Introduction
A reduction in the number of blood cells, including
red blood cells, leukocyte and platelets, is a common complication
of liver cirrhosis (LC), and may exacerbate the progression of the
disease and severely affect the patient's quality of life (1). The mechanisms underlying the observed
reduction in the number of blood cells in LC are unknown, however,
several mechanisms have been proposed for this anomaly. For
instance, hypersplenism and reduced liver thrombopoietin leads to
platelet deficiency (2). In
addition, portal hypertension may lead to gastrointestinal
bleeding, hemolysis and reduced hematopoietic substances, such as
iron and folic acid, which may induce anemia (3). Furthermore, hepatitis viruses,
excessive alcohol consumption and the intake of certain drugs,
including methotrexate and amiodarone, often lead to LC (4,5). If
bone marrow production is suppressed, these factors may lead to
deficiencies in bone marrow function, which may subsequently affect
the function of the hematopoietic system (6,7). A
previous study demonstrated that bone marrow endothelial cells
(BMECs) derived from rats with liver fibrosis (LF) exhibited
ultrastructural changes in vivo, and in vitro results
demonstrated that the serum of patients with LC may induce
apoptosis in BMECs (8,9).
BMECs are an important component of the
hematopoietic microenvironment, where they generate hematopoietic
stem cells and serve an important role in regulating the
self-renewal, differentiation, homing and migration of
hematopoietic stem cells (10–12).
Therefore, damage to the bone marrow microenvironment during LC by
serum inhibitory factors such as endotoxin and inflammatory
cytokines, may subsequently result in damage to hematopoietic stem
cells. Thus, determining the mechanisms underlying the destructive
actions of LC on the bone marrow microenvironment, and the
identification of an effective drug therapy that inhibits this
process, is important for the study of hematological abnormalities
during LC, as well as for the clinical treatment of LC.
Previous studies have demonstrated that protein
kinases, which are intermediate molecules in signal transduction
pathways, can regulate the activity of metabolic enzymes or the
expression of genes by phosphorylating target proteins (13). Protein kinases are one of the most
important regulatory factors of cell behavior, and are associated
with almost all cellular functions. The central role of protein
kinases in controlling cellular behavior demonstrates their
potential as a therapeutic target for a number of diseases,
including cancer, inflammation and eye diseases (14). Thus, protein kinases have been
widely studied as potential therapeutic targets. In addition, with
the increase in the number of studies investigating protein kinases
as therapeutic targets for different diseases, the development of
novel kinase inhibitors is increasing rapidly. To date, 30 kinase
inhibitors have been approved by the US Food and Drug
Administration for clinical treatment or testing, and these
developments have promoted the advancement of laboratory results to
clinical practice (15).
In the present study, whole genome microarray
results obtained from previous studies were used to screen for
differentially expressed kinase genes in BMECs treated with serum
derived from patients with LC and normal healthy controls (8,9).
Bioinformatics tools were used to predict the functions of
differentially expressed kinases, and the signaling pathways that
they may regulate. Finally, a kinase inhibitor was used to inhibit
the activity of a candidate protein kinase in a rat model of LF, in
order to determine its effect on bone marrow tissue function.
Materials and methods
Bioinformatics analysis
In a previous study (9), the sera from 26 patients with LC and
10 healthy volunteers were used to treat BMECs for 48 h, resulting
in identification of 1,872 differentially expressed genes by
screening whole genome microarray chips, with 1,106 overexpressed
genes and 766 underexpressed genes. Reverse
transcription-quantitative polymerase chain reaction analysis was
used to verify the results of the whole genome microarray chips in
a previous study (9). Patient
clinical data, such as the number of blood cells in the 26 patients
with LC, was described previously (9). In the present study, pathway and gene
ontology analyses of these differentially expressed genes were
performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG;
http://www.genome.jp/kegg/pathway.html) and the
Database for Annotation, Visualization and Integrated Discovery
(DAVID; https://david.ncifcrf.gov/) databases
in order to identify their predicted functions and the signaling
pathways that they may regulate. Differentially expressed protein
kinase genes were identified using the Kinase Substrate Database
(http://kinasource.co.uk/Database/substrates.html),
and were selected for further screening. The search terms used to
generate the protein kinase list was obtained from kinase library
(https://www.cellsignal.com/common/content/content.jsp?id=kinases).
The predicted pathways of differentially expressed kinase genes
were identified using KEGG, and Cytoscape software (version 2.8.2;
http://www.cytoscape.org/) was used to generate a
kinase-pathway network. ActivePerl software (version 5.16.2;
http://www.activestate.com/activeperl/) was used to
retrieve literature data from the National Center for Biotechnology
Information (U.S. National Library of Medicine, Bethesda, MD, USA)
PubMed database (http://www.ncbi.nlm.nih.gov/pubmed). The search
results included the titles and abstracts of publications, and the
search keywords were the names of the differentially expressed
genes and ‘bone marrow apoptosis’ (for example, ‘AKT and bone
marrow apoptosis’).
Animal model
All procedures involving animals, including animal
feeding, operation and sacrifice, were performed after receiving
the approval of the institutional ethics review board of the First
Affiliated Hospital of Harbin Medical University (Harbin, China). A
total of 36 healthy male Wistar rats (weight, 230–250 g; age, 7
weeks; purchased from Changchun Yisi Laboratory Animal Co., Ltd.,
Changchun. China) were used in the present study, maintained on a
12-h light/dark cycle in a temperature- and humidity-controlled
room with access to water and food ad libitum. The rats were
divided into a sham group (control), consisting of 13 rats with a
bile duct that was separated but not ligated, and an LF group,
consisting of 23 rats with a bile duct that was ligated. To achieve
this, the rats were weighed, and 10% chloral hydrate was injected
intraperitoneally (0.3 ml/100 g body weight) to anesthetize the
rat, which normally required 2–3 min. The animal was then fixed on
the experiment bench, and the hair from the abdomen was shaved to
prepare the skin, which was disinfected and surgical drapes were
placed over the abdomen. A 2–3 cm incision was made along the
midline section of the abdomen below the xiphoid, and retractors
were used to open the abdominal walls and fix them in place. The
abdominal organs were examined and were observed to be normal. The
duodenum at the pylorus was then identified, and in the middle
section of the duodenum, the light yellow bile duct 4 cm in length
and with a diameter of 2 mm was observed. A glass dissecting needle
was used to isolate 1 cm of bile duct from its upper section. For
the LF group, a 3–0 thread was used to ligate the short duct at the
proximal and distal ends, and the duct was cut in the middle. For
the control group, there was no further treatment after the bile
duct was isolated. The abdomen was closed, and a 2–0 thread was
used as a simple continuous suture of the peritoneum and muscle,
which was applied at an interval of 0.5 cm, and as a discontinuous
suture of the skin. Following surgery, 5 ml of saline solution was
administered in the tail vein for rehydration. The rats were placed
in a warm location until they recovered, which occurred after ~3 h.
The rats were provided with food and water ad libitum, and
their condition was monitored every day including mental condition,
feeding state, and skin and urine color. At 3 weeks following
surgery, three rats were selected at random from each group for
liver pathological examinations to verify the status of the rat
model.
Aside from the 10 rats in the control group, the 14
rats in the LF group that survived after the model was established,
were randomly divided into two subgroups: The kinase inhibitor
group, consisting of seven rats that were treated with a kinase
inhibitor, and the LF group, consisting of seven rats that were
treated with the solvent solution without the kinase inhibitor.
Based on the bioinformatics analysis results, p38a
was selected as the target kinase, and the p38a kinase inhibitor
SB203580 (Selleck Chemicals, Houston, TX, USA) was used to perform
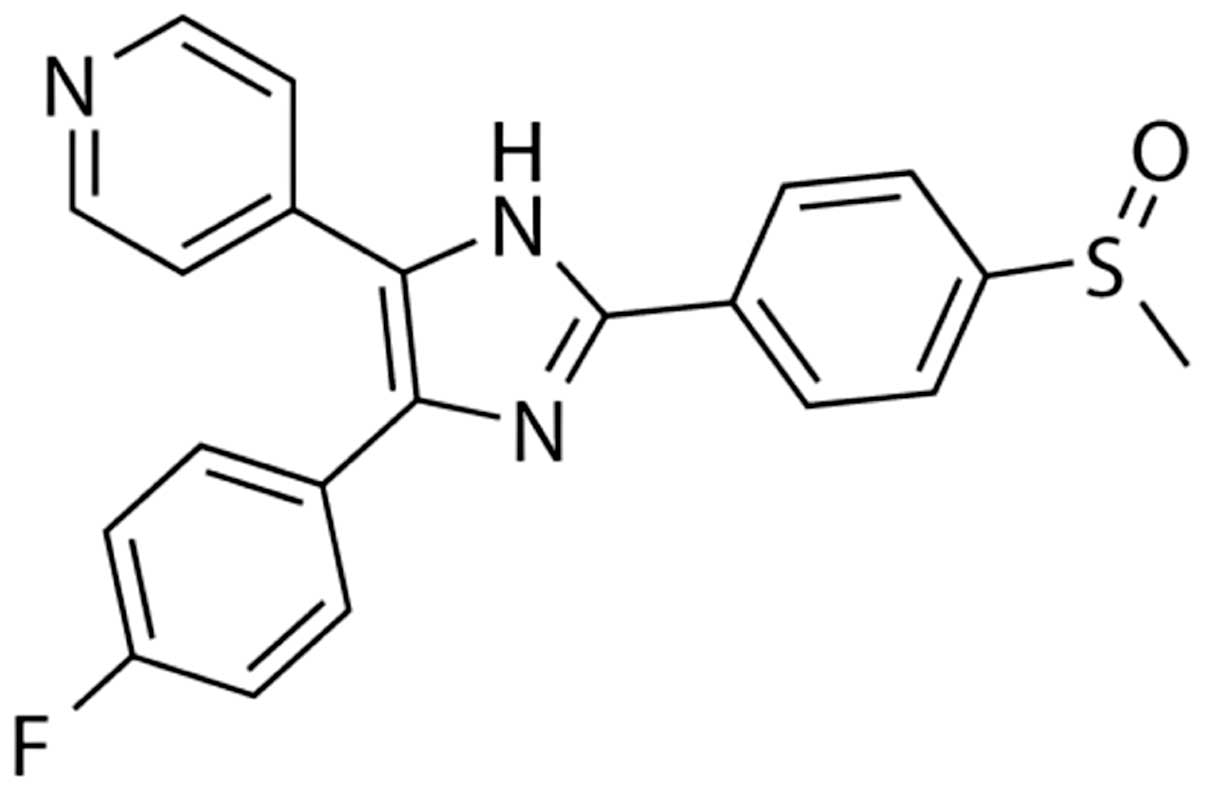
subsequent experiments. The chemical formula of SB203580 is shown
in Fig. 1. The kinase inhibitor
group was treated with SB203580 (hereafter referred to as the
SB203580 group). To achieve this, 50 mg SB203580 was dissolved in 1
ml dimethyl sulfoxide (DMSO) prior to the addition of 19 ml sterile
saline solution to produce a 5% DMSO inhibitor solution. At 3 weeks
following surgery, the LF rats were treated with SB203580 for 7
days. A total of 1 mg inhibitor solution/kg body weight was
injected intravenously each day into rats in the SB203580 group.
The LF and control groups were given an equal volume of 5% DMSO
solution. After 1 week, 10% chloral hydrate was injected
intraperitoneally (0.3 ml/100 g body weight) to terminally
anesthetize the rat. Following anesthesia, the femur and tibia were
isolated, and the muscle and tendon were removed, then the rats
were sacrificed by bloodletting from the abdominal aorta. Samples
were collected for blood analysis, pathological examination of the
liver, a bone marrow smear, and apoptosis-associated tests.
Blood counts
Whole blood (3 ml) from all rats was obtained from
the abdominal aorta immediately after the femurs were removed.
Whole blood samples were analyzed using a Cell-Dyn 3500 (Abbott
Diagnostics, Abbott Park, IL, USA) programmed for rat peripheral
blood cells. The absolute number of leukocytes and platelets, and
concentration of hemoglobin were determined.
Hematoxylin and eosin (H&E)
staining of the liver
The rat liver was fixed in 10% neutral formalin for
24 h, followed by dehydration and embedding in paraffin. Sections
were prepared using a microtome (Labsun China Co. Ltd., Shanghai,
China) to slice the paraffin wax. Slice thickness was set to 7 µm.
The slices were washed in phosphate buffered saline (PBS) for 5
min, and hematoxylin was used to stain the nuclei for 5 min,
followed by destaining with hydrochloric acid and ethanol for 30
sec. The slices were then washed using tap water and distilled
water for 1 min. Eosin staining was performed for 30 sec, followed
by a 70% ethanol rinse for 30 sec, a 90% ethanol rinse for 30 sec,
a 95% ethanol rinse for 30 sec, two 100% ethanol rinses for 2 min
each time and xylene clearing twice for 5 min each time. The
stained slices were observed under an inverted light
microscope.
Massons trichrome staining of the
liver
Paraffin-embedded slices were dewaxed and
rehydrated, before the slices were washed in tap water and
distilled water. Regaud's hematoxylin staining solution or
Weigert's hematoxylin staining solution was used to stain the
nuclei for 5–10 min before washing. The slices were thoroughly
washed thoroughly with water. If the tissues were over-stained,
they were destained with 1% hydrochloric acid in ethanol and washed
with distilled water. Masson Ponceau-acidic fuchsin staining
solution was used to stain the slices for 5–10 min, and 2% acetic
acid was then used to briefly wash the slice. The slices were
subsequently destained with a 1% phosphomolybdic acid aqueous
solution for 3–5 min before they were stained with aniline blue or
green staining solution for 5 min. The slices were briefly rinsed
with a 0.2% glacial acidic acid solution and then rinsed in 95%
ethanol and absolute ethanol, cleared in xylene and sealed with
neutral resin.
Preparation of bone marrow smears
After the rats were anesthetized, the femur and
tibia were isolated, and the muscle and tendon were removed. The
metaphysis of the long bone was removed, and rat serum was used to
repeatedly wash the marrow cavity until no marrow remained. A
pipettor was used to mix the bone marrow fluid until uniform. One
drop of bone marrow fluid was then placed at one end of a slide,
and the frosted end of another glass slide was used to flatten the
drop and spread it along the slide. The slide was air-dried at room
temperature for subsequent bone marrow staining.
Apoptosis detection in the bone
marrow
DNA damage and cell death in rat bone marrow samples
was detected using the ApopTag Plus Peroxidase in situ
Apoptosis detection kit (cat. no. S7101; EMD Millipore, Billerica,
MA, USA) according to manufacturer's instructions. Briefly, tissue
sections were deparaffinized and pretreated with Proteinase-K
solution (20 µg/ml) at room temperature for 15 min. Endogenous
peroxidase activity was quenched using 3% hydrogen peroxide in PBS
at room temperature for 15 min. Following incubation with terminal
deoxynucleotidyl transferase at 37°C for 1 h, the apoptotic cells
were visualized under a bright-field microscope using a
diaminobenzidine (DAB)-based detection system supplied with the
kit, and sections were counterstained with the methyl green nuclear
stain (Trevigen, Gaithersburg, MD, USA). Terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL)-positive cells were
counted in five high-power fields of view (magnification, ×40)
selected at random, and counts from three sections for each rat
were used for statistical analysis.
Detection of caspase 3 and von
Willebrand factor (vWF) in the bone marrow
Immunohistochemical staining was conducted using the
streptavidin-peroxidase method (16). Tissue sections were first prepared
using the procedures described above. Endogenous peroxidase
activity was inhibited by incubating tissue sections in 3% hydrogen
peroxide. Antigen retrieval was achieved by autoclaving tissue
samples at 120°C for 15 min in 0.01 M citrate buffer (pH 6.0). The
sections were incubated overnight at 4°C with rabbit polyclonal
anti-caspase 3 (dilution, 1:65; cat. no. WL0146; Wanleibio Co.,
Ltd., Shenyang, China) and anti-vWF (dilution, 1:65; cat. no.
sc-365712; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary
antibodies. The biotinylated goat anti-rabbit IgG secondary
antibody (dilution, 1:1,000; cat. no. A0277; Beyotime Co. Ltd.,
Shanghai, China) was then applied for 30 min at room temperature.
Sections were treated with horseradish peroxidase-labeled
streptavidin (Beyotime Co. Ltd.) for 30 min at 37°C. Then sections
were visualized by 3,30-DAB-tetrahydrochloride horseradish
peroxidase color development kit staining (cat. no. P0203; Beyotime
Co. Ltd.). The expression of caspase-3 and vWF were quantified
using Image-Pro Plus software (Media Cybernetics, Inc., Rockville,
MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between two groups were performed using the Student's
t-test. Based on information provided by the company that
conducted the mRNA microarray analysis (KangChen Bio-tech Inc.,
Shanghai, China), a ≥2-fold alteration in gene expression was
considered to be significant (9).
Statistical analyses were performed using SPSS statistics software
(version, 15.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Screening of differentially expressed
protein kinases in bone marrow endothelial cells during LC
Additional pathway and functional analyses were
performed on the 1,106 overexpressed genes and 776 underexpressed
genes identified in a previous study, whereby BMECs were treated
with serum from patients with LF or normal healthy individuals
(9). In the present study, a total
of 14 differentially expressed kinase genes were identified through
screening, of which nine were overexpressed [Janus kinase 3 (JAK3),
G-protein-coupled receptor kinase 6 (GRK6), AKT serine/threonine
kinase 1 (AKT1), protein kinase, X-linked (PRKX), myosin light
chain kinase (MYLK), calcium/calmodulin-dependent protein kinase
type II β-chain (CAMK2B), Erb-B2 receptor tyrosine kinase 3
(ERBB3), Src and p38a] and five were underexpressed
[3-phosphoinositide-dependent kinase 1 (PDPK1),
receptor-interacting serine/threonine-protein kinase 3 (RIPK3),
casein kinase 2α-1 (CSNK2A1), epidermal growth factor receptor
(EGFR) and neurotrophic receptor tyrosine kinase 1 (NTRK1)]. In the
present study, KEGG pathway analysis demonstrated that these
differentially expressed kinases may regulate a number of important
signaling pathways (Fig. 2A). The
results of the literature mining demonstrated that >153 reports
regarding p38a and ‘bone marrow apoptosis’ were identified, which
was the greatest number of reports identified when compared with
the other kinases that were searched (Fig. 2B). Therefore, p38a was selected as
the candidate kinase for further investigation, and the p38a
inhibitor SB203580 was employed in subsequent experiments.
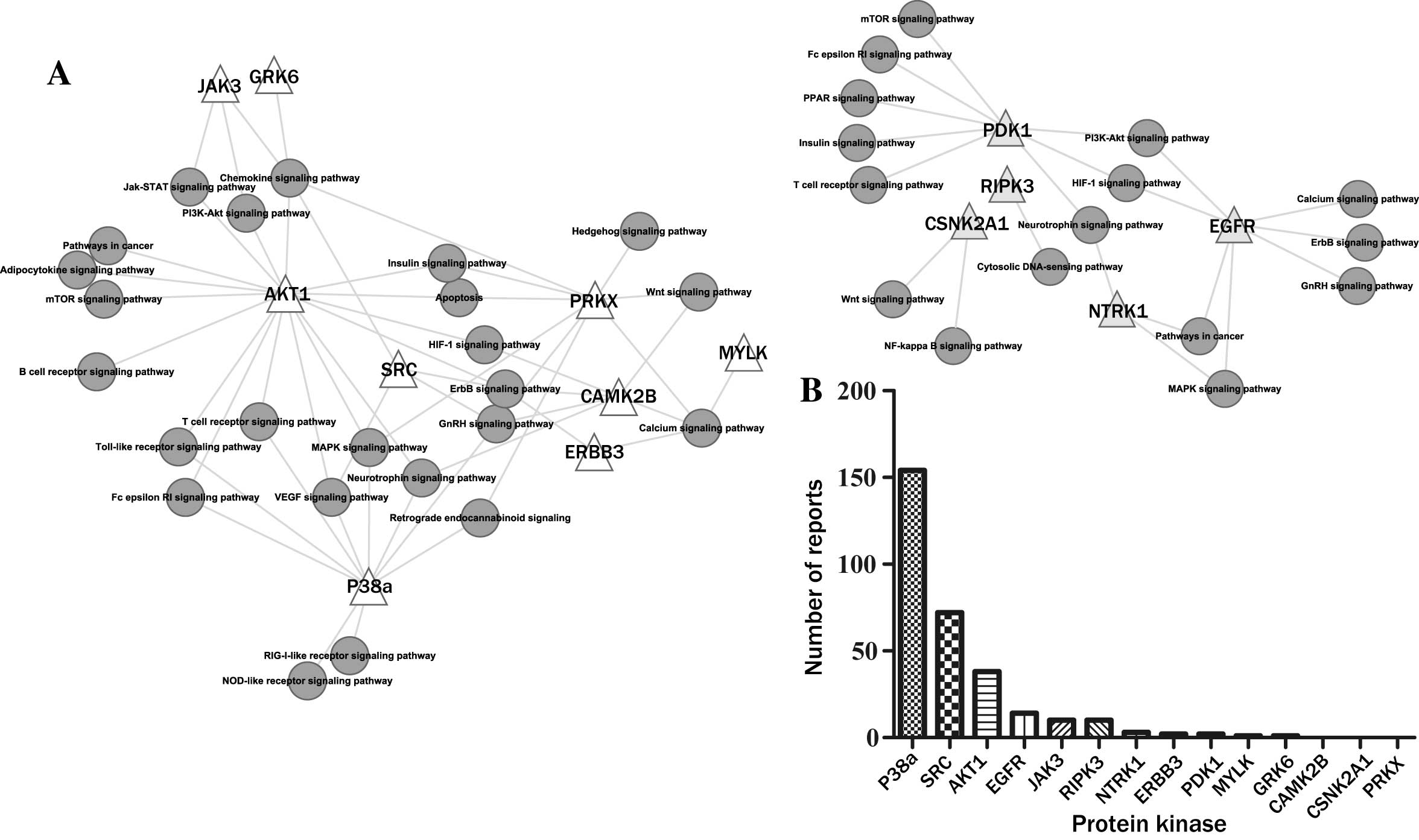 | Figure 2.Differentially expressed protein
kinases in BMECs treated with serum from patients with LF. (A)
Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg/pathway.html) pathway
analysis of 14 differentially expressed protein kinases. A total of
nine protein kinases were overexpressed (JAK3, GRK6, AKT1, PRKX,
MYLK, CAMK2B, ERBB3, SRC, p38a) and five were underexpressed
(PDPK1, RIPK3, CSNK2A1, EGFR, NTRK1). (B) The number of reports
identified regarding individual protein kinases and bone marrow
apoptosis (search term, e.g., ‘AKT1 + bone marrow apoptosis’) as
determined using the national center for biotechnology information
PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) database. BMECs,
bone marrow endothelial cells; LF, liver fibrosis. See the main
text for the abbreviations of the kinases. |
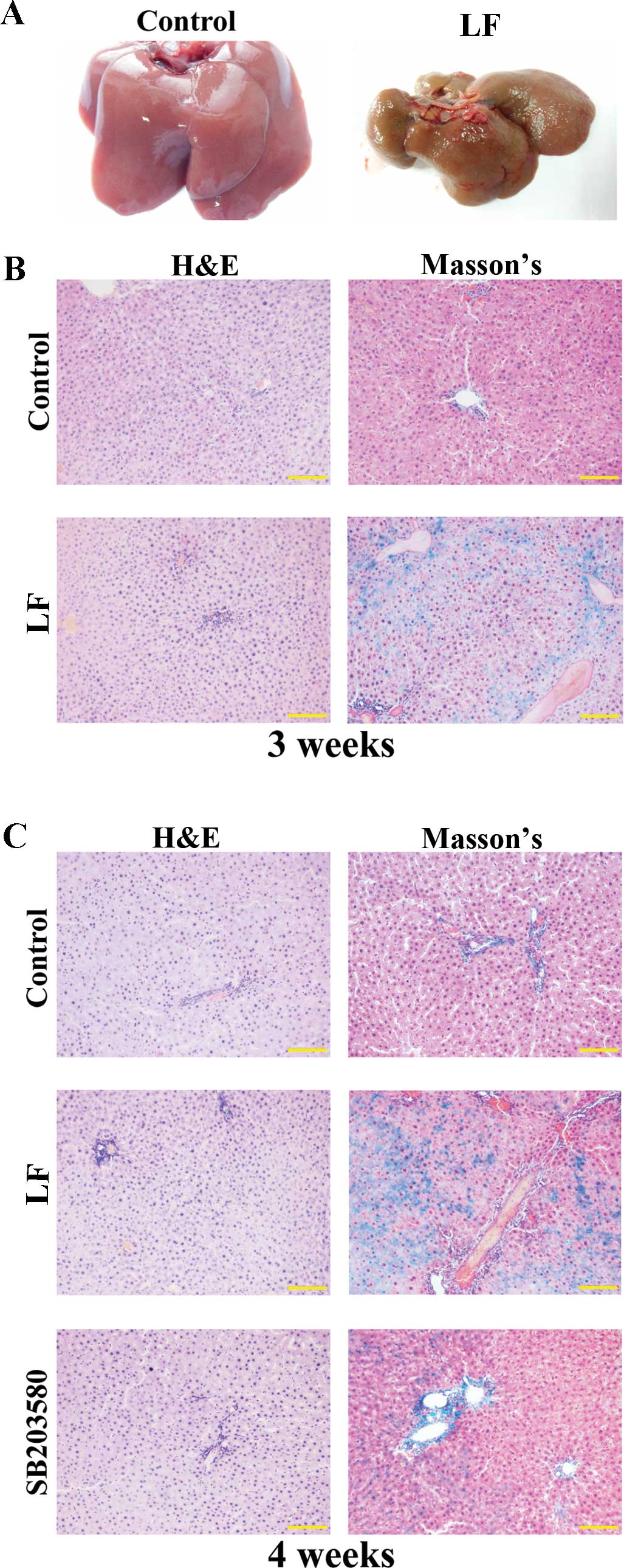
Liver fibrosis model
As shown in Fig.
3A, the rat livers in the control group were reddish in color,
with a smooth surface and a soft texture. In contrast, rat livers
in the LF group exhibited a dull yellow color, and the surface
possessed uniform granules with a hard texture when handled.
Compared with the control group, livers from the LF group were
visibly reduced in volume and weight. In addition, the livers from
the LF group were observed to frequently adhere to other organs,
and there were difficulties when isolating the organ (Fig. 3A).
As demonstrated by H&E staining, the liver
tissue in control rats at three weeks following surgery exhibited a
normal structure, and the hepatocytes were intact and aligned in
order (Fig. 3B). The liver tissue
did not present with lesions, the liver lobular structure was
normal, and the hepatic cord exhibited a regular structure without
abnormal fibrosis. At 3 weeks following surgery in the LF group,
the liver tissue exhibited a disorganized structure, and several
striped regions of fibrosis were observed (Fig. 3B). As determined using Masson's
trichrome staining, no collagen was observed in the livers from the
control group at three weeks following surgery (Fig. 3B). However, in the LF group,
collagen fiber hyperplasia was observed, the portal area was
expanded, and the over-proliferated fibers extended out from the
central vein or portal area (Fig.
3B).
As demonstrated by H&E staining at 4 weeks
following surgery, the livers from LF group rats exhibited an
abnormal lobular structure. The presence of fibrous connective
tissue hyperplasia, pseudolobule formation and damaged lobular
structures were separated and formed hepatocyte groups of different
sizes (Fig. 3C). The degree of
liver fibrosis in the SB203580 group was markedly reduced compared
with that of the LF group. Masson's trichrome staining of liver
samples at 4 weeks following surgery demonstrated that collagen
fiber hyperplasia was more pronounced in the LF group, with several
samples demonstrating fibrous septa formation, and separation of
the liver lobules to form pseudolobules. In contrast, the fiber
hyperplasia in the SB203580 group was reduced compared with that of
the LF group (Fig. 3C).
Analysis of blood cell number
As shown in Table
I, analysis of blood cells at 4 weeks following surgery
demonstrated that the amount of hemoglobin was 157.66±8.19 g/l, the
number of white blood cells was 17.08±1.47×109 cells/l, and the
number of platelets was 527.44±49.77×109 cells/l in the control
group. In the LF group, the amount of hemoglobin was 143.40±8.26
g/l, the number of white blood cells was 15.29±2.65×109 cells/l,
and the number of platelets was 422.01±43.80×109 cells/l. In the
SB203580 group, the amount of hemoglobin was 153.80±3.75 g/l, the
number of white blood cells was 16.53±2.59×109 cells/l, and the
number of platelets was 487.72±48.04×109 cells/l. A comparison of
the three groups including comparison between any two groups
demonstrated that there was no significant difference in the amount
of hemoglobin or the number of white blood cells among the groups.
However, a significantly higher number of platelets were observed
in the SB203580 group and the control group when compared with the
LF group (SB203580 group vs. LF group, P=0.0303; control group vs.
LF group, P<0.0001). No significant difference in platelet
number was observed between the SB203580 and the control
groups.
 | Table I.Analysis of blood cells in control,
LF and SB203580 rats at 4 weeks following surgery. |
Table I.
Analysis of blood cells in control,
LF and SB203580 rats at 4 weeks following surgery.
| Group | n | Hemoglobin
(g/l) | Leukocytes
(×109/l) | Platelets
(×109/l) |
|---|
| SB203580 | 7 | 153.80±3.75 | 16.53±2.59 |
487.72±48.04a |
| LF | 7 | 143.40±8.26 | 15.29±2.65 | 422.01±43.80 |
| Control | 10 | 157.66±8.19 | 17.08±1.47 |
527.44±49.77a |
vWF expression in bone marrow
smear
Positive immunohistochemical staining of vWF was
indicated by the presence of brown-colored cells. The positive
expression rate was 12.67±2.40% in the control group, 30.86±6.85%
in the LF group and 17.73±4.37% in the SB203580 group (Fig. 4). vWF expression was significantly
increased (P=0.0122) in the LF group compared with the control
group. Compared with the SB203580 group, vWF expression in the LF
group was significantly decreased (P=0.0488), however, no
significant difference was observed between the control and
SB203580 groups (Fig. 4).
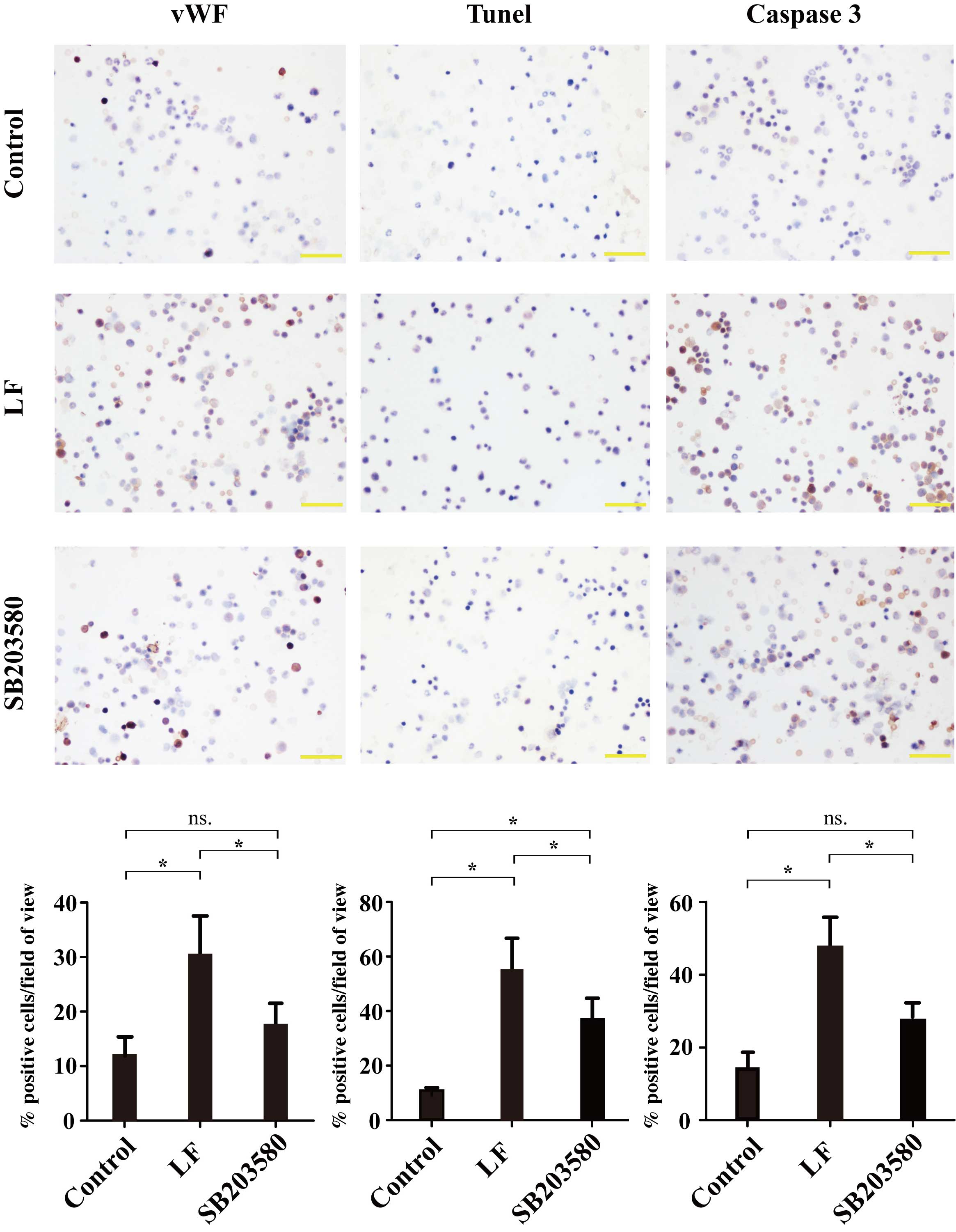 | Figure 4.Immunohistochemical analysis of bone
marrow smears from control, LF and SB203580 groups for vWF, TUNEL
and caspase 3-positive cells. For vWF, the positive expression rate
was 12.67±2.40% in the control group, 30.86±6.85% in the LF group
and 17.73±4.37% in the SB203580 group. For TUNEL-positive cells,
the positive expression rate was 12.57±2.86% in the control group,
57.28±11.03% in the LF group and 33.61±9.24% in the SB203580 group.
For caspase 3, the positive expression rate was 13.37±4.40% in the
control group, 48.13±13.67% in LF group and 23.78±4.80% in the
SB203580 group. Magnification, ×400; Scale bars represent 50 µm.
*P<0.05 vs. control, LF or SB203580 groups as indicated; ns,
P≥0.05. LF, liver fibrosis; vWF, von Willebrand factor; TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling; ns,
not significant; control group, sham-operated rats; LF group, rats
that underwent ligation of the bile duct; SB203580 group, LF rats
that underwent treatment with p38a inhibitor SB203580. |
Detection of bone marrow
apoptosis
Positive TUNEL detection is indicated by the
presence of brown-yellow colored cells. As shown in Fig. 4, the positive expression rate was
12.57±2.86% in the control group, 57.28±11.03% in the LF group and
33.61±9.24% in the SB203580 group. Bone marrow apoptosis was
significantly higher in the LF group compared with the control
group (P=0.0024). In addition, bone marrow apoptosis was
significantly decreased in the SB203580 group compared with the LF
group (P=0.0197), whereas a significant increase in apoptosis was
observed between the SB203580 group and the control group
(P=0.0464; Fig. 4).
Caspase 3 expression in the bone
marrow
Positive immunohistochemical staining of caspase 3
is indicated by the presence of brown-colored cells. The positive
expression rate of caspase 3 was 13.37±4.40% in the control group,
48.13±13.67% in the LF group and 23.78±4.80% in the SB203580 group
(Fig. 4). Compared with the
control group, caspase 3 expression was significantly increased in
the LF group (P=0.0138). Caspase 3 expression was significantly
reduced in the SB203580 group compared with the LF group
(P=0.0436), whereas no significant difference was observed between
the SB203580 and the control group (Fig. 4).
Discussion
In the present study, bioinformatic analysis was
performed on the whole genome array data obtained from a previous
study (9), and 14 differentially
expressed genes that encode protein kinases were observed in BMECs
following exposure to serum from patients with LC. Out of these,
nine genes were overexpressed, and five genes were underexpressed.
Based on these results, combined with the literature-mining
results, the p38a protein kinase and its inhibitor, SB203580, were
selected for in vivo experiments. The results of the in
vivo experiments indicated that the bone marrow apoptotic rate,
and the level of caspase 3 and vWF expression in rats with LF, was
significantly increased compared with controls. This demonstrated
that endothelial cell damage may be increased in rats with LF
compared with normal rats. However, following administration of the
p38a inhibitor, SB203580, the rate of apoptosis, caspase 3 and vWF
expression levels were significantly decreased.
Numerous studies have demonstrated that protein
kinases are the most important factors for regulating cell behavior
and function. The central role of protein kinases in regulating
cell behavior indicates their potential for use as therapeutic
targets in cancer, inflammation and numerous additional diseases,
which has been studied widely (17–19).
Among the methods used to screen target kinases, whole genome
arrays provide a significant amount of information about protein
kinases, and have become an important method for identifying and
analyzing kinases and kinase families. For example, Hofberger et
al (20) used whole genome and
bioinformatics analysis tools to study the expression and function
of the L-type lectin receptor kinase gene family in the
Brassicaceae flowering plant family. Draghetti et al
(21) used whole genome analysis
and determined that polo-like kinase 2 functions to regulate the
differentiation of neurons. In the present study, whole genome and
bioinformatics analyses were performed to screen for differentially
expressed genes that encode protein kinases in BMECs following
exposure to serum derived from patients with LC. Based on the
results of bioinformatics and literature mining as shown in
Fig. 2, the p38a kinase was
identified as a potentially important factor in LC and was subject
to further investigation. The in vivo experimental results
confirmed that the use of whole genome methods to screen for target
kinases is practical and effective.
In a previous study, the ultrastructure of BMECs in
rats with LF was observed to be altered (22). Furthermore, vWF, an endothelial
cell injury biomarker, was increased compared with normal controls
(22). In the process of vascular
endothelial damage, vWF mediates leukocyte and platelet adhesion
(23). As the production of vWF is
increased during vascular endothelial cell injury, it has been
proposed to be an indicator of endothelial dysfunction (24). Consistent with these observations,
the expression of vWF was observed to increase in the bone marrow
smears from rats with LF compared with those of the controls in the
current study. Additional studies involving a rat model of LF have
also demonstrated an increased vWF expression in bone marrow smear
tests (8,9). Previous in vitro experiments
have demonstrated that the serum from patients with LC may induce
BMEC apoptosis (9). The in
vivo results from the present study indicated that the
apoptosis rate and the expression of caspase 3 in the bone marrow
tissues of rats with LF were increased when compared with those of
normal rats. These results suggest that LF may affect bone tissue
survival and function, which may provide an explanation for the
abnormal number of blood cells observed during LF. As an important
component of the bone marrow microenvironment, BMECs support the
growth and adhesion of hematopoietic cells, and secrete a number of
cytokines to regulate hematopoietic cell functions. However, injury
to, and apoptosis of, these cells may exacerbate the damage to bone
marrow tissues during LC (25).
Previous studies have demonstrated that p38a is highly expressed in
BMECs during LC (9). p38a is an
important member of the mitogen-activated protein kinase (MAPK)
family, is involved in cell survival, differentiation and
apoptosis, and is important in several cellular signaling pathways
(26). Studies have demonstrated
that p38a is closely associated with injury and apoptosis of the
vascular endothelium. p38a regulates the production of tumor
necrosis factor alpha (TNFα), interleukin 1 (IL-1) and other
inflammatory cytokines, and the subsequent signaling cascade leads
to the release of a large amount of proinflammatory cytokines that
may cause injury to endothelial cells (27). In addition, p38a is involved in the
expression of the inducible isoform of nitric oxide synthase (iNOS)
in endothelial cells, as well as the production of nitric oxide
(28). Inhibiting this signaling
pathway may reduce the production of iNOS and additional cytokines,
as well as preventing the transport of the apoptotic protein BAX
into mitochondria, thereby reducing injury to endothelial cells
(28). In addition, p38a may
promote apoptosis by activating the tumor suppressor gene p53, the
proto-oncogene c-myc and additional important genes,
including stat1 and PKB (29).
Taking these observations into account, the authors hypothesize
that the p38a kinase, which is highly expressed in BMECs during LC,
may be an important factor in the injury and apoptosis of these
cells. In support of this notion, Zhou et al (30) demonstrated that p38a was highly
expressed in patients with bone marrow hypoplasia, and it was found
to regulate apoptosis in hematopoietic stem cells. These results
are consistent with those presented in the current study.
Studies have indicated that specific cytokines may
function as critical regulators of p38a expression in BMECs during
LC. For example, transforming growth factor-β (TGFβ) has been
observed to be an important factor that activates the p38-MAPK
signaling pathway (31). Induction
of TGFβ expression is one of the most important factors for
promoting the onset of LC, and is maintained at a higher level in
the blood during LC (32).
Therefore, the blood environment during LC may provide favorable
conditions for the activation of the p38-MAPK signaling pathway in
BMECs. In addition, TNFα is a potential factor that may activate
p38a. Studies have demonstrated that serum TNFα levels in LC
patients are significantly higher when compared with normal healthy
individuals, and TNFα is reportedly capable of regulating bone
repair following injury by activating p38a (33). Of particular note, TNFα and p38a
interact and activate caspase 3 to induce endothelial cell
apoptosis (34). Nagila et
al (35) demonstrated that
p38a inhibitors may reduce TNFα-induced apoptosis during dengue
fever-induced liver damage. Therefore, TNFα may serve an important
role in p38a-mediated BMEC apoptosis during LC. In addition,
endotoxins may exacerbate bone marrow damage during LC (22). Portal hypertension during LC leads
to reduced intestinal blood perfusion and damage to intestinal
mucosa barrier function, which results in bacterial translocation
(25,36). As a consequence, a large quantity
of intestinal toxins enter the blood, and lipopolysaccharide (LPS),
the major component of endotoxin, may damage the vascular
endothelial system and affect hematopoiesis in the bone marrow
(37). However, whether TGFβ,
TNFα, LPS or additional factors induce the high expression levels
of p38a kinase observed in BMECs during LC requires further
investigation and verification.
In recent years, increased research has focused on
protein kinases as therapeutic targets for a number of diseases,
and the development of novel kinase inhibitors is growing rapidly.
In addition, research into p38a inhibitors has progressed rapidly,
and SB203580 is the most representative and widely used p38a
inhibitor. Furthermore, compared with other p38a inhibitors,
SB203580 exhibits a more specific inhibitory effect (38). Therefore, SB203580 was employed as
a p38a kinase inhibitor for the purposes of the present study.
Following the administration of SB203580, vWF expression in bone
marrow smear samples was significantly decreased compared with that
of the LF group, which indicates that inhibition of p38a kinase may
reduce injury to the bone marrow endothelium during LF. In
addition, the bone marrow tissue apoptotic rate and caspase 3
expression in the SB203580 group were significantly reduced
compared with that of the control group, which suggests that p38a
kinase may be an important factor that induces bone marrow tissue
apoptosis during LF. Consistent with these results, Zhou et
al (30) demonstrated that use
of the p38a inhibitor may reduce hypoplastic bone marrow tissue
apoptosis and stimulate hematopoiesis. The results of the present
study associated with the application of SB203580 suggest the
ability of p38a kinase to promote bone marrow tissue injury and
apoptosis during LF, and the p38 kinase may therefore be a novel
therapeutic target for treating the abnormal blood cell count
observed during LC.
In conclusion, the present study performed whole
genome and bioinformatics analyses to identify 14 differentially
expressed genes that encode protein kinases in BMECs exposed to
serum from patients with LC. Using a common bile duct ligation
method, a rat model of LF was established successfully.
Immunohistochemical analytical methods demonstrated that the
expression levels of vWF, which is an indicator of endothelial cell
injury, were significantly increased in the bone marrow smears of
rats with LF compared with those of normal rats. In addition, the
rate of apoptosis in rats with LF was also significantly increased
compared with the controls. However, administration of the p38a
inhibitor, SB203580, in rats with LF may improve these abnormal
phenotypes to a certain degree. These results suggest that p38a may
serve an important role in the damage and apoptosis of BMECs and
bone marrow tissue during LF. However, further extensive
experiments are required to elucidate the specific mechanisms
involved in these processes.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81370566, 81170397,
81570579 and 81570581) and the Foundation of The First Affiliated
Hospital of Harbin Medical University (grant no. 2011BS018).
References
|
1
|
Qamar AA, Grace ND, Groszmann RJ,
Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R,
Escorsell A, et al: Incidence, prevalence, and clinical
significance of abnormal hematological indices in compensated
cirrhosis. Clin Gastroenterol Hepatol. 7:689–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rois R, Sangro B, Herrero I, Quiroga J and
Prieto J: The role of thrombopoietin in the thrombocytopenia of
patients with live cirrhosis. Am J Gastroenterol. 100:1131–1136.
2005.
|
|
3
|
Sheehy T and Berman A: The anemia of
cirrhosis. J Lab Clin Med. 56:72–82. 1960.PubMed/NCBI
|
|
4
|
Mas VR, Fassnacht R, Archer KJ and Maluf
D: Molecular mechanisms involved in the interaction effects of
alcohol and hepatitis C virus in liver cirrhosis. Mol Med.
16:287–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramachandran R and Kakar S: Histological
patterns in drug-induced liver disease. J Clin Pathol. 62:481–492.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sullivan LW and Herbert V: Suppression of
hematopoiesis by ethanol. J Clin Invest. 43:2048–2062. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenfeld SJ and Young NS: Viruses and
bone marrow failure. Blood Rev. 5:71–77. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao B, Wang D, Ma W, Jia X, Ma B, Zhang W
and Xue D: MicroRNA-mRNA regulatory network study and apoptosis
analysis on bone marrow endothelial cells induced by liver
cirrhosis serum. Clin Res Hepatol Gastroenterol. 38:451–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao B, Sun W, Wang X, Jia X, Ma B, Chang
Y, Zhang W and Xue D: Whole genome expression profiling and
screening for differentially expressed cytokine genes in human bone
marrow endothelial cells treated with humoral inhibitors in liver
cirrhosis. Int J Mol Med. 32:1204–1214. 2013.PubMed/NCBI
|
|
10
|
Kopp HG, Avecilla ST, Hooper AT and Rafii
S: The bone marrow vascular niche: Home of HSC differentiation and
mobilization. Physiology (Bethesda). 20:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafii S, Shapiro F, Rimarachin J, Nachman
RL, Ferris B, Weksler B, Moore MA and Asch AS: Isolation and
characterization of human bone marrow microvascular endothelial
cells: Hematopoietic progenitor cell adhesion. Blood. 84:10–19.
1994.PubMed/NCBI
|
|
12
|
Li WM, Huang WQ, Huang YH, Jiang DZ and
Wang QR: Positive and negative haematopoietic cytokines produced by
bone marrow endothelial cells. Cytokine. 12:1017–1023. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hubbard SR and Till JH: Protein tyrosine
kinase structure and function. Annu Rev Biochem. 69:373–398. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein complement of the human genome.
Science. 298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheung CH, Coumar MS, Chang JY and Hsieh
HP: Aurora kinase inhibitor patents and agents in clinical testing:
An update (2009–10). Expert Opin Ther Pat. 21:857–884. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zempleni J and Mock DM: Chemical synthesis
of biotinylated histones and analysis by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis/streptavidin-peroxidase.
Arch Biochem Biophys. 371:83–88. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiu CT, Chen JH, Chou FP and Lin HH:
Hibiscus sabdariffa leaf extract inhibits human prostate cancer
cell invasion via down-regulation of Akt/NF-kB/MMP-9 pathway.
Nutrients. 7:5065–5087. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Xia W, Lu M, Gao B, Qiao X, Sun
B, Zhang W, Zhang Y and Xue D: Role of kinase epidermal growth
factor receptor and SRC in the caerulein-induced acute pancreatitis
in mice. Pancreas. 44:152–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Ma B, Lu M, Qiao X, Sun B, Zhang W
and Xue D: Construction of network for protein kinases that play a
role in acute pancreatitis. Pancreas. 42:607–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hofberger JA, Nsibo DL, Govers F,
Bouwmeester K and Schranz ME: A complex interplay of tandem- and
whole-genome duplication drives expansion of the L-type lectin
receptor kinase gene family in the brassicaceae. Genome Biol Evol.
7:720–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Draghetti C, Salvat C, Zanoguera F,
Curchod ML, Vignaud C, Peixoto H, Di Cara A, Fischer D, Dhanabal M,
Andreas G, et al: Functional whole-genome analysis identifies
Polo-like kinase 2 and poliovirus receptor as essential for
neuronal differentiation upstream of the negative regulator
alphaB-crystallin. J Biol Chem. 284:32053–32065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao S, Fu YM, Li XF, Jin ZF, Zhao RB,
Huang Q, Zhang FM and Zhang WH: Alterations of bone marrow
sinusoidal endothelium in rat and patients with liver cirrhosis.
Dig Dis Sci. 55:654–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagner DD and Frenette PS: The vessel wall
and its interactions. Blood. 111:5271–5281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blann AD: von Willebrand factor and the
endothelium in vascular disease. Br J Biomed Sci. 50:125–134.
1993.PubMed/NCBI
|
|
25
|
Gao B, Li ZT, Xue DB and Zhang WH: A novel
mechanism of abnormal hematological indices in liver cirrhosis:
Bone marrow endothelial cell dysfunction caused by humoral
inhibitor affects the hematopoietic function of bone marrow. Med
Hypotheses. 82:282–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji RR, RW IV Gereau, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Rev. 60:135–148.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kevil CG, Oshima T and Alexander JS: The
role of p38 MAP kinase in hydrogen peroxide mediated endothelial
solute permeability. Endothelium. 8:107–116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: Apigenin induces c-Myc-mediated apoptosis in FRO
anaplastic thyroid carcinoma cells. Mol Cell Endocrinol.
369:130–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, Opalinska J and Verma A: p38 MAP
kinase regulates stem cell apoptosis in human hematopoietic
failure. Cell Cycle. 6:534–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo YH, Ouyang PB, Tian J, Guo XJ and Duan
XC: Rosiglitazone inhibits TGF-β 1 induced activation of human
Tenon fibroblasts via p38 signal pathway. PLoS One. 9:e1057962014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang M, Crisostomo PR, Herring C, Meldrum
KK and Meldrum DR: Human progenitor cells from bone marrow or
adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a
p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp
Physiol. 291:R880–R884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu J, Eto M, Akishita M, Okabe T and Ouchi
Y: A selective estrogen receptor modulator inhibits
TNF-alpha-induced apoptosis by activating ERK1/2 signaling pathway
in vascular endothelial cells. Vascul Pharmacol. 51:21–28. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagila A, Netsawang J, Suttitheptumrong A,
Morchang A, Khunchai S, Srisawat C, Puttikhunt C, Noisakran S,
Yenchitsomanus PT and Limjindaporn T: Inhibition of p38MAPK and
CD137 signaling reduce dengue virus-induced TNF-α secretion and
apoptosis. Virol J. 10:1052013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Douhara A, Moriya K, Yoshiji H, Noguchi R,
Namisaki T, Kitade M, Kaji K, Aihara Y, Nishimura N, Takeda K, et
al: Reduction of endotoxin attenuates liver fibrosis through
suppression of hepatic stellate cell activation and remission of
intestinal permeability in a rat non-alcoholic steatohepatitis
model. Mol Med Rep. 11:1693–1700. 2015.PubMed/NCBI
|
|
37
|
Meng F, Meliton A, Moldobaeva N, Mutlu G,
Kawasaki Y, Akiyama T and Birukova AA: Asef mediates HGF protective
effects against LPS-induced lung injury and endothelial barrier
dysfunction. Am J Physiol Lung Cell Mol Physiol. 308:L452–L463.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bühler S and Laufer SA: p38 MAPK
inhibitors: A patent review (2012–2013). Expert Opin Ther Pat.
24:535–554. 2014. View Article : Google Scholar : PubMed/NCBI
|