Introduction
A potential role of optineurin (OPTN) when it was
first established in 1998 in the GLCE1 region at locus p15-14 on
chromosome 10 (1). Our previous
study suggested that a novel optineurin genetic mutation encoding a
K322E amino acid substitution was associated with primary
open-angle glaucoma in a Chinese family (2). A number of additional disease-causing
amino acid substitutions, including E50K, H486R and R545Q have also
been confirmed (3–5). The E50K mutation is considered to be
the predominant hereditary cause of normal tension glaucoma
(1,3,6), and
has been found to selectively destroy retinal ganglion cells (RGCs)
through oxytosis and apoptosis, whereas other mutants do not have a
similar effect (7). Our previous
study showed that the mutant E50K optineurin induced the apoptosis
of RGCs in transgenic mouse models, however, the precise mechanisms
remain to be fully elucidated (8).
Regulation of the expression of brain-derived
neurotrophic factor (BDNF) appears central to the mechanism of E50K
OPTN-induced RGC apoptosis. Transcription factors, including the
repressor, RE1-silencing transcription factor (REST), are essential
for the development and function of the nervous system, in part
through the regulation of BDNF (9,10).
Increasing the level of endogenous BDNF exerts a neuroprotective
effect against tumor necrosis factor-α-induced axonal loss
(11). The periocular delivery of
hydrogels containing Leu-Ile induce the expression of BDNF by
increasing the levels of phosphorylated cAMP-responsive element
binding protein in the retina, an effect which may enhance the
survival of RGCs following optic nerve injury (12). The above findings demonstrate that
the expression of BDNF is critical for RGC survival.
Animal genomes encode an abundance of small
regulatory RNAs of ~22 nucleotides in length (13). These microRNAs (miRNAs) represent a
class of gene regulatory molecules in multicellular organisms,
which are involved in post-transcriptional regulation through
sequence complementarity to the 3′ untranslated regions (UTRs) of
mRNAs (14). The binding of miRNA
to target mRNAs can result in translational repression through mRNA
degradation or translational inhibition.
The miRNA, miR-9, is an important regulator of REST,
and is considered to contribute to the regulation of the levels of
BDNF (9), therefore, miR-9 may be
involved in the regulation of RGC apoptosis. miR-9 directs the
post-transcriptional repression of REST by pairing with conserved
sites in the 3′ UTR of the REST gene (15,16).
A previous investigation provided evidence for a double negative
feedback loop between the REST silencing complex and the miRNAs it
regulates (17). It was suggested,
by software prediction, that miR-9 is the miRNA associated with
OPTN (16). These findings
suggested that the mutation of E50K OPTN may affect the levels of
miR-9, leading to the disruption of REST and subsequent abrogation
of the expression of BDNF, which is essential for the survival of
RGC-5 cells. The primary aim of the present study was to evaluate
whether the expression of E50K OPTN contributes to RGC-5 cell
apoptosis through the disruption of miR-9. The present study may
aid development of novel ideas for drug development.
Materials and methods
Cell culture
The RGC-5 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences) and 1% penicillin and
streptomycin in a humidified 37°C incubator with 5%
CO2.
TargetScan
TargetScan (www.targetscan.org/vert_71/) is an online database
that matches miRNAs and their target mRNAs. It was used to
determine target genes of miR-9.
Plasmid construction
The coding region of the human OPTN cDNA was
amplified by PCR using a placental cDNA library (GeneCopoeia, Inc.,
Rockville, MD, USA) as a template. The reaction mixture (25 µl)
contained 2.5 µl buffer, 2.5 µl dNTPs, 2 µl cDNA, 0.5 µl forward
primer, 0.5 µl reverse primer, 0.3 µl Taq polymerase and 16.7 µl
double distilled water and the thermocycling conditions were as
follows: 95°C for 5 min; 30 cycles 95°C for 15 sec, 65°C for 30 sec
and 72°C for 30 sec; 72°C for 10 min; followed by 4°C to stop the
reaction. The nucleotide sequence of the cloned OPTN was identical
with that reported in GenBank (accession no. NM_021980; www.ncbi.nlm.nih.gov/nuccore/BC032762.1). The PCR
product was cloned into the pcDNA3.1 expression vector (Invitrogen;
Thermo Fisher Scientific, Inc.) by GeneCopoeia, Inc., which places
a His tag in-frame with the 3′-end of the cDNA. Mutations in the
OPTN cDNA were created using a PCR-based, site-directed mutagenesis
strategy (also conducted by GeneCopoeia, Inc.) to introduce
nucleotide changes encoding the E50K amino acid substitution. The
nucleotide sequences of the mutant E50K and wild-type OPTN cDNA
constructs were confirmed using automated DNA sequencing.
RNA interference and plasmid
transfection
The mmu-miR-9-5p (accession no. MIMAT0000142)
mimic/inhibitor and negative control/inhibitor (GenePharma,
Shanghai, China) were dissolved in RNase-free H2O. The
cells were plated in 6-well plates (2×105 cells/well)
for 18–24 h at 37°C in 5% CO2 prior to transfection. The
cells were co-transfected with the miRNA and expression plasmids
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol, when the cells
were ~70% confluent. RNA-lipid complexes were introduced into each
well of cells (4 µl/well for mimic and duplex for inhibitor), with
4 µg/well plasmids. Between 48 and 72 h at 37°C in 5%
CO2 post-transfection, the levels of target genes were
assessed using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analyses.
Analysis of mRNA and miRNA
expression
The expression levels of mRNA and miRNA were
determined using RT-qPCR analysis. Total RNA was extracted from the
transfected cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The RT-qPCR analysis for miRNAs was performed
using an All-in-One™ miRNA qPCR detection kit (GeneCopoeia, Inc.,
Rockville, MD, USA) and SYBR® Premix Ex
Taq II (Takara Bio, Inc., Otsu, Japan) for coding genes on a
Lightcycler 480 system (Roche, Mannheim, Germany), according to the
manufacturer's protocol. The thermocycling conditions were as
follows: 95°C for 10 min; followed by 40 cycles of 95°C for 10 sec,
62°C for 20 sec and 72°C for 20 sec. Ratios to indicate the
relative quantities were automatically exported by the Lightcycler
480 version 1.5.0 software.
Western blot analysis
The total protein was extracted from the cultured
RGC-5 cells. Samples were homogenized at 4°C in lysis buffer,
containing 50 mM Tris (pH 8.0), 150 mM NaCl, 50 mM EDTA, 0.5%
sodium deoxycholate and 1% Triton X-100, containing a protease
inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), 2 mM DTT
and 0.1 mM PMSF. Protein content was determined using a Bio-Rad DC
protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Subsequently, 50 µg total protein was resuspended in loading buffer
(20% glycerol, 10% SDS and 0.1% bromophenol blue), incubated for 5
min at 95°C and loaded onto a 10% polyacrylamide gel (Invitrogen;
Thermo Fisher Scientific, Inc.). Following electrophoresis, protein
was transferred onto a PVDF membrane and blocked in 5% nonfat milk.
The membrane was then incubated overnight at 4°C with rabbit
anti-BDNF antibody (1:500; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA; cat. no. sc-20981), rabbit anti-REST antibody
(1:1,000; Abcam, Hong, China; cat. no. ab21635) or mouse
anti-β-actin antibody (1:1,000; Beyotime Institute of
Biotechnology, Shanghai, China; cat. no. AA128) as an internal
control. Goat horseradish peroxidase (HRP)-conjugated anti-rabbit
(cat. no. ZB2308) and anti-mouse (cat. no. ZB2305) secondary
antibodies (1:2,000; Zhongshan Goldenbridge Biotechnology Co.,
Ltd., Beijing, China) was added to the membrane for 2 h at room
temperature. The blots were washed several times with saline buffer
(Tris-buffered saline with Tween-20 (25 mM Tris-HCl, 150 mM NaCl
and 0.1% Tween-20), incubated with ECL (HRP; 100 µl
ECL/cm2 of membrane; Pierce Biotechnology, Inc.,
Rockford, IL, USA) for 1 min at room temperature, and evaluated
using a LAS-3000 luminescent image analyzer (Fujifilm; Tokyo,
Japan). Band intensity was measured using Image J software version
2.1.4.7 (imagej.nih.gov/ij).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments, each performed
in triplicate. Statistical evaluation was performed using an
unpaired Student's t-test. *P<0.05 was considered to
indicate a statistically significant differences. Calculations were
performed using standard statistical software (SigmaPlot; Systat
Software, Inc, San Jose, CA, USA).
Results
E50K OPTN decreases the expression of
miR-9 in transfected RGC-5 cells
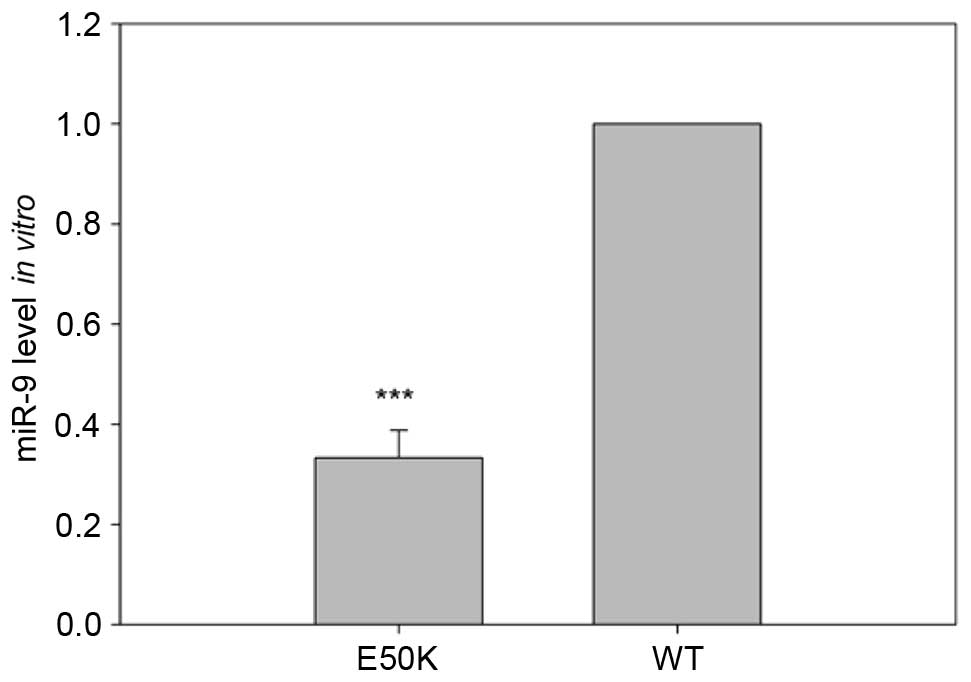
The present study first evaluated the effect of the
expression of mutant E50K OPTN on the mRNA levels of miR-9. The
levels of miR-9 were measured using RT-qPCR analysis following the
isolation of total RNA from the transfected RGC-5 cells. The
transfection-mediated overexpression of E50K OPTN in the RGC-5
cells led to a statistically significant decrease in the levels of
miR-9, compared with the RGC-5 cells transfected with a construct
overexpressing wild-type OPTN (P<0.001; Fig. 1). The reduced expression of miR-9
due to E50K OPTN suggested a potential role of miR-9 in RGC-5
cells.
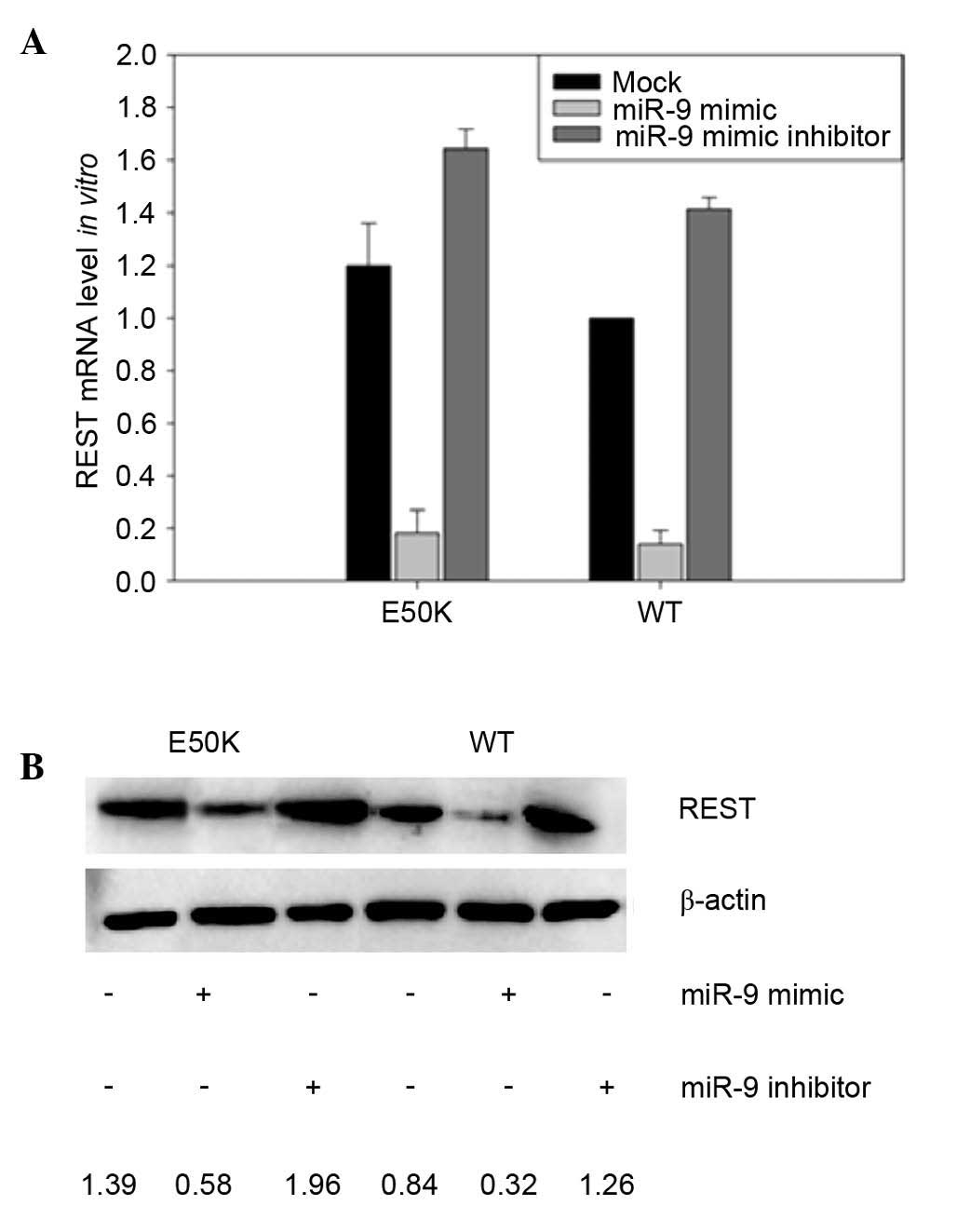
Expression of REST is increased in
E50K OPTN-transfected RGC-5 cells
miR-9 is known to regulate the expression of the
transcription factor, REST, and it has been previously shown that
the transfection of miR-9 significantly decreased the activity of a
reporter construct driven by the 3′ UTR of REST (17). As overexpression of E50K OPTN
significantly reduced the expression levels of miR-9, the present
study subsequently evaluated the levels of REST in RGC-5 cells
transfected to overexpress E50K OPTN. The transfection-mediated
expression of E50K OPTN led to a modest increase in the mRNA levels
of REST in the mock-treated RGC-5 cells, although this difference
was not statistically significant (Fig. 2A). Consistent with previous
studies, the in vitro transfection of RGC-5 cells with miR-9
mimic led to a marked reduction in the mRNA levels of REST, whereas
the introduction of an miR-9 mimic inhibitor led to modest
increases in the mRNA levels of REST. Evaluation of the protein
levels of REST in the transfected RGC-5 cells closely correlated
with the mRNA levels of REST mRNA (Fig. 2B). The protein levels of REST were
markedly increased in the mutant E50K OPTN models, compared with
the wild-type RGC-5 cells (Fig.
2B).
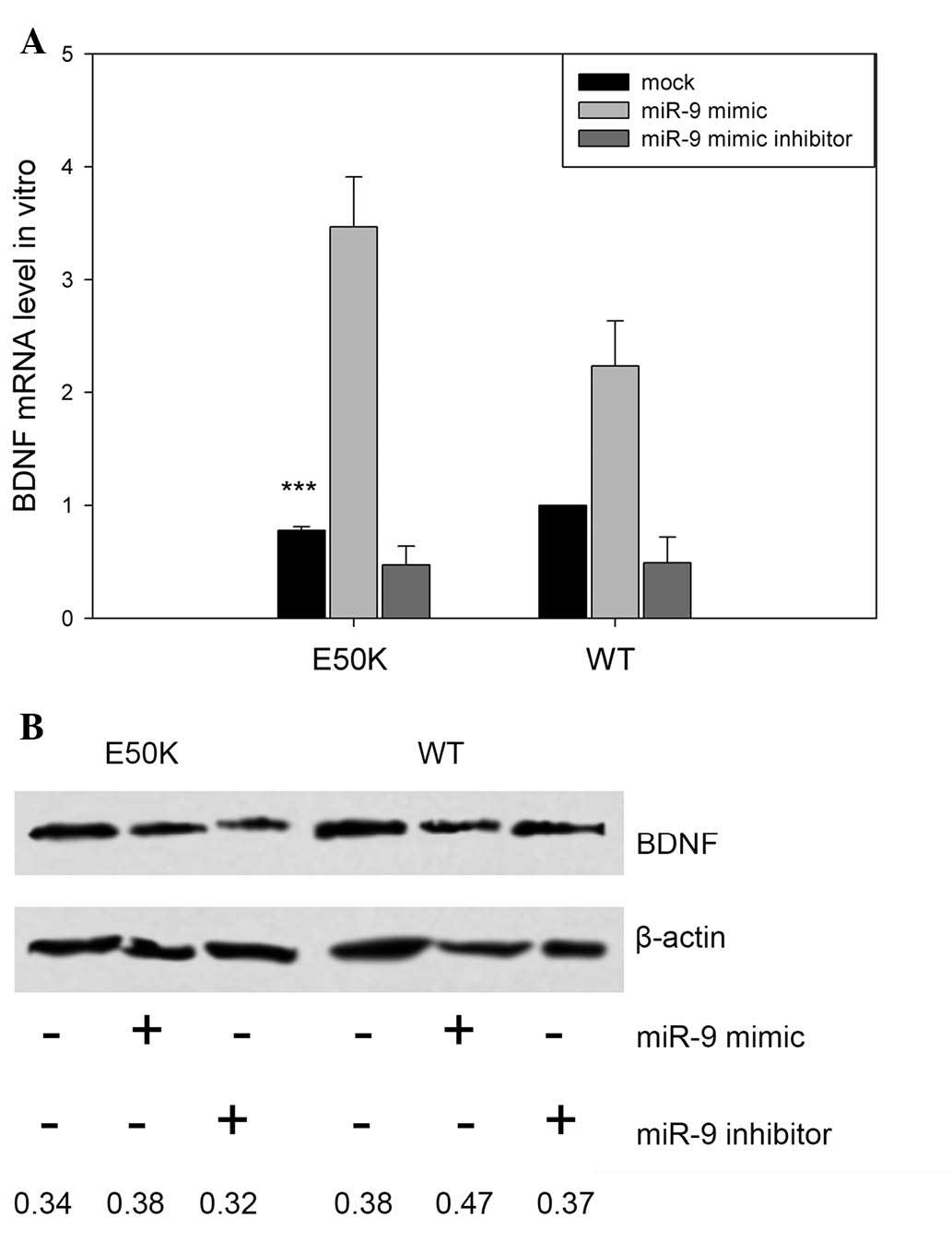
Expression of BDNF is decreased in
E50K OPTN-transfected RGC-5 cells
Our previous study suggested that BDNF is essential
for the survival of RGC-5 cells (18). Therefore, the present study
compared the levels of BDNF in E50K OPTN the RGC-5 cells
transfected to express wild-type OPTN. The mock-treated RGC-5 cells
transfected to express E50K OPTN exhibited decreased mRNA levels of
BDNF, compared with mock-treated RGC-5 cells transfected with
wild-type OPTN (***P<0.001; n=3), as shown in Fig. 3A. Co-transfection with the miR-9
mimic led to increased mRNA expression levels of BDNF in the E50K
and wild-type OPTN RGC-5 cells, whereas treatment with the miR-9
mimic inhibitor led to decreased mRNA levels of BDNF, compared with
the mock-treated cells (Fig. 3A).
Evaluation of the protein levels of BDNF in the E50K transfected
RGC-5 cells showed that the protein expression levels were also
attenuated, compared with the wild-type controls, and exhibited
patterns that correlated closely with the mRNA levels of BDNF
(Fig. 3B).
Discussion
The results of the present study showed that the
E50K OPTN mutation is associated with markedly reduced expression
levels of miR-9 using the cultured RGC-5 cells. E50K OPTN-dependent
reductions in miR-9 led to increased expression of the
transcriptional repressor, REST, suggesting a potential mechanism
by which the E50K OPTN mutation may lead to increased RGC-5 cell
apoptosis.
The transcriptional repression of miR-9 by REST has
been suggested. The present study provided evidence that the E50K
OPTN mutation downregulated the expression of miR-9 in RGC-5 cell
models (Fig. 1). miR-9 has been
implicated in development of the nervous system, and in
physiological and pathological processes in several organisms
(19).
miRNAs can also bind to protein factors and recruit
them to specific mRNAs to indirectly control the binding of other
regulatory factors (13).
TargetScan prediction of miRNA targets identified REST as a target
gene of miR-9. The results of the present study directly
demonstrate a repressive function of miR-9 on the expression of
REST (Fig. 2), consistent with a
previous report, which suggested a network of REST and miRNAs in
neuronal gene expression (9).
A series of studies have confirmed that BDNF
prevents the apoptotic death of neurons during development
(20–22). Caspase inhibitors can support
neurite regeneration by preventing RGC apoptosis and allowing the
rescue of adult neurons of the central nervous system, and BDNF may
have a protective effect against apoptosis in addition to promoting
neurite regeneration by the surviving cells (20). The analysis of polymorphisms of the
gene has revealed a statistically significant association of BDNF
with the risk of RGC apoptosis (21). BDNF results in the activation of
pro-survival cell-signaling pathways, which can afford
neuroprotection to the retina (22). REST act to coordinate neuronal gene
expression and promote neuronal identity, in part through BDNF
(9).
The cells used in the present study were those of
the RGC-5 line, and these are reported to be the same as the 661W
cell line, which is reported to be of cone photoreceptor origin. A
prior investigation also used HEK 293 cells to examine the
expression of miR-9, and the regulation of REST and BDNF, however,
the difference between the miR-9 mimic group and inhibitor group
were not statistically significant, suggesting that this mechanism
may be specific for diseases of the nervous system.
In conclusion, the E50K OPTN mutation markedly
reduced the levels of miR-9, which led to alterations in REST and
reduced expression levels of BDNF in RGC-5 cells.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81271000) and the Major
State Basic Research Development Program of China (973 Program;
grant no. 2011CB707502). The current study was directed by
Professor Renke Li of the Division of Cardiovascular Surgery and
Toronto General Research Institute, University Health Network and
Department of Surgery, Division of Cardiac Surgery, University of
Toronto (Toronto, Canada).
References
|
1
|
Rezaie T, Child A, Hitchings R, Brice G,
Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, et
al: Adult-onset primary open-angle glaucoma caused by mutations in
optineurin. Science. 295:1077–1079. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao Z, Meng Q, Tsai JC, Yuan H, Xu N and
Li Y: A novel optineurin genetic mutation associated with
open-angle glaucoma in a Chinese family. Mol Vis. 15:1649–1654.
2009.PubMed/NCBI
|
|
3
|
Aung T, Rezaie T, Okada K, Viswanathan AC,
Child AH, Brice G, Bhattacharya SS, Lehmann OJ, Sarfarazi M and
Hitchings RA: Clinical features and course of patients with
glaucoma with the E50K mutation in the optineurin gene. Invest
Ophthalmol Vis Sci. 46:2816–2822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung YF, Fan BJ, Lam DS, Lee WS, Tam PO,
Chua JK, Tham CC, Lai JS, Fan DS and Pang CP: Different optineurin
mutation pattern in primary open-angle glaucoma. Invest Ophthalmol
Vis Sci. 44:3880–3884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuse N, Takahashi K, Akiyama H, Nakazawa
T, Seimiya M, Kuwahara S and Tamai M: Molecular genetic analysis of
optineurin gene for primary open-angle and normal tension glaucoma
in the Japanese population. J Glaucoma. 13:299–303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alward WL, Kwon YH, Kawase K, Craig JE,
Hayreh SS, Johnson AT, Khanna CL, Yamamoto T, Mackey DA, Roos BR,
et al: Evaluation of optineurin sequence variations in 1,048
patients with open-angle glaucoma. Am J Ophthalmol. 136:904–910.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chalasani ML, Swarup G and Balasubramanian
D: Optineurin and its mutants: Molecules associated with some forms
of glaucoma. Ophthalmic Res. 42:176–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chi ZL, Akahori M, Obazawa M, Minami M,
Noda T, Nakaya N, Tomarev S, Kawase K, Yamamoto T, Noda S, et al:
Overexpression of optineurin E50K disrupts Rab8 interaction and
leads to a progressive retinal degeneration in mice. Hum Mol Genet.
19:2606–2615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J and Xie X: Comparative sequence
analysis reveals an intricate network among REST, CREB and miRNA in
mediating neuronal gene expression. Genome Bio. 7:R852006.
View Article : Google Scholar
|
|
10
|
Koch JM, Hinze-Selch D, Stingele K,
Huchzermeier C, Goder R, Seeck-Hirschner M and Aldenhoff JB:
Changes in CREB phosphorylation and BDNF plasma levels during
psychotherapy of depression. Psychother Psychosom. 78:187–192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujino H, Kitaoka Y, Hayashi Y, Munemasa
Y, Takeda H, Kumai T, Kobayashi S and Ueno S: Axonal protection by
brain-derived neurotrophic factor associated with CREB
phosphorylation in tumor necrosis factor-alpha-induced optic nerve
degeneration. Acta Neuropathol. 117:75–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakatani M, Shinohara Y, Takii M, Mori H,
Asai N, Nishimura S, Furukawa-Hibi Y, Miyamoto Y and Nitta A:
Periocular injection of in situ hydrogels containing Leu-Ile, an
inducer for neurotrophic factors, promotes retinal ganglion cell
survival after optic nerve injury. Exp Eye Res. 93:873–879. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: microRNAs: Tiny regulators with
great potential. Cell. 107:823–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao F, Zuo Z, Cai G, Kang S, Gao X and Li
T: miRecords: An integrated resource for microRNA-target
interactions. Nucleic Acids Res. 37:(Database issue). D105–D110.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Q, Wang Y, Hao Y, Juan L, Teng M,
Zhang X, Li M, Wang G and Liu Y: miR2 Disease: A manually curated
database for microRNA deregulation in human disease. Nucleic Acids
Res. 37:(Database issue). D98–D104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Packer AN, Xing Y, Harper SQ, Jones L and
Davidson BL: The bifunctional microRNA miR-9/miR-9* regulates REST
and CoREST and is downregulated in Huntington's disease. J
Neurosci. 28:14341–14346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Shan L, Song W, Xiao Z, Shi L and
Yuan H: Copolymer-1 immunization reduces damage in retinal ganglion
cells under high intraocular pressure through altering the
expression of retinal neurotrophins. J Ocul Pharmacol Ther.
26:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leucht C, Stigloher C, Wizenmann A, Klafke
R, Folchert A and Bally-Cuif L: MicroRNA-9 directs late organizer
activity of the midbrain-hindbrain boundary. Nat Neurosci.
11:641–648. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oshitari T and Adachi-Usami E: The effect
of caspase inhibitors and neurotrophic factors on damaged retinal
ganglion cells. Neuroreport. 14:289–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nowak A, Majsterek I, Przybyłowska-Sygut
K, Pytel D, Szymanek K, Szaflik J and Szaflik JP: Analysis of the
expression and polymorphism of APOE, HSP, BDNF, and GRIN2B genes
associated with the neurodegeneration process in the pathogenesis
of primary open angle glaucoma. Biomed Res Int. 2015:2582812015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta V, You Y, Li J, Golzan M, Klistorner
A, van den Buuse M and Graham S: BDNF impairment is associated with
age-related changes in the inner retina and exacerbates
experimental glaucoma. Biochim Biophys Acta. 1842:1567–1578. 2014.
View Article : Google Scholar : PubMed/NCBI
|