Introduction
Cellular therapy has been widely researched, and is
commonly used to treat cardiovascular disease. Bone marrow-derived
mesenchymal stem cells (BM-MSCs) are nonhematopoietic multi-potent
stem cells and have an intrinsic ability to differentiate into
functional cell types able to repair diseased or injured tissue
(1). BM-MSCs can be readily
obtained, rapidly proliferate in culture, and display a capacity to
differentiate towards endothelial (2,3) or
vascular smooth muscle cells (VSMCs) (4), and they serve an important role in
postnatal neovascularization in various tissue contexts. Neointimal
hyperplasia in response to arterial injury is a complex process,
and it has been previously suggested that neointimal lesions also
contain BM-MSCs attracted to the vascular injury site (5). However, the effect of BM-MSCs in
vascular injury remains to be fully elucidated. Certain studies
have demonstrated the capacity of BM-MSCs to restoring the
endothelial lining and reduce neointimal formation following injury
(6–8). However, contradictory studies have
demonstrated that the transplantation of BM-MSCs was unable to
reduce neointimal hyperplasia (9),
and potentially aggravated neointimia formation (10,11).
It is widely accepted that BM-MSCs can be induced to
differentiate toward endothelial-like cells (ELCs) in vitro
(2). In the present study, a
method for the isolation, growth and ex vivo expansion of
ELCs differentiated from BM-MSCs was described, and it was
hypothesized that ELCs could attenuate neointimal hyperplasia
following arterial injury.
Materials and methods
Animals
The animal use in this study was approved by the
Animal Care and Use Committee of Shanghai Jiaotong University
School of Medicine (Shanghai, China), and all procedures were
conducted in accordance with institutional guidelines. A total of
12 young male Sprague-Dawley (SD) rats (weight, 100–150 g; age, 4
weeks) were used to isolate the MSCs. A total of 26 adult male SD
rats (weight, 300–350 g; age, 16 weeks) were used in the carotid
balloon-injury model and cell transplantation experiments. Rats
were given ad libitum access to food and water, unless
otherwise specified. The rats were maintained under controlled
temperature (20–24°C), humidity (30–70%) and lighting conditions
(12:12 h ligh/dark cycle). Subsequently, rats were sacrificed
following abdominal injection of 10% chloral hydrate (600
mg/kg).
MSC isolation and culture
BM-MSCs were collected from the bone marrow of young
male SD rat femurs and tibias, as previously described (12). The femoral and tibial bones were
obtained from donor rats under anesthesia with 10% chloral hydrate
(300 mg/kg; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany).
Bone marrow was flushed with low glucose Dulbecco's modified
Eagle's medium (L-DMEM; Gibco; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 15% (v/v) fetal
bovine serum (FBS; Gibco; Invitrogen; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 U/ml streptomycin using a
syringe with a 21-gauge needle. Cells from one rat were plated into
two 35 mm dishes at 37°C in a humidified atmosphere of 5% CO2.
Non-adherent cells were removed by changing the medium after 48 h.
The cells were then incubated for 5–7 days at 37°C in a humidified
atmosphere to reach confluence. Once the cells had grown to near
confluence, they were passaged two to three times, being detached
with 0.25% trypsin/1 mM EDTA, and were re-plated at a density of
1×106/ml. Finally, MSCs were propagated for 3–6 passages for
further experiments.
BM-MSCs differentiated into ELCs
Confluent cells were cultivated in the presence of
endothelial cell growth medium-2 (EGM-2; Cambrex Corporation, East
Rutherford, NJ, USA) with 2% FBS and 50 ng/ml rVEGF164 (R&D
Systems, Inc., Minneapolis, MN, USA) for 7 days. The medium was
changed every 2 days. Cells were propagated for 3–6 passages after
7 days.
Flow cytometric analysis of MSCs and
ELCs
Cells were trypsinized, washed with
phosphate-buffered saline (PBS), and incubated with the following
antibodies: Phycoerythrin (PE)-mouse anti-rat CD31 (cat. no.
555027), PE-mouse anti-rat CD54 (cat. no. 554970), PE-mouse
anti-rat CD71 (cat. no. 554891), PE-mouse anti-rat CD90 (cat. no.
554898), PE-Cy™5 mouse anti-rat CD45 (cat. no. 559135), and
fluorescein isothiocyanate (FITC)-mouse anti-rat CD34 (cat. no.
555821; BD Biosciences, Franklin Lakes, NJ, USA). Analysis was
performed using a FACSCalibur flow cytometer (BD Biosciences).
Fluorescence immunocytochemistry
Immunofluorescence analysis was used to detect the
expressions of ELC surface antigens von Willebrand factor (vWF) and
vascular endothelial growth factor (VEGF) receptor 2 (R2). Cultured
MSCs grown in slide chambers were fixed in 4% buffered
paraformaldehyde for 10 min, incubated with blocking solution [2%
bovine serum albumin (BSA; Sigma-Aldrich; Merck Millipore)] for 1 h
at room temperature, and then incubated with the primary antibody
(mouse anti-rat vWF, cat. no. sc-365712, 1:50; mouse anti-rat
VEGFR2, cat. no. sc-393179, 1:50; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) in blocking solution overnight at 4°C.
Negative controls were incubated with blocking solution only. The
samples were subsequently incubated with monoclonal anti-mouse
FITC-conjugated secondary antibodies (1:100; cat. no. sc-358943;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The
nuclei were stained with Hoechst 33,258 (Sigma-Aldrich; Merck
Millipore) for 5 min and the samples examined under a fluorescence
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Cell labeling
The third and sixth passaged cells were collected
and labeled with 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich; Merck
Millipore,). The cells were incubated in complete medium with 20
µmol/l BrdU to label cells in the S phase of the cell cycle during
a 48 h period.
Rat carotid balloon-injury model and
cell transplantation
Adult SD rats were anesthetized with 10% chloral
hydrate (300 mg/kg) and underwent a balloon catheter injury to the
right carotid artery, as previously described (13). A 2F Fogarty balloon catheter
(Edwards Life Sciences Corporation, Irvine, CA, USA) was inserted
through the right external carotid artery. The balloon was inflated
with 0.1 ml saline to distend the common carotid artery (CCA) and
passed 3 times along the isolated segment length (1 to 1.5 cm in
length), and then the catheter was removed.
All rats (n=18) were randomly divided into three
groups. In group 1 (control), rats were given PBS alone (without
donor cell administration) injected on the day of the carotid
injury operation, immediately subsequent to balloon injury (n=6).
In group 2, rats were administered with rat MSCs
(2×106/ml) in 1 ml of total fluid volume (L-DMEM)
injected through the femoral vein (n=6). In group 3, rats were
administered with ELCs (2×106/ml) that were injected
through the femoral vein (n=6).
Ultrasound biomicroscopy imaging
An ultrasound biomicroscopy (UBM) system (Vevo 770;
VisualSonics, Inc., Toronto, Canada) equipped with a 40 MHz
transducer was used for all examinations. The current study
involved 18 rats, and UBM was performed on days 7, 14 and 28
subsequent to vascular injury. The same researcher conducted all
examinations and performed the measurements in a blinded
fashion.
Tissue processing and histological
analysis
A total of 28 days subsequent to injury, the CCA was
excised on both sides (injured and non-injured contralateral side
artery). Each CCA was fixed with 10% buffered formalin and embedded
in paraffin. Sections 5 µm thick were taken from the middle portion
of the balloon-injured segment, stained with a Masson's trichrome
stain to evaluate general morphology, and photographed at a
magnification of ×100. Morphometric analyses of the digital images
were performed using Leica QWin V3 software (Leica Microsystems
GmbH). The intima and media areas were determined, and the ratio
between these two areas was calculated.
Immunohistochemistry and
immunofluorescence
In order to detect the donor cells ex vivo,
sections were dried at 60°C for 20 min, deparaffinized and
dehydrated, and treated with 0.3% hydrogen peroxide in methanol to
block endogenous peroxidase activity. Subsequent to washing with
PBS, tissues were incubated with BSA (5%) at room temperature for
30 min, then incubated with anti-BrdU (1:100; cat. no. ab125306;
Abcam, Cambridge, UK) at 4°C overnight. Slides were rinsed in PBS,
incubated in horseradish peroxidase-conjugated anti-mouse IgG
(1:100; cat. no. sc-2373; Santa Cruz Biotechnology, Inc.) in BSA
(1%) for 1 h, stained with 3,3′-diaminobenzidine, and analyzed
using a Zeiss Axioskop 50 light microscope (Carl Zeiss AG,
Oberkochen, Germany).
To detect donor cell differentiation in vivo,
endothelial cells and VSMCs were identified using immunostaining
with mouse anti-rat CD31 antibody (1:50; cat. no. sc-52713; Santa
Cruz Biotechnology, Inc.) and rabbit anti-α-smooth muscle actin
(α-SMA) antibody (1:100; cat. no. ab15734; Abcam), respectively.
Nuclear staining was conducted using anti-BrdU. Slides were
pre-incubated with 5% BSA for 30 min each. The primary antibody was
then applied at 4°C overnight, followed by application of the
appropriate tetramethylrhodamine-conjugated IgG (1:200 dilution;
cat. no. sc-3827; Santa Cruz Biotechnology, Inc.) and
FITC-conjugated IgG (1:200 dilution; cat. no. sc-358943; Santa Cruz
Biotechnology, Inc.). Sections were washed with PBS and then
mounted at room temperature.
Statistical analysis
All results were expressed as the mean ± standard
deviation. Statistical significance was evaluated by one-way
analysis of variance followed by Bonferroni post-hoc tests.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
software, version 13 (SPSS, Inc., Chicago, IL, USA).
Results
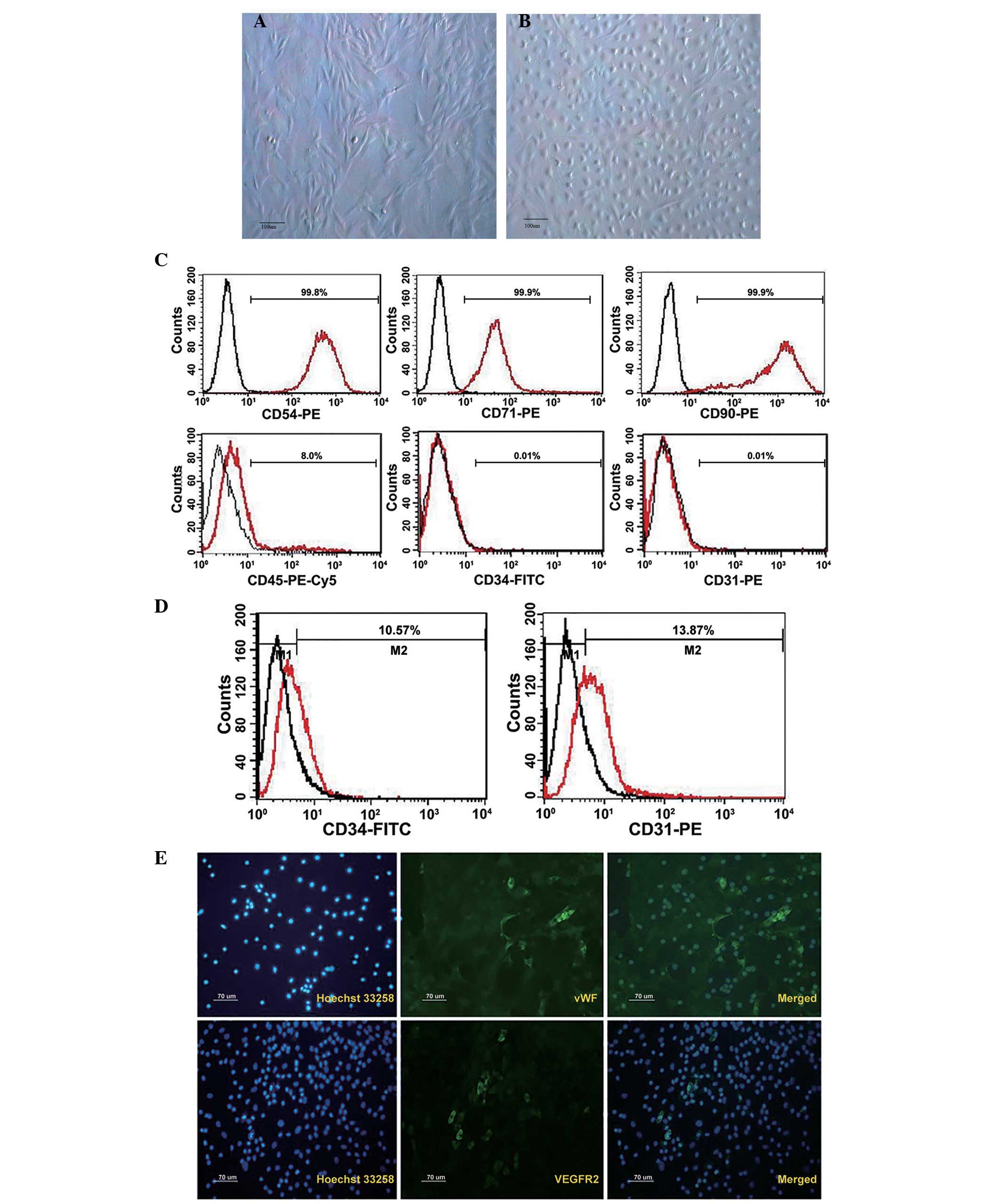
Cultured BM-MSCs can differentiate to
endothelial cell phenotypes
BM-MSCs were cultured as plastic adherent cells
in vitro, and were subsequently analyzed (Fig. 1). Their morphological features are
presented in Fig. 1A.
Characteristic flattened and spindle-shaped cells were recognized.
Therefore, endothelial experiments were performed on cells from
passage 3–6. Cells were tested with flow cytometry for the presence
or absence of characteristic hematopoietic and endothelial markers.
MSCs typically expressed the antigens CD54, CD71 and CD90. They
were negative for the early hematopoietic marker CD34, endothelial
cell marker CD31 and 8% expressed leukocyte marker CD45 (Fig. 1C).
Subsequent to 7 days of culture, the outgrowth cells
cultivated by EGM-2 exhibited the typical ‘cobblestone’ endothelial
cell morphology (Fig. 1B), and
approximately 10% of cells expressed CD34 and CD31 (Fig. 1D). Immunofluorescence analysis
indicated the expression of endothelial-specific markers such as
vWF and VEGFR2 in ELCs, although the positive rate was less than 1%
(Fig. 1E).
Ultrasound biomicroscopy imaging for
detecting neointima formation
A comparative image series presented the neointimal
site subsequent to balloon injury at 7, 14 and 28 days,
respectively (Fig. 2A). PBS (n=6),
MSCs (2×106/ml) (n=6) and ELCs (2×106/ml) (n=6) were injected,
respectively, through the femoral vein. After 4 weeks, the ELCs
group attenuated intimal hyperplasia subsequent to vascular injury
compared with the PBS and MSCs groups. Ultrasound biomicroscopy
exhibited significant IMT increases in the PBS and BM-MSCs groups
compared with the ELCs group at 14 days after arterial injury
(0.14±0.03 mm vs. 0.13±0.02 mm vs. 0.10±0.02 mm; P<0.01), and
IMT was significantly increased at 28 days subsequent to injury
(0.23±0.04 mm vs. 0.22±0.05 vs. 0.16±0.02 mm; P<0.01) (Fig. 2B).
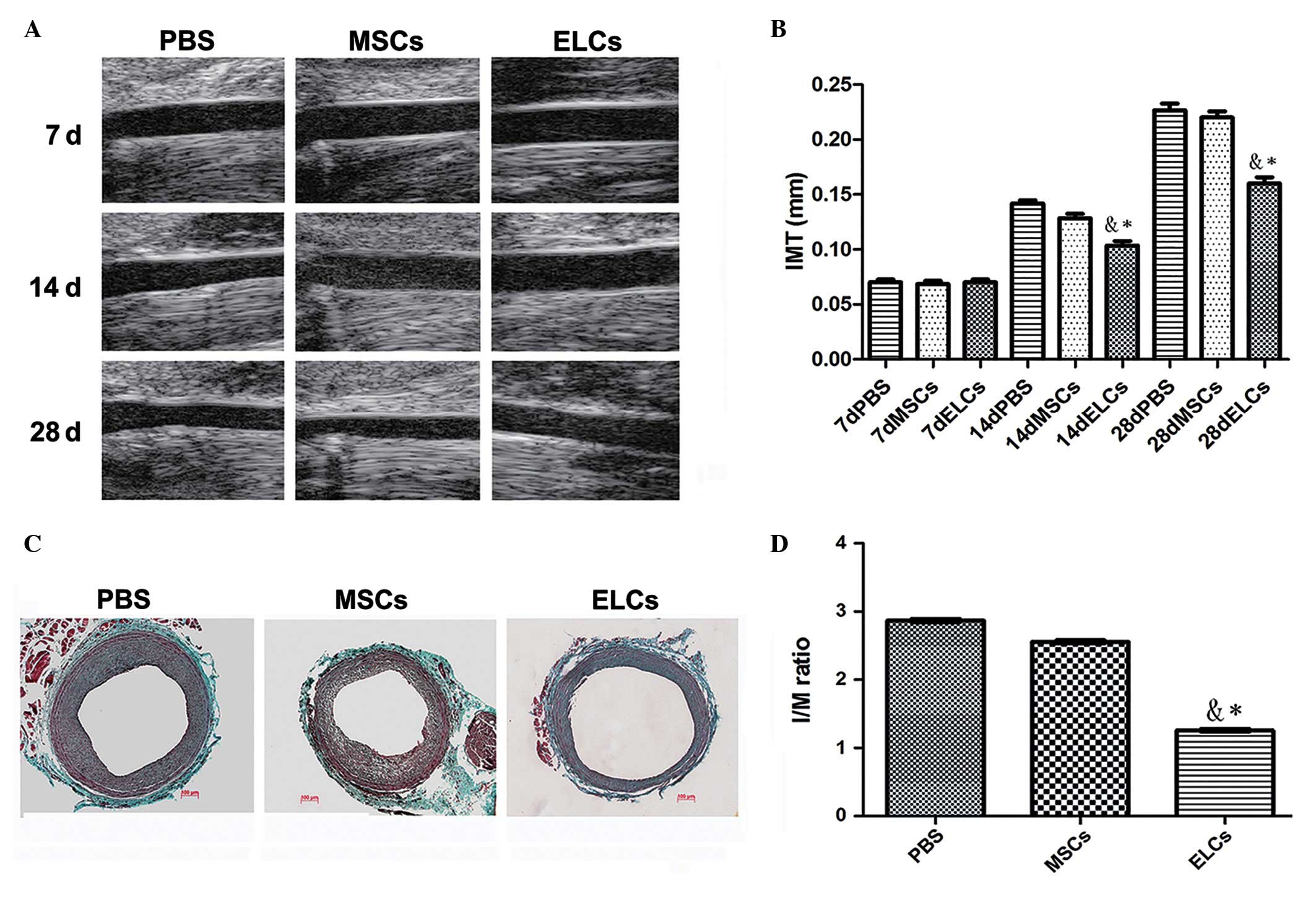 | Figure 2.(A) A series of comparative images
showing the site of the neointima subsequent to balloon injury at
7, 14, and 28 days. (B) PBS (n=6), MSCs (2×106/ml) (n=6)
and ELCs (2×106/ml) (n=6) were injected through the
femoral vein, respectively. Ultrasound biomicroscopy identified
significant increases of IMT in the PBS and MSCs groups compared
with the ELCs group 14 days subsequent to arterial injury
(0.14±0.03 mm vs. 0.13±0.02 mm vs. 0.10±0.02 mm; P<0.01), and
IMT significantly increased 28 days subsequent to injury (0.23±0.04
mm vs. 0.22±0.05 vs. 0.16±0.02 mm; P<0.01). (C) Masson's
trichrome staining for the contribution of MSCs and ELCs to
neointimal formation. (D) The I/M ratio was significantly reduced
in the ELCs group compared with the PBS and MSCs groups (1.22±0.03
vs. 2.52±0.04 vs. 2.87±0.04; P<0.001) (n=6). *P<0.05 vs. PBS
group; &P<0.05 vs. MSCs group. PBS,
phosphate-buffered saline; MSCs, bone marrow-derived mesenchymal
stem cells; ELCs, endothelial-like cells; IMT, intima-media
thickness; I/M, intima/media. |
Contribution of MSCs and ELCs to
neointimal formation
Masson's trichrome-stained sections from the PBS,
MSCs, and ELCs groups indicated that attenuated intimal hyperplasia
was present in the ELCs group (Fig.
2C). The intima/media ratio was significantly reduced with
therapy in the ELCs group (1.22±0.02) when compared with the MSCs
(2.51±0.03) and PBS groups (2.87±0.04) (P<0.01). There was no
significant difference between the MSCs and PBS groups following
implantation (P>0.05; Fig.
2D).
Differentiation and homing of the MSCs
and ELCs towards the site of injured carotids
Subsequent to intravenous transplantation of either
BM-MSCs (Fig. 3C and D) or ELCs
(Fig. 3E and F), the BrdU+- cells
were identified at the site of injury on the arterial surface,
predominantly in the adventitial area and particularly in the zone
of the vasa vasorum, with few cells present in the neointima
(Fig. 3). Numerous
α-SMA/BrdU+-cells were identified in the BM-MSCs group at 28 days
subsequent to arterial injury (Fig.
3G; upper panel). However, when using CD31 as a marker of
highly differentiated ELCs, few neointima cells were BrdU+-MSCs
(Fig. 3G). When ELCs were
transplanted, numerous CD31+/BrdU+-cells were present in the
adventitia (Fig. 3G; lower panel),
and few α-SMA(+)/BrdU+-cells were observed.
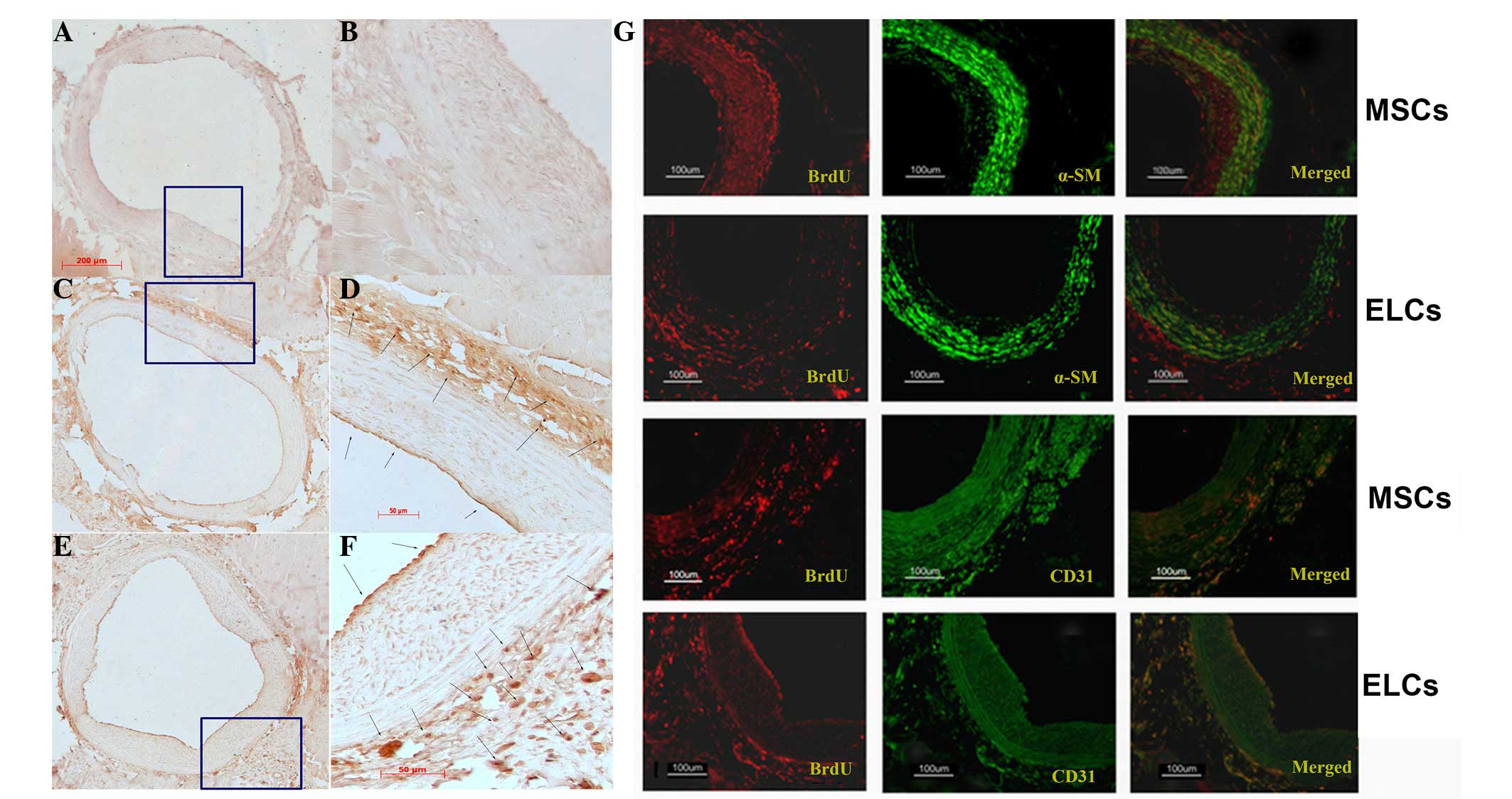 | Figure 3.(A and B) No BrdU+ cells were visible
in the control injured artery vascular wall (A, ×100; B, ×400).
(C-F) Immunohistochemistry staining with anti-BrdU highlighted
numerous BrdU-labeling BM-MSCs (C, ×100; D, ×400) and ELCs (E,
×100; F, ×400) in the adventitia (arrow), particularly in the zone
around the vasa vasorum, and few in the neointimal area (arrow)
(F). (G) In the MSCs group, immunofluorescence analysis indicated
that the majority of α-SMA was expressed in the adventitia area,
suggesting that MSCs differentiated into vascular smooth muscle
cells. By contrast, few CD31+ transplanting cells were observed. In
the ELCs group, numerous BrdU+-ELCs expressed CD31 in the surface
and adventitia, indicating that ELCs homed to the injured artery
and differentiated into endothelial cells. BrdU,
5-bromo-2-deoxyuridine; BM-MSCs, bone marrow-derived mesenchymal
stem cells; ELCs, endothelial-like cells; α-SMA; α-smooth muscle
actin. |
Discussion
ELCs were derived from BM-MSCs their therapeutic
effects in treating attenuating neointimal hyperplasia were
demonstrated. A sufficient number of ELCs were consistently
obtained to allow transplantation for ex vivo applications
within 2 weeks of initial plating. Furthermore, these cells were
demonstrated to be home towards injury sites and significantly
reduce neointimal hyperplasia associated with balloon-induced
carotid injury in rats.
BM-MSCs can be induced to differentiate toward ELCs
in vitro and in vivo (3). In the present study, their ability to
differentiate into ELCs was confirmed by treating them with EGM-2
and VEGF. Subsequent to a 7-day differentiation, it was identified
that the treatment resulted in endothelial cell marker expression
of CD31, vWF, VEGFR2 and the early hematopoietic marker CD34,
whereas in other studies ELCs did not express CD31 under the same
culture conditions (2). One
previous study demonstrated that MSCs cultured in the same medium
differentiated into high amounts of DiI-AcLDL-positive cells and
enhanced the presence of endothelial cell markers, including vWF
(90%), vascular endothelial-cadherin-(60%), and platelet
endothelial cell adhesion molecule 1 (48%) (14). All results demonstrated the ability
of BM-MSCs to differentiate into ELCs under in
vitro-induction conditions. ELCs were identified not to be
mature endothelial cells due to expression of endothelial
progenitor cell marker CD34. MSCs, due to their clear ex
vivo proliferation and multipotent differentiation, are ideal
candidates for cell-based therapy (15) and may be a good potential option
for ELCs to re-endothelialize following interventional procedures
and tissue engineering.
In the current study, UBM, a highly feasible,
non-invasive and simple technique to assess the neointima, was
used. Previous studies reported that UBM-measured IMT, maximum
plaque thickness and plaque area were highly correlated with
histological data (16,17). In the current study, UBM, which
allowed for accurate real-time imaging of the continuously changing
neointima in injured carotids was used, and no significant
differences among the three groups were observed at 7 days. Between
7 and 14 days of age, growth in the neointima was observed. IMT in
the ELCs group was significantly reduced compared with the PBS and
BM-MSCs groups at 14 and 28 day subequent to injury, and appeared
to increase in a time-dependent manner with maximal neointima at
the injury artery identified after 28 days. This is, to the best of
our knowledge, the first study to employ UBM real-time non-invasive
assessment of the alterations of the injured artery, and identified
that ELCs can attenuate the neointima.
The ability of MSCs to differentiate into either
endothelial cells or VSMCs in vitro has been previously well
characterized and documented (18–21).
In comparison, differentiation to endothelial cells or vascular
muscle cells in vivo subsequent to arterial injury remains
unclear. Previous studies reported that transplanting BM-MSCs
aggravates neointimal hyperplasia by affecting vasculature and
differentiating into VSMCs (18,19).
However, other studies indicate that engrafted MSCs appear to
differentiate into endothelial cells, diminish neointimal formation
and improve endothelial function (20,21).
BM-MSCs have two different effects on vascular cells subsequent to
arterial injury, although their differentiated direction remains
controversial. Thus, it is suggested that VEGF may be used to
direct MSCs towards an endothelial lineage in vitro prior to
transplantation in vivo. This is the first time, to the best
of our knowledge, that ELCs derived from BM-MSCs in vitro
were reported to reduce neointimal hyperplasia. The precise
mechanism of ELCs remains unclear, however data from the current
study indicated that ELCs can home to the surface of the injured
vessel and re-endothelize it. It is suggested that ELCs derived
from BM-MSCs may be a endothelial progenitor cell source, which is
able to re-endothelialize the injured artery during the early
stages and attenuate neointima formation via intravenous
transfusion.
A previous study identified that the majority of
transplanted stem cells homed towards the lungs, and were retained
in the lung alveoli via intravenous planting (22). The data indicated that BM-MSCs can
home towards the injured artery, predominantly in the adventitia,
with few in the intima and numerous cells in the vasa vasorum area,
which was in agreement with a previous study (6). Notably, the majority of BM-MSCs and
ELCs home towards the adventitia, and seldom MSCs and ELCs
contribute to the neointima. Studies havew suggested that the
arterial wall is a recipient and source of MSCs, and it has been
demonstrated that a large population of vascular progenitor cells
exist in the adventitia of the artery (23–25),
which may be a niche of stem cells.
BM-MSCs were able to differentiate into ELCs in
vitro. These cells presented with characteristics of
endothelial markers however were not mature endothelial cells.
Overall, the results suggested that transplantation not with
BM-MSCs, however with ELCs, significantly suppressed intimal
hyperplasia following vascular injury. These results indicate that
ELCs differentiated from BM-MSCs can reduce neointimal formation
subsequent to vascular lesions, which indicates important
therapeutic implications for cardiovascular diseases and a new cell
source for cell-based vascular engineering and repair in the
future. Additional experiments are required in order to address the
mechanisms of reduced neointimal formation by ELCs.
Acknowledgements
The current study was supported financially by
grants from the Fund of Shanghai Municipal Commission of Health and
Family Planning (grant no. 201440023) and Medical-Engineering Cross
Fund (grant no. 2014128) of Shanghai Jiao Tong University. The
funders had no role in study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
References
|
1
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oswald J, Boxberger S, Jørgensen B,
Feldmann S, Ehninger G, Bornhäuser M and Werner C: Mesenchymal stem
cells can be differentiated into endothelial cells in vitro. Stem
Cells. 22:377–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silva GV, Litovsky S, Assad JA, Sousa AL,
Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki S, Narita Y, Yamawaki A, Murase Y,
Satake M, Mutsuga M, Okamoto H, Kagami H, Ueda M and Ueda Y:
Effects of extracellular matrix on differentiation of human bone
marrow-derived mesenchymal stem cells into smooth muscle cell
lineage: Utility for cardiovascular tissue engineering. Cells
Tissues Organs. 191:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu
JS, Helms JA and Li S: Differentiation of multipotent vascular stem
cells contributes to vascular diseases. Nat Commun. 3:8752012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forte A, Finicelli M, Mattia M, Berrino L,
Rossi F, De Feo M, Cotrufo M, Cipollaro M, Cascino A and Galderisi
U: Mesenchymal stem cells effectively reduce surgically induced
stenosis in rat carotids. J Cell Physiol. 217:789–799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forte A, Rinaldi B, Sodano L, Berrino L,
Rossi F, Finicelli M, Grossi M, Cobellis G, Botti C, De Feo M, et
al: Stem cell therapy for arterial restenosis: Potential parameters
contributing to the success of bone marrow-derived mesenchymal
stromal cells. Cardiovasc Drugs Ther. 26:9–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shoji M, Oskowitz A, Malone CD, Prockop DJ
and Pochampally R: Human mesenchymal stromal cells (MSCs) reduce
neointimal hyperplasia in a mouse model of flow-restriction by
transient suppression of anti-inflammatory cytokines. J Atheroscler
Thromb. 18:464–474. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng J, Liu JP, Miao L, He GX, Li D, Wang
HD and Jing T: Conditional expression of the type 2 angiotensin ii
receptor in mesenchymal stem cells inhibits neointimal formation
after arterial injury. J Cardiovasc Transl Res. 7:635–643. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu
CM, Yeh HI, Lan YJ, Yeh CH and Stanford WL: Late-outgrowth
endothelial cells attenuate intimal hyperplasia contributed by
mesenchymal stem cells after vascular injury. Arterioscler Thromb
Vasc Biol. 28:54–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao J, Chen X, Li Y, Ge Z, Duan H, Zou Y
and Ge J: Transfer of bone-marrow-derived mesenchymal stem cells
influences vascular remodeling and calcification after balloon
injury in hyperlipidemic rats. J Biomed Biotechnol.
2012:1652962012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang FB, Li L, Fang B, Zhu DL, Yang HT
and Gao PJ: Passage-restricted differentiation potential of
mesenchymal stem cells into cardiomyocyte-like cells. Biochem
Biophys Res Commun. 336:784–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim S, Kawamura M, Wanibuchi H, Ohta K,
Hamaguchi A, Omura T, Yukimura T, Miura K and Iwao H: Angiotensin
II type 1 receptor blockade inhibits the expression of
immediate-early genes and fibronectin in rat injured artery.
Circulation. 92:88–95. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pankajakshan D, Kansal V and Agrawal DK:
In vitro differentiation of bone marrow derived porcine mesenchymal
stem cells to endothelial cells. J Tissue Eng Regen Med. 7:911–920.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gan LM, Grönros J, Hägg U, Wikström J,
Theodoropoulos C, Friberg P and Fritsche-Danielson R: Non-invasive
real-time imaging of atherosclerosis in mice using ultrasound
biomicroscopy. Atherosclerosis. 190:313–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu DJ, Xu JZ, Wu YJ, Jean-Charles L, Xiao
B, Gao PJ and Zhu DL: Effects of fasudil on early atherosclerotic
plaque formation and established lesion progression in
apolipoprotein E-knockout mice. Atherosclerosis. 207:68–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hillebrands JL, Klatter FA, van den Hurk
BM, Popa ER, Nieuwenhuis P and Rozing J: Origin of neointimal
endothelium and alpha-actin-positive smooth muscle cells in
transplant arteriosclerosis. J Clin Invest. 107:1411–1422. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grimm PC, Nickerson P, Jeffery J, Savani
RC, Gough J, McKenna RM, Stern E and Rush DN: Neointimal and
tubulointerstitial infiltration by recipient mesenchymal cells in
chronic renal-allograft rejection. N Engl J Med. 345:93–97. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Li S, Yu L, Wu J, She T, Gan Y, Hu
Z, Liao W and Xia H: Bone mesenchymal stem cells contributed to the
neointimal formation after arterial injury. PLoS One. 8:e827432013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yue WM, Liu W, Bi YW, He XP, Sun WY, Pang
XY, Gu XH and Wang XP: Mesenchymal stem cells differentiate into an
endothelial phenotype, reduce neointimal formation, and enhance
endothelial function in a rat vein grafting model. Stem Cells Dev.
17:785–793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerkelä E, Hakkarainen T, Mäkelä T, Raki
M, Kambur O, Kilpinen L, Nikkilä J, Lehtonen S, Ritamo I, Pernu R,
et al: Transient proteolytic modification of mesenchymal stromal
cells increases lung clearance rate and targeting to injured
tissue. Stem Cells Transl Med. 2:510–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Psaltis PJ, Puranik AS, Spoon DB, Chue CD,
Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, et
al: Characterization of a resident population of adventitial
macrophage progenitor cells in postnatal vasculature. Circ Res.
115:364–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Wong MM, Campagnolo P, Simpson R,
Winkler B, Margariti A, Hu Y and Xu Q: Adventitial stem cells in
vein grafts display multilineage potential that contributes to
neointimal formation. Arterioscler Thromb Vasc Biol. 33:1844–1851.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grudzinska MK, Kurzejamska E, Bojakowski
K, Soin J, Lehmann MH, Reinecke H, Murry CE, Soderberg-Naucler C
and Religa P: Monocyte chemoattractant protein 1-mediated migration
of mesenchymal stem cells is a source of intimal hyperplasia.
Arterioscler Thromb Vasc Biol. 33:1271–1279. 2013. View Article : Google Scholar : PubMed/NCBI
|