Introduction
Approximately 80% of the aging population suffer
from lower back pain at some point in their lives (1–3).
Back pain associated with intervertebral disc degeneration (IDD)
clearly affects quality of life and work productivity, and
significantly impacts health care spending (4). However, the currently used first-line
treatments, including activity modification, analgesic and
anti-inflammatory medications, physical therapy and surgery only
relieve symptoms temporarily and do not target repair or
deceleration of the underlying degenerative progress (5,6).
Alternative and less invasive treatment methods such as gene
therapy are being developed, aiming to slow or reverse the
degenerative process of IDD.
Developing approaches to stimulating regeneration of
disc tissue or slowing the degeneration progression requires an
understanding of the causes of the degeneration. Previous studies
have demonstrated that the number of nucleus pulposus (NP) cells
reduces and the composition of the extracellular matrix associated
with these cells is altered in degenerative discs (7–9).
In vitro and in vivo studies have suggested that the
cellular loss attributed to the excessive apoptosis of disc cells
serves an important role in disc degeneration (10,11).
These mechanisms provide direction and fundamental information for
gene therapy.
Survivin protein, a baculoviral inhibitor of
apoptosis, serves an important role in the regulation of mitosis
progression and apoptosis inhibition (12). In osteoarthritis and rheumatoid
arthritis, survivin stimulates chondrocyte proliferation and
inhibits apoptosis (13,14). Furthermore, preliminary studies
have indicated that survivin is expressed in fetal disc tissue, and
that there is a differential expression of survivin between
degenerated NP tissue and comparatively normal NP tissue (15,16).
In a previous study, it was demonstrated that survivin is
re-expressed in disc degeneration disease and is required for
degenerated NP cell proliferation and anti-apoptosis in
vitro (17). Thus, it is
suggested that the use of survivin as a target gene for gene
therapy for IDD may be efficacious. However, to the best of our
knowledge, at present no studies investigating gene therapy with
survivin via a lentiviral vector (LV) and the course of
intervertebral disc degeneration in vivo have been
conducted.
The present study investigated the use of gene
therapy in improving IDD by administering LV-survivin into disc
cells. This validated IDD model was used to determine whether
treatment with LV-survivin can ameliorate the course of NP cell
apoptosis. The results provided information for gene therapy to
decelerate IDD in future clinical application.
Materials and methods
Materials
A total of 15 skeletally mature female New Zealand
White rabbits (3-months-old, weighing 2–2.5 kg; Chongqing Kangda
Juxin Rabbit Co., Ltd., Qingdao, China) were randomly divided into
three groups: Group A, punctured blank control group (degeneration
and placebo injection, n=5); group B, punctured empty vector
control group (degeneration and an empty LV injection, n=5); and
group C, treatment group (degeneration and LV-survivin injection,
n=5). LV-survivin and empty LV were purchased from Genechem Co.,
Ltd. (Shanghai, China). The titers of LV-survivin and empty LV were
2×108 and 1×109 TU/ml, respectively. The
Animal Care and Use Committee of the Affiliated Hospital of Qingdao
University (Shandong, China), approved all animal experimental
protocols, which followed the principles expressed in the National
Institute of Health Guide. The rabbits were maintained separately
at room temperature with 16:8 h light:dark cycle, and were fed with
rabbit feed (Qingdao Kangda Foodstuffs Co., Ltd., Qingdao,
China).
Puncture surgery
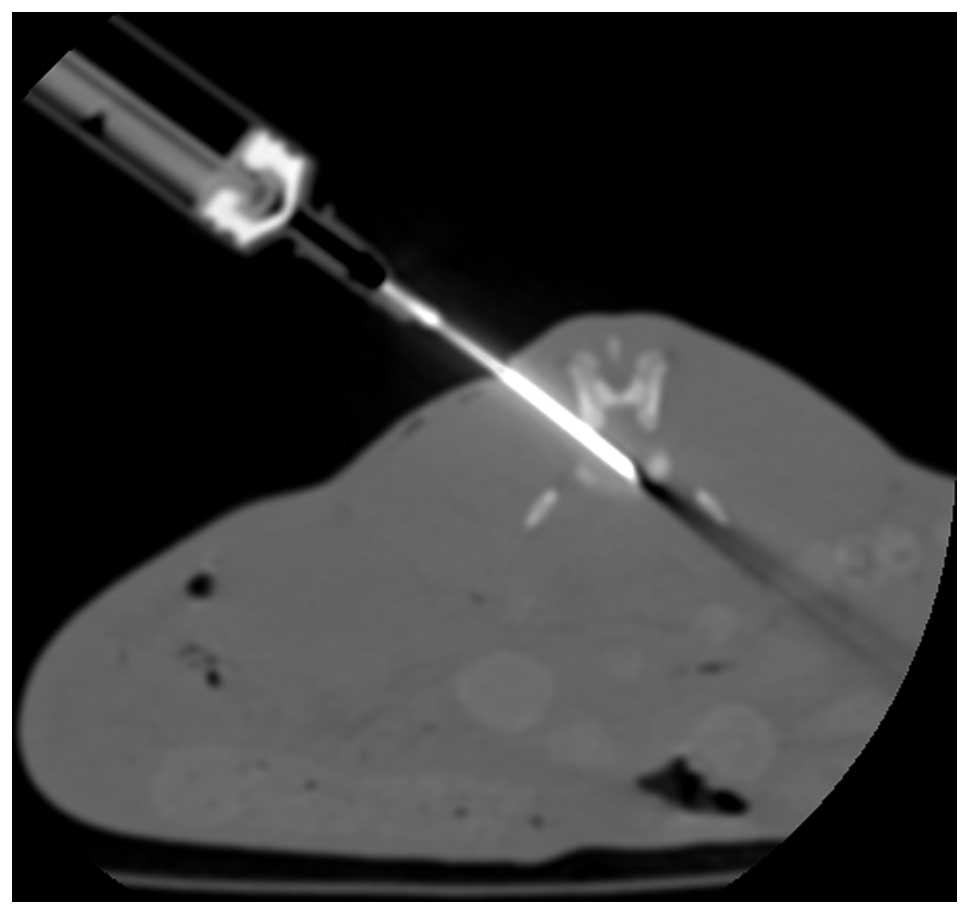
Computed tomography (CT)-guided percutaneous needle
puncture technology was used to establish this model (18). Magnetic resonance imaging (MRI) and
histology highlighted alterations that resembled the hallmarks of
human disc degeneration. In brief, rabbits were anesthetized with
Su Mian Xin II (0.15 ml/kg intramuscularly; Kangda Medical Products
Co., Ltd., Shanghai, China) and atropine sulfate (0.05 mg/kg
intramuscularly; Jiangsu Lianshui Pharmaceutical Co., Ltd.,
Lianshui, China), the position of the target discs (L3-L4 and
L4-/L5) were identified using CT, and then a 16-gauge needle was
inserted toward the center of the disc, with CT guidance. The
needle pinpoint was confirmed to be positioned in the disc center
by CT (Fig. 1). Postoperatively,
the rabbits were housed in individual cages and injected
intramuscularly with penicillin at 80×105 U to prevent
infection.
Injection surgery
At 3 weeks post-puncture surgery, groups A, B and C
were injected with equivalent phosphate-buffered saline (PBS; 50
µl), empty LV (5×106/50 µl; Genechem Co., Ltd.,
Shanghai, China), and LV-survivin (5×106/50 µl; Genechem
Co., Ltd.), respectively, using CT-guided percutaneous needle
injection technology. This time point was selected due to the fact
that it is the earliest that MRI could identify changes of
degeneration with this model (18). An 100 µl microinjector (Hamilton
Bonaduz AG, Bonaduz, Switzerland) was used for therapeutic
injections into the center of the NP.
MRI
MRIs were obtained at time-point 0 (prior to
puncture), 3 weeks post-puncture (prior to injection), and 12 weeks
post-puncture (prior to sacrifice) for all rabbits. A 3-T standard
human knee coil was used to obtain T2-weighted images (repetition
time=2120 ms, echo time=113 ms, slice thickness=0.6 mm). The
rabbits were anesthetized and placed in the knee coil in the supine
position.
Histological processing
All rabbits were sacrificed by air injection into
the ear vein 12 weeks subsequent to the initial puncture surgery,
following the final MRI scan. Immediately after sacrifice, spines
were dissected out en bloc. The L3-L4 discs (n=5 per group)
were prepared for histology. Two discs from each group were fixed
and then dehydrated using a histology tissue processor and embedded
in paraffin. Next, the discs were sectioned at a thickness of 5 µm
in the coronal plane. The sections were stained with hematoxylin
and eosin (H&E; Beijing Leagene Biotechnology Co., Ltd.,
Beijing, China). The other discs were embedded in Tissue-Tek™
(Sakura, CA, USA) and then sectioned at a thickness of 5 µm in the
coronal plane in a freezing microtome (Leica CM1950; Leica
Microsystems GmbH, Wetzlar, Germany). The sections were stained for
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL; Nanjing KeyGEN Biotech Co., Ltd., Nanjing, China) and were
imaged using an Olympus BX51 microscope (Olympus Corporation,
Tokyo, Japan).
Gene expression and protein content of
survivin
A total of 3 L4-L5 discs from each group (n=5 per
group) were prepared for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis. mRNA
was extracted from NP tissue using TRIzol™ (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and the absorbance at
was measured at 260 and 280 nm for quantification and quality
control. A total of 1 µg mRNA was reverse transcribed to cDNA using
PrimeScript™ RT reagent kit (cat no. DRR037A; Takara Bio, Inc.,
Otsu, Japan), and the reaction product was treated with RNAse-free
DNase I. PCR was conducted using the cycling conditions
(LightCycler 480 Instrument II) as stated by the manufacturer's
instructions. Primers were designed and purchased from Shanghai
Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). Another specific primer pair for
human GAPDH was used as an internal control. In each experiment,
samples were analyzed in duplicate. The normalized target gene
expression was determined through the comparative Cq method (ΔΔCq
method) (19).
 | Table I.Details of nucleotide sequences of
sense and antisense primers, RT-qPCR amplification product. |
Table I.
Details of nucleotide sequences of
sense and antisense primers, RT-qPCR amplification product.
| Gene | Primer | PCR product (bp) |
|---|
| Survivin | F:
CAGATGACGACCCCATAGAGGA | 141 bp |
|
| R:
CCTTTGCAATTTTGTTCTTGGC |
| GAPDH | F:
GGATTTGGTCGTATTGGG | 205 bp |
|
| R:
GGAAGATGGTGATGGGATT |
|
NP tissues were washed with ice-cold PBS, weighed
and then ground using a pestle and mortar. Lysis was performed on
ice for 45 min using 200 µl radioimmunoprecipitation assay buffer
with 2 µl phenylmethanesulfonylfluoride for every 100 µg of tissue.
Subsequently, the lysis solution was centrifuged at 5,700 ×
g at 4°C for 20 min. For the western blot analysis of
survivin, GAPDH was used as an internal control, and proteins were
resolved through sodium dodecyl sulfate-polyacrylamide gel
electrophoresis using a 10% gel, prior to transferring onto
Immobilon P membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% fat-free dried milk and probed with
a monoclonal rabbit anti-survivin antibody (1:1,000; ab76424;
Abcam, Cambridge, MA) and anti-GAPDH antibody (1:2,000; ab9485;
Abcam) for 8 h at 4°C. Subsequent to incubation with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; CW0103;
Beijing Kangwei Century Biotechnology Co., Ltd., Beijing, China)
for 4 h at room temperature, the positive bands were visualized
using chemiluminescence (Pierce Biotechnology, Inc., Rockford, IL,
USA).
Cell culture and caspase-3 activity
assay
The remaining L4-L5 discs were prepared for
measuring caspase-3 activity. NP cells were cultured as described
in a previous study (20). In
brief, the NP tissue was cut into small pieces (~1 mm2)
and then digested with 0.25% trypsinase (HyClone™; GE Healthcare
Life Sciences, Logan, UT, USA) at 37°C under gentle agitation for
20 min. Subsequently, 0.5% collagenase type II (MP Biomedicals,
LLC, Santa Ana, CA, USA) was used at 37°C for approximately 4 h.
The cells were transferred to a 12.5 cm2 culture flask
at a density of 105 cells/cm2. The cells were
cultured in a CO2 incubator (SANYO Electric Co., Ltd.,
Osaka, Japan) at 37°C with humidity, and grown in Dulbecco's
modified Eagle's medium (DMEM)-F12 containing 15% fetal calf serum.
The experiments were analyzed in duplicate. For ischemic
conditions, NP cells were cultured in DMEM culture medium (glucose
deprivation) in a CO2 incubator at 37°C with 1% oxygen
and 95% humidity. These cells were subsequently used for analyzing
caspase-3 activity. The caspase-3 activity was measured using the
Caspase-3 Colorimetric Assay kit (BioVision, Inc., Milpitas, CA,
USA). The NP cells were counted and pelleted at 1.5×106
cells. The cells were then re-suspended in cell lysis buffer, and
50 µl 2X reaction buffer (containing 10 mM DTT) and 5 µl of
DEVE-pNA were added. The samples were incubated for 90 min at 37°C
and their optical densities were read at 405 nm using a microtiter
plate reader (Sunrise™; Tecan Group, Ltd., Männedorf,
Switzerland).
Statistical analysis
All values were presented as the mean ± standard
error. Student's t-test and one-way analysis of variance
with a post hoc Fisher's least significant difference test were
applied to measure the statistical significance of the differences.
Statistical analyses were performed using SPSS software for Windows
(version 19; SPSS, Inc., Chicago, IL, USA).
Results
Imaging
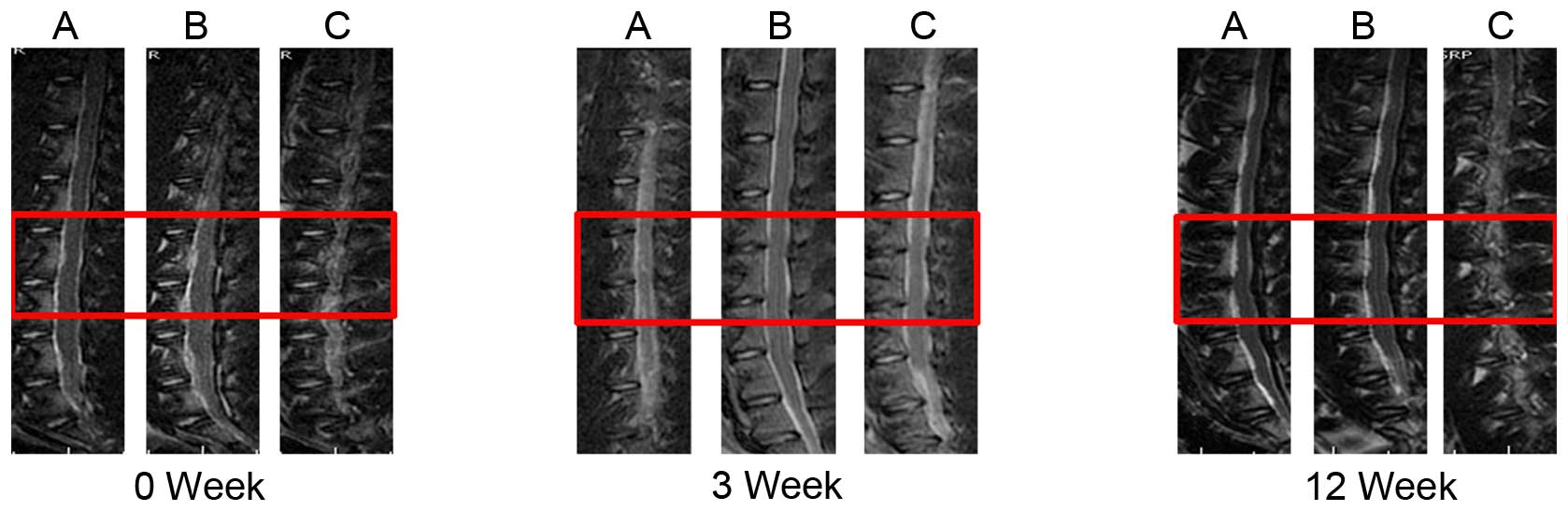
The T2-weighted midsagittal MRI scans of groups A
and B were darkened and reduced in area from 0–12 weeks, consistent
with the degeneration. Group C demonstrated reduced qualitative
evidence of degeneration when compared with groups A and B; treated
NPs did not darken and reduce in area as much as the punctured
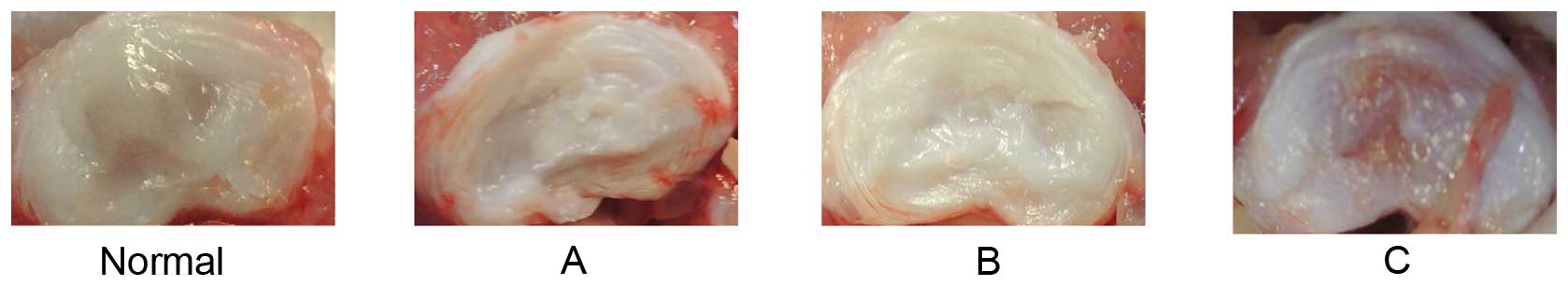
discs (Fig. 2). The morphology of
normal discs indicated an abundance of water and ECM. Discs from
groups A and B were reduced in the quantity of both water and ECM,
and exhibited fibrotic morphology, consistent with the degree of
degeneration. However, the quantity of water and ECM in group C
were between that of the normal group (group A) and the punctured
group (group B) (Fig. 3).
Gene expression and protein content of
survivin
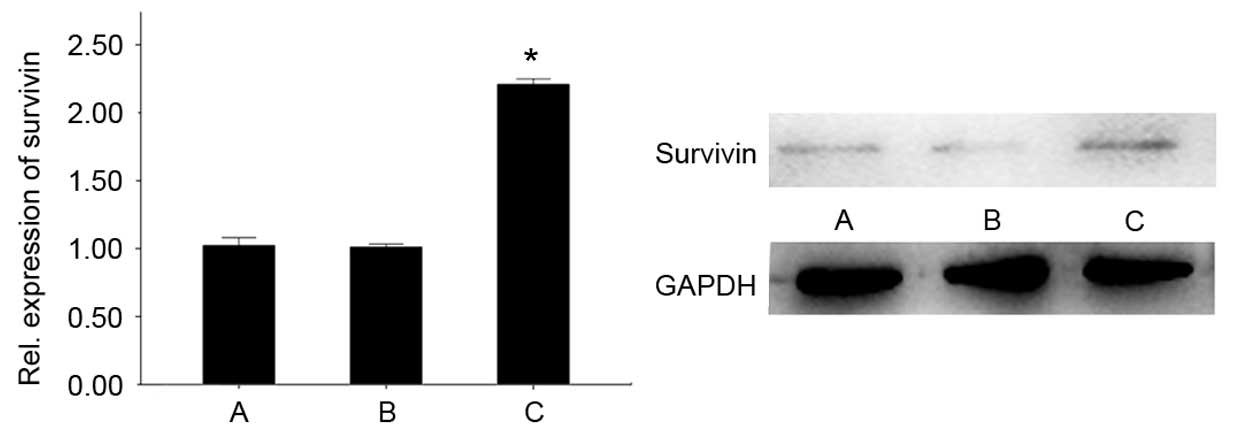
The gene and protein content of each group were
analyzed by RT-qPCR and western blot analysis. Gene expression and
protein content of survivin in group C demonstrated a significant
increase compared with groups A and B (P<0.05). No significant
difference was observed between groups A and B (Fig. 4).
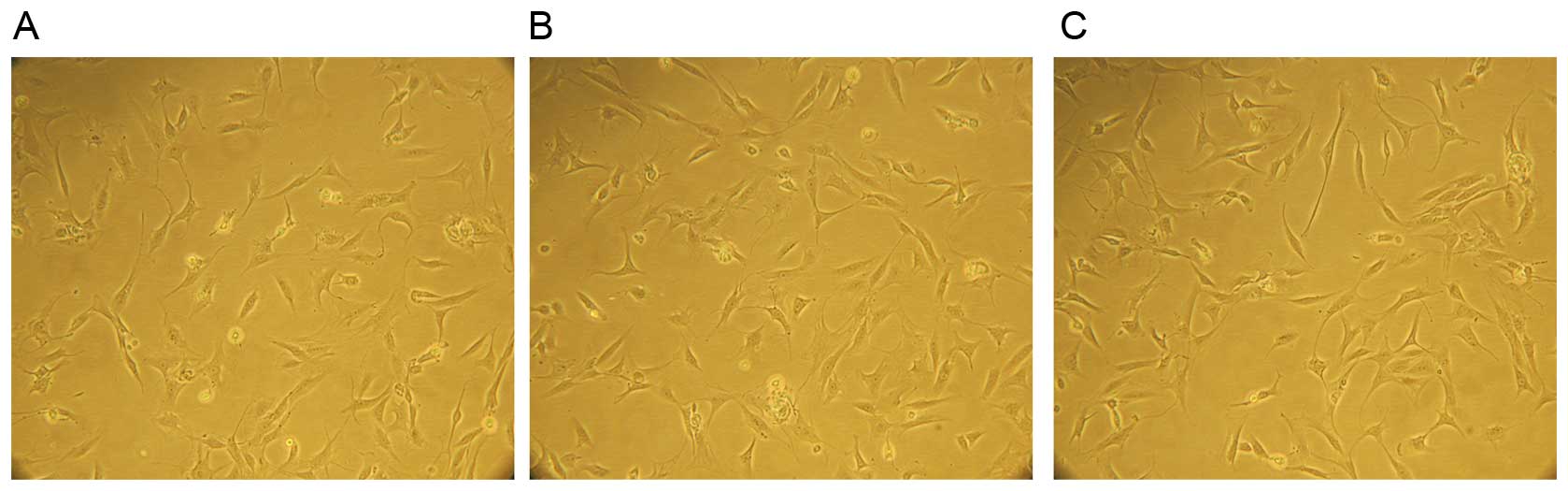
The morphology of NP cells
The NP cells were attached to the culture dish after
3–5 days of culture. The cell morphology gradually elongated and
became triangular or polygonal, and the cytoplasm became plump and
equally distributed, and the attached cells exponentially
increased. Subsequently, 10–15 days later, 90% of the cells formed
colonies. No significant differences of cell morphology were
observed among the three groups (Fig.
5).
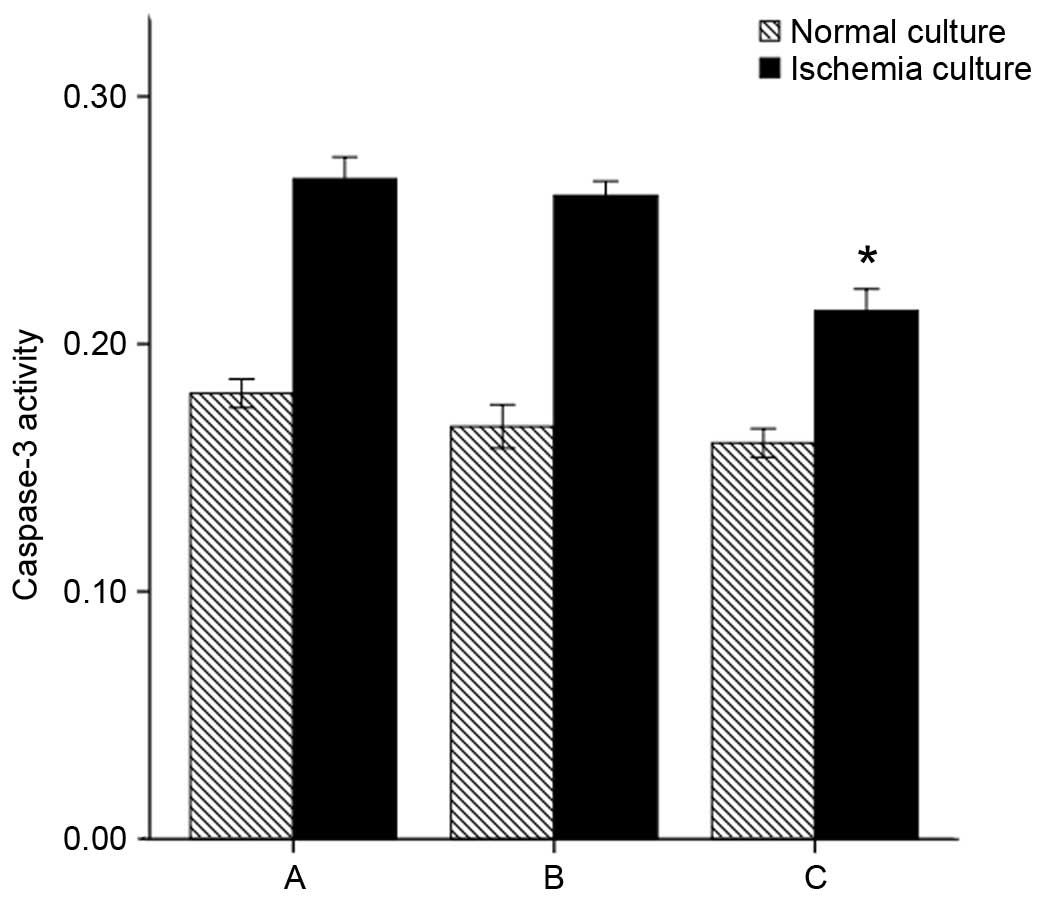
Caspase-3 activity reflected apoptosis
of NP cells
The caspase-3 activity of NP cells was assayed.
Under the normal culture conditions, group C did not exhibit
significantly altered caspase-3 activity compared with groups A and
B (P=0.082 and P=0.539, respectively). When exposed to ischemia
in vitro (1% oxygen, glucose deprivation), the caspase-3
activity of all groups increased compared with the normal culture
conditions (P=0.00). However, caspase-3 activity remained
significantly reduced overall for group C (group C + in
vitro ischemia vs. group A + in vitro ischemia and group
B + in vitro ischemia: P=0.00 and P=0.01, respectively)
(Fig. 6).
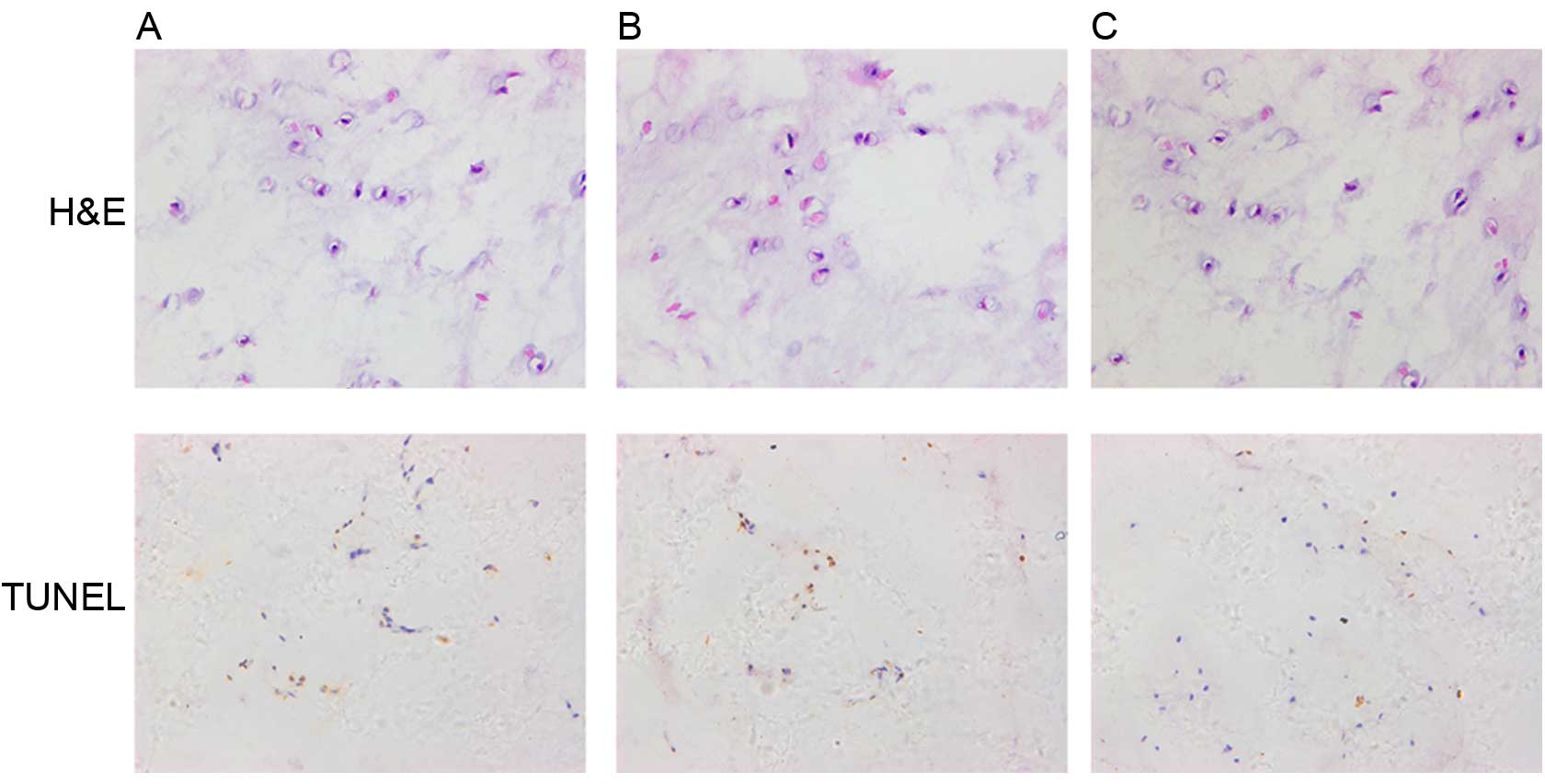
Histological staining
The histological analysis of NP tissues was
performed using H&E staining. Under the highest magnification,
the number of NP cells in group C was significantly increased
compared with groups A and B (Fig.
7).
Apotosis analysis of NP tissues was performed using
TUNEL staining. The TUNEL-positive NP cells (brown cells) in group
C were markedly reduced compared with groups A and B (Fig. 7).
Discussion
The current study demonstrated that LV-survivin
injection into the intervertebral discs may slow the process of IDD
in vivo. Results from MRI, histology, gene expression,
protein content and apoptosis analyses all demonstrated a slowing
of the course of injury-induced degeneration.
The degeneration model was established using
CT-guided percutaneous needle puncture technology. Although an
injury model for disc degeneration does not truly reflect the
complex biochemical cascade of human degenerative disc disease, it
does suggest that LV-survivin may be a possible treatment method to
ameliorate the process of injury-induced degeneration. Similarly,
injection surgery was accomplished with CT guidance; however, a
microinjector with a needle small enough not to exacerbate further
degeneration was used (21).
Furthermore, 3 weeks subsequent to the puncture, there was an
alteration in the NP visible by MRI, and the degeneration was in an
early phase. In the phase, the cellular state might be able to
mount a healing response for LV injection, so this time point was
chosen for the LV injection (22).
In the current study, the MRI imaging of all groups
at the 0 week time point exhibited normal intervertebral disc
morphology. A total of 3 weeks after the puncture, the imaging of
all groups demonstrated the same degree of degeneration. A total of
12 weeks after the puncture, treated NPs did not darken and reduce
in area as much as the punctured discs. The anatomical morphology
of the discs following sacrifice were consistent with that of MRI
imaging. These results indicated that LV-survivin injection aided
in the preservation of the intervertebral discs.
The gene expression and protein content of survivin
in NP tissues of group C were significantly increased compared with
that of groups A and B. These results indicated that there was a
stable expression of survivin in NP cells following in vivo
LV-survivin injection into the rabbits. Thus, based on the effect
and function of survivin, a reduction in caspase-3 activity and
apoptosis of NP cells, and an increase in the number of NP cells
was detected, compared with the punctured control groups. This
observation may elucidate the effect of LV-survivin gene
transfection on the deceleration of the degeneration process in
vivo.
Caspase-3 activity was analyzed using the caspase-3
activity assay, and no alteration between the caspase-3 activities
of groups A, B and C was observed in the unstressed NP cells.
However, in in vitro ischemiic cultures (1% oxygen, glucose
deprivation), the caspase-3 activity of the NP cells significantly
increased compared with unstressed NP cells, and the increase of
group C caspase-3 activity was statistically reduced compared with
that of groups A and B. These results were consistent with a
previous study (17), and
indicated that ischemiic culture conditions induced NP cell
apoptosis, and that overexpression of survivin may contribute in
the reduction of caspase-3 activity for the anti-apoptotic function
in the degeneration process.
In order to identify NP cell numbers and apoptotic
alterations occurring in the different groups, H&E staining and
TUNEL were used. These results indicated that LV injection with
survivin slowed NP cell apoptosis according to the results of
caspase-3 activity, and similarly demonstrated that overexpression
of survivin may serve a role under ischemic conditions in
vivo or vitro.
Furthermore, in the primary culture of NP cells, no
significant different of cell morphology were observed among the
three groups. The results were different from the previous studies,
with a previous study reporting that in vitro, the
morphology of degenerative NP cell subsequent to transfection with
LV-survivin was significantly changed (23). The difference may have resulted
from the different transfection conditions. The in vitro
condition of NP cells is complex, and numerous factors and pathways
influence each other, thus the regulation of cell morphology is
complicated.
Although the current study identified a number of
statistically significant observations, there were limitations. All
imaging, gene expression, protein content, apoptosis and histology
results demonstrated that the LV injection with survivin had a weak
effect on slowing degeneration.
It is also possible that the 12-week course of the
present study may have been insufficient to demonstrate the full
therapeutic effect in the degenerative cascade. This may be due to
the fact that in the model used, degeneration has been demonstrated
to continue through to a minimum of 24 weeks (24), therefore longer time points may
have demonstrated more statistically significant alterations in
signal intensity between the treated and punctured discs.
The present study demonstrated, to the best of our
knowledge, for the first time that LV injection may slow disc
degeneration. Although the results may not necessarily translate to
human degenerative disc disease, and the results are preliminary
compared with clinical treatment, they provide a potential
direction for gene therapy as a treatment for disc
degeneration.
Acknowledgements
The current study was supported by a research grant
award from the National Natural Science Foundation of China (grant
no. 81371998).
References
|
1
|
Hoy D, Brooks P, Blyth F and Buchbinder R:
The Epidemiology of low back pain. Best Pract Res Clin Rheumatol.
24:769–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Aoki Y and Ohtori S:
Resolving discogenic pain. Eur Spine J. 17:(Suppl 4). S428–S431.
2008. View Article : Google Scholar
|
|
3
|
Hillman M, Wright A, Rajaratnam G, Tennant
A and Chamberlain MA: Prevalence of low back pain in the community:
Implications for service provision in Bradford, UK. J Epidemiol
Community Health. 50:347–352. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McMeeken J, Tully E, Stillman B, Nattrass
C, Bygott IL and Story I: The experience of back pain in young
Australians. Man Ther. 6:213–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fritzell P, Hägg O, Wessberg P and
Nordwall A: Swedish Lumbar Spine Study Group: 2001 Volvo award
winner in clinical studies: Lumbar fusion versus nonsurgical
treatment for chronic low back pain: A multicenter randomized
controlled trial from the Swedish Lumbar Spine Study Group. Spine
(Phila Pa 1976). 26:2521–2534; discussion 2532–2534. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park P, Garton HJ, Gala VC, Hoff JT and
McGillicuddy JE: Adjacent segment disease after lumbar or
lumbosacral fusion: Review of the literature. Spine (Phila Pa
1976). 29:1938–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith LJ, Nerurkar NL, Choi KS, Harfe BD
and Elliott DM: Degeneration and regeneration of the intervertebral
disc: Lessons from development. Dis Model Mech. 4:31–41. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rannou F, Lee TS, Zhou RH, Chin J, Lotz
JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A and Shyy JY:
Intervertebral disc degeneration: The role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao CQ, Liu D, Li H, Jiang LS and Dai LY:
Interleukin-1beta enhances the effect of serum deprivation on rat
annular cell apoptosis. Apoptosis. 12:2155–2161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F and Ling X: Survivin study: An update
of ‘what is the next wave’? J Cell Physiol. 208:476–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lechler P, Balakrishnan S, Schaumburger J,
Grässel S, Baier C, Grifka J, Straub RH and Renkawitz T: The
oncofetal gene survivin is re-expressed in osteoarthritis and is
required for chondrocyte proliferation in vitro. BMC Musculoskelet
Disord. 12:1502011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bokarewa M, Tarkowski A and Magnusson M:
Pathological survivin expression links viral infections with
pathogenesis of erosive rheumatoid arthritis. Scand J Immunol.
66:192–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang KS, Yue B and Ma XX: The expression
of survivin and its significance in fetal intervertebral disc.
Qingdao Daxue Yixueyuan Xuebao. 49:205–206. 2013.
|
|
16
|
Yang KS: The expression of survivin and
its significance inintervertebral disc (dissertation). Qingdao
University; 2013
|
|
17
|
Lin Yazhou Yue, Bin Xiang Hongfei, et al:
Survivin is re-expressed in disc degeneration disease and is
required for degenerated nucleus pulposus cell proliferation and
anti-apoptosis in vitro. Mol Med Rep. (In press).
|
|
18
|
Zhou RP, Zhang ZM, Wang L, Huang MJ, Zheng
XC, Cui YN, Yin M, Wang XK, Yao NZ, Chen TY, et al: Establishing a
disc degeneration model using computed tomography-guided
percutaneous puncture technique in the rabbit. J Surg Res.
181:e65–e74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kluba T, Niemeyer T, Gaissmaier C and
Gründer T: Human anulus fibrosis and nucleus pulposus cells of the
intervertebral disc: Effect of degeneration and culture system on
cell phenotype. Spine (Phila Pa 1976). 30:2743–2748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elliott DM, Yerramalli CS, Beckstein JI,
Johannessen W and Vresilovic EJ: The effect of relative needle
diameter in puncture and sham injection animal models of
degeneration. Spine (Phila Pa 1976). 33:588–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwon YJ: A minimally invasive rabbit model
of progressive and reproducible disc degeneration confirmed by
radiology, gene expression, and histology. J Korean Neurosurg Soc.
53:323–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Lin Y, Yang K, Yue B, Xiang H and
Chen B: Effect of lentivirus-mediated survivin transfection on the
morphology and apoptosis of nucleus pulposus cells derived from
degenerative human disc in vitro. Int J Mol Med. 36:186–194.
2015.PubMed/NCBI
|
|
24
|
Sowa G, Westrick E, Pacek C, Coelho P,
Patel D, Vadala G, Georgescu H, Vo N, Studer R and Kang J: In vitro
and in vivo testing of a novel regulatory system for gene therapy
for intervertebral disc degeneration. Spine. 36:E623–E628. 2011.
View Article : Google Scholar : PubMed/NCBI
|