Introduction
Gastric cancer is one of the most common malignant
tumors in China. It has the highest morbidity and mortality rates
of malignant tumors of the digestive system. Lymph node metastasis
often represents the first step in the metastasis process. A
previous study has indicated that the rate of metastatic lymph
nodes was 5% in early stages, and can reach 70% in advanced gastric
cancer (1).
Therefore, elucidation of the carcinomatous
metastasis mechanism may enable development of more effective
measures to suppress tumor metastasis, thus, improving the
prognosis of patients with gastric cancer.
Previous studies have also demonstrated that the
inflammatory response is an important feature of malignant
neoplasms, and it is important in the genesis, growth and
metastasis of tumors (1,2). Chemokines are a large family,
including numerous members, researchers have identified >50
types of chemokines (3).
Chemokines mediate directional chemotactic movement during the
inflammatory process as the predominant inflammatory factors. CXCLl
belongs to the chemotactic superfamily and is expressed in
neutrophils, macrophages, and epithelial cells. It has been
observed in melanoma tumors, and are involved in the carcinogenesis
of melanoma. CXCL1 specifically binds to the CXC chemokine
receptor, CXC motif chemokine receptor 2 (CXCR2), which is a member
of the G protein-coupled receptor family (4).
Previous studies have demonstrated CXCLl is
important in the oncogenesis and development of malignant tumors.
CXCL1 is upregulated in melanoma, ovarian, colorectal and bladder
cancer, and other tumors (5–8).
Numerous studies investigating the expression of CXCLl in the
occurrence and development process of gastric cancer have produced
consistent results (9–11). By comparing gene expression of the
gastric cancer and adjacent tissues, it was observed that CXCLl is
expressed in a high percentage of gastric cancer tissue samples,
and it is associated with tumor stage and the prognosis of patients
(12,13).
A previous study (12) also observed a high expression of
CXCR2 in gastric cancer tissue, and its positive correlation with
TNM classification of gastric cancer and lymphatic vessel density
(LMVD) indicates CXCLl and its receptor CXCR2 may be important in
lymphatic metastasis. Furthermore, tumor cells may promote CXCLl
expression in the endothelial cells of efferent lymphatic vessels,
in order to promote a suitable microenvironment for tumor
metastasis. Tumor cells may express CXCR2 and metastasize to
lymphatic vessels under the attraction of CXCL1. Few studies have
investigated the mechanism of the chemokine CXCLl/CXCR2 signaling
pathway, thus, identifying specific underlying mechanisms may offer
novel ideas for the treatment of gastric cancer with lymph node
metastasis.
Materials and methods
Cell lines
The human gastric cancer cell lines, including
HGC803, BGC823, AGS, MGC803, SGC7901 and MKN45 cells, were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and streptomycin/penicillin (100 mg/ml and 100
U/ml, respectively) at 37°C in 5% CO2 humidified atmosphere.
The normal human gastric cell line, GES-1 gastric
epithelial cells were purchased from the Cell Bank of Chinese
Academy of Sciences and used as the control for the
experiments.
Patients
Informed consent was obtained from 100 patients
(age, 18 to 75) diagnosed with gastric cancer at the First
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China)
between 2007 to 2008 who were enrolled in the current study. Each
patient had undergone gastrectomy with extended (D2) removal of
regional lymph nodes. No patients had received adjuvant
chemotherapy, radiation therapy or other biological therapy. The
present study was approved by the Ethics Committee of the First
Affiliated Hospital, Sun Yat-sen University (Guangzhou, China).
Exclusive criteria were as follows: i) <18 or
>75 years of age; ii) patients with severe medical diseases or
heart, lung, liver and kidney disorders; iii) patients with major
postoperative complications; and iv) dissected lymph node number of
<15. All the patients were followed until December 31, 2013. All
specimens were fixed by formalin and embedded in paraffin. The
clinicopathological characteristics and prognosis of patients were
recorded in the hospital database.
Trial grouping in vivo and in vitro
experiments
For the in vitro experiment, three groups
were designed, including the CXCL1-siRNA group (with the siRNA to
silence the CXCL1 gene), the HGC803 group (blank cells) and the
CXCL1 stimuli group [human CXCL1 α; obtained from R&D Systems
(Minneapolis, MN, USA)]. For the CXCL1-siRNA group, the HGC803
cells were treated with 0.5 µg CXCL1 siRNA. For the CXCL-1 stimuli
group, the HGC803 cells were treated with 10 µg human CXCL1 α.
For the in vivo experiment, the CXCL1
expression levels in 100 gastric cancer patients were analyzed
using an immunohistochemistry assay, and overall survival analysis
and correlation analysis were conducted.
Western blotting
The HGC803 cells were homogenized in ice-cold
radioimmunoprecipitation lysis buffer (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) containing the cocktail of protease
inhibitor (Roche Diagnostics, Basel, Switzerland). Lysates were
centrifuged at 500 × g for 5 min at room temperature to
obtain the protein. The extracted protein was quantified using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China). The quantified protein was separated
by 15% SDS-PAGE (0.2 µg protein per well) and transferred to
nitrocellulose membranes and blocked overnight at 4°C in 5% milk.
The blocked membranes were incubated at 4°C overnight with the
mouse anti-human CXCL1 monoclonal antibody (1:4,000; cat. no.
20326R; BD Biosciences, San Jose, CA, USA). Subsequently, the
membranes were incubated with goat anti-rabbit IgG (1:2,000; cat.
no. sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
rabbit anti-mouse IgG (1:2,000; cat no. sc-358920; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. Immunodetection was performed
using the Amersham ECL Plus (GE Healthcare Life Sciences, Chalfont,
UK). The blots were scanned and the pixel count and intensity of
each band was quantified using the Scion image software (version
4.2.3.2; Scion Corporation, Frederick, MD, USA). The signals were
normalized to GAPDH levels using a mouse anti-human GAPDH
monoclonal antibody (cat. no. sc-365062; Santa Cruz Biotechnology,
Inc.).
Wound healing assay
The HGC803 cells were plated in 6-well plates at a
density of 1×105 cells/well and grown to ~80% confluency. The
monolayer was scraped with a sterile 200 µl pipette tip following
removal of the culture medium. Subsequently, the culture was washed
twice with serum-free medium. Cells were maintained in DMEM. Images
of the scratched areas were captured at 0 and 24 h after wounding
using computer-assisted optical microscopy. Cell migration was
calculated as the percentage of cell coverage compared to the
initial cell-free zone.
Transwell invasion assay
HGC803 cell invasion was evaluated using a Transwell
chamber (Costar; Corning Incorporated, Corning, NY, USA) equipped
with a Matrigel-coated filter membrane (8 µm pores). Briefly, the
filters were pre-coated with 0.5 µg basement membrane proteins
(Matrigel; BD Biosciences) and allowed to dry overnight at room
temperature. Cells in FBS-free medium were seeded in the upper
chambers, and lower wells contained medium with 10% FBS. Following
incubation at 37°C for 24 h, non-migratory cells on the upper side
of the insert were removed with a cotton swab. Cells that had
passed through the filter were fixed in methanol and stained with
hematoxylin. For quantification, images were captured of six
randomly selected fields on the lower side of the insert using
computer-assisted optical microscopy.
Immunohistochemistry
The tissues were frozen and then sliced into 4-µm
sections. In the sections, cell nuclei or cytoplasm stained yellow
to yellow-brown were considered as positive. The cells were
incubated with rabbit anti-human CXCL1 monoclonal antibody (1:3,000
dilution; cat. no. sc-2778; Santa Cruz Biotechnology, Inc.) and
mouse anti-human D2-40 monoclonal antibody (1:3,000 dilution; cat.
no. 182410; Invitrogen; Thermo Fisher Scientifc, Inc.) at 37°C for
2 h. Content determination results were analyzed by HMIAS-2000
automatic medical color image analysis system (Qianping Image
Technology Co., Ltd., Wuhan, China). Sections were observed using
light microscopy, 5 randomly selected high power fields were
observed and grey values of specimens were obtained following image
segmentation, editing and statistics scoring criteria. Stained
area: 0, stained cell area of ≤10% of overall cell area; 1, 11–25%;
2, 26–50%; 3, 51–75%; 4, >75%. Staining intensity: 0, without
staining; 1, yellow or light brown; 2, brown; 3, dark brown. The
total score of each section was defined as the product of the
stained area score and the staining intensity score, a score of ≤3
was negative, while 3–12 was positive.
Determination of lymphatic vessel
density in gastric cancer and peri-carcinoma region
Immunohistochemical staining (as described above)
with the D2–40 monoclonal antibody (1:200) was used for detection
of lymphatic vessels. LMVD in gastric cancer and the peri-carcinoma
region was determined by counting lymph vessels. D2-40-positive
lymphatic vessel endothelial cells were observed by microscope and
appeared brown. Microlymphatic vessels were counted by Weidtler
calculation standard, first, a comprehensive observation of the
section was conducted at a lower magnification (×100) to observe
new vessels, the most densely populated areas were determined to be
‘hot spots’, which were subsequently stained purple (by the
hematoxylin-eosin staining method) (6,8) and
observed at the single cell and cell cluster level at high
magnification (×200). The mean value was determined to be the
section LMVD value.
Statistical analysis
Analysis of data was conducted using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA), the association between expression
of CXCL1, D2-40 and clinicopathological features were investigated
by χ2 test. The patient 5-year survival rate was
determined by Kaplan-Meier analysis. The Cox regression model was
established to analyze the peaks of metastasis and relapse, and the
life curves of patients. The group data were presented as the mean
± standard error of the mean. P<0.05 was considered to indicate
a statistically significant difference.
Results
CXCL1 protein expression was increased
in gastric cancer cell lines
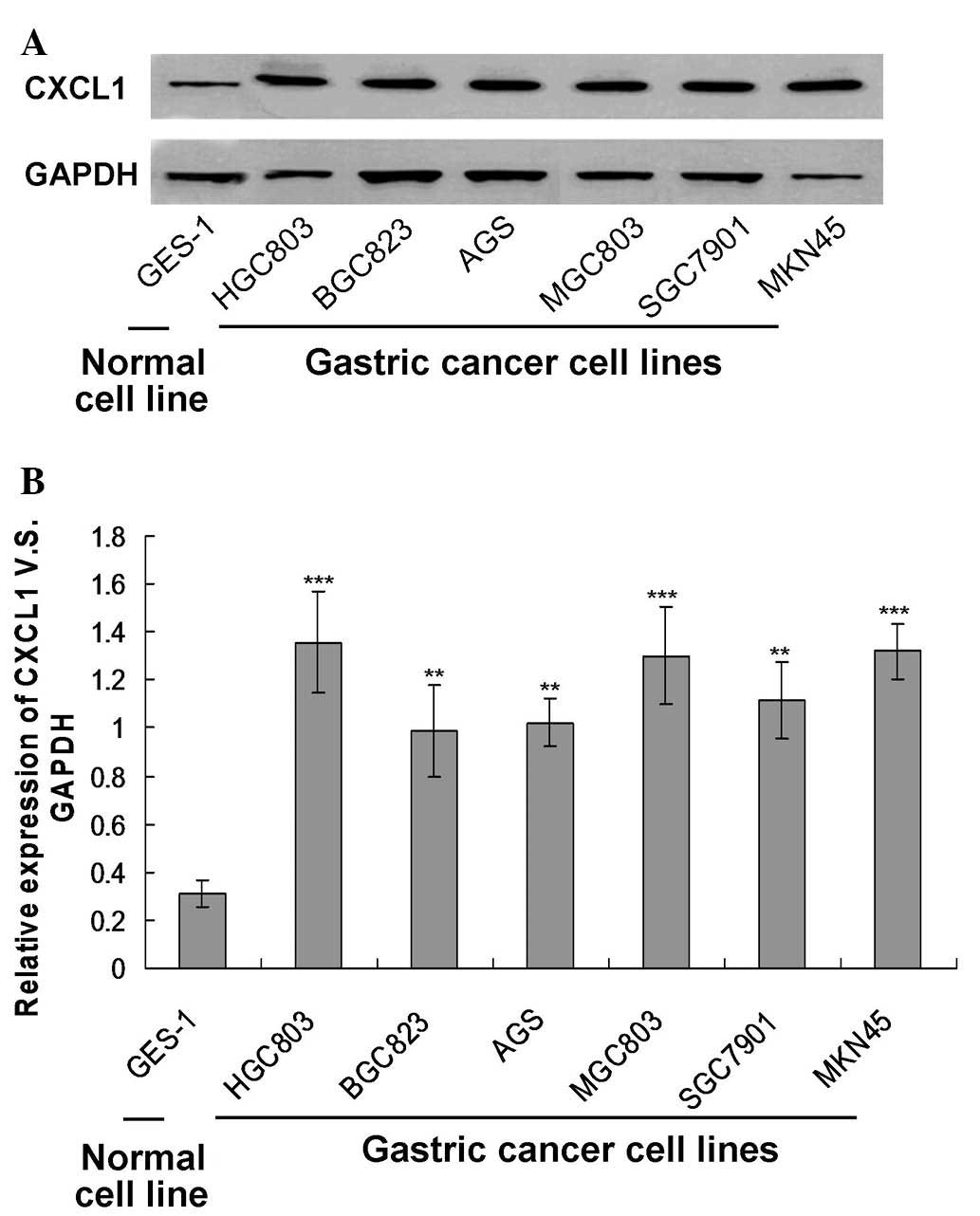
In order to investigate the association between
CXCL1 protein expression and the gastric cancer cell lines, protein
expression was examined by western blotting (Fig. 1A). The results indicated that the
CXCL1 protein was expressed in all the gastric cancer cell lines,
and the levels were significantly higher compared to the levels in
the GES-1 normal cell line (Fig.
1B; P<0.01). Furthermore, it was observed that the HGC803
cell line expressed the highest level of CXCL1. Thus, in the
subsequent in vitro experiments, the HGC803 cell line was
used.
Silencing of the CXCL1 gene reduced
migratory and invasive ability of HGC803 cells
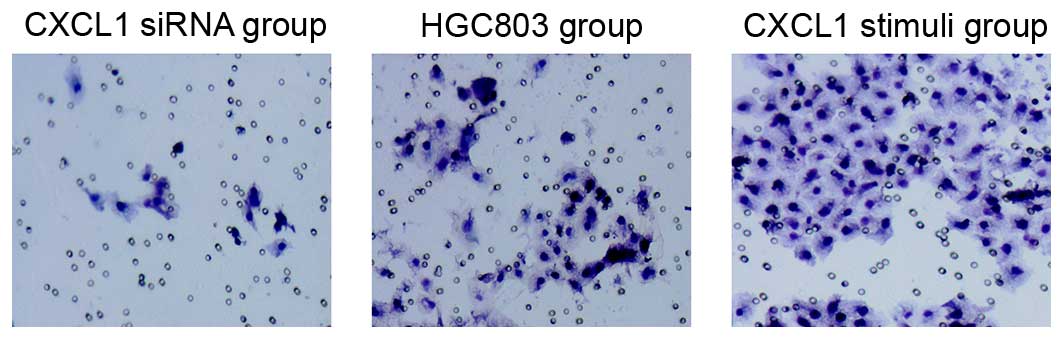
To assess the migratory ability of CXCL1 siRNA
silenced HGC803 cells, a wound healing and a Transwell migration
assay were used. The Transwell migration assay demonstrated that
the number of migrated cells in the HGC803 control group were
significantly higher when compared with the CXCL1 stimuli cells
(Fig. 2). Few invaded cells were
observed in the CXCL1-siRNA groups compared with the HGC803 control
group (Fig. 2).
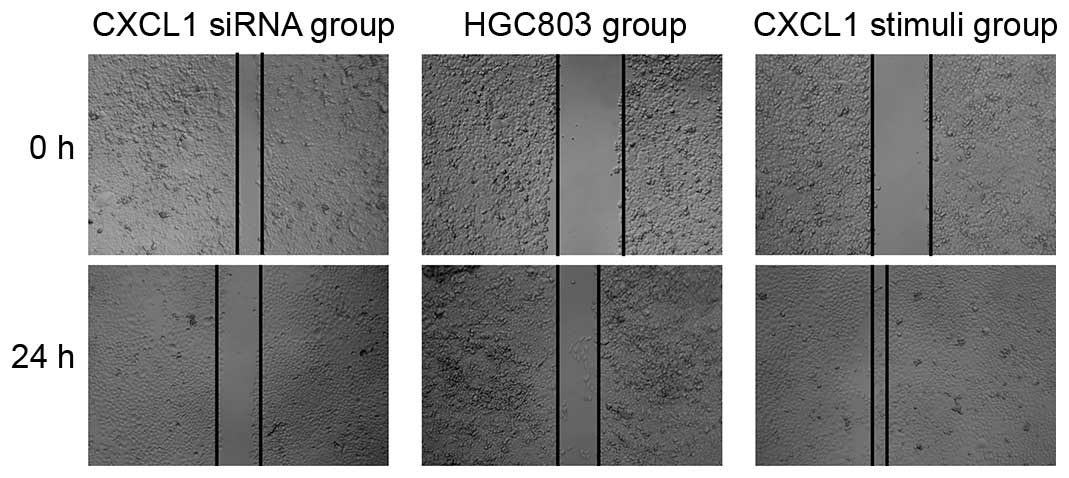
The results demonstrated that the wounds in the
CXCL1 stimuli group were almost closed at the 24 h time point, yet
the wounds in the HGC803 control group remained observable
(Fig. 3). Notably, the wounds in
the CXCL1-siRNA group were enlarged after 24 h treatment (Fig. 3). These results suggest knockdown
of CXCL1 suppressed cell invasion.
Clinical pathology data for gastric
cancer patients
In the present study, tumor size, tumor location,
classification and differentiation were observed. The
clinicopathological correlations of plasma CXCL1 expression and
lymphatic vessel density are presented in Table I.
 | Table I.Clinicopathological associations
between plasma CXCL1 expressions and LMVD in 100 gastric cancer
patients. |
Table I.
Clinicopathological associations
between plasma CXCL1 expressions and LMVD in 100 gastric cancer
patients.
|
|
| Plasma CXCL1 | LMVD |
|---|
|
|
|
|
|
|---|
| Parameters | n | Negative | Positive | P-value |
| P-value |
|---|
| Gender |
|
|
| 0.601 |
| 0.970 |
| Male | 65 | 39 | 26 |
| 8.23±3.47 |
|
|
Female | 35 | 20 | 15 |
| 9.31±2.23 |
|
| Age (years) |
|
| 0.523 |
|
| 0.440 |
|
<60 | 40 | 25 | 15 |
| 9.24±2.34 |
|
| ≥60 | 60 | 34 | 26 |
| 9.03±1.68 |
|
| Tumor size (cm) |
|
| 0.290 |
|
| 0.003 |
|
<4 | 48 | 31 | 17 |
| 5.55±2.56 |
|
| ≥4 | 52 | 28 | 24 |
| 10.38±2.88 |
|
| Location |
|
| 0.195 |
|
| 0.876 |
| Proximal
gastric cancer | 18 | 11 | 7 |
| 11.53±3.23 |
|
| Gastric
body cancer | 29 | 18 | 11 |
| 9.34±1.38 |
|
| Distal
gastric cancer | 53 | 30 | 23 |
| 8.78±2.56 |
|
| T classification |
|
| 0.031 |
|
| 0.002 |
|
T1/T2 | 36 | 31 | 5 |
| 4.56±1.23 |
|
|
T3/T4 | 64 | 28 | 36 |
| 10.23±2.31 |
|
| WHO
classification |
|
| 0.062 |
|
| 0.890 |
|
Adenocarcinoma | 71 | 43 | 28 |
| 8.45±2.35 |
|
| Mucinous
adenocarcinoma | 18 | 10 | 8 |
| 9.56±1.23 |
|
| Signet
ring cell carcinoma | 11 | 6 | 5 |
| 10.12±3.78 |
|
| Differentiated
degree |
|
| <0.001 |
|
| 0.04 |
|
High/middle
differentiation | 38 | 30 | 8 |
| 6.03±1.39 |
|
| Low/no
differentiation | 62 | 29 | 33 |
| 11.86±3.67 |
|
| N classification |
|
| <0.001 |
|
| 0.02 |
|
Negative | 42 | 34 | 9 |
| 5.37±2.11 |
|
|
Positive | 58 | 25 | 32 |
| 12.04±3.88 |
|
| TNM
classification |
|
| <0.001 |
|
| <0.001 |
|
I/II | 43 | 33 | 10 |
| 4.06±1.56 |
|
|
III/IV | 57 | 26 | 31 |
| 12.23±3.31 |
|
CXCL1 expression in gastric tumor
tissues
The present study included a total of 100 patients,
and the expression levels of CXCL1 and the clinicopathological
characteristics are presented in Table
I. The rate of patients with positive CXCL1 expression is 41%.
Expression of CXCL1 has no marked association with gender, age,
tumor location or tumor diameter, however, it is closely associated
with degree of tumor differentiation, lymph node metastasis and TNM
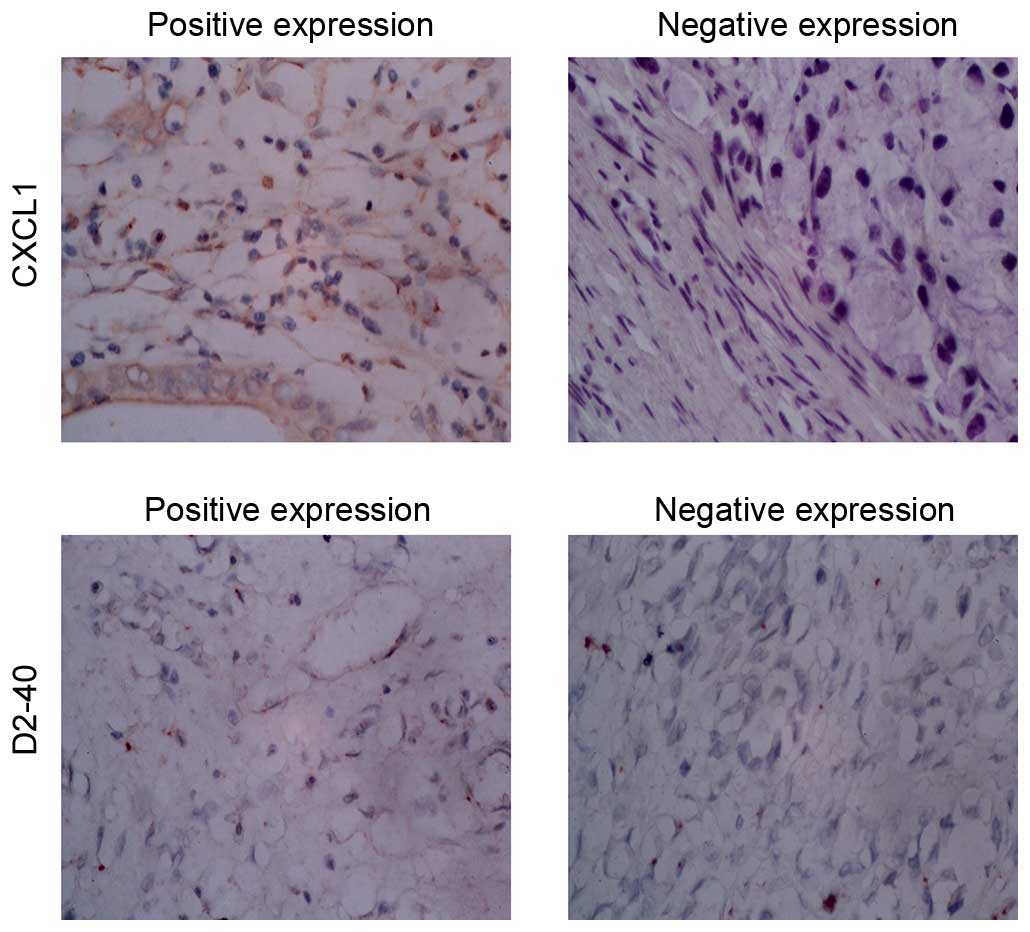
classification (P<0.05). Immunohistochemical staining
demonstrated that expression of CXCL1 was detected in the cell
cytoplasm (Fig. 4). The CXCL1
positive group had significantly higher TNM stage and poorer
pathological differentiation.
Expression of D2-40 in gastric cancer
and the peri-carcinoma region
D2-40 positive staining of lymphatic vessels in the
normal gastric mucosa were located in the deeper layers of the
mucosa and close to submucosal muscular layer, with larger lumen
and thicker walls. New lymphatic vessels were only observed between
the inherent glands of the mucosa lamina propria, as presented in
Fig. 4. New D2-40 positive
staining of lymphatic vessels was yellow brown, located in the
tumor stroma and no red blood cells observed. At low magnification
(×100), five intense staining areas were selected and 100 positive
cells in each field were observed at high magnification. The
average percentage of positive cells from the five areas indicate
the LMVD results and are presented in Table I.
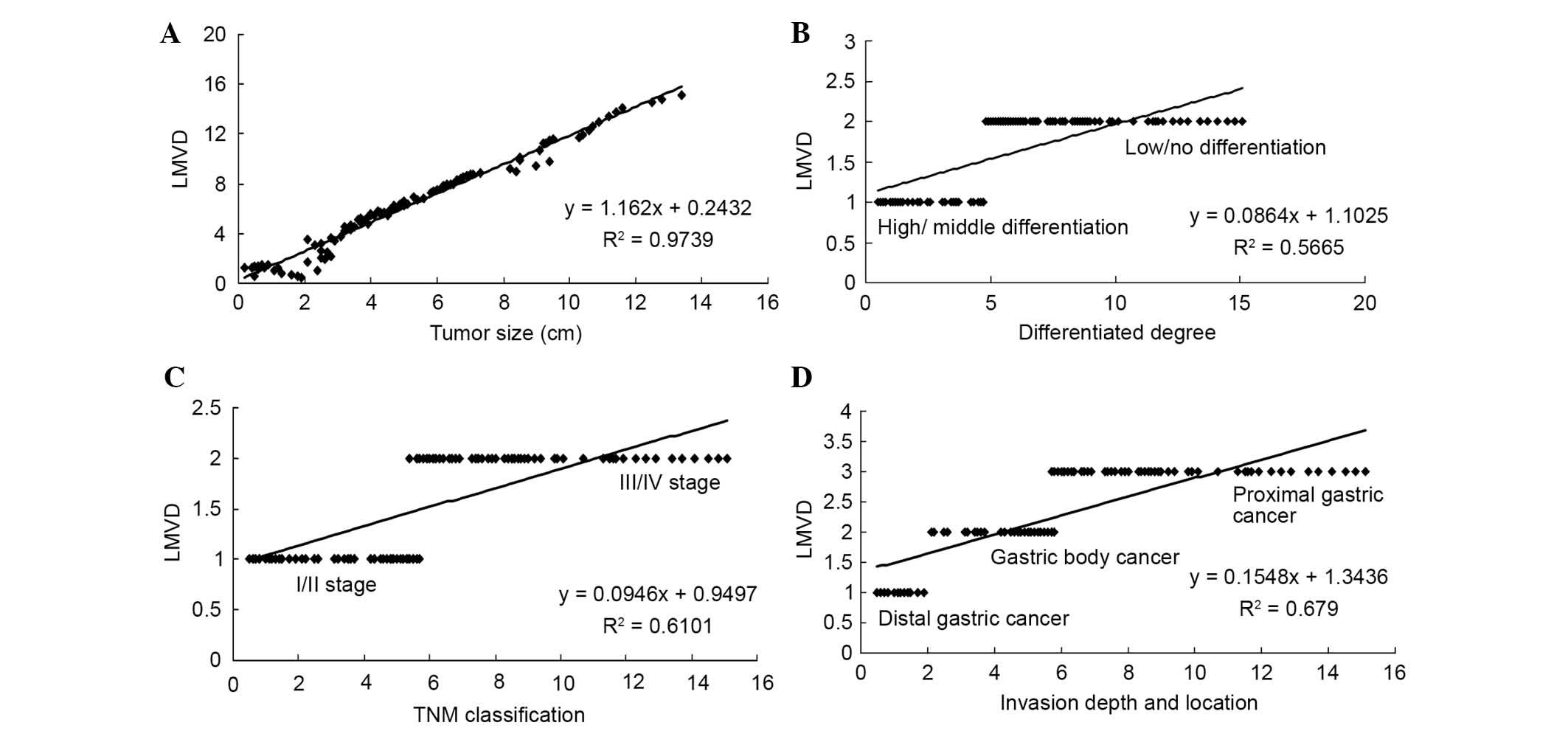
Correlation between LMVD and
pathological biological characteristics
LMVD positively correlated with gastric cancer
lesion size, degree of differentiation, clinical TNM stage and
depth of invasion (Fig. 5;
P<0.05), while there is no notable correlation between lymphatic
vessel density (LMVD) and age, gender, WHO classification or tumor
location.
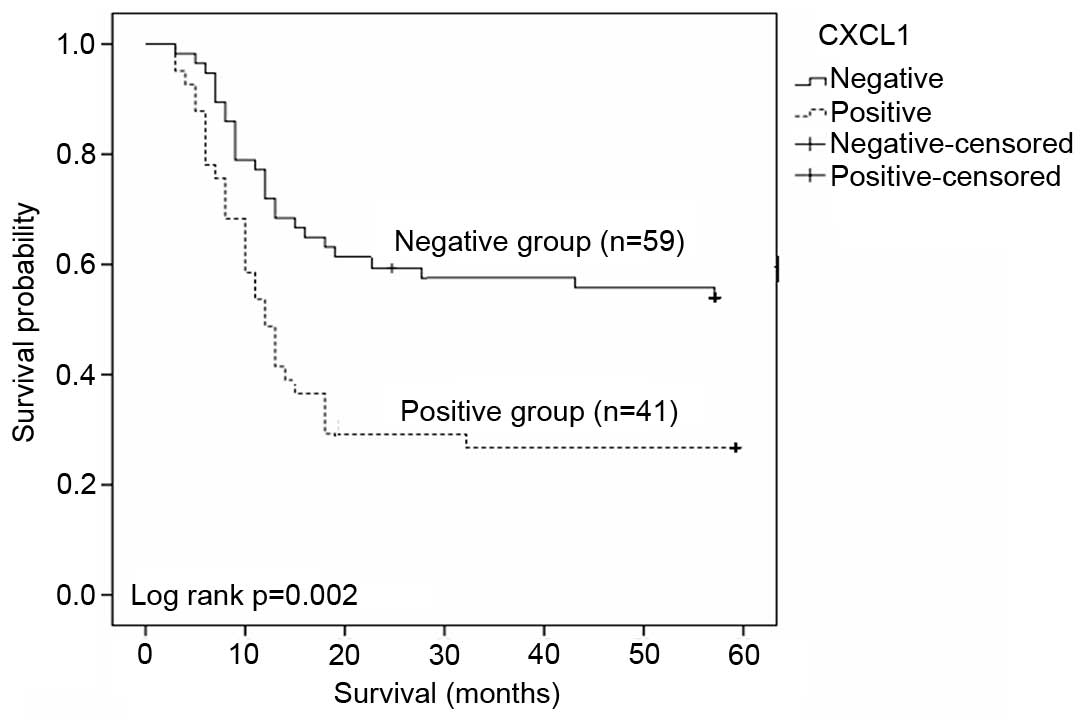
CXCL1 protein expression reflects
gastric cancer survival rate
The 5-year survival rate was determined by
Kaplan-Meier analysis. CXCL1 positive group (n=41) exhibited poorer
survival rates in 5 years than the negative group (n=59) as
presented in Fig. 6 (P=0.002). The
total mean survival time of the negative group was 40 months, with
that of positive group typically ~23.2 months. This is a
statistically significant difference.
Analysis of independent prognostic
factor of patients with radical gastrectomy of gastric cancer
The Cox regression model was established to analyze
the peaks of metastasis and relapse, and the life curves of
patients (Table II). Following
multivariate analysis, CXCL1, differentiation degree, TNM stage and
LMVD may be used as independent prognostic factors.
 | Table II.Results from multivariate analysis of
Cox regression models. |
Table II.
Results from multivariate analysis of
Cox regression models.
| Parameters | P-value | Relative risk | 95% CI |
|---|
| Differentiation
degree |
0.04 | 2.204 | 0.818–3.048 |
| TNM
classification | 0.005 | 2.236 | 1.468–4.379 |
| CXCL1 | 0.003 | 1.904 | 1.406–4.148 |
| LMVD |
0.02 | 1.616 | 1.168–3.779 |
Discussion
Lymphatic metastasis is a major mechanism of
metastasis of malignant tumors, and key in determination of
prognosis (14). Lymphangiogenes
is a process of developing new capillary lymphatic vessels by
budding and further differentiation. Vasculature are at a stable
state following maturity, new blood vessels or the lymphatic
vessels are formed only in certain conditions, such as embryonic
development, inflammation, wound healing, and tumors. No
development of lymph node metastases is generally due to lack of
lymphatic vessels at the tumor site in earlier stages of the
neoplastic process.
Chemokines are members of the cytokine superfamily,
which are small molecule proteins that induce chemotaxis (15). According to different positions of
four conservative cysteine residues in its N terminal region,
chemokines may be divided into A, 3, Y and S chemokine subfamilies.
A subfamily (also called CXC subfamily) includes IL-8, CXCL1,
CXCL2, and CXCL3. Via interaction with their receptors, chemokines
induce chemotactic migration of target cells, enhance the adhesive
capacity of endothelial cells to target cells, and participate in
cellular functions, including proliferation, apoptosis, invasion
and differentiation. Previous studies have demonstrated that
chemokines are involved in pathogenesis, progression, and
metastasis of carcinoma (16,17).
For example, IL-8 may regulate tumor angiogenesis, induce release
of proteolytic enzymes and stimulate the proliferation of tumor
cells by degradation of the extracellular matrix and basement
membrane, and promote the invasion and metastasis of gastric cancer
via autocrine or paracrine modes of action. At present, there has
been no investigation into the association between CXCL1 expression
and LMVD.
The present study analyzed the association between
CXCL1 expression and LMVD in clinical specimens of gastric cancer
tissue and clinicopathological features. Kaplan-Meier analysis and
the Cox regression model were used to analyze the association
between CXCL1 expression, LMVD and prognosis in gastric cancer. In
addition, the relevant independent prognostic factors were
investigated. Experimental results indicate that the CXCL1 positive
expression in patients with gastric cancer have higher LMVD, which
is positively correlated with tumor invasion, lymph node
metastasis, TNM stage and the degree of differentiation, indicating
the CXCL1 may participate in the pathogenesis and development of
gastric cancer. This finding was consistent with our previous study
(12). A number of previous
studies (15–17) have demonstrated that CXCL1 is
overexpressed in gastric cancer tissue, which is associated with
infiltration depth, and lymph node involvement, thus, affecting the
prognosis of patients, and that CXCL1 may promote lymphatic and
blood metastasis of gastric carcinoma. The current study also
observed that the D2-40-positive staining was predominantly
localized in lymphatic endothelial cells, while no expression of
D2-40 was observed in tumor cells. LMVD was higher in poorly
differentiated carcinomas than in well or moderately differentiated
cases. LMVD in gastric cancer with lymph node metastasis was
significantly higher than in those without lymph node metastasis.
Under similar conditions, LMVD in gastric cancer with positive
expression of CXCL1 is significantly higher than in gastric cancer
with negative expression of CXCL1. Significant positive correlation
was observed between expression of CXCL1 and LMVD in gastric cancer
tissues (P<0.05). These results indicate LMVD was closely
associated with gastric cancer, and has a significant correlation
with the size of carcinoma, classification, clinical TNM stages,
depth of invasion and lymph node metastasis. Thus, LMVD may be used
as an indicator for differential diagnosis and determination of
malignancy of gastric cancer, and may aid prediction of prognosis
and treatment decisions for patients with gastric cancer.
Furthermore, results of the Kaplan-Meier survival
curves demonstrated that the expression of CXCL1 and LMVD may
worsen prognosis as patients with positive expression exhibit a
significantly reduced 5-year survival rate than those with negative
expression. The experimental results obtained are consistent with
results of our previous study (12). Results of the further Cox survival
analysis indicated that expression of CXCL1, TNM stage and
differentiation degree and LMVD all can be used as independent
prognostic factors.
The results of the present study suggest that high
expression of CXCL1 in gastric cancer tissue was closely associated
with tumor differentiation and staging, indicating that CXCL1 may
be a potential tumor marker. At the earlier stages of the
neoplastic process, CXCL1 may affect the tumor microenvironment and
then promote the growth of tumor cells and angiogenesis, suggesting
that CXCL1 may also be used as a biomarker for monitoring clinical
effect. The current study identified the association between
expression of CXCL1 and gastric cancer growth, prognosis and the
clinicopathological features, which may aid elucidation of the
underlying mechanism of the CXCL1/CXCR2 signaling pathways and have
potential for therapeutic agent intervention.
In conclusion, CXCL1 gene silencing inhibited the
migration and invasion of HGC803 cells. The positive expression of
CXCL1 is correlated with advanced TNM stage, LMVD, tumor
differentiation, lymph node metastasis and poor survival. LMVD was
correlated with advanced TNM stage, size of tumor, lymph node
metastasis, tumor differentiation and poor survival. CXCL1 may be
an independent prognostic factor for gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Fund (grant no. 81272637).
References
|
1
|
Hua D, Shen L, Xu L, Jiang Z, Zhou Y, Yue
A, Zou S, Cheng Z and Wu S: Polypeptide
N-acetylgalactosaminyltransferase 2 regulates cellular
metastasis-associated behavior in gastric cancer. Int J Mol Med.
30:1267–1274. 2012.PubMed/NCBI
|
|
2
|
Bernal C, Aguayo F, Villarroel C, Vargas
M, Díaz I, Ossandon FJ, Santibáñez E, Palma M, Aravena E,
Barrientos C and Corvalan AH: Reprimo as a potential biomarker for
early detection in gastric cancer. Clin Cancer Res. 14:6264–6269.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhawan P and Richmond A: Role of CXCL1 in
tumorigenesis of melanoma. J Leukoc Biol. 72:9–18. 2002.PubMed/NCBI
|
|
4
|
Ravindran A, Sawant KV, Sarmiento J,
Navarro J and Rajarathnam K: Chemokine CXCL1 dimer is a potent
agonist for the CXCR2 receptor. J Biol Chem. 288:12244–12252. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yonemura Y, Fonseca L, Tsugawa K, Ninomiya
I, Matsumoto H, Sugiyama K, Ohoyama S, Fushida S, Kimura H and
Miyazaki I: Prediction of lymph node metastasis and prognosis from
the assay of the expression of proliferating cell nuclear antigen
and DNA ploidy in gastric cancer. Oncology. 51:251–257. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wallace AE, Sales KJ, Catalano RD,
Anderson RA, Williams AR, Wilson MR, Schwarze J, Wang H, Rossi AG
and Jabbour HN: Prostaglandin F2alpha-F-prostanoid receptor
signaling promotes neutrophil chemotaxis via chemokine (C-X-C
motif) ligand 1 in endometrial adenocarcinoma. Cancer Res.
69:5726–5733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang G, Rosen DG, Zhang Z, Bast RC Jr,
Mills GB, Colacino JA, Mercado-Uribe I and Liu J: The chemokine
growth-regulated oncogene 1 (Gro-1) links RAS signaling to the
senescence of stromal fibroblasts and ovarian tumorigenesis. Proc
Natl Acad Sci USA. 103:16472–16477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng WL, Wang CS, Huang YH, Liang Y, Lin
PY, Hsueh C, Wu YC, Chen WJ, Yu CJ, Lin SR and Lin KH:
Overexpression of a secretory leukocyte protease inhibitor in human
gastric cancer. Int J Cancer. 123:1787–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu CC, Chien KY, Tsang NM, Chang KP, Hao
SP, Tsao CH, Chang YS and Yu JS: Cancer cell-secreted proteomes as
a basis for searching potential tumor markers: Nasopharyngeal
carcinoma as a model. Proteomics. 5:3173–3182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goulding H, Pinder S, Cannon P, Pearson D,
Nicholson R, Snead D, Bell J, Elston CW, Robertson JF, Blamey RW,
et al: A new immunohistochemical antibody for the assessment of
estrogen receptor status on routine formalin-fixed tissue samples.
Hum Pathol. 26:291–294. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawanishi H, Matsui Y, Ito M, Watanabe J,
Takahashi T, Nishizawa K, Nishiyama H, Kamoto T, Mikami Y, Tanaka
Y, et al: Secreted CXCL1 is a potential mediator and marker of the
tumor invasion of bladder cancer. Clin Cancer Res. 14:2579–2587.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eck M, Schmausser B, Scheller K, Brändlein
S and Müller-Hermelink HK: Pleiotropic effects of CXC chemokines in
gastric carcinoma: Differences in CXCL8 and CXCL1 expression
between diffuse and intestinal types of gastric carcinoma. Clin Exp
Immunol. 134:508–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh S, Sadanandam A, Nannuru KC, Varney
ML, Mayer-Ezell R, Bond R and Singh RK: Small-molecue antagonists
for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing
tumor cell proliferation, survival, and angiogenesis. Clin Cancer
Res. 15:2380–2386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitadai Y, Haruma K, Mukaida N, Ohmoto Y,
Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ
and Tahara E: Regulation of disease-progression genes in human
gastric carcinoma cells by interleukin 8. Clin Cancer Res.
6:2735–2740. 2000.PubMed/NCBI
|
|
15
|
Zhi Y, Lu H, Duan Y, Sun W, Guan G, Dong Q
and Yang C: Involvement of the nuclear factor-kB signaling pathway
in the regulation of CXC chemokine receptor-4 expression in
neuroblastoma cells induced by tumor necrosis factor-α. Int J Mol
Med. 35:349–357. 2015.PubMed/NCBI
|
|
16
|
Salazar N, Castellan M, Shirodkar SS and
Lokeshwar BL: Chemokines and chemokine receptors as promoters of
prostate cancer growth and progression. Crit Rev Eukaryot Gene
Expr. 23:77–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wakabayashi S, Yamaguchi K, Kumakura S,
Murakami T, Someya A, Kajiyama Y, Nagaoka I and Inada E: Effects of
anesthesia with sevoflurane and proporol on the cytokine/chemokine
production at the airway epithelium during esophagectomy. Int J Mol
Med. 34:137–144. 2014.PubMed/NCBI
|