Introduction
In mammals, the SLIT-ROBO-GTPase activating protein
(srGAP) family consists of four members, srGAP1, 2, 3 and 4. These
proteins were identified as the downstream regulators of the Slit
and Robo receptor system and are important in numerous
developmental processes in diverse cell types (1–3).
During neural development, srGAP1, 2 and 3 are widely expressed in
the nervous system and function as multifunctional adaptor proteins
involved in neuronal migration, neuronal morphogenesis, neurite
outgrowth and synaptic plasticity (1,3–8).
srGAP1 binds to the CC3 conserved cytoplasmic domain
of Robo1 and mediates the blocking effect of Slit on neural
progenitor migration via inactivated Ras homologue gene family,
member A and cell division control protein 42 homolog (Cdc42), but
not Ras-related C3 botulinum toxin substrate 1 (Rac1) (3). srGAP3 regulates Rac1 and Cdc42 and is
involved in neuronal morphogenesis and neurite outgrowth (3,9). By
contrast, srGAP2 negatively regulates cortical neuronal migration
via its IF-BAR domain and promotes neurite outgrowth and branching
(7). In addition, the srGAP2 gene
has recently been implicated in a severe neurodevelopmental
syndrome resulting in infantile epileptic encephalopathy, and
srGAP2 knockout mice are prone to epileptic seizures. Similarly,
the srGAP3 gene has been associated with mental retardation, and
the knockout mice develop lethal hydrocephalus or
‘schizophrenia-associated’ behaviors (6,10–13).
The underlying mechanisms that are involved in the differences
between the various srGAPs remain to be elucidated.
NSCs/NPCs are a group of undifferentiated
mulitpotent cells which give rise to three predominant types of
cells in the nervous system (14–15).
They act as valuable tools for the study of neural development.
During development, NSCs/NPCs develop different cellular
morphologies whilst retaining their basic properties. Our previous
findings have demonstrated that the knockdown of srGAP3 attenuated
NSC/NPC survival, proliferation and differentiation and arrested
their morphological alteration in vitro (16). The influence of srGAP2 on NSCs/NPCs
development requires further elucidation.
As srGAP2 was expressed in the developmental rat
brain until the 14th postnatal day (17), the present study hypothesizes that,
similarly to srGAP3, srGAP2 may exert its effect on neural
development via alteration of NSC/NPC proliferation and
differentiation. No direct association has yet been indicated
between the expression of srGAP2 and the differentiation or
morphological maturation of NSCs/NPCs. In addition, the expression
pattern of srGAP2 during postnatal brain development changed
dynamically (17). The altered
expression in the cytoplasm and nuclei may be associated with its
particular function over time. In the present study, the expression
of endogenous srGAP2 in NSCs/NPCs during differentiation in
vitro was detected, and the proportion of srGAP2 positive cells
within the differentiated cell population was analyzed, to
elucidate the possible association between the dynamic expression
of srGAP2 and the differentiation of NSCs/NPCs.
Materials and methods
Brian tissue preparation
Six male Sprague-Dawley rats (weight, 250±15 g) were
purchased from the Experimental Animal Center, Xi'an Jiaotong
University College of Medicine (Xi'an, China). The environment was
controlled with a 12:12-h light/dark cycle, 45–65% humidity, and
room temperature of 20±2°C, and the rats had access to food and
water ad libitum. All procedures involving animals conformed
to the ethical guidelines of the National Institutes of Health
(NIH) Guide for the Care and Use of Laboratory Animals (NIH
publication no. 85–23, revised 1996), and those set out by the
Xi'an Jiaotong University. Rats were fixed by trans-cardiac
perfusion with 4% paraformaldehyde (PFA) in 0.1 M
phosphate-buffered saline (PBS) under anesthesia by intraperitoneal
injection of 10% (w/v) chloral hydrate solution (300 mg/kg). Brains
were dissected from the skull and then post-fixed with 4% PFA at
4°C overnight. Following gradient elution by sucrose solutions,
brain tissue was cut in 15 µm sections for immunohistochemical
staining.
Culture of rat embryonic
NSCs/NPCs
NSCs were isolated from the cerebral cortex of rat
embryos on embryonic day 14 and cultured in serum-free growth
medium following the protocol of Gage et al (18) and optimized in our laboratory
(19). NSC/NPC growth medium
contained Dulbecco's modified Eagle's medium/nutrient mixture F-12
(DMEM/F12), 10 ng/ml basic fibroblast growth factor, 20 ng/ml
epidermal growth factor, 100 U/ml penicillin, 100 µg/ml
streptomycin, 1% N-2, and 2% B-27 supplement (all from Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 0.4 IU/ml
heparin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Cells
were sub-cultured at 5–7 days in vitro (DIV). Upon passage,
spheres were trypsinized and mechanically triturated into single
cells and replaced at 1×105 cells/ml and cultured in growth medium
as mentioned above.
Induced differentiation of NSCs/NPCs
in vitro
Serum was used to induce the spontaneous
differentiation of NSCs/NPCs in vitro. Following passage, a
sample of 5,000 NSCs/NPCs was suspended in 500 µl differentiation
medium that contained DMEM/F12 with 1% fetal bovine serum
(Invitrogen; ThermoFisher Scientific, Inc.). Cells were cultured on
poly-L-lysine coated glass coverslips in 24-well plates and cell
differentiation was observed at different time points. Glass
coverslips with cells were washed with 0.01 M PBS at 1, 3, 7 and 14
days, respectively, followed by fixation with 4% PFA for 30 min at
room temperature.
Immunocytochemistry staining
Immunocytochemistry staining was performed following
the standard protocol (19).
Monoclonal antibodies (EMD Millipore; Billerica, MA, USA) including
mouse anti-nestin (1:200; cat. no. MAB353), mouse anti-β-tubulin
III (1:200; cat. no. MAB5564), mouse anti-glial fibrillary acidic
protein (GFAP; 1:500; cat. no. MAB360) and mouse
anti-oligodendrocytes (1:2,000; cat. no. MAB1580) were used to
identify NSCs, neurons and astrocytes, respectively. Polyclonal
rabbit anti-srGAP2 (1:50) (17)
was raised in Dr Jin's laboratory and used to perform double
staining with nestin, β-tubulin III and GFAP. Primary antibodies
were diluted in 0.01 M PBS. Blocking solution contains 10% normal
goat serum (CWBIO, Beijing, China) and 0.3% Triton X-100 in PBS.
Tetramethylrhodamine and fluorescein isothiocyanate-conjugated goat
anti-mouse IgG/anti-rabbit IgG (1:400; CWBIO; cat. nos. CW0152S and
CW0113S) were used as secondary antibodies. Cell nuclei were
counterstained with DAPI-containing mounting media (Vector
Laboratories, Inc., Burlingame, CA, USA) and visualized under a
fluorescent microscope (Olympus BX57; Olympus Corporation, Tokyo,
Japan) equipped with a DP70 digital camera and the DPManager
(DPController) software (Olympus Corporation). For the negative
control, the primary antibody was replaced by 0.01 M PBS.
Quantification and statistical
analysis
Cell counting was performed using a 20x objective
lens. Immunoreactive cells from 6 random fields (2 cover slips, 3
fields from each coverslip) were counted. All the quantitative data
were obtained from three independent experiments. They were
presented as the mean ± standard error of the mean, and the
statistical analysis was performed with SPSS 13.0 software (SPSS,
Inc. Chicago, IL, USA). A one-way analysis of variance followed by
Tukey's test was used and P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of srGAP2 in the
subventricular zone (SVZ) in vivo
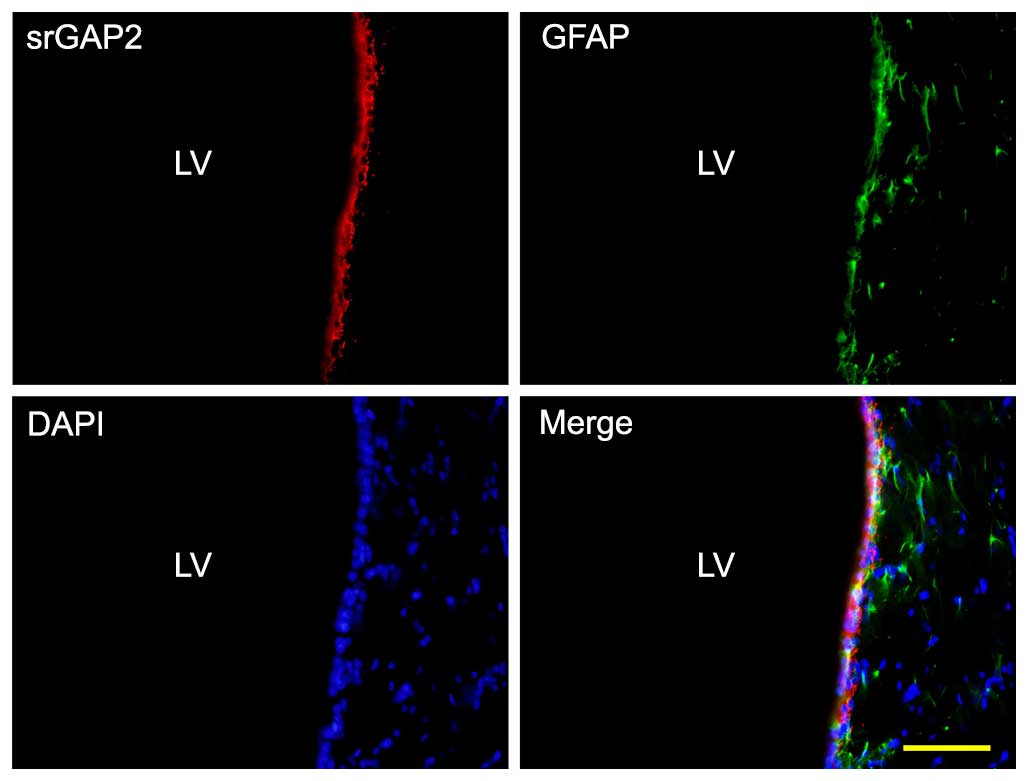
NSCs/NPCs are distributed in the SVZ and can be
stained by GFAP. Double staining immunohistochemistry was used to
identify srGAP2 expression in NSCs/NPCs in the SVZ. The results
demonstrated that endogenous srGAP2 is detectable in the
ventricular zone (VZ)/SVZ of the adult brain and that it
co-localizes with GFAP+ cells in vivo (Fig. 1).
Culture and identification of rat
embryonic NSCs
Cells were isolated from the cerebral cortex of rat
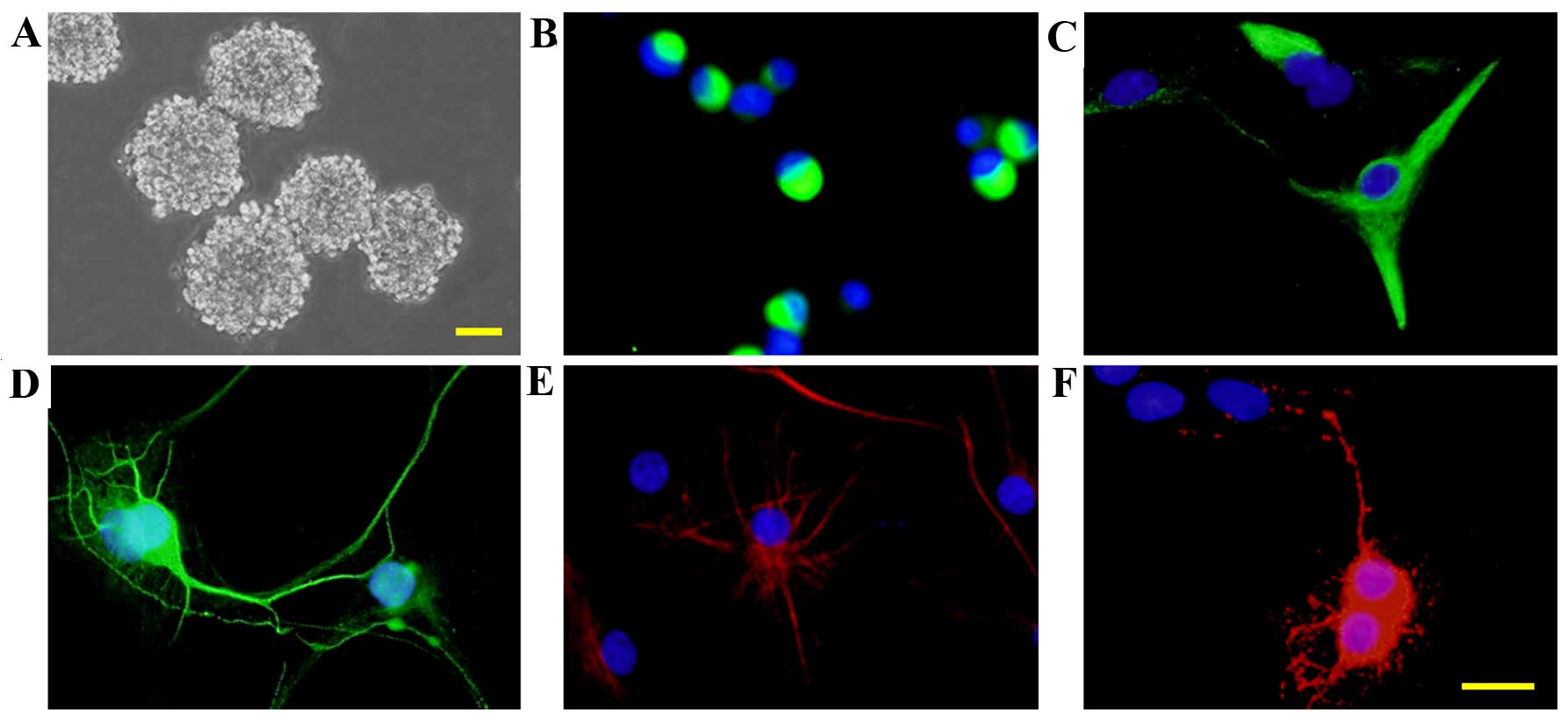
embryos and cultured in standard growth medium. Neurospheres were
observed at 5 DIV (Fig. 2A) and
immunocytochemistry staining indicated that the majority of the
cells were nestin+ (Fig. 2B).
After 7 days culturing in a differentiation medium, β-tubulin III+
neurons (Fig. 2D), GFAP+
astrocytes (Fig. 2E) and
oligodendrocytes+ oligodendrocytes (Fig. 2F) were detected. However a few of
the cells did remain nestin+ (Fig.
2C). The data suggests that the cells cultured were
NSCs/NPCs.
Dynamic expression of srGAP2 during in
vitro differentiation of NSCs/NPCs
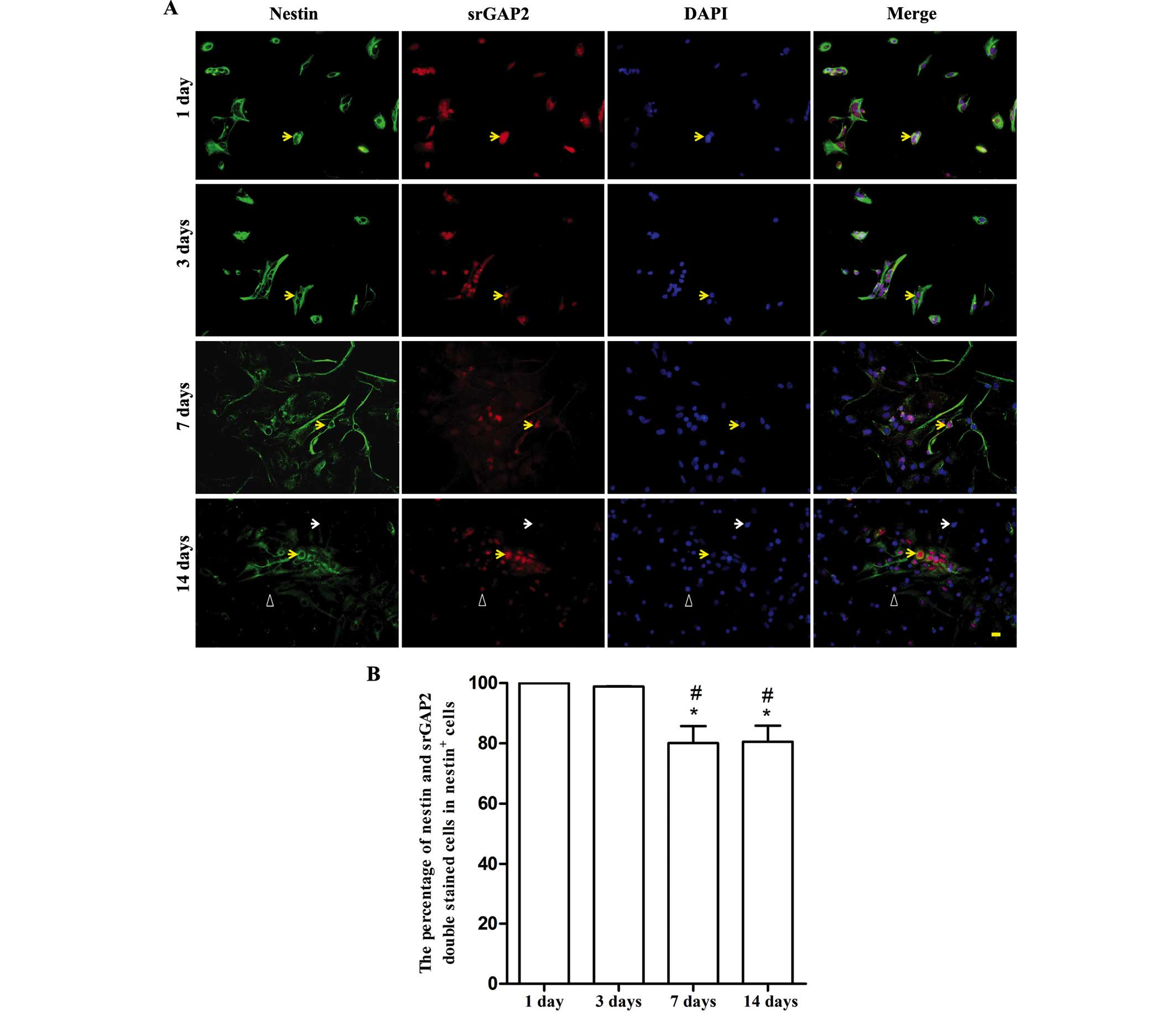
With the spontaneous differentiation of NSCs in
vitro, the number of nestin+ NSCs/NPCs markedly reduced. It was
noted that over a period of 7 days, ~56.33±10.32% of cells were
nestin+ (data not shown). srGAP2 was predominantly expressed in the
cell nucleus (Fig. 3A). The
expression of srGAP2 dynamically changed along with the decreased
expression of nestin. The ratio of srGAP2+/nestin+ cells from the
total nestin+ cells was ~80% at 7 and 14 days. It was significantly
lower than that at 1 and 3 days (almost 100%, P<0.05). No
difference was observed between 7 and 14 days (Fig. 3B).
Dynamic expression of srGAP2 in
GFAP+ cells
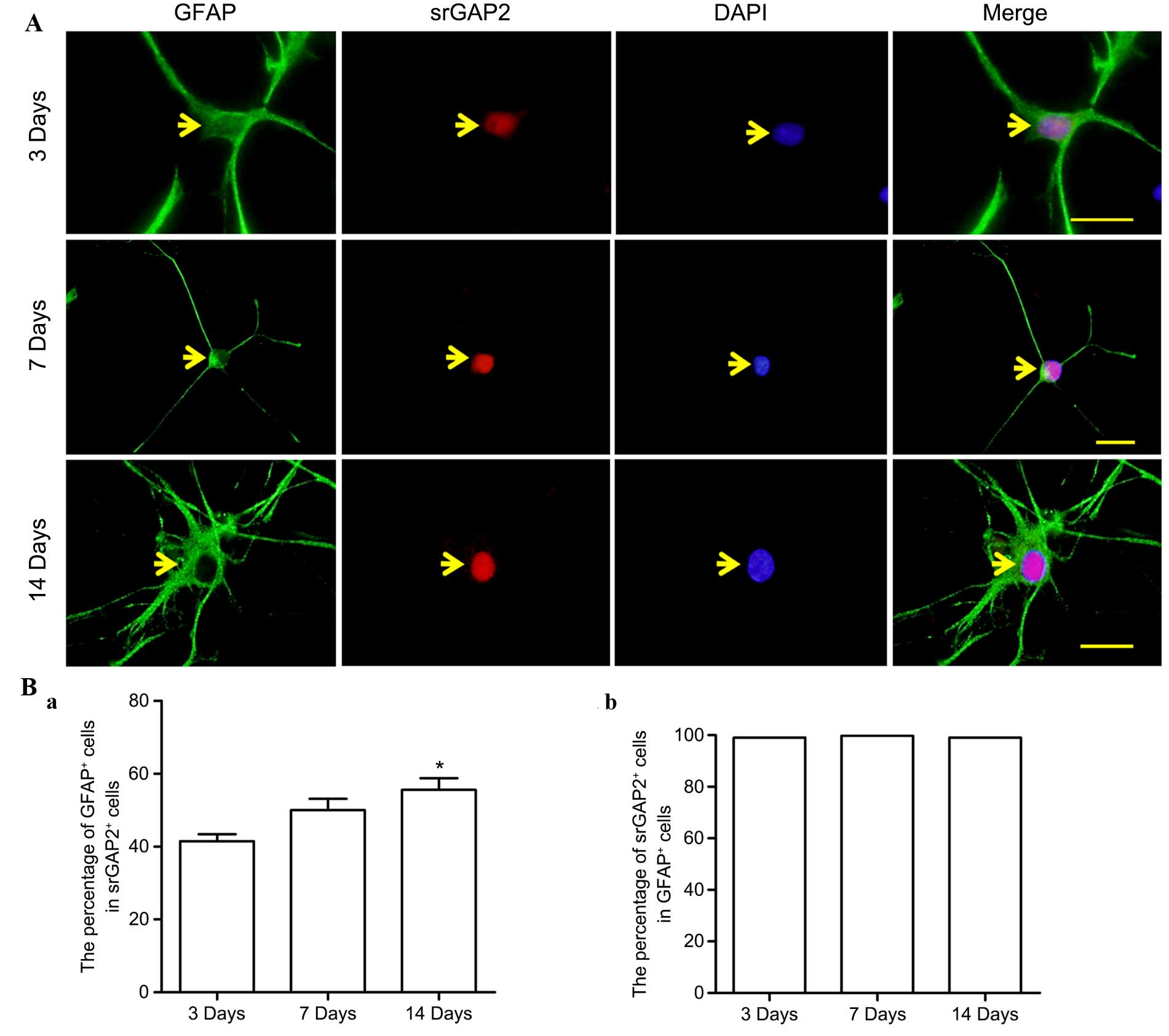
Along with the differentiation of NSCs/NPCs in
vitro, the expression of srGAP2 in GFAP+ cells was observed to
further elucidate the association between srGAP2 and GFAP+ cells.
srGAP2 was predominantly expressed in the cell nucleus (Fig. 4A), a similarity also observed in
nestin+ cells. During differentiation, with the slightly enhanced
expression of srGAP2 in cell nuclei, the ratio of srGAP2+/GFAP+
cells from the total srGAP2+ cells significantly increased
(Fig. 4Ba; 41.48±3.37 to
51.02±5.47%; P<0.05), while within the population of GFAP+
cells, this ratio was maintained at a similar level (Fig. 4Bb).
Altered expression of srGAP2 in
β-tubulin+ cells in vitro
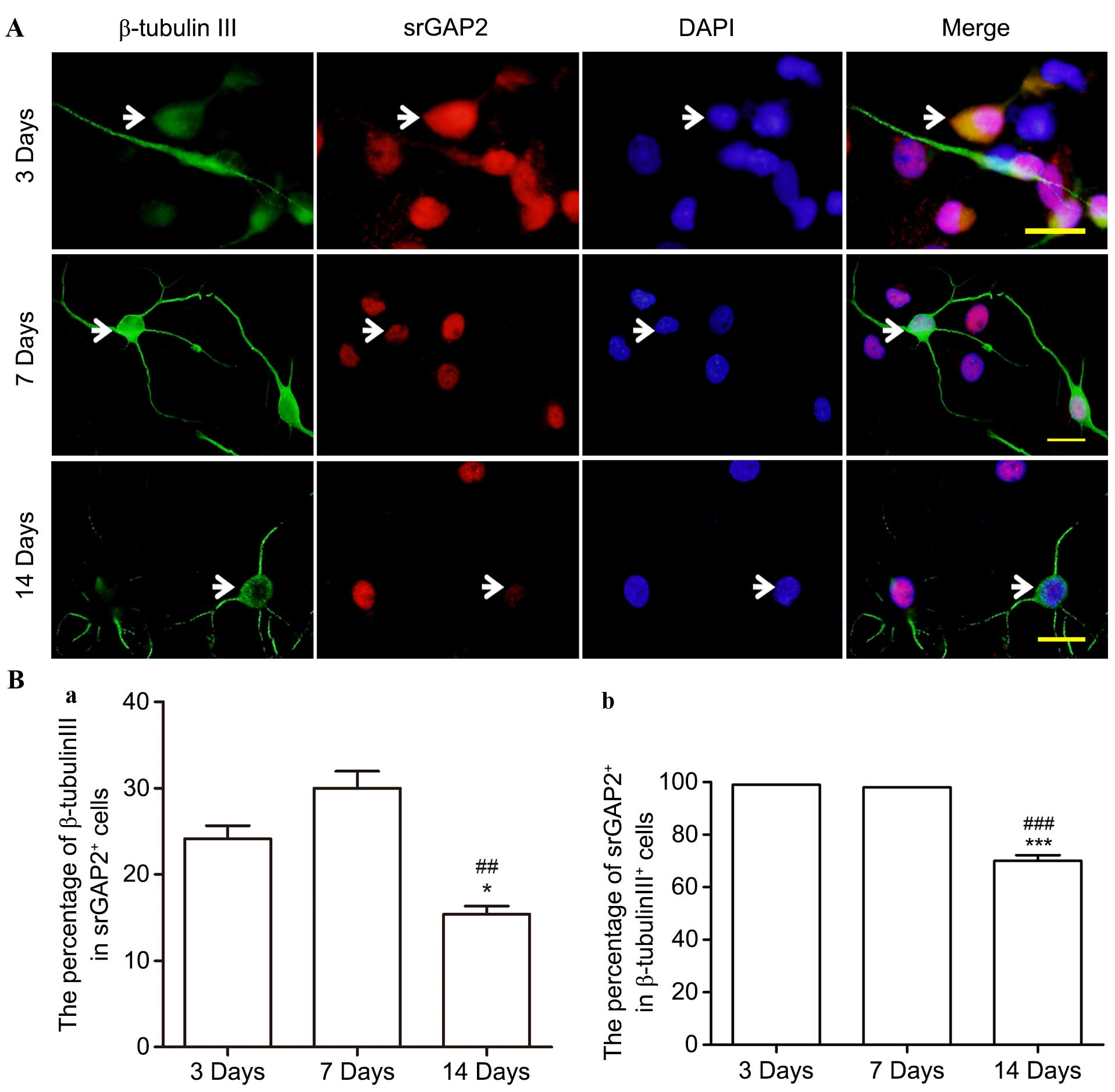
During culture in the differentiation medium,
~28.9±3.06% of NSCs/NPCs differentiated into β-tubulin III+
neuronal progenitors/neurons at 7 days (data not shown). srGAP2 was
observed to be expressed in almost all the β-tubulin III+ cells at
3 and 7 days, specifically in the cell nuclei (Fig. 5A and B). However, the percentage of
srGAP2+/β-tubulin III+ cells compared with the total number of
srGAP2+ and β-tubulin III+ cells was significantly reduced from
30.02±3.41 and almost 100% on the 3rd day to 15.38±1.66 and
68.25±2.75% on the 14th day, (P<0.05). By contrast, no srGAP2
was observed in the cell cytoplasm of nestin+ cells on the 14th
day.
Discussion
The current study demonstrated that along with the
differentiation of NSCs/NPCs in vitro, expression patterns
of srGAP2 changed dynamically. It was expressed predominantly in
cell nuclei and markedly decreased with the differentiation of
NSCs/NPCs, particularly in β-tubulin III+ neuronal
progenitors/neurons.
All members of the srGAP family are widely expressed
in the nervous system and work as multifunctional adaptor proteins,
however, different srGAPs have diverse patterns of expression that
are often distinct from each other. This indicates that different
srGAPs are likely to be important for different aspects of central
nervous system development (1,4).
Although srGAP2 is detectable during rat neural development until
postnatal day 14 (17), it is
notably absent from the site of neurogenesis, but often strongly
detected in the region of neuronal migration and differentiation
(4). This demonstrates that srGAP2
may not be essential for the production of neuronal precursors. The
immunohistochemistry staining of adult rat brain tissue conducted
in the present study, demonstrated that srGAP2 is weakly expressed
in the SVZ where neurogenesis occurs and that it co-localizes with
GFAP+ type B cells, which refers to slowly proliferating
cells. Furthermore, it has been confirmed that srGAP2 is involved
in neural development, however it may not be involved in neuronal
genesis.
Nestin is an intermediate filament protein expressed
in dividing cells during the early stages of development. srGAP2
was expressed in nestin+ NSCs/NPCs throughout their
in vitro differentiation. With the downregulation of nestin
upon cell differentiation, the ratio of srGAP2/nestin double
positive cells compared with total nestin positive cells declined
significantly at 7 and 14 days. srGAP2 was expressed predominantly
in the cell nucleus. Weak expression of srGAP2 in the cytoplasm
markedly reduced after 7 days. This suggested that srGAP2 in cell
cytoplasm may be involved in maintaining the stemness, or
undifferentiated state, of the NSCs/NPCs. The results of the
present study are partially supported by observations in the rat
brain described by Yao et al (17).
During neuro- and gliogenesis, nestin is gradually
replaced by cell type-specific intermediate filaments, including
neurofilaments and GFAP. It was observed that with astrocytic
differentiation, srGAP2 was detectable in the cell nucleus only and
significantly stronger at 14 days than 3 days. Although the
percentage of srGAP2+/GFAP+ cells compared
with the total GFAP+ cells is stable during gliogenesis,
it is notable that the ratio of srGAP2+/GFAP+
cells to total srGAP2+ cells is significantly increased
at 14 days. This indicated that srGAP2 in the cell nucleus may
promote astrocytic differentiation of NSCs/NPCs in
vitro.
By contrast, srGAP2 was expressed weakly in the
cytoplasm but strongly in the cell nucleus in β-tubulin
III+ cells at 3 days. The srGAP2 levels in the cell
nucleus were markedly downregulated during neuronal
differentiation. This is in contrast with the srGAP2 expression
pattern in cultured neurons (17).
In addition, the percentage of srGAP2+/β-tubulin
III+ cells from the total β-tubulin+ and the
total srGAP2+ cells was significantly reduced at 14
days. This suggested that srGAP2 may negatively regulate NSCs/NPCs
to differentiate into neurons in vitro. The srGAP3-Rac1
signal pathway may be involved in the attenuation (20).
In conclusion, the present study suggests that
srGAP2 is expressed in nestin+, GFAP+ and
β-tubulin III+ cells at different time points throughout
the life cycle of the cell, with distinct localization patterns. It
suggests that srGAP2 is associated with cellular development of the
nervous system. Throughout the differentiation period of the
NSCs/NPCs in vitro, srGAP2 was demonstrated to be expressed
at varying levels in different cell types. The translocation of
srGAP2 in the cytoplasm and cell nucleus in different cell types
may function as a director during cell fate decision. srGAP2 levels
in the cell cytoplasm are involved in maintaining the stemness, or,
undifferentiated state of NSCs/NPCs, whilst in the cell nucleus, it
may promote astrocytic differentiation and attenuate neuronal
differentiation of the NSCs/NPCs in vitro. As Rho-GTPases
are known to have diverse roles in neurogenesis, srGAP2 will have
different functional consequences. In addition, the srGAP2 gene has
recently been implicated in a severe neurodevelopmental syndrome
that causes early infantile epileptic encepholopathy and srGAP2
knockout mice are prone to epileptic seizures. Thus, srGAP2 may
also be associated with neuronal functions. Further investigation
into the underlying mechanisms of the diverse functions of srGAP2
in different cell types and different developmental periods is
required.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31070943,
81070998, 31271151 and 30960107) and partially supported by the
State Key Laboratory of Neuroscience, Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences (grant no.
SKLN-210204).
References
|
1
|
Wong K, Ren XR, Huang YZ, Xie Y, Liu G,
Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, et al: Signal
transduction in neuronal migration: Roles of GTPase activating
proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell.
107:209–221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aspenström P: Roles of F-BAR/PCH proteins
in the regulation of membrane dynamics and actin reorganization.
Int Rev Cell Mol Biol. 272:1–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ypsilanti AR, Zagar Y and Chédotal A:
Moving away from the midline: New developments for Slit and Robo.
Development. 137:1939–1952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacon C, Endris V and Rappold G: Dynamic
expression of the Slit-Robo GTPase activating protein genes during
development of the murine nervous system. J Comp Neurol.
513:224–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guerrier S, Coutinho-Budd J, Sassa T,
Gresset A, Jordan NV, Chen K, Jin WL, Frost A and Polleux F: The
F-BAR domain of srGAP2 induces membrane protrusions required for
neuronal migration and morphogenesis. Cell. 138:990–1004. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charrier C, Joshi K, Coutinho-Budd J, Kim
JE, Lambert N, de Marchena J, Jin WL, Vanderhaeghen P, Ghosh A,
Sassa T and Polleux F: Inhibition of SRGAP2 function by its
human-specific paralogs induces neoteny during spine maturation.
Cell. 149:923–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soderling SH, Guire ES, Kaech S, White J,
Zhang F, Schutz K, Langeberg LK, Banker G, Raber J and Scott JD: A
WAVE-1 and WRP signaling complex regulates spine density, synaptic
plasticity, and memory. J Neurosci. 27:355–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu H, Jiao Q, Wang Y, Yang Z, Feng M, Wang
L, Chen X, Jin W and Liu Y: The mental retardation-associated
protein srGAP3 regulates survival, proliferation, and
differentiation of rat embryonic neural stem/progenitor cells. Stem
Cells Dev. 22:1709–1716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soderling SH, Binns KL, Wayman GA, Davee
SM, Ong SH, Pawson T and Scott JD: The WRP component of the WAVE-1
complex attenuates Rac-mediated signalling. Nat Cell Biol.
4:970–975. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saitsu H, Osaka H, Sugiyama S, Kurosawa K,
Mizuguchi T, Nishiyama K, Nishimura A, Tsurusaki Y, Doi H, Miyake
N, et al: Early infantile epileptic encephalopathy associated with
the disrupted gene encoding Slit-Robo Rho GTPase activating protein
2 (SRGAP2). Am J Med Genet A 158A. 199–205. 2012. View Article : Google Scholar
|
|
11
|
Carlson BR, Lloyd KE, Kruszewski A, Kim
IH, Rodriguiz RM, Heindel C, Faytell M, Dudek SM, Wetsel WC and
Soderling SH: WRP/srGAP3 facilitates the initiation of spine
development by an inverse F-BAR domain, and its loss impairs
long-term memory. J Neurosci. 31:2447–2460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim IH, Carlson BR, Heindel CC, Kim H and
Soderling SH: Disruption of wave-associated Rac GTPase-activating
protein (Wrp) leads to abnormal adult neural progenitor migration
associated with hydrocephalus. J Biol Chem. 287:39263–39274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waltereit R, Leimer U, von Bohlen Und
Halbach O, Panke J, Hölter SM, Garrett L, Wittig K, Schneider M,
Schmitt C, Calzada-Wack J, et al: Srgap3−/−
mice present a neurodevelopmental disorder with
schizophrenia-related intermediate phenotypes. FASEB J.
26:4418–4428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parati EA, Pozzi S, Ottolina A, Onofrj M,
Bez A and Pagano SF: Neural stem cells: An overview. J Endocrinol
Invest. 27:(6 Suppl). S64–S67. 2004.
|
|
15
|
Price J and Williams BP: Neural stem
cells. Curr Opin Neurobiol. 11:564–567. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao Q, Xie WL, Wang YY, Chen XL, Yang PB,
Zhang PB, Tan J, Lu HX and Liu Y: Spatial relationship between
NSCs/NPCs and microvessels in rat brain along prenatal and
postnatal development. Int J Dev Neurosci. 31:280–285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao Q, Jin WL, Wang Y and Ju G: Regulated
shuttling of Slit-Robo-GTPase activating proteins between nucleus
and cytoplasm during brain development. Cell Mol Neurobiol.
28:205–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gage FH, Coates PW, Palmer TD, Kuhn HG,
Fisher LJ, Suhonen JO, Peterson DA, Suhr ST and Ray J: Survival and
differentiation of adult neuronal progenitor cells transplanted to
the adult brain. Proc Natl Acad Sci USA. 92:11879–11883. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu HX, Hao ZM, Jiao Q, Xie WL, Zhang JF,
Lu YF, Cai M, Wang YY, Yang ZQ, Parker T and Liu Y: Neurotrophin-3
gene transduction of mouse neural stem cells promotes proliferation
and neuronal differentiation in organotypic hippocampal slice
cultures. Med Sci Monit. 17:BR305–BR311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Mi YJ, Dai YK, Fu HL, Cui DX and Jin
WL: The inverse F-BAR domain protein srGAP2 acts through srGAP3 to
modulate neuronal differentiation and neurite outgrowth of mouse
neuroblastoma cells. PLoS One. 8:e578652013. View Article : Google Scholar : PubMed/NCBI
|