most common
type of cancer worldwide and is the fourth most common cause of
cancer-associated mortality (1).
To treat cancer, multiple interventions are required, including
surgery, chemotherapy and radiotherapy, with the goal to cure the
disease or prolong and improve quality of life (2). Chemotherapy has been demonstrated to
be effective in cancer treatment, and has helped to save lives of
millions of patients worldwide (3). There are numerous chemotherapeutic
drugs that are commonly used, which can be effective in certain
cancer types, however, chemotherapy does not specifically target
cancer cells, therefore results in numerous side effects (4). The majority of drugs used for the
treatment of cancer are cytotoxic drugs interfering with the
operation of the cancer cells. Cytotoxic drugs can be harmful
unless they are specific to cancer cells. Specificity is a
challenge, due to the fact that the modifications required to
convert a noncancerous cell into a cancerous cell are slight. To
design novel selective drugs for cancer cells, with reduced side
effects, is a key challenge (5).
An important class of compounds with a variety of biological and
clinical applications are Schiff bases (6). Copper is a metal long used for
medicinal applications and its potential anticancer activity has
been recently reported (7).
Copper-based complexes have become a focus, due to the assumption
that endogenous metals may be less toxic for normal cells compared
with cancer cells. Differential response between normal and tumor
cells to copper in addition to the altered metabolism of cancer
cells are the basis for development of copper complexes with
antineoplastic characteristics (8). Salicylaldehyde-derived copper (II)
Schiff base complexes are promising anticancer agents as a result
of their manifold properties such as anticancer and antifungal
properties (9) antiviral,
anti-inflammatory, antimicrobial and antioxidant activities
(10). In addition, their
transition metal complexes exhibit enhanced pharmacological
properties (11).
In the current study, four different copper (II)
complexes containing Schiff bases derived from salicylaldehyde and
glutamic acid were used, in addition to molecular O-or
N-donor ligands, in order to examine the anticancer
activities.
Materials and methods
Cell cultures
Human colon cancer cells (HT-29), non-cancerous
human cells (VH10), human embryonal non-cancerous cells (HEK-293T),
mouse cells (NIH-3T3) and mouse leukemia cells (L1210) were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; Life Technologies; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal bovine serum, 100 µg/ml streptomycin
and 100 U/ml penicillin G at 37°C in a humidified atmosphere of 5%
CO2/95% air.
Cu (II) complexes
Complexes 1,
[Cu2(sal-D,L-glu)2(isoquinoline)2]·2C2H5OH; 2,
[Cu(sal-5-met-L-glu)(H2O)].H2O; 3,
[Cu(ethanol)2(imidazole)4][Cu2(sal-D, L-glu)2(imidazole)2]; and 4,
[Cu(sal-D,L-glu)(2-methylimidazole)] were prepared, where
(sal-D,L-glu) or (sal-L-glu) is N-salicylidene-D, L- or L-glutamate
and (sal-5-met-L-glu) is N-salicylidene-5-methylester-L-glutamate.
The synthesis and the structure of the complexes are described in
Nakao et al (12),
Krätsmár-Šmogrovič et al (13) and Langer et al (14–17).
Cytotoxicity analysis
The effects of Cu (II) complexes on viability of
cancerous and noncancerous cells were determined by direct counting
of viable cells by the trypan blue exclusion assay in addition to
using the 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium
bromide (MTT) colorimetric technique. Cells were placed (8×103
cells/200 µl well) in individual wells of 96-multiwell plates. Each
concentration was tested four times and all dye exclusion tests
were performed in triplicate. Cu (II) complexes were diluted with
distilled water and final concentrations of the complexes added to
the cells were 0.001, 0.01, 0.1, 1, 10, 50 and 100 µmol/l.
Following 72 h exposure to 7 concentrations of Cu (II) complexes
(37°C, humidified atmosphere of 5% CO2/95% air), cells were treated
with MTT solution [5 mg/ml in phosphate-buffered saline (PBS), 20
µl] for 4 h. The dark crystals of formazan formed in intact cells
were dissolved in dimethyl sulfoxide (200 µl). The plates were
shaken for 15 min and the optical density was determined at 595 nm
using a MicroPlate Reader (Biotek Instruments, Inc., Winooski, VT,
USA).
Staining of actin filaments
The cell suspension (2×103 cells/well) was seeded
into 6-well chamber slides. Cells were then treated with Cu (II)
complexes (IC50 concentration) for 24 h. Non-treated cells were set
as the controls. For staining of the actin filaments, the following
procedures were conducted at room temperature. Cells were washed
with PBS and were fixed with 4% formaldehyde in PBS for 20 min,
followed by washing with PBS. Cells were then stained with 40
µl/slide phalloidine (1 µg/ml) for 30 min in dark. Subsequent to
incubation, cells were washed with distilled water. Actin fibers
were visualized by fluorescence microscopy (Zeiss, Oberkochen,
Germany) and images were captured under a magnification of
×600.
Detection of apoptosis
Untreated (control) and drug-treated (0.1–100
µmol/l) cancerous HT-29 cells (1×105), and noncancerous NIH-3T3
(6×104) and VH10 (1×105) cells, were incubated for 24, 48 and 72 h,
then harvested, washed in PBS, centrifuged at 1,500 × g for 10 min
at 4°C and lysed with 50 µl of lysis solution (10 mmol/l Tris, 10
mmol/l ethylenediaminetetraacetic acid and 0.5% Triton X-100)
supplemented with proteinase K (1 mg/ml). Samples were incubated at
37°C for 1 h and then heated at 70°C for 10 min. Following lysis,
2.5 µl RNase (200 µg/ml) was added followed by repeated incubation
at 37°C for 1 h. The samples were subjected to electrophoresis at
40 V for 2.5 h in 2% agarose gel with ethidium bromide. As a
positive control, L1210 cells were incubated with 6 µmol/l
cis-platin (Sigma-Aldrich, St. Louis, MO, USA) for 24 h and
processed according to the protocol described above. Separated DNA
fragments were visualized using an Ultra-Lum Electronic UV
transilluminator at 254 nm.
Caspase 3 activity
Caspase 3 activity was analyzed using Caspase 3
colorimetric assay kit (CaspACE™ Assay system Colorimetric; Promega
Corporation, Madison, WI, USA). The cells were treated with Cu (II)
complexes (1–100 µmol/l) for 60 h. Cell lysates were isolated and
caspase 3 activity was measured according to the manufacturer's
protocol. Protein concentrations were determined by the Bradford
method as previously described by Buzdar et al (18). Samples were added to the reaction
mixtures containing colorimetric substrate peptides specific for
caspase 3 N-acetyl-Asp-Glu-Val-Asp-p-nitroanilin (Ac-DEVD-pNA). The
plate was incubated at 37°C for 2, 4, 6 and 8 h. Each sample
containing 200 µg proteins was incubated with caspase 3 substrate,
Ac-DEVD-pNA, in the reaction buffer in a 96-well flat bottomed
microplates. Absorbance was read at 405 nm using a
spectrophotometric microplate reader (Humareader; HUMAN Diagnostics
Worldwide, Wiesbaden, Germany).
DNA constructs
The pCMVHA ubiquitin construct [ubiquitin DNA
sequence ligated into pCMV-hemagglutinin (Ha) vector; Clontech
Laboratories, Inc., Mountain View, CA, USA] and pCMVcMyc ubiquitin
construct (ubiquitin DNA sequence ligated into the pCMV-Myc vector;
Clontech Laboratories, Inc.) were prepared at the Institute of
Chemical Technology (Prague, Czech Republic) by Dr Markéta Landová
and Dr Anna Lounková,. They were allowed to express ubiquitin fused
to either the c-Myc or HA epitope tag for detection with the
appropriate antibodies: mouse monoclonal anti-HA tag antibody (cat.
no. ab18181); mouse monoclonal anti-c-myc antibody (cat. no.
ab11917); dilutions 1:5,000; purchased from Abcam (Cambridge, UK).
The pRK5-HA ubiquitin construct was provided by Addgene, Inc.
(Cambridge, MA, USA), used according to previous studies by Chung
et al (19) and Lim et
al (20). c-Myc and HA have
been previously characterized and are highly immunoreactive tags,
thus are easily detected via western blotting.
Spectrophotometric measurement of
caspase 8/9 activities
The Caspase Glo 8/9 Assay kit was used to measure
caspase 8 and 9 activities according to the manufacturer's protocol
(Promega Corporation). Cells were incubated with complexes 1–4
(1–100 µmol/l) for 60 h. Briefly, 100 µl Caspase Glo™ 8 reagent for
measuring caspase 8 activity, containing specific substrate
Ac-LETD-pNA (N-acetyl-Leu-Glu-Thr-Asp-p-nitroanilin) and 100 µl
Caspase Glo™ 9 reagent for measuring caspase 9 activity, containing
the specific substrate Ac-LEHD-pNA
(N-acetyl-Leu-Glu-His-Asp-p-nitroanilin), were added to the test
tube with 100 µl cell suspension containing 50,000 cells. The cells
were then mixed, and the luminescent signal was measured after 0,
10, 30, 60, 90 and 120 min in the GloMax®-96 Luminometer
(Turner Biosystems, Sunnyvale, CA, USA).
Detection of anti- and proapoptotic
proteins by western blotting
Cells were grown in 6-well microplates and treated
with Cu (II) complexes (IC50 concentration) for
different time periods (24, 48 and 72 h). Subsequent to treatment,
cells were resuspended in lysis buffer and boiled for 3 min at
100°C. Proteins in the cell lysates were separated using 10%
SDS-PAGE and blotted onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.). Subsequent to blocking overnight in 5% milk,
the membranes were incubated with mouse monoclonal anti-cytochrome
c (cyt c; 1:500; cat. no. sc-13560), mouse monoclonal
anti-Bax (1:500; cat. no. sc-7480) or rabbit polyclonal Bcl-2
(1:500; cat. no. sc-492) antibodies (Santa Cruz Biotechnologies,
Inc., Dallas, TX, USA) for 3 h at 4°C. The membranes were then
washed and incubated for 1 h with the appropriate anti-mouse (cat.
no. sc-2005) or anti-rabbit (cat. no. sc-2004) horseradish
peroxidase-conjugated secondary antibody (1:4,000 or 1:5,000
respectively; Santa Cruz Biotechnology, Inc.). Immunoreactive bands
were visualized with SuperSignal West Femto and images were
captured using the Alliance 4.7 UVITEC Imaging system.
Transfection and the proteasome
activity
HT-29 human colon cancer cells and human healthy
HEK-293T cells were seeded into a single 6-well cell culture plate.
Transfection with the aforementioned plasmids was conducted using
FuGene HD reagent (Promega Corporation) according to manufacturer's
instructions. After 4–5 h, the medium was changed and Cu (II)
complexes were added to the final concentration of the
IC50. After 24 h, cells were washed with PBS.
Non-treated cells were used as a negative control and cells treated
with the commercially available inhibitor of proteasome MG132 (2.5
mol/l; Sigma-Aldrich) were used as a positive control.
Statistical analysis
Results are expressed as the mean ± standard
deviation three separate experiments (each experiment was performed
with five replicates). GraphPad Prism 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). The data without deviation from normality were
evaluated using two-way analysis of variance with Tukey test (for
caspase 3) or Dunnett´s T3 (for caspases 8 and 9) as post-hoc
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxicity analysis
Effect of tested Cu (II) complexes (1–4) on
the HT-29 human colon carcinoma, NIH-3T3 mouse noncancerous and the
VH10 human noncancerous fibroblast cell lines was evaluated using
the MTT assay and a direct counting of viable cells by the trypan
blue exclusion assay during 72 h of treatment. The concentration
range of the complexes was 0.001–100 µmol/l.
Table I presents
the effects of different concentrations of Cu (II) complexes on the
tested cell lines. The results of the current study indicate the
cytotoxic effects of Cu (II) complexes on the HT-29 cell line.
IC50 values were reduced with treatment duration.
Complexes 3 and 4 exhibited the highest efficacy against HT-29
cells.
 | Table I.Inhibitory effects of Cu(II) complexes
on the proliferation of human cancer (HT-29) and noncancerous cells
(NIH-3T3 and VH10). |
Table I.
Inhibitory effects of Cu(II) complexes
on the proliferation of human cancer (HT-29) and noncancerous cells
(NIH-3T3 and VH10).
|
| HT-29
(IC50) | NIH-3T3
(IC50) | VH10
(IC50) |
|---|
|
|
|
|
|
|---|
| Complex | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| 1 | 10.00±0.32 | 4.00±0.12 | 1.00±0.05 | 10.00±0.25 | 25.00±0.34 | 0.550±0.001 | >100 | 10.00±0.36 | 18.5±0.10 |
| 2 | 31.00±0.28 | 25±0.42 | 6.00±0.11 | 50.00±0.48 | >100 | 1.00±0.06 | >100 | >100 | 10.00±0.35 |
| 3 | 0.17±0.06 | 13.00±0.25 | 0.007±0.002 | >100 | 5.50±0.34 | 1.50±0.02 | >100 | >100 | 30.50±0.21 |
| 4 | 0.57±0.01 | 0.07±0.01 | 0.17±0.01 | 0.075±0.009 | 30.00±0.48 | 1.50±0.07 | >100 | 34.5±0.24 | 40.0±0.33 |
Of the healthy cell lines (Table I) the most sensitive noncancerous
cells were identified to be NIH-3T3 when compared with VH10. VH10
cells were sensitive only following 48 h of exposure (complexes 1
and 4).
In order to ascertain the influence of Cu (II) ions
and free ligands on cell proliferation,
CuSO4.5H2O, salicylaldehyde and L-glutamic
acid (0.001–100 µmol/l) were additionally tested. All tested
compounds exhibited an IC50>100 µmol/l.
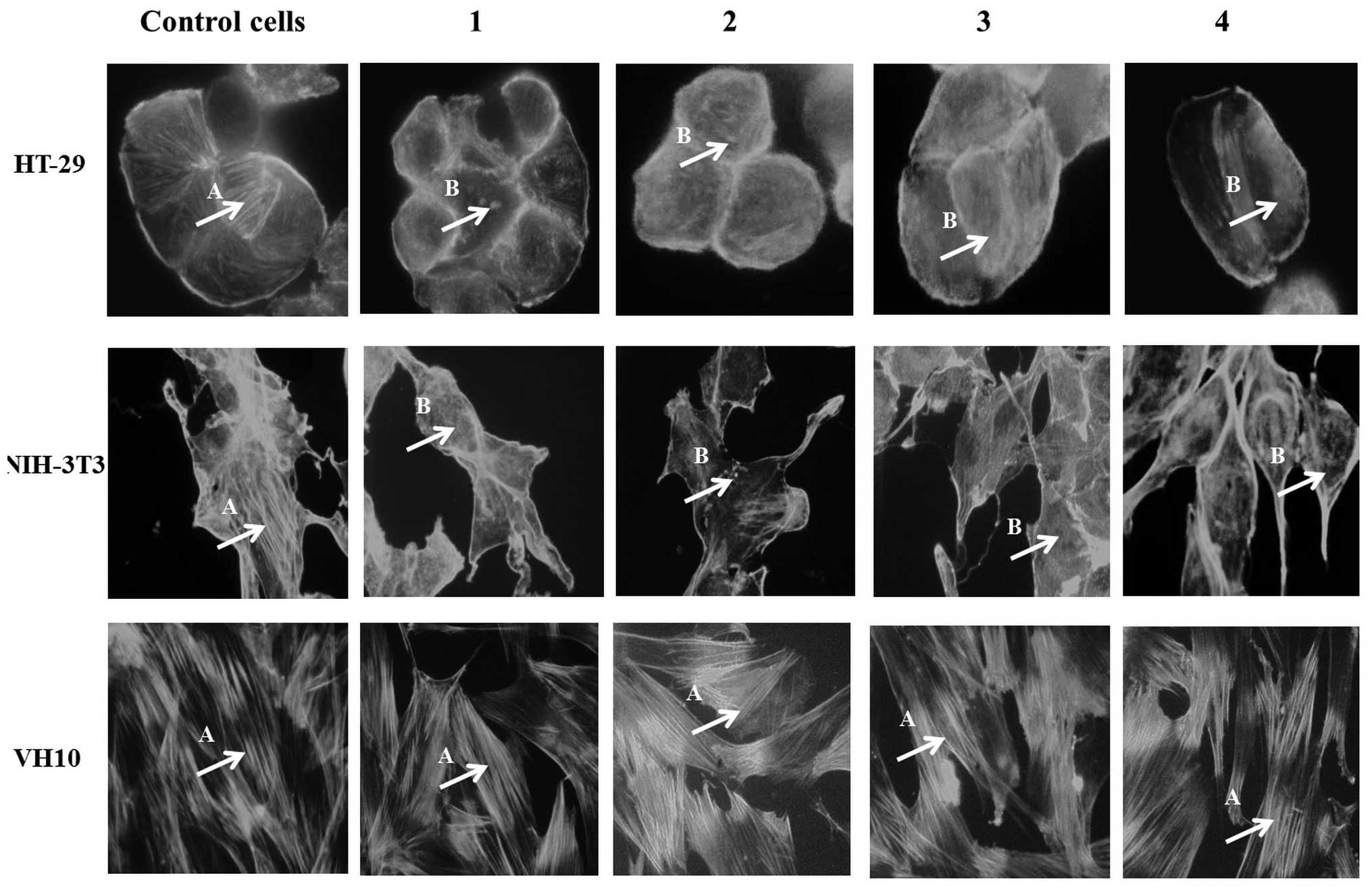
Fluorescence microscopy
Analysis of the morphological alterations of
individual actin filaments was conducted using fluorescence
microscopy to identify a correlation with filament bending
mechanics. In order to investigate the structural alterations of
the F-actin filaments making up the actin cytoskeleton, fluorescent
phalloidine was used to mark the F-actin filaments of the HT-29
human colon carcinoma cells, NIH-3T3 mouse noncancerous fibroblast
cells and VH10 human noncancerous fibroblast cells treated with Cu
(II) complexes at concentrations of IC50 for 24 h (Fig. 1).
In the control non-treated cells, actin filaments
were without morphological changes. On the other hand, subsequent
to treatment with Cu (II) complexes, cytoskeletal alterations were
observed, with breakdown of the actin network. These results
suggested that an IC50 concentration of the Cu (II)
complexes resulted in damage to the cytoskeletal network of cancer
cells and may be associated with a reduction in mobility. However,
the actin fibers of noncancerous cells (NIH-3T3 and VH10) were
comparable with those of control cells (without complexes),
exhibiting no cytoskeletal morphological alterations.
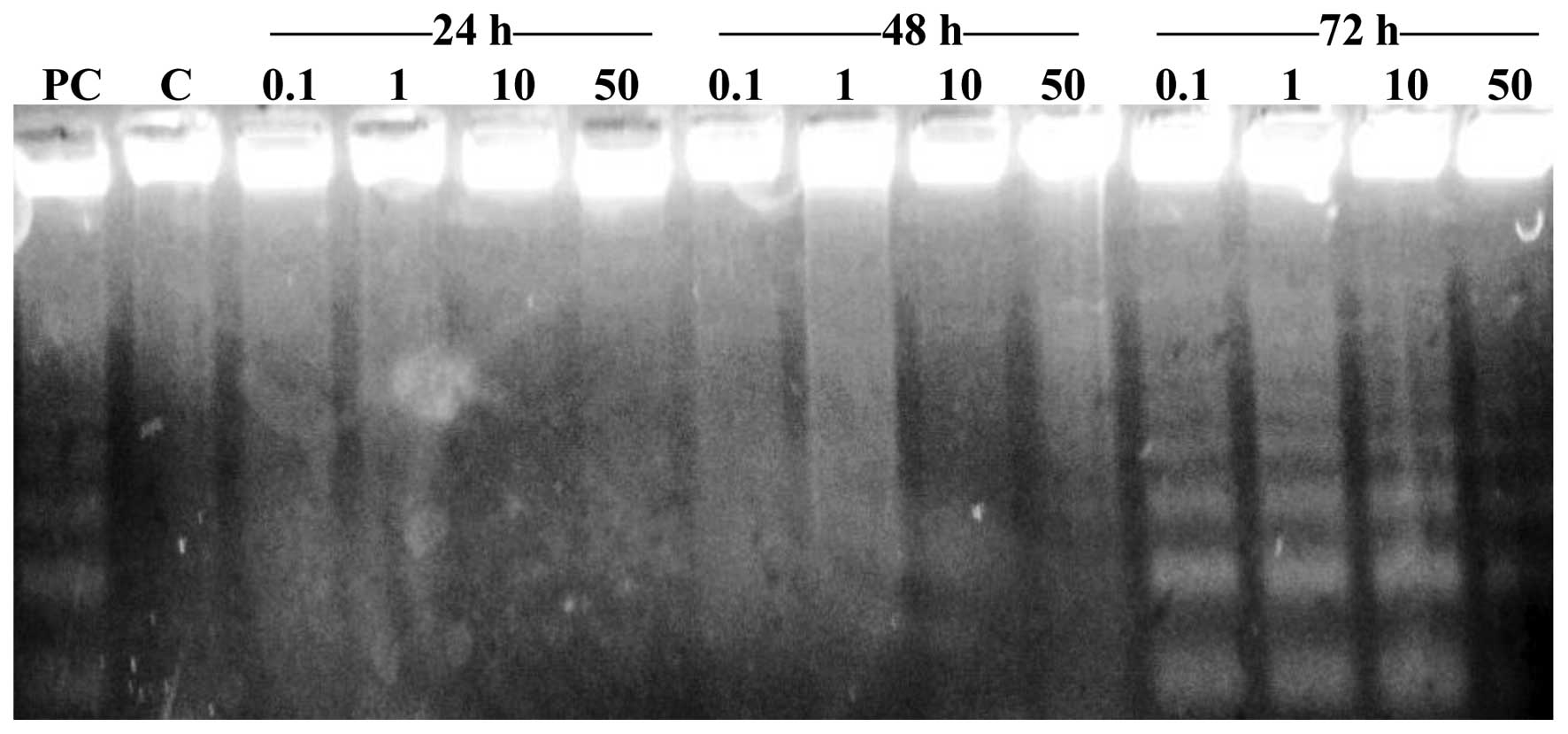
Detection of apoptosis in HT-29
cells
In order to distinguish the type of cell death
induced by Cu (II)-complexes in HT-29 human colon carcinoma cells,
in NIH-3T3 mouse noncancerous fibroblast cells and in VH10 human
noncancerous fibroblast cells (Fig.
2), electrophoretic analysis was used. A total of 4
concentrations of the complexes were incubated with the cells for
72 h. Cell nuclei of HT-29 cells collapsed and disintegrated,
indicating that apoptosis was induced by Cu (II) complexes. In
samples exposed to complexes 1 (0.1, 1, 10 and 50 µmol/l), 2 (1,
10, 50 and 100 µmol/l), 3 (0.01, 1, 10 and 50 µmol/l) and 4 (0.01,
0.1, 1 and 10 µmol/l), apoptosis was identified following 72 h of
incubation.
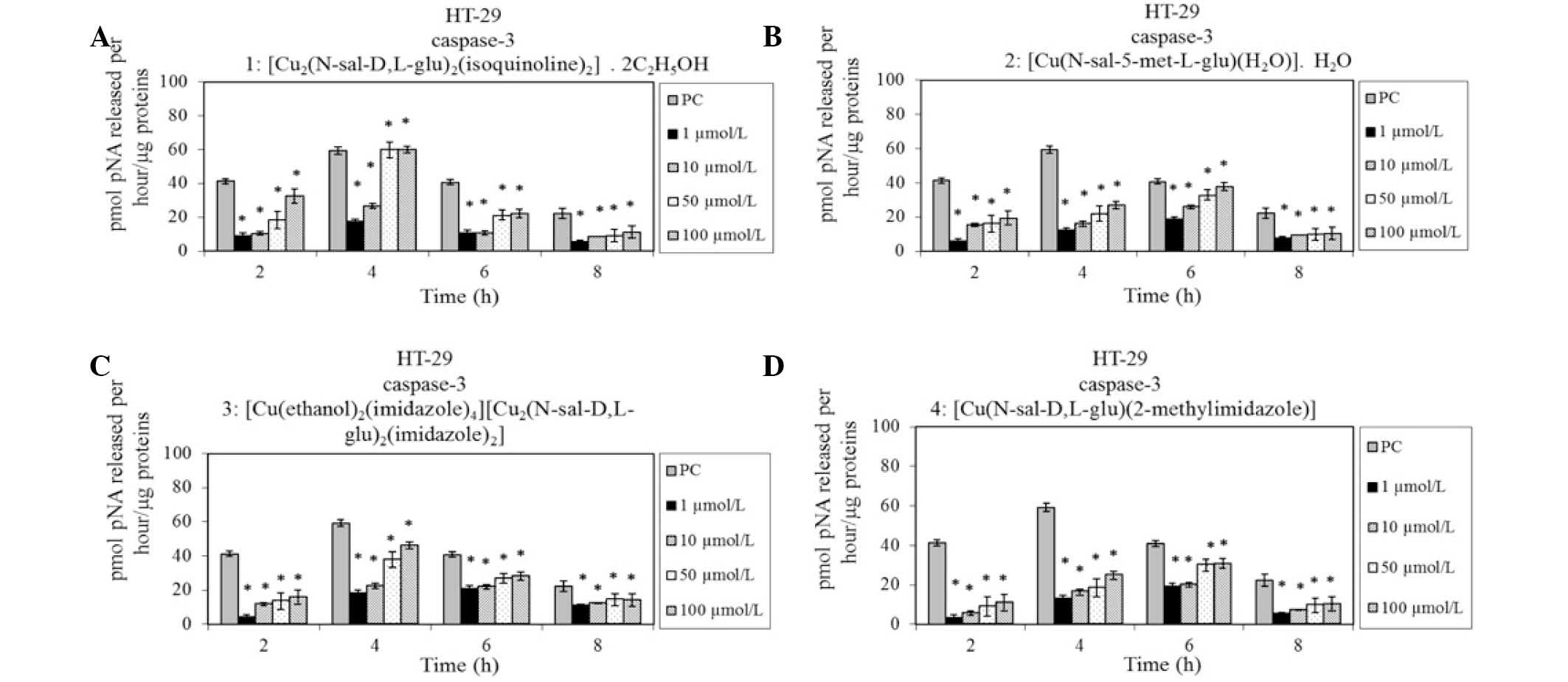
To validate the apoptotic type of death induced by
Cu (II) complexes (0.1–100 µmol/l) in HT-29 human colon carcinoma
cells, caspase 3 activity was examined following 60 h of exposure.
The activity of caspase 3 was measured every 2 h during 8 h
incubation with the complex. It was identified that the Cu (II)
complexes induced an increase in activity of caspase 3 (Fig. 3). As a positive control, L1210
murine leukemia cells influenced with cis-platin were used (6
µmol/l).
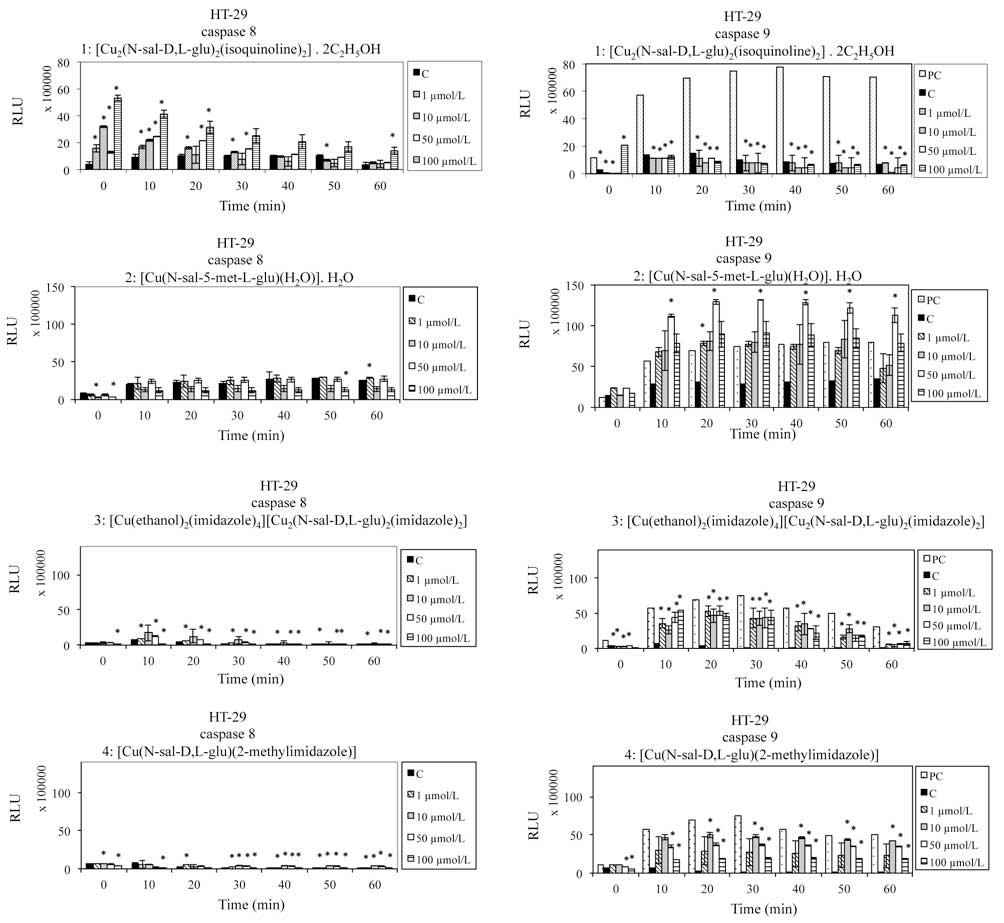
Apoptosis can be triggered through two signaling
pathways, the extrinsic (with active caspase 8) and intrinsic
mitochondria-dependent (with active caspase 9) pathways (21). The aim of the curent study was to
identify which of these pathways is activated by the Cu (II)
complexes. Therefore, the activity of caspase 8/9 was
monitored.
Activity of caspase 8/9 in HT-29
cells. HT-29 human colon carcinoma cells were treated with Cu
(II) complexes (0.1–100 µmol/l) for 60 h. To detect caspase 8/9
activities, a luminometric assay was used. Activation of caspase 9
observed with exposure to complexes 2, 3 and 4 (Fig. 4) and activation of caspase 8 with
exposure to complex 1 (Fig. 4).
The results indicate that complexes 2, 3 and 4 are able to induce
the mitochondria-dependent pathway of apoptosis in HT-29 cells,
while complex 1 activates the extrinsic pathway of apoptosis.
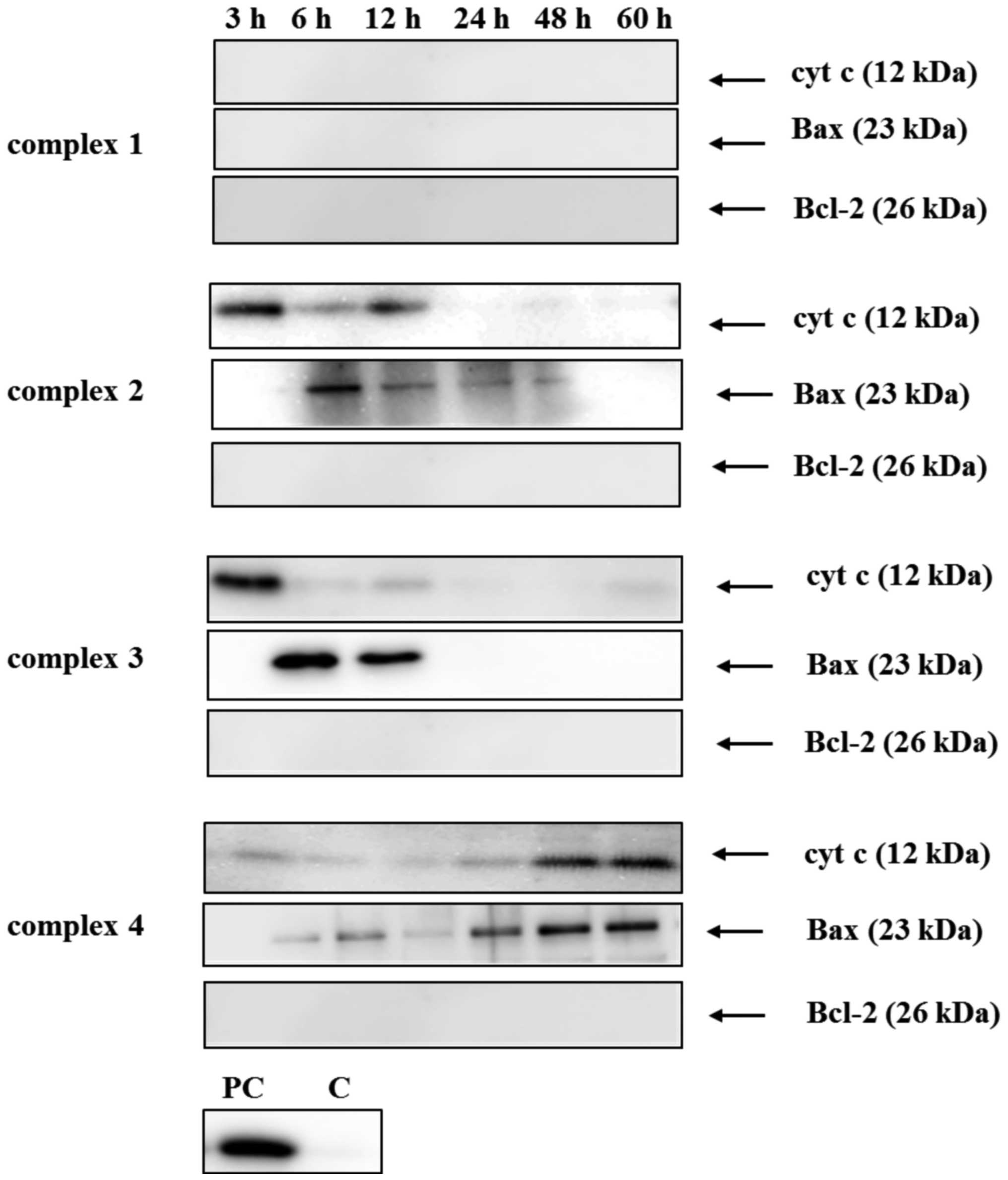
Levels of anti/pro-apoptotic proteins
in HT-29 cells and release of cyt c
To further investigate the mechanism of apoptosis,
the expression levels of Bcl-2 and Bax proteins were measured, and
the release of cyt c was analyzed by western blotting subsequent to
exposure of cells to the Cu (II) complexes (IC50) for 60 h
(Fig. 5). The anti-apoptotic
protein Bcl-2 was not detected in HT-29 cells. In contrast, the
quantity of the pro-apoptotic protein Bax was increased following
treatment with complexes 2, 3 and 4, and this increase was
time-dependent, with the maximum at 6 h (complexes 2 and 3) and 60
h (complex 4). Complex 1 alone did not induce Bax protein
expression. The release of cyt c from mitochondria was also
investigated. The elevation of cyt c levels by complex 4 was
time-dependent with the maximum at 60 h. By contrast, complexes 2
and 3 induced the maximal cyt c release at 3 h-incubation. No cyt c
was detected in HT-29 cells influenced with complex 1, suggesting
that this Cu (II) complex activated the extrinsic pathway of
apoptosis.
Effect of Cu (II) complexes on
proteasome activity
The ubiquitin-proteasome system serves an important
role in cell growth and apoptosis and has been demonstrated as a
novel target for cancer therapy (22). The inhibition of the tumor
proteasomal activity results in the accumulation of ubiquitinated
proteins, the proteasome target proteins p27 and Bax and
ubiquitinated form of inhibitor of κB-α, a natural proteasome
substrate, followed by the induction of apoptosis. The ability of
Cu (II) complexes to inhibit the proteasome was investigated.
Proteasome inhibition was detected by western blot analysis in
HT-29 human colon carcinoma cells (Fig. 6A) and HEK-293T human healthy
embryonal cells (Fig. 6B). Cells
were transfected with plasmid DNA (pCMVHA ubiquitin) and treated
with the Cu (II) complexes at the IC50 concentration (µmol/l) for
24 h. No ubiquitin bands (10 kDa) were detected in the samples, and
were not detected in the sample containing the commercially used
inhibitor of proteasome MG132 (Fig.
6A). Therefore, alternative DNA plasmids (pCMVcMyc ubiquitin
and pRK-5HA Ubiquitin) were used, however the results were the
same. In order to examine whether the plasmid DNAs used were
correct, healthy embryonic HEK-293T were transfected with the first
plasmid DNA construct (pCMVHA ubiquitin) and these cells were
treated with Cu (II) complexes under the same conditions as for the
HT-29 cells. Fig. 6B indicates
that Cu (II) complexes inhibited the proteasome, and a ubiquitin
band (10 kDa) was detecte in all samples (1–4)
containing Cu (II) complexes. Ubiquitin levels in the HEK-293T
cells were comparable with cells incubated with the commercially
used inhibitor of proteasome MG132 (line C2). The results suggest
that HT-29 cells are not suitable for transfection with the DNA
constructs used in the current experiment.
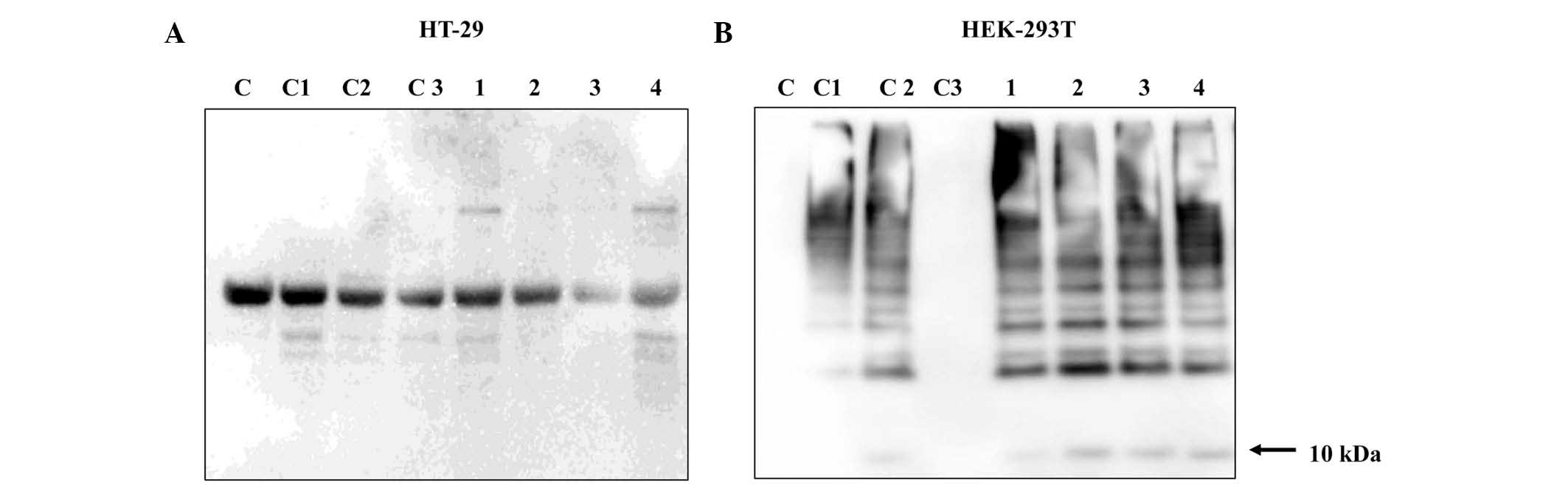 | Figure 6.Detection of proteasome inhibition in
(A) HT-29 and (B) HEK-293T cells by western blot analysis
subsequent to treatment with Cu (II) complexes at the
IC50 concentration for 24 h. The cell groupings were as
follows: C, no transfection, not treated with the commercial
inhibitor MG123 or with Cu (II) complexes; C1, underwent
transfection, not treated with the commercial inhibitor MG123 or
with Cu (II) complexes; C2, underwent transfection, treated
commercial inhibitor MG123, not treated with Cu (II) complexes; C3,
cells without transfection, treated with the commercial inhibitor
MG123, not treated with Cu (II) complexes; 1, 2, 3 and 4, underwent
transfection, treated with Cu (II) complexes at the IC50
concentration for 24 h. |
Discussion
A total of 4 Schiff base Cu (II) complexes were
investigated in HT-29 human colon carcinoma cells and VH10 and
NIH-3T3 noncancerous cells. The most effective Cu (II) complex
against HT-29 cells (with an imidazole ligand in its structure) had
an IC50 of 0.007 µmol/l (0.008 µg/ml) subsequent to 72 h
incubation with cells. In order to improve understanding of the
biological effects of novel potential anticancer drugs, it is
important to study the molecular mechanisms involved. Two types of
caspases have been reported to serve key roles in mediating
apoptosis: Initiator caspases, caspases 2, 8, 9 and 10; and
effector caspases, caspases 3, 6 and 7. Activation of initiator
caspases is required to activate specific effector caspases that
subsequently proteolytically degrade a host of intracellular
proteins to mediate the cell death program/apoptosis. In the
current study, the types of apoptotic pathway activated by tested
Schiff base Cu (II) complexes were reported. The only complex
inducing the extrinsic apoptotic pathway was complex 1, which
contains isoquinoline as a ligand. The remaining complexes
activated the intrinsic (mitochondrial) pathway of apoptosis.
Rajalakshmi et al (23) tested two copper (II) complexes
([Cu(bitpy)2](ClO4)2•2H2O;
[Cu(bitpy)(phen)](NO3)2•3H2O). It
was identified that these two complexes (10 µmol/l) activated the
intrinsic pathway of apoptosis in NIH-3T3 and MG63 cells. Thati
et al (24) additionally
identified that the intrinsic pathway of apoptosis was activated in
A498 and HepG2 cells as a result of exposure to the Cu (II) complex
bis [phenanthroline-4-methylcoumarin-6,7-dioxacetatocopper (II)] at
concentrations of 12.5 and 25 µmol/l, however not at the lower
concentration (6.25 µmol/l). They detected increased activities of
caspase 3 and 9 following 24 h incubation (24).
An additional Cu (II) complex
[Cu(BMA)Cl2]•(CH3OH) [BMA=N, N'-bis
(benzimidazol-2-yl-methyl) amine] also activated apoptosis through
the intrinsic (mitochondrial) pathway by activation of caspases 9
and 3 subsequent to its 48 h incubation with HeLa cells. Enzyme
activities were increased in a dose-dependent manner (25).
The current study identifed that complex 1 alone,
with the isoquinoline ligand, activated the extrinsic pathway of
apoptosis. All other Cu (II) complexes tested in the present study
activated the intrinsic pathway of apoptosis. The intrinsic pathway
of apoptosis results in mitochondrial dysfunction, and mitochondria
are semi-autonomous organelles that serve essential roles in
cellular metabolism and programmed cell death pathways (26).
Major resistance mechanisms of mitochondria that are
regulated by Bcl-2 family proteins and potential strategies to
circumvent the resistance have additionally been examined (27). In the current study, it was
identified that Cu (II) complexes inhibited the release of Bcl-2
(antiapoptotic) protein and induced the release of Bax
(proapoptotic) protein. Liu et al (28) exposed GBM cells to disulfiran plus
Cu (II) (1 µmol/l) for 48 h and observed the release of Bax
protein.
Proliferation and apoptosis pathways are tightly
regulated in a cell by the ubiquitin-proteasome system (UPS).
Alterations in the UPS may result in cellular transformation or
additional pathological conditions. A previous study suggested that
Cu (II) complexes can inhibit proteasome activity and induce
apoptosis in certain human cancer cells (29). However, using the cancer cell line
HT-29, the current study was unable to confirm these results.
Schiff base Cu (II) complexes were able to suppress
tumor cell growth via the direct inhibition of proteosomal
activity. Zhang et al (30)
studied the complex
[Cu(tssb)(phen)H2O]•C2H5OH•0.5H2O
(H2tssb=Schiff base derived from salicylaldehyde and
taurine, phen=1,10-phenanthroline) and confirmed that it inhibits
the activity of purified 20S and 26S proteasome in human breast
cancer MDA-MB-231 and leukemia Jurkat T cells.
A study by Xiao et al (31) used a newly synthesized
L-glutamine-containing copper complex
(L-glutamine-o-vanillin-copper) on tumor cells. This copper complex
had proteasome-inhibitory activity in human breast cancer and
leukemia cells (31).
With an increased understanding of the intrinsic and
extrinsic pathways of apoptosis in recent years, novel approaches
of targeting the apoptotic pathways have been tested in
pre-clinical and clinical models (32). The results of the current study,
together with those from previous studies suggest that Cu (II)
complexes containing Schiff bases have potential as novel
anticancer drugs.
Acknowledgements
The current study was conducted with the financial
support of the Ministry of Education (grant no. VEGA 1/0752/13),
the Operational Programme Prague (grant no. CZ.2.16/3.1.00/24503),
NPU I (LO1601 grant no. MSMT-43760/2015). We wish to thank to Ľuboš
Kuračka for his assistance with figure preparations.
References
|
1
|
Nielsen DL, Palshof JA, Larsen FO, Jensen
BV and Pfeiffer P: A systematic review of salvage therapy to
patients with metastatic colorectal cancer previously treated with
fluorouracil, oxaliplatin and irinotecan +/− targeted therapy.
Cancer Treat Rev. 40:701–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Funakoshi T, Latif A and Galsky MD: Safety
and efficacy of addition of VEGFR and EGFR-family oral
small-molecule tyrosine kinase inhibitors to cytotoxic chemotherapy
in solid cancers: A systematic review and meta-analysis of
randomized controlled trials. Cancer Treat Rev. 40:636–647. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lyman GH, Abella E and Pettengell R: Risk
factors for febrile neutropenia among patients with cancer
receiving chemotherapy: A systematic review. Crit Rev Oncol
Hematol. 90:190–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miltenburg NC and Boogerd W:
Chemotherapy-induced neuropathy: A comprehensive survey. Cancer
Treat Rev. 40:872–882. 2004. View Article : Google Scholar
|
|
5
|
Damia G and Garattini S: The
pharmacological point of view of resistance to therapy in tumors.
Cancer Treat Rev. 40:909–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zayed EM, Zayed MA and El-Desawy M:
Preparation and structure investigation of novel Schiff bases using
spectroscopic, thermal analyses and molecular orbital calculations
and studying their biological activities. Spectrochim Acta A Mol
Biomol Spectrosc. 134:155–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emam SM, Sayed IETE and Nassar N:
Transition metal complexes of neocryptolepine analogues. Part I:
Synthesis, spectroscopic characterization and in vitro anticancer
activity of copper(II) complexes. Spectrochim Acta A Mol Biomol
Spectrosc. 138:942–953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santini C, Pellei M, Gandin V, Porchia M,
Tisato F and Marzano C: Advances in copper complexes as anticancer
agents. Chem Rev. 114:815–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Creaven BS, Duff B, Egan DA, Kavanaghc K,
Rosaird G, Thangella VR and Walsh M: Anticancer and antifungal
activity of copper(II) complexes of quinolin-2(1H)-one-derived
Schiff bases. Inorganica Chim Acta. 363:4048–4058. 2010. View Article : Google Scholar
|
|
10
|
Khoo TJ, Break MK, Crouse KA, Tahirc MIM,
Alid AM, Cowleye AR, Watkine DJ and Tarafderf MTH: Synthesis,
characterization and biological activity of two Schiff base ligands
and their nickel (II), copper(II), zinc(II) and cadmium(II)
complexes derived from S-4-picolyldithiocarbazate and X-ray crystal
structure of cadmium (II) complex derived from
pyridine-2-carboxaldehyde. Inorganica Chim Acta. 413:68–76. 2014.
View Article : Google Scholar
|
|
11
|
Fei BL, Xua WS, Tao HW, Li W, Zhang Y,
Long JY, Liu QB, Xia B and Sun WY: Effects of copper ions on DNA
binding and cytotoxic activity of a chiral salicylidene Schiff
base. J Photochem Photobiol B. 132:36–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakao Y, Sakurai K and Nakahara A:
Copper(II) chelates of Schiff bases derived from salicylaldehyde
and various α-amino acids. Bull Chem Soc Jpn. 40:1536–1538. 1967.
View Article : Google Scholar
|
|
13
|
Krätsmár-Šmogrovič J, Pavelčík F,
Soldánová J, Sivýa J, Seressová V and Žemlička M: The crystal and
molecular structure and properties of diaqua
(N-salicylidene-L-glutamato)copper (II) monohydrate. Z Naturforsch.
46b:1323–1327. 1991.
|
|
14
|
Langer V, Gyepesová D, Kohútová M and
Valent A: Dimeric (isoquinoline) (N-salicylidene-D,
L-glutamato)copper(II) ethanol solvate. Acta Cryst. C65:208–210.
2009.
|
|
15
|
Langer V, Gyepesová D, Scholtzová E, Mach
P, Kohútová M, Valent A and Smrčok Ľ: Crystal and electronic
structure of aqua(N-salicylidene-methylester-L-glutamato)Cu(II)
monohydrate. Z Kristallogr. 219:112–116. 2004.
|
|
16
|
Langer V, Mach P, Gyepesová D, Andrezálová
L and Kohútová M: X-ray and DFT studies of a mono- and binuclear
copper (II) ionic compound containing a schiff base. Acta Cryst.
C68:M326–M328. 2012.
|
|
17
|
Langer V, Scholtzová E, Gyepesová D,
Kohútová M and Valent A: (N-salicylidene-D, L-glutamato)
(2-methylimidazole)copper(II). Acta Cryst. E60:129–132. 2004.
|
|
18
|
Buzdar AU, Marcus C, Smith TL and
Blumenschen GR: Early and delayed clinical cardiotoxicity of
doxorubicin. Cancer. 55:2761–2765. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang
H, Gao J, Ross CA, Dawson VL and Dawson TM: Parkin ubiquitinates
the alpha-synuclein-interacting protein, synphilin-1: Implications
for lewy-body formation in parkinson disease. Nat Med. 7:1144–1150.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim KL, Chew KC, Tan JM, Wang C, Chung KK,
Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, et al: Parkin
mediates nonclassical, proteasomal-independent ubiquitination of
synphilin-1: Implications for lewy body formation. J Neurosci.
25:2002–2009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho YT, Lu CC, Yang JS, Chiang JH, Li TC,
Ip SW, Hsia TC, Liao CL, Lin JG, Wood WG and Chung JG: Berberine
induced apoptosis via promoting the expression of Caspase 8, −9 and
−3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anti Res. 29:4063–4070.
2009.
|
|
22
|
Xiao Y, Chen D, Zhang X, Cui Q, Fan Y, Bi
C and Dou QP: Molecular study on copper-mediated tumor proteasome
inhibition and cell death. Int J Oncol. 37:81–87. 2010.PubMed/NCBI
|
|
23
|
Rajalakshmi S, Kiran MS and Nair BU: DNA
condensation by copper(II) complexes and their anti-proliferative
effect on cancerous and normal fibroblast cells. Eur J Med Chem.
80:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thati B, Noble A and Creaven SBS:
Apoptotic cell death: A possible key event in mediating the in
vitro anti-proliferative effect of a novel copper(II) complex,
[Cu(4-Mecdoa)(phen)2] (phen=phenanthroline,
4-Mecdoa=4-methylcoumarin-6,7-dioxactetate), in human malignant
cancer cells. Europ J Pharm. 569:16–28. 2007. View Article : Google Scholar
|
|
25
|
Qiao X, Ma ZY, Shao J, Bao WG, Xu JY,
Qiang ZY and Lou JS: Biological evaluation of a cytotoxic
2-substituted benzimidazole copper (II) complex: DNA damage,
antiproliferation and apoptotic induction activity in human
cervical cancer cells. Biometals. 27:155–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barbosa IA, Machado NG, Skildum AJ, Scott
PM and Oliveira PJ: Mitochondrial remodeling in cancer metabolism
and survival: Potential for new therapies. Biochim Biophys Acta.
1826:238–254. 2012.PubMed/NCBI
|
|
27
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu P, Brown S, Goktug T, Channathodiyil
P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL,
Darling JL and Wang W: Cytotoxic effect of disulfiram/copper on
human glioblastoma cell lines and ALDH-positive cancer-stem-like
cells. Br J Cancer. 107:1488–1497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuo J, Bi C, Fan Y, Buac D, Nardon C,
Daniel KG and Dou QP: Cellular and computational studies of
proteasome inhibition and apoptosis induction in human cancer cells
by amino acid schiff base-copper complexes. J Inorg Biochem.
118:83–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Bi C, Fan Y, Cui Q, Chen D, Xiao
Y and Dou QP: Induction of tumor cell apoptosis by taurine schiff
base copper complex is associated with the inhibition of
proteasomal activity. Int J Mol Med. 22:677–682. 2008.PubMed/NCBI
|
|
31
|
Xiao Y, Bi C, Fan Y, Cui C, Zhang X and
Dou QP: L-glutamine schiff base copper complex as a proteasome
inhibitor and an apoptosis inducer in human cancer cells. Int J
Oncol. 33:1037–1079. 2008.PubMed/NCBI
|
|
32
|
Khan KH, Codesido MB and Molife LR: Cancer
therapeutics: Targeting the apoptotic pathway. Crit Rev Oncol
Hematol. 90:200–219. 2014. View Article : Google Scholar : PubMed/NCBI
|