Introduction
Bladder cancer, which is the second most common
malignant tumor of the urinary system, is ranked as the fourth most
common cancer in males and the eighth leading cause of
cancer-associated deaths worldwide (1). Intravesical administration of
chemotherapy drugs and adjuvant chemotherapy after surgery have
been the standard treatment of bladder cancer for >20 years
(2). The five-year survival rate
of patients with bladder cancer is 72.8% in men and 69.3% in women
(3). Although chemotherapeutic
drugs, including doxorubicin, vincristine, cisplatin and
methotrexate, are widely used in the radical and palliative
treatment of human bladder cancer, multidrug resistance (MDR) is
one of the major drawbacks of bladder cancer chemotherapy. MDR is a
significant obstacle to successful cancer treatment, which
contributes to >90% of all treatment failures in patients with
bladder cancer (4). Therefore,
further studies regarding MDR in bladder cancer are urgently
required.
MDR is a complex process associated with numerous
factors in various types of cancer. In several instances,
resistance is achieved by an increased efflux of chemotherapeutic
agents out of the tumor. For example, P-glycoprotein (P-gp) and
multidrug resistance-associated protein (MRP) are two classical
efflux proteins, which result in drug resistance by pumping drugs
out of the cell. Alterations to the drug target enzyme activity are
also associated with drug resistance. Dysregulation of
topoisomerase II (Topo II) results in the dissociation of cleavable
complexes and reduction of DNA damage, which consequently induces
drug resistance. Apoptotic cell death is the most common underlying
mechanism of antineoplastic drugs; however, the excessive
expression of anti-apoptotic genes, such as p53, may lead to
chemotherapy resistance. Microenvironmental resistance is another
important factor; changes to the tumor microenvironment due to the
action of vascular endothelial growth factor (VEGF) may also result
in MDR (5–9).
Our laboratory has successfully established an
adriamycin (ADM)-resistant human bladder cancer cell line
(pumc-91/ADM) from the parental cell line pumc-91. According to
drug resistance spectrum analysis, the pumc-91/ADM cell line
exhibited the characteristics of MDR (10). In the present study, the expression
levels of the five markers were analyzed in pumc-91/ADM and pumc-91
cells in order to determine the relationship between protein
expression and drug resistance. In addition, the possible pathways
that may lead to bladder cancer MDR were evaluated. The results may
provide the basis for the knockdown or transfection of a target to
reverse MDR in the future.
Materials and methods
Cell culture
The pumc-91 human bladder cancer cell line was
generously provided by the Peking Union Medical College Hospital
(Beijing, China). The increasing concentration gradient method was
adopted in vitro to induce resistance. Increasing ADM
concentrations (0.3, 0.6 and 1.0 µg/ml) were used to establish
pumc-91/ADM multidrug-resistant bladder cell lines. The half
maximal inhibitory concentration (IC50) values for ADM treatment in
pumc-91 and pumc-91/ADM cells were 0.61 and 6.02 µM, respectively.
The IC50 for pumc-91/ADM was 9.86-fold higher than that of pumc-91,
thus indicating that the ADM-resistant cell line pumc-91/ADM (1.0
µg/ml) was successfully established. Pumc-91 and pumc-91/ADM cells
were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10 and 18% fetal bovine
serum (Beijing Dingguo Biotechnology Co., Ltd., Beijing, China),
respectively. Cells were maintained at 37°C in an atmosphere
containing 5% CO2.
Drug cytotoxicity analysis
To analyze drug cytotoxicity, 2.0×104 cells/well
were cultured with a concentration gradient (0.3, 0.6 and 1.0
µg/ml) of ADM (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
in 96-well plates at 37°C. Following 72 h of drug treatment, 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was
added to the cells. Following a 4 h incubation at 37°C, absorbance
was measured in the presence of dimethyl sulfoxide at 450 nm. Cell
survival in the absence of the drug was defined as 100% cell
survival. The IC50 values of ADM were determined from the
corresponding absorbance.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) as previously described (11). Total RNA (2 µg) was then reverse
transcribed to cDNA using the Reverse Transcription system
according to the manufacturer's protocol (Promega Corporation,
Madison, WI, USA). TransStart Top Green qPCR SuperMix (Beijing
TransGen Biotech Co., Ltd., Beijing, China) and Roche LightCycler
480 Real Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) were used to conduct RT-qPCR, according to the
manufacturers' protocols. The PCR conditions were as follows: 45
cycles of annealing at 55°C for 1 min, denaturation at 95°C for 40
sec, and elongation at 72°C for 1 min. The PCR primer sequences are
presented in Table I. The
2-∆∆Cq method was applied for relative quantitative
analysis (12). All samples were
independently analyzed at least three times in duplicate.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|
| P-gp |
5′-GATATTGCCTGGTTTGATGA-3′ |
5′-TGCATTTTGTGTTAAGACGC-3′ |
| MRP |
5′-TTGCCGTCTACGTGACCATT-3′ |
5′-AGGCGTTTGAGGGAGACACT-3′ |
| Topo II |
5′-AGGCATCGCATCTTGTTTAG-3′ |
5′-CTGTCTCCGGTCTTCCATAA-3′ |
| p53 |
5′-CACTAAGCGAGCACTGTCCA-3′ |
5′-TTCAGCTCTCGGAACATCTC-3′ |
| VEGF |
5′-CAATCGAGACCCTGGTGGACA-3′ |
5′-TGTTGGACTCCTCAGTGGGCA-3′ |
| GAPDH |
5′-TTTGGTATCGTGGAAGGACT-3′ |
5′-AGTAGAGGCAGGGATGATGT-3′ |
Western blot analysis
Pumc-91 and pumc-91/ADM cells were grown to 80–90%
confluence. Cells were harvested, washed three times with
phosphate-buffered saline (PBS), and lysed with
radioimmunoprecipitation assay cell lysis buffer (Beijing Dingguo
Biotechnology Co., Ltd.). The protein was quantified using
Bicinchoninic Acid kit (Sigma-Aldrich; Merck Millipore) and protein
samples (60 µg) were loaded onto an 8% sodium dodecyl
sulfate-polyacrylamide gel and were electrotransferred to
polyvinylidene fluoride membranes. After blocking with 5% nonfat
dry milk for 2 h at room temperature, the membranes were incubated
with the following primary antibodies: P-gp (1:2,000; cat. no.
ab170903; Abcam, Cambridge, MA, USA), Topo II (1:10,000; cat. no.
ab12318; Abcam), MRP (1:500; cat. no. sc-18835; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), p53 (1:500; cat. no. sc-126;
Santa Cruz Biotechnology, Inc.), VEGF (1:500; cat. no. sc-65617;
Santa Cruz Biotechnology, Inc.) and β-actin (1:400; cat. no. TA-09;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China)
at 4°C overnight. Subsequently, the membranes were incubated with
goat anti-mouse immunoglobulin (Ig)G (1:1,000; Beijing Dingguo
Biotechnology Co., Ltd.; cat. no. IH-0031) or goat anti-rabbit IgG
(1:1,000; Beijing Dingguo Biotechnology Co., Ltd.; cat. no.
IH-0011) for 30 min at 37°C. After extensive washing with PBS with
Tween 20, proteins were visualized using an enhanced horseradish
peroxidase-3,3′-diaminobenzidine (DAB) chromogenic kit (Tiangen
Biotech Co., Ltd., Beijing, China), according to the manufacturer's
protocol. Lane 1D gel analysis software (Beijing Sage Creation
Science Co., Ltd., Beijing, China) was used to analyze the
bands.
Immunohistochemistry
For staining, the pumc-91 and pumc-91/ADM cell
slides were fixed with 4% paraformaldehyde for 15 min at room
temperature. After washing with PBS, the slides were treated with
50 µl hydrogen peroxide (Beijing Dingguo Biotechnology Co., Ltd.)
for 20 min at room temperature to block the action of endogenous
peroxidases. Subsequently, the slides were blocked in nonimmune
serum (Beijing Dingguo Biotechnology, Co., Ltd.) at 37°C for 30
min. The specimens were then incubated with the following primary
antibodies: P-gp (1:400), Topo II (1:10,000), MRP (1:400), p53
(1:500), VEGF (1:500) and β-actin (1:200) at 37°C for 1 h, followed
by incubation with secondary antibodies (1:1,000; EnVision
Detection systems, Peroxidase/DAB, Rabbit/Mouse; Dako, Carpinteria,
CA, USA) for 30 min at 37°C. A DAB kit was used to visualize the
immunohistochemical staining, and slides were counterstained with
hematoxylin. Finally, specimens were dehydrated in gradient
alcohol. The slides were observed using an Olympus CX31 microscope
(Olympus Corporation, Tokyo, Japan) and the staining intensity and
percentage of stained cells were quantified according to a
previously described approach (13). Professional image analysis
software, Image Pro Plus 6.0 (Media Cybernetics, Inc., Rockville,
MD USA) was used to evaluate the results.
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments. The
differences between groups were assessed by Student's t-test.
Statistical analysis was carried out using SPSS software version
16.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Differential expression of mRNA in the
two cell lines
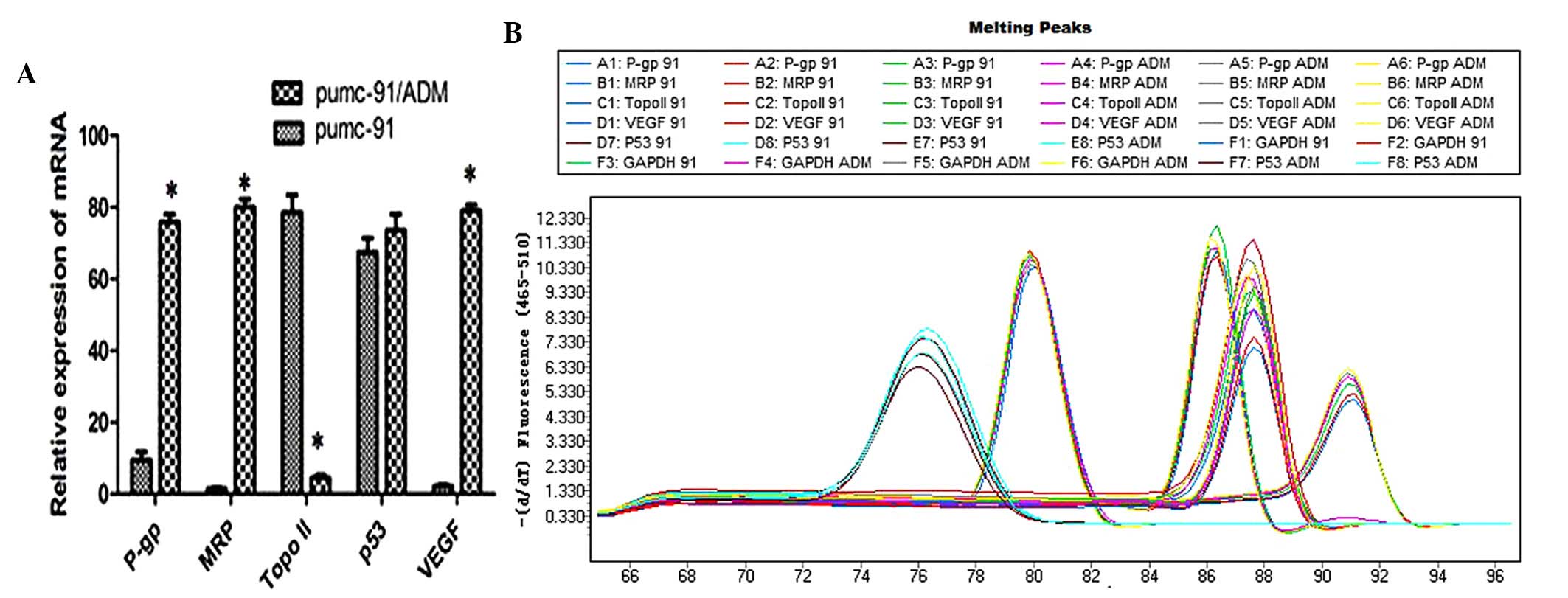
The mRNA expression levels of P-gp, MRP, Topo II,
p53 and VEGF were detected by RT-qPCR. Glyceraldehyde 3-phosphate
dehydrogenase was used as an endogenous control. The mRNA
expression levels of P-gp, MRP and VEGF were markedly upregulated,
whereas Topo II was downregulated in the pumc-91/ADM cells compared
with the pumc-91 cell line (P=0.017, 0.045, 0.029 and 0.004,
respectively; Fig. 1). These
differences were statistically significant. However, no difference
was detected in p53 expression at the genetic level between the two
cell lines (P=0.722; Fig. 1).
Differential expression of proteins in
the two cell lines
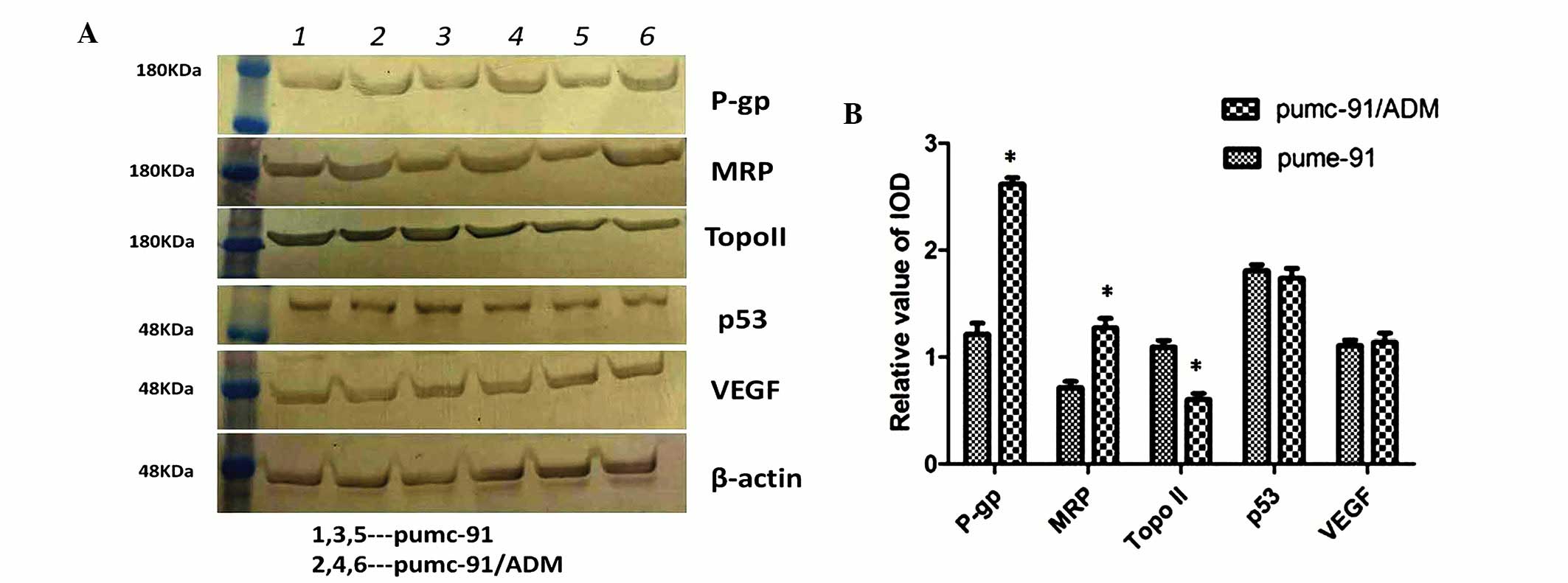
The corresponding protein expression levels were
confirmed by western blot analysis in the two cell lines. Compared
with pumc-91 cells, a significant increase in P-gp and MRP, and
decrease in Topo II was detected in the pumc-91/ADM drug-resistant
cell line (P=0.008, 0.031 and 0.014, respectively; Fig. 2). These differences were
statistically significant. However, no evident differences in p53
and VEGF expression were detected between the two cell lines at the
protein level (P=0.103 and 0.700, respectively; Fig. 2).
Immunohistochemical protein expression
in the two cell lines
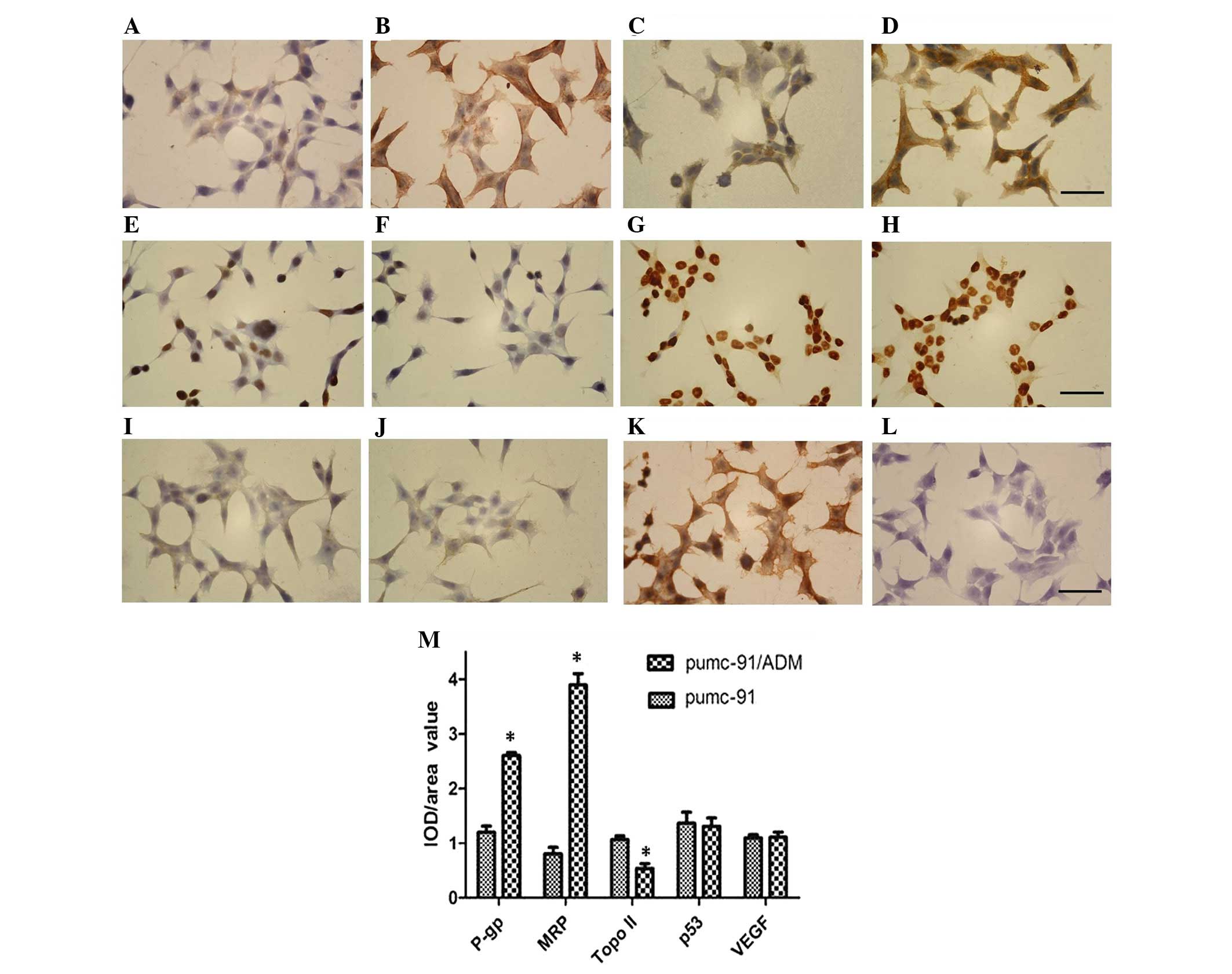
The results of the expression analyses were further
confirmed by localization of P-gp, MRP, Topo II, p53 and VEGF by
immunohistochemistry in the two cell lines. Image Pro Plus 6.0 was
used to evaluate the results. β-actin was used as a positive
control, whereas PBS was used instead of the primary antibodies as
a negative control. The cytoplasmic and cell membrane localization
of P-gp and MRP, the cytoplasmic localization of VEGF, and the
nuclear localization of p53 and Topo II were observed in the two
cell lines. Consistent with the results of western blotting, P-gp
and MRP were upregulated, and Topo II was downregulated in
pumc-91/ADM cells compared with in pumc-91 cells (P=0.014, 0.048
and 0.039, respectively; Fig. 3).
No significant difference in p53 and VEGF expression was detected
between the two cell lines (P=0.316 and 0.113, respectively;
Fig. 3).
Discussion
Adjuvant chemotherapy following surgery is
considered necessary for the treatment of bladder cancer; however,
long-term chemotherapy decreases the response of cancer cells to
anticancer agents, thus leading to the occurrence of tumor escape.
In the majority of cancer types, several key molecules participate
in the development of MDR, including P-gp, MRP, Topo II, p53 and
VEGF (14–19). The present study investigated the
relationship between the expression levels of these five markers
and drug resistance in ADM-resistant (pumc-91/ADM) and parental
(pumc-91) human bladder cancer cell lines. The results provided
evidence regarding the possible pathways that lead to bladder
cancer MDR. In addition, the results may provide useful information
for the reversal of MDR in bladder cancer.
Among the five molecules evaluated in the present
study, aberrant expression of P-gp and MRP has been the most
extensively reported. P-gp and MRP are two important members of the
ATP-binding cassette transporter superfamily, which have been
reported to confer resistance against various chemotherapeutic
agents (20). By affecting the
intracellular drug concentration through drug efflux alterations,
P-gp and MRP are associated with drug resistance in various types
of cancer. The results of the present study suggested that P-gp and
MRP were upregulated in pumc-91/ADM cells compared with in pumc-91
cells. Notably, MRP demonstrated a more significant increasing
trend. The mechanisms underlying the effects of MRP on MDR include
effects on drug efflux and microenvironment alterations. MRP is
able to redistribute intracellular drugs, via altering the pH
microenvironment of the cytoplasm and organelles, which may trigger
resistance (21). Therefore, in
further studies regarding the reversal of MDR, researchers have
selected MRP as the first factor to target by transfection or
expression knockdown. Copsel et al reported that knockdown
of MRP by short hairpin RNA could regulate drug resistance in the
U937 acute myeloid leukemia cell line (22). In addition, Su and Pasternak
revealed that downregulation of MRP significantly enhanced the
analgesic potency of systemic morphine in MRP knockout mice
(23).
Topo II is a nuclear protein that is usually highly
expressed during active cell proliferation; therefore,
overexpression of Topo II is common in malignant tumors. However,
it is generally believed that decreased expression of Topo II is
associated with tumor drug resistance. Yu et al investigated
the expression of Topo II in gastric cancer following chemotherapy
using immunohistochemistry; Topo II staining was negative whereas
other MDR-related proteins exhibited positive staining (24). Tsang et al hypothesized that
drug resistance could be induced by downregulation of Topo II in
A431 human squamous carcinoma cells (25). Notably, in the present study, Topo
II was downregulated in pumc-91/ADM cells compared with in pumc-91
cells. The detailed mechanism underlying the participation of Topo
II downregulation in bladder cancer MDR remains to be elucidated.
Notably, Topo II has a role in formation of DNA complexes in the
S-G2-M phase of the cell cycle. Understanding the mode
of action of Topo II in MDR in other types of cancer may provide
certain possibilities for its underlying mechanism in bladder
cancer MDR. Topo II has been previously reported to be
significantly decreased in gastric cancer cells resistant to ADM
and mitomycin (26). The
downregulation of Topo II may alter the crosslinking and production
of DNA complexes, resulting in a decline in chemosensitivity. In
addition, treatment with a Topo II inhibitor resulted in MDR in
lung cancer cells via the epidermal growth factor receptor
signaling pathway (27).
P53 has an important role in cell cycle regulation
and apoptosis induction in bladder cancer. Chin and Kong reported
that p53 markedly reduced the sensitivity of tumor cells towards
chemotherapy by activating the initiator of MDR-1, inducing the
duplication of MDR-1 (28). Garufi
and D'Orazi demonstrated that inactivation of p53 reduced the tumor
cell response to drugs, thus inducing drug resistance (29). However, discordance exists between
the results of the present study and the aforementioned reports
with regards to the expression of p53. In the present study, the
expression levels of p53 did not differ between the drug-resistant
pumc-91/ADM cell line and the pumc-91 cell line.
There have been few reports regarding the
relationship between VEGF and drug resistance in tumors. Lin et
al decreased the mRNA and protein expression of the critical
target gene VEGF-A and VEGFR2 in hepatic cancer xenograft tumor
tissues (30). In vitro
studies conducted by Mésange et al revealed that
bevacizumab-resistant cells displayed intrinsically higher VEGF
signaling intensity and tolerance compared with their
bevacizumab-sensitive counterparts in colorectal cancer (31). In the present study, differential
expression of VEGF was detected between the protein and gene level.
It may be hypothesized that methylation of cytosine induced the
abnormal gene expression, whereas the DNA sequence and gene product
remained normal (32). Besides
methylation, acetylation may regulate gene transcription, leading
to differential expression between the protein and gene (33).
In conclusion, the present study detected a
significant upregulation of MRP and downregulation of Topo II in
ADM-resistant human bladder cancer cells (pumc-91/ADM) compared
with in the parental cells (pumc-91). Further studies are required
to explore the specific mechanisms underlying MDR in bladder
cancer. In addition, how to reverse MDR based on the targets
examined in the present study will be our future aim.
Acknowledgements
The present study was supported by the Beijing
Administration of Traditional Chinese Medical (grant no.
2014-ZYJ04) and Beijing Key Laboratory of Urinary Cellular
Molecular Diagnostics (grant no. Z151100001615060).
References
|
1
|
Lei T, Zhao X, Jin S, Meng Q, Zhou H and
Zhang M: Discovery of potential bladder cancer biomarkers by
comparative urine proteomics and analysis. Clin Genitourin Cancer.
11:56–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meng Q, Lei T and Zhang M, Zhao J, Zhao XH
and Zhang M: Identification of proteins differentially expressed in
adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human
bladder cancer cell lines by proteome analysis. J Cancer Res Clin
Oncol. 139:509–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sacerdote C, Guarrera S, Ricceri F,
Pardini B, Polidoro S, Allione A, Critelli R, Russo A, Andrew AS,
Ye Y, et al: Polymorphisms in the XRCC1 gene modify survival of
bladder cancer patients treated with chemotherapy. Int J Cancer.
133:2004–2009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeekpudsa P, Kukongviriyapan V,
Senggunprai L, Sripa B and Prawan A: Suppression of NAD(P)H-quinone
oxidoreductase 1 enhanced the susceptibility of cholangiocarcinoma
cells to chemotherapeutic agents. J Exp Clin Cancer Res. 33:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zandvliet M, Teske E and Schrickx JA:
Multi-drug resistance in a canine lymphoid cell line due to
increased P-glycoprotein expression, a potential model for
drug-resistant canine lymphoma. Toxicol In Vitro. 28:1498–1506.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W and Song M: Expression of multidrug
resistance proteins in invasive ductal carcinoma of the breast.
Oncol Lett. 8:2103–2109. 2014.PubMed/NCBI
|
|
7
|
Semiglazova TIu, Klimenko VV, Filatova LV,
Chubenko VA, Krivorot'ko PV, Ivanov VG, Turkevich EA, Ivantsov AO,
Novikov SN, Semiglazov VV, et al: Markers of effectiveness of
preoperative taxane-based chemotherapy for locally advanced breast
cancer. Vopr Onkol. 59:363–367. 2013.(In Russian). PubMed/NCBI
|
|
8
|
Grosset AA, Labrie M, Gagné D, Vladoiu MC,
Gaboury L, Doucet N and St-Pierre Y: Cytosolic galectin-7 impairs
p53 functions and induces chemoresistance in breast cancer cells.
BMC Cancer. 14:8012014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ki CS, Lin TY, Korc M and Lin CC:
Thiol-ene hydrogels as desmoplasia-mimetic matrices for modeling
pancreatic cancer cell growth, invasion, and drug resistance.
Biomaterials. 35:9668–9677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang M, Jin S and Zhang M: Establishing
human multi-drug resistant bladder cancer cell lines Pumc-91/ADM
and evaluating its biological characteristics. J Med Res. 38:70–72.
2009.
|
|
11
|
Yu S, Meng Q, Hu H and Zhang M:
Correlation of ANXA1 expression with drug resistance and relapse in
bladder cancer. Int J Clin Exp Pathol. 7:5538–5548. 2014.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu H, Meng Q, Lei T and Zhang M:
Nucleophosmin1 associated with drug resistance and recurrence of
bladder cancer. Clin Exp Med. 15:361–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pappas JJ, Petropoulos S, Suderman M,
Iqbal M, Moisiadis V, Turecki G, Matthews SG and Szyf M: The
multidrug resistance 1 gene Abcb1 in brain and placenta:
Comparative analysis in human and guinea pig. PLoS One.
9:e1111352014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu P, Du Y, Cheng X, Yu Q, Huang L and
Dong R: Expression of multidrug resistance-associated proteins and
their relation to postoperative individualized chemotherapy in
gastric cancer. World J Surg Oncol. 12:3072014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kodiakov DS, Klimachëv VV, Avdalian AM,
Bobrov IP, Lazarev AF, Lushnikova EL and Nepomiashchikh LM:
Topoisomerase IIa expression in correlation with clinical and
morphological parameters and proliferation (based on argyrophilic
proteins of nucleolar organizer regions and Ki-67 antigen) in lung
adenocarcinoma. Vopr Onkol. 60:63–68. 2014.(In Russian). PubMed/NCBI
|
|
17
|
Llovet JM: Focal gains of VEGFA: Candidate
predictors of sorafenib response in hepatocellular carcinoma.
Cancer Cell. 25:560–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tonigold M, Rossmann A, Meinold M, Bette
M, Märken M, Henkenius K, Bretz AC, Giel G, Cai C, Rodepeter FR, et
al: A cisplatin-resistant head and neck cancer cell line with
cytoplasmic p53(mut) exhibits ATP-binding cassette transporter
upregulation and high glutathione levels. J Cancer Res Clin Oncol.
140:1689–1704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
An J, Wang X, Guo P, Zhong Y, Zhang X and
Yu Z: Hexabromocyclododecane and polychlorinated biphenyls increase
resistance of hepatocellular carcinoma cells to cisplatin through
the phosphatidylinositol 3-kinase/protein kinase B pathway. Toxicol
Lett. 229:265–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kovalev AA, Tsvetaeva DA and Grudinskaja
TV: Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the
development of primary and acquired multiple drug resistance in
patients with early and metastatic breast cancer. Exp Oncol.
35:287–290. 2013.PubMed/NCBI
|
|
21
|
Lee YK, Lin TH, Chang CF and Lo YL:
Galectin-3 silencing inhibits epirubicin-induced ATP binding
cassette transporters and activates the mitochondrial apoptosis
pathway via β-catenin/GSK-3β modulation in colorectal carcinoma.
PLoS One. 8:e824782013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Copsel S, Garcia C, Diez F, Vermeulem M,
Baldi A, Bianciotti LG, Russel FG, Shayo C and Davio C: Multidrug
resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels
and controls human leukemia cell proliferation and differentiation.
J Biol Chem. 286:6979–6988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su W and Pasternak GW: The role of
multidrug resistance-associated protein in the blood-brain barrier
and opioid analgesia. Synapse. 67:609–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu P, Du Y, Cheng X, Yu Q, Huang L and
Dong R: Expression of multidrug resistance-associated proteins and
their relation to postoperative individualized chemotherapy in
gastric cancer. World J Surg Oncol. 12:3072014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsang WP, Kong SK and Kwok TT: Epidermal
growth factor induction of resistance to topoisomerase II toxins in
human squamous carcinoma A431 cells. Oncol Rep. 16:789–793.
2006.PubMed/NCBI
|
|
26
|
Campiglio M, Somenzi G, Olgiati C, Beretta
G, Balsari A, Zaffaroni N, Valagussa P and Ménard S: Role of
proliferation in HER2 status predicted response to doxorubicin. Int
J Cancer. 105:568–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fillmore CM, Xu C, Desai PT, Berry JM,
Rowbotham SP, Lin YJ, Zhang H, Marquez VE, Hammerman PS, Wong KK
and Kim CF: EZH2 inhibition sensitizes BRG1 and EGFR mutant lung
tumours to TopoII inhibitors. Nature. 520:239–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chin KV and Kong AN: Application of DNA
microarrays in pharmacogenomics and toxicogenomics. Pharm Res.
19:1773–1778. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garufi A and D'Orazi G: High glucose
dephosphorylates serine 46 and inhibits p53 apoptotic activity. J
Exp Clin Cancer Res. 33:792014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Shen A, Chen H, Liao J, Xu T, Liu
L, Lin J and Peng J: Nitidine chloride inhibits hepatic cancer
growth via modulation of multiple signaling pathways. BMC Cancer.
14:7292014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mésange P, Poindessous V, Sabbah M,
Escargueil AE, de Gramont A and Larsen AK: Intrinsic bevacizumab
resistance is associated with prolonged activation of autocrine
VEGF signaling and hypoxia tolerance in colorectal cancer cells and
can be overcome by nintedanib, a small molecule angiokinase
inhibitor. Oncotarget. 5:4709–4721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baranasic D, Oppermann T, Cheaib M, Cullum
J, Schmidt H and Simon M: Genomic characterization of variable
surface antigens reveals a telomere position effect as a
prerequisite for RNA interference-mediated silencing in paramecium
tetraurelia. MBio. 5:e013282014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo B, Ju S, Muneri CW and Rui R: Effects
of histone acetylation status on the early development of in vitro
porcine transgenic cloned embryos. Cell Reprogram. 17:41–48. 2015.
View Article : Google Scholar : PubMed/NCBI
|