Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) remain leading causes for morbidity and
mortality in critically ill patients (1,2).
According to data from Acute Lung Injury Verification of
Epidemiology, ALI/ARDS affects approximately 7% of patients in
intensive care units, and approximately 54% of those develop ARDS
within 24 h (3). Similar to stress
situations such as trauma, burns and sepsis, seawater aspiration
can induce ALI/ARDS, with hypoxemia being the major
pathophysiological change that occurs. Furthermore, lung edema is
prominent in seawater exposure-induced lung injury, as highly
osmotic and alkaline seawater can force water to leave the blood
vessels and flood into the alveolar spaces (4). Progress has been made, however, there
remains a requirement to investigate the underlying
pathophysiological mechanisms of seawater aspiration-induced ALI,
in order to improve the prevention and treatment of this
condition.
Nuclear factor of activated T cells 5 (NFAT5), also
termed osmotic-response element binding protein (OREBP) or
tonicity-responsive enhancer binding protein (TonEBP), is a member
of the NFAT family of transcription factors (5). When NFAT5 is activated by high NaCl
and other hypertonic stresses, it increases transcription of
osmoprotective genes, including those involved in increased
expression of organic osmolytes (6) and heat shock proteins (7–9).
Several mechanisms contribute to hypertonicity-induced activation
of TonEBP/OREBP, including its translocation from the cytoplasm to
nucleus (10,11), transactivation (12), and increased TonEBP/OREBP protein
expression (11). In the kidney,
NFAT5 additionally controls expression of a urea transporter (UT-A)
(13) and of aquaporin-2 (6,7),
thus regulating the mechanisms of urinary concentration. NFAT5 has
been previously observed to be expressed in the majority of tissue
types, suggesting that the function of NFAT5 is not limited to the
renal medulla. Several additional genes regulated by NFAT5 that are
not directly involved in cellular osmoadaptation have additionally
been identified (14). These
include genes involved in embryogenesis and development, tumor
metastasis and hepatic detoxification enzymes, suggesting
biological importance of NFAT5 that is distinct from osmoadaptation
(14). However, it remains unclear
whether NFAT5 serves a pathophysiological role in seawater
aspiration-induced ALI.
Materials and methods
Drugs and reagents
Monoclonal anti-NFAT5 (cat. no. ab3446) and
monoclonal β-actin (cat. no. ab8226) antibodies were purchased from
Abcam (Cambridge, UK). Anti-phosphorylated (P)-nuclear factor
(NF)-κB p65 (cat. no. 8242) and anti-NF-κB p65 (cat. no. 3033)
monoclonal antibodies were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Enzyme-linked immunosorbent assay (ELISA)
kits for tumor necrosis factor (TNF)-α, interleukin (IL)-1β and
IL-8 were purchased from (R&D Systems, Inc., Minneapolis, MN,
USA). TurboFect transfection reagent was obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Seawater (osmolality
1300 mmol/l, pH 8.2, specific weight 1.05, NaCl2 6.518
g/l, MgSO4 3.305 g/l, MgCl2 2.447 g/l, CaCl2
1.141 g/l, KCl 0.725 g/l, NaHCO3 0.202 g/l and NaBr 0.083 g/l) was
prepared according to the major composition of the East China Sea
provided by the Chinese Ocean Bureau. All the other chemical
reagents were obtained from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany).
Animals and procedures
Adult male Sprague-Dawley rats (200–250 g weight; 9
weeks old; n=48) were obtained from the animal center of the Fourth
Military Medical University (Xi'an, China). Rats were maintained in
a temperature-controlled house with 12-h light-dark cycles. All
experiments were approved by the Animal Care and Use Committee of
the Fourth Military Medical University and were in accordance with
the Declaration of the National Institutes of Health Guide for Care
and Use of Laboratory Animals.
All rats were randomly divided into the following 4
groups: i) Control group (N, n=12), rats were anesthetized with
1.5% sodium pentobarbital (50 mg/kg; Sigma-Aldrich; Merck
Millipore) and followed by a sham operation; ii) seawater
aspiration 3 h group (S3, n=12), rats were anesthetized with 1.5%
sodium pentobarbital followed by intratracheal administration of
seawater (4 ml/kg body weight) into both lungs via a 20-gauge
intravenous catheter through the tracheae over 4 min, rats were
maintained in a supine position and 30° head-up tilt during the
experiments and were euthanized at 3 h; iii) seawater aspiration 6
h group (n=12), rats received seawater aspiration as for the S3
group, and were euthanized at 6 h; and iv) seawater aspiration 12 h
group (n=12), rats received seawater aspiration as for the S3
group, and rats were euthanized at 12 h. Euthanasia was conducted
with a pentobarbital overdose at the time points indicated, then
the lungs were harvested for the experiments described below.
Arterial blood gas analysis
Subsequent to seawater administration, a PE-50
catheter was inserted in the right carotid artery to obtain blood
samples. Arterial blood gas analysis was performed at 3, 6 and 12 h
subsequent to seawater aspiration, respectively.
Lung wet-to-dry (W/D) weight
ratio
Subsequent to removal of the trachea by blunt
dissection, the right lungs (n=6) were weighed immediately (wet
weight). Subsequently, the lungs were dried to a constant weight at
50°C for 72 h and weighed again (dry weight). The ratios of lung
wet-to-dry weight were calculated to evaluate the severity of
pulmonary edema.
Histopathological evaluation
At the end of experiments, the lung tissues of the
lower right lung of each rat (n=6) were fixed with 10% formalin for
24 h. Subsequent to fixation, the tissues were embedded in paraffin
and cut into 5 µm sections, and then stained with
hematoxylin-eosin. Microscopic evaluation was performed to
characterize lung injury.
Immunohistochemistry assessment of
NFAT5 in rat lungs
To investigate the expression level of NFAT5, the
lung tissue harvested previously was embedded in paraffin and cut
into 5-µm sections. Subsequent to dewaxing, rehydration and antigen
retrieval, the sections were stained with the anti-NFAT5 antibody
(1:100) following a standard procedure (15). Staining was detected using
3,3′-diaminobenzidine and observed under a light microscope.
Preparation of bronchoalveolar lavage
fluid (BALF)
BALF was performed using 3 ml ice-cold
phosphate-buffered saline three times) in all groups. In each rat,
90% (2.7 ml) of the total injected volume was consistently
recovered. Subsequently, BALF was centrifuged at 520 × g for 20 min
at 4°C, then the supernatant was stored at −70°C for subsequent
use.
NR8383 cell culture
The alveolar macrophage cell line, NR8383, was
maintained in Ham's F12 medium supplemented with 10% fetal calf
serum, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2 and 95% air. Different
volume ratios of seawater (12.5, 25 and 37.5%) were prepared by
adding seawater into the medium immediately prior to use. The cells
and supernatant were harvested at 6 h subsequent to exposure to
seawater.
Small interfering RNA (siRNA)
transfection
The primer sequences of the NFAT5 siRNA were
5′-GCAGCAGUCUCCUCUUUAUTT-3′ and 5′-AUAAAGUGGAGACUGCUGCTT-3′. The
primers sequences of the negative control were
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT −3′. The
NFAT5 siRNA and negative control siRNA were transfected into NR8383
cells using TurboFect transfection reagent according to the
manufacturer's instructions.
ELISA
Levels of TNF-α, IL-1 and IL-8 in BALF and the cell
supernatants were determined using the commercially available ELISA
kits according to the manufacturer's instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA were isolated from lung tissues or NR8383
cells homogenate using TRIzol (Takara Bio, Inc., Otsu, Japan).
Samples with a 260:280 nm absorbance ratio of 1.9 or greater were
used for subsequent reverse transcription using PrimeScript RT
reagent kit (Takara Bio, Inc.). The sequences of the rat NFAT5
primers were 5′-CGACAGTGCCAAAGCACCTC-3′ (forward) and
5′-AACCGGATACTGTCCACACAACATA-3′ (reverse). The sequences of the rat
β-actin primers were 5′-GCACTGTGTTGGCATAGAGGTC-3′ (forward) and
5′-ACGGTCAGGTCATCACTATCGG-3′ (reverse). SYBR Premix Ex Taq II
(TaKaRa Bio Inc.) and 50 µg cDNA were used for PCR. The PCR
reaction conditions for NFAT5 were as follows: 2 min initial
denaturation procedure at 94°C, followed by 35 cycles of 94°C for
45 sec, 62°C for 45 sec and 72°C for 1 min, and a final extension
step at 72°C for 8 min. The PCR products were analyzed by agarose
gel electrophoresis. An invariant mRNA quantity of β-actin was used
as an internal control to quantify PCR products.
In addition, the mRNA expression levels of three
NF-κB-dependent genes [TNF-α, monocyte chemoattractant protein 1
(MCP-1) and inhibitor of κB (IκBα)] were detected in order to
reveal the changes of NF-κB activation by RT-quantitative PCR
(RT-qPCR) analysis, as previously described (16). The sequences of the rat TNF-α
primers were 5′-TGAACTTCGGGGTGATCG−3′ (forward) and
5′-GGGCTTGTCACTCGAGTTTT-3′ (reverse). The sequences of the rat
MCP-1 primers were 5′-AGCATCCACGTGCTGTCTC-3′ (forward) and
5′-GATCATCTTGCCAGTGAATGAG-3′ (reverse). The sequences of the rat
IκBα primers were 5′-CTGGCCAGTGTAGCAGTCTT-3′ (forward) and
5′-GTCACCAAGTGCTCCACGAT-3′ (reverse).
Western blot analysis
Total proteins from lung tissues or NR8383 cells
were extracted as previously reported (17). Protein concentrations were
determined using the coomassie brilliant blue assay. Samples were
separated on a denaturing 12% SDS-polyacrylamide gel and
transferred to a nitrocellulose membrane followed by incubation
with the primary antibodies for NFAT5 (1:500), P-NF-κB p65 (1:500),
NF-κB p65 (1:500) and β-actin (1:8,000). Immunoreactivity was
visualized with the corresponding peroxidase-conjugated secondary
antibody (cat. no. ab6721; Abcam); the relative content of target
proteins was detected by chemiluminescence using Chemiluminescent
HRP Substrate (EMD Millipore, Billerica, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard error, and
statistical analysis was performed with a one-way analysis of
variance, followed by a Tukey test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Seawater inhalation induced severe ALI
and increased inflammatory cytokines in rats
As presented in Table
I, seawater aspiration markedly reduced PaO2, and an clear
increase in PaCO2 was observed at 3 and 6 h, which was partially
reduced at 12 h. We next observed the pulmonary histological
alterations at 3, 6 and 12 h subsequent to seawater inhalation by
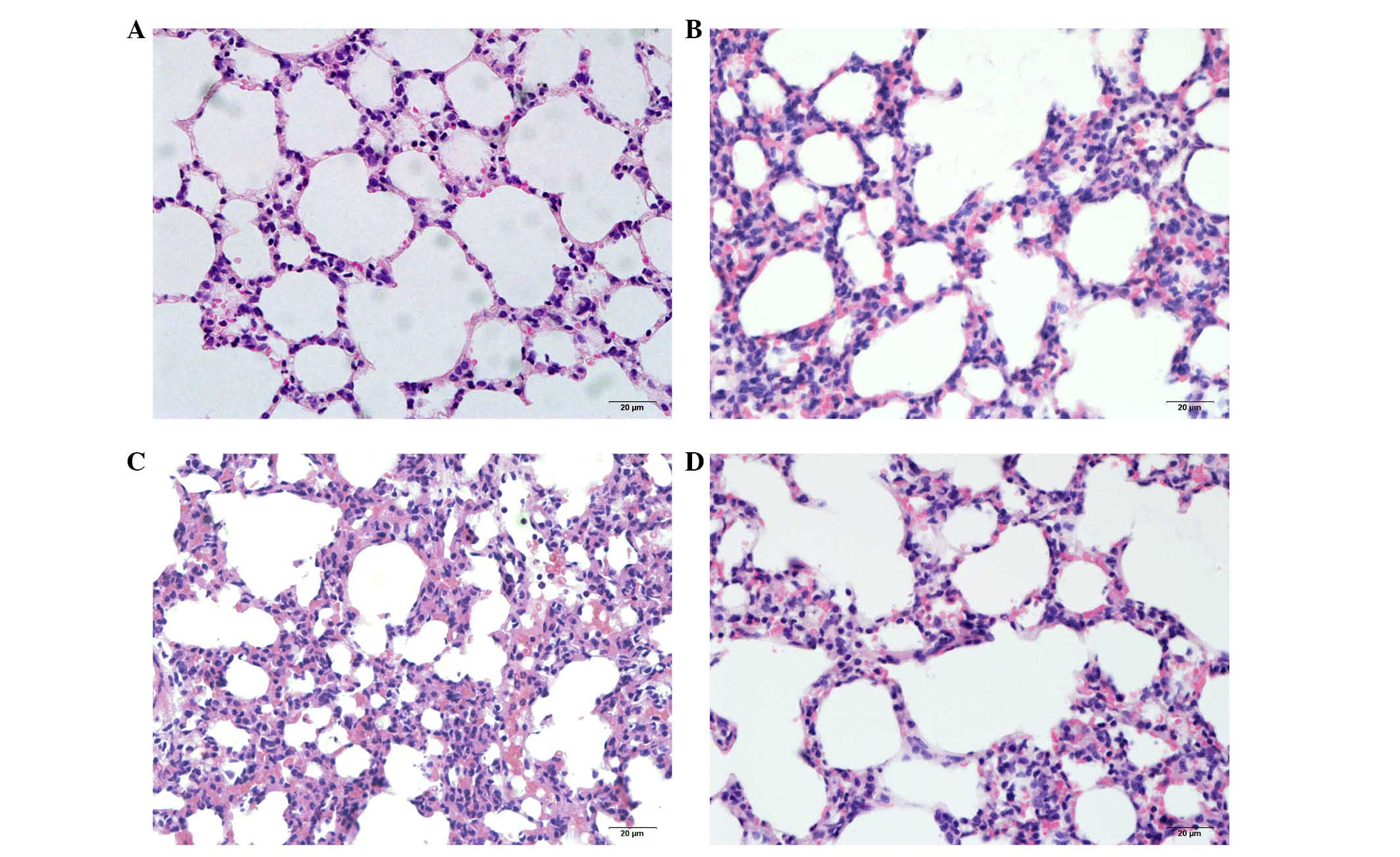
microscopy. The pulmonary tissue structure and alveoli were
observed to be normal in the control group (Fig. 1A). Seawater inhalation
time-dependently resulted in pulmonary edema, infiltration of
inflammatory cells and alveolar damage (Fig. 1B-D).
 | Table I.Effect of seawater inhalation on
changes to arterial blood-gas analysis. |
Table I.
Effect of seawater inhalation on
changes to arterial blood-gas analysis.
| Group | PO2
(mmHg) | PCO2
(mmHg) |
|---|
| N | 95.0±1.3 | 31.3±2.6 |
| S3 | 53.8±7.1b | 58.8±4.3b |
| S6 | 74.3±4.7a,c | 47.2±3.1a |
| S12 | 83.7±4.8d | 40.5±2.7d |
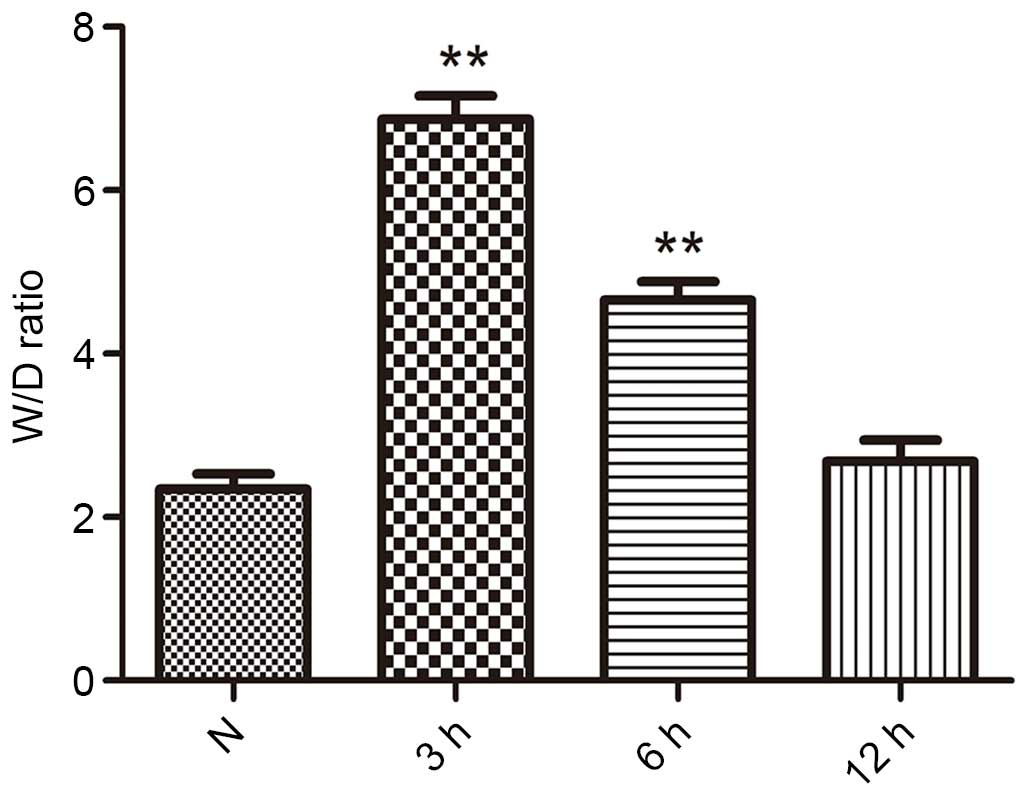
The lung W/D weight ratios was an index of lung
edema, and is presented in Fig. 2.
Subsequent to seawater inhalation, the W/D weight ratios were
significantly increased compared with that of the control group at
3 and 6 h (P<0.01). However, seawater inhalation did not
increase the W/D weight ratios of the 12 h group.
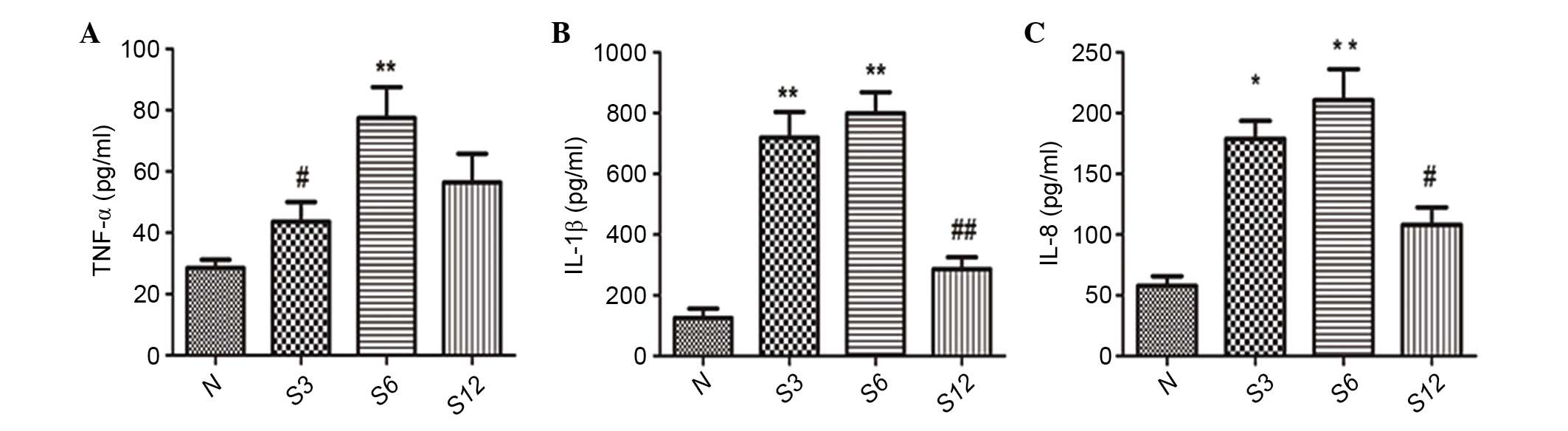
Regarding the inflammatory cytokines, TNF-α, IL-1β
and IL-8 in BALF were only minimally expressed in the control
groups, as presented in Fig. 3.
Subsequent to seawater administration, the content of TNF-α, IL-1β
and IL-8 were significantly increased at 3 and 6 h.
Seawater inhalation enhanced the
expression of NFAT5 and the activation of NF-κB in rats
Subsequent to seawater inhalation, the mRNA
expression of NFAT5 was markedly increased at different time points
(P<0.05; Fig. 4A), and the
immunohistochemical assessment of NFAT5 expression confirmed the
results above (Fig. 4B).
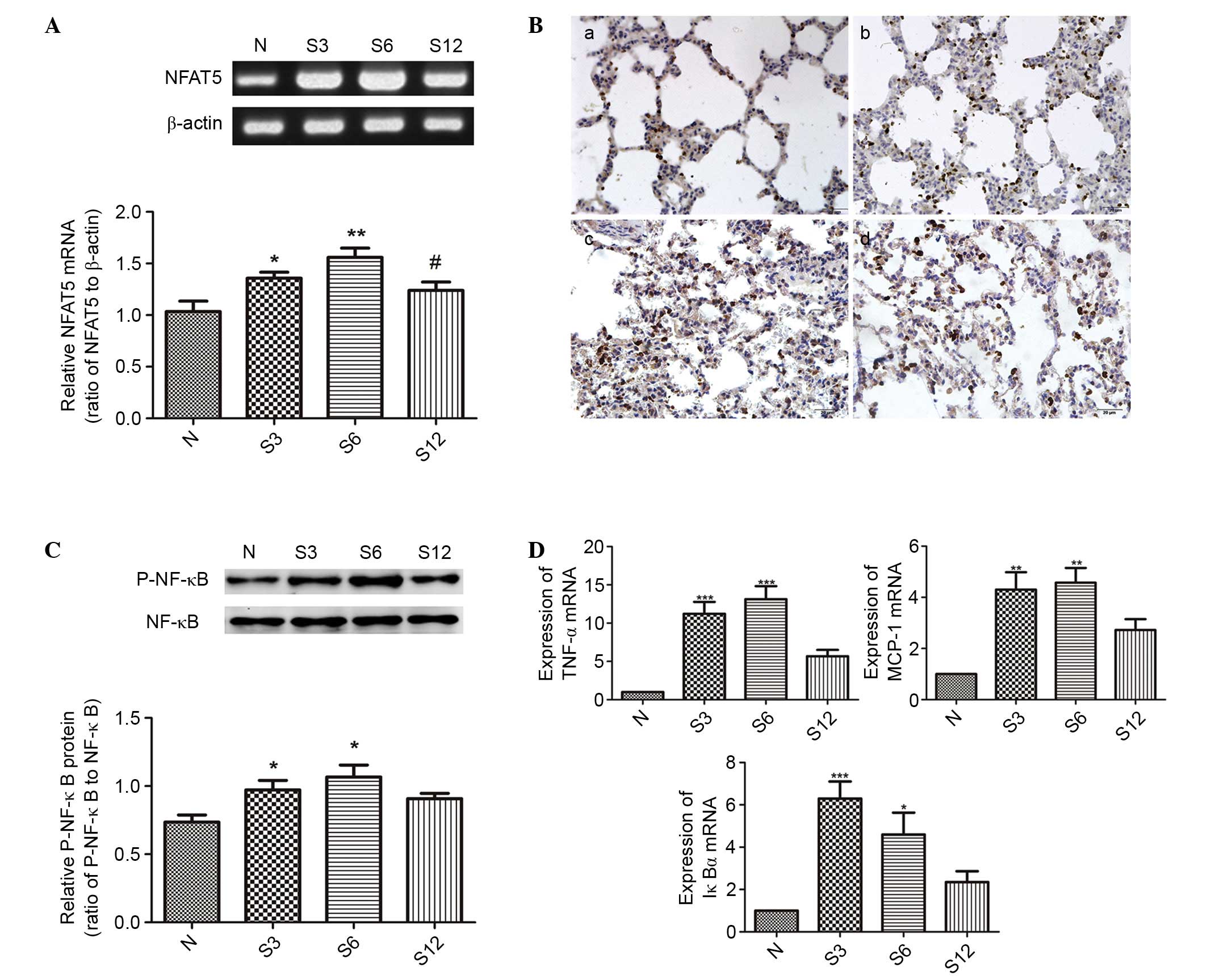 | Figure 4.The effects of seawater on NFAT5
expression and activation of NF-κB in lung tissue (n=6). (A)
RT-PCR, (B) immunohistochemistry and (C) western blotting results
indicated that seawater inhalation upregulated NFAT5 and P-NF-κB
expression, however no significant alteration was observed for
NF-κB levels. (D) The transcription of three NF-κB-dependent genes
by RT-quantitative PCR (TNF-α, MCP-1 and IκBα), which demonstrated
that the NF-κB activity increased 4- to 10-fold subsequent to 6 h
of seawater challenge, corresponding to the phosphorylation of
NF-κB. *P<0.05, **P<0.01, ***P<0.001 vs. the control
group; #P<0.05 vs. the seawater inhalation 6 h group.
NFAT5, nuclear factor of activated t cells 5; NF-κB, nuclear factor
κB; RT-PCR, reverse transcription-polymerase chain reaction; P-,
phosphorylated; TNF-α, tumor necrosis factor α; MCP-1, monocyte
chemoattractant protein 1; IκBα, inhibitor of κB; N, control; S3, 3
h seawater treatment group; S6, 6 h seawater treatment group; S12,
12 h seawater treatment group. |
In addition, the expression of P-NF-κB was also
significantly increased at 3 and 6 h (P<0.05; Fig. 4C). In addition, the transcription
of three NF-κB-dependent genes (TNF-α, MCP-1 and IκBα) was detected
in order to assess the changes to NF-κB activation. As presented in
Fig. 4D, the NF-κB activity
increased 4- to 10-fold following 6 h of seawater challenge, which
corresponded to the phosphorylation of NF-κB.
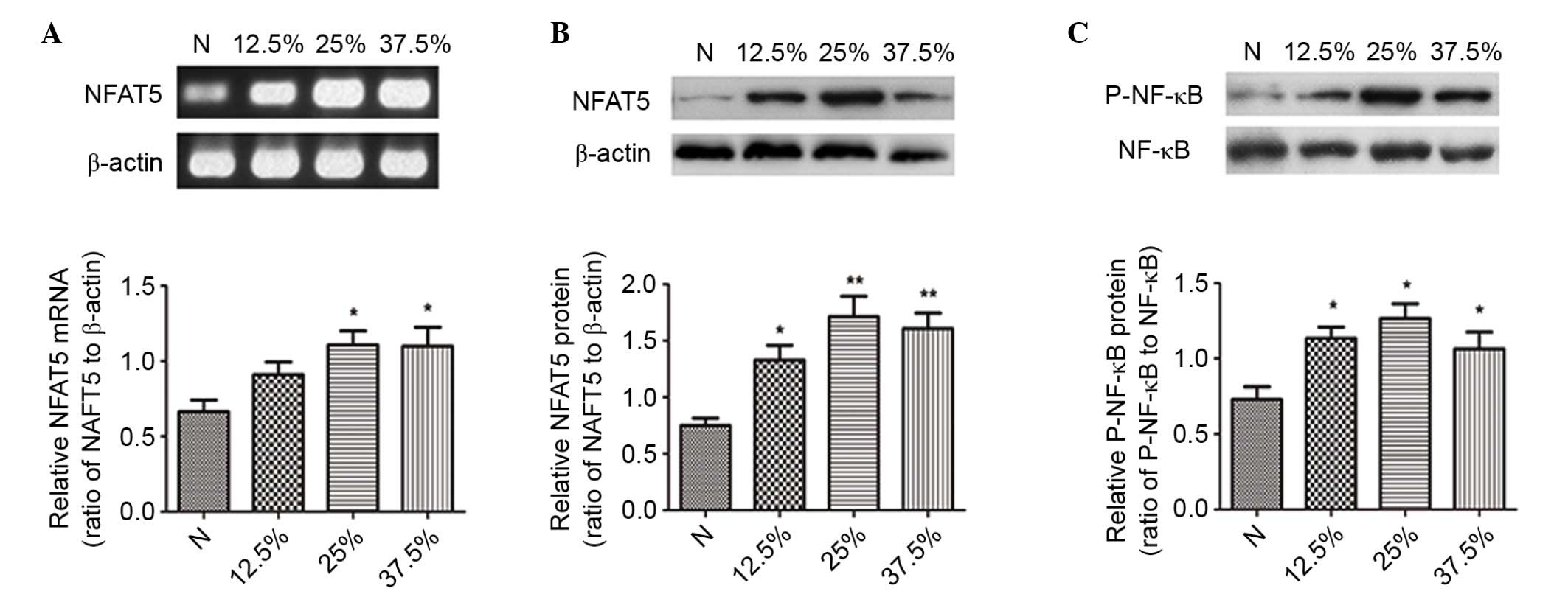
Different levels of seawater increased
the expression of NFAT5 and the activation of NF-κB in NR8383
cells
Subsequently, the effects of different
concentrations of seawater on NR8383 cells were assessed. The
results demonstrated that seawater concentration-dependently
promoted the mRNA and protein expression levels of NFAT5 (Fig. 5A and B).
The activation of NF-κB was significantly increased
at the different concentrations (Fig.
5C). The mRNA expression of all three NF-κB-dependent genes
(TNF-α, MCP-1 and IκBα) further confirmed the stimulatory effect of
seawater on the NF-κB pathway (Fig.
6D-F).
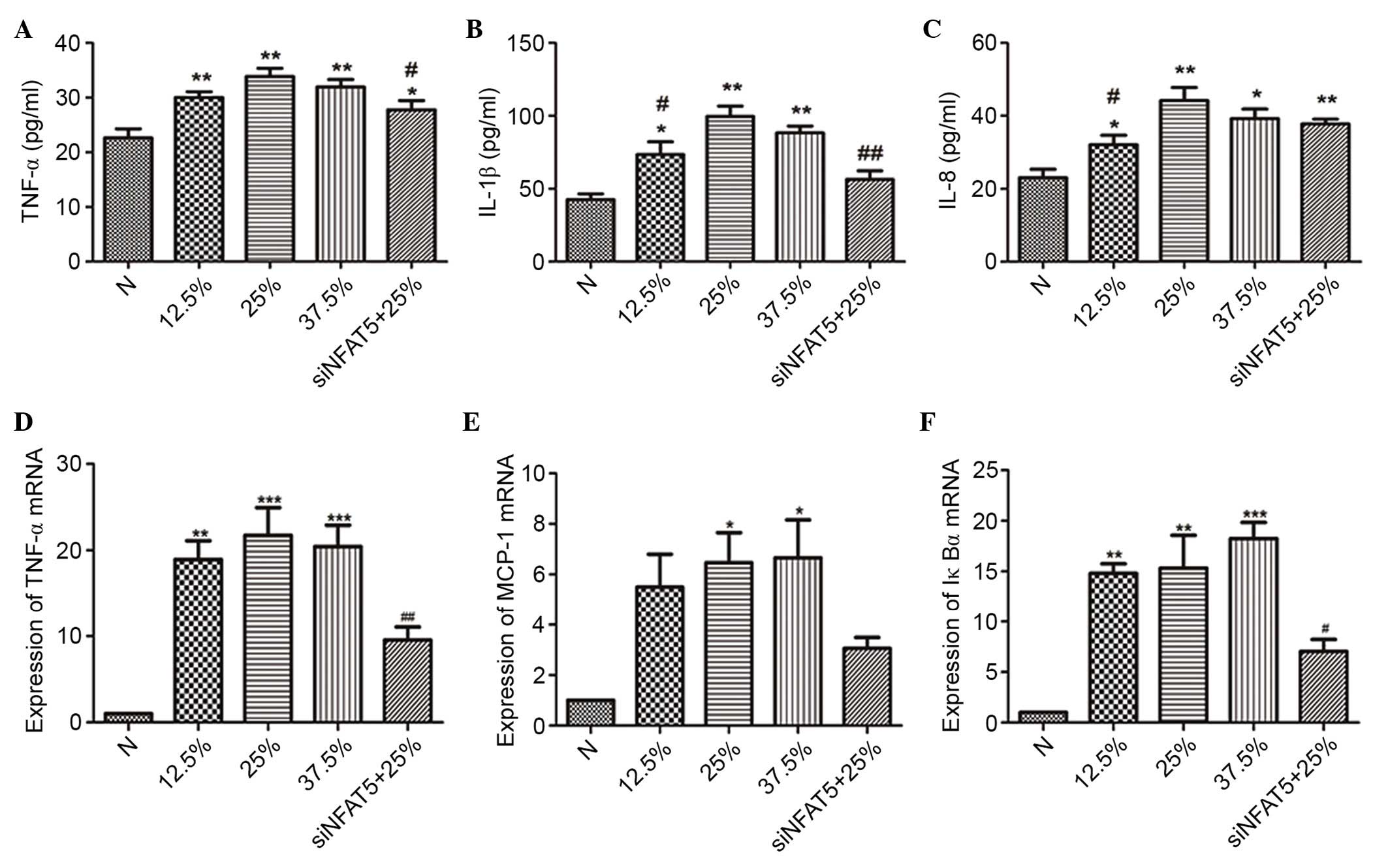 | Figure 6.The effect of seawater and siNFAT5 on
NR8383 cells cytokine expression and transcription of
NF-κB-dependent genes (TNF-α, MCP-1 and IκBα) (n=6). The ELISA
results demonstrated that the highest expression of the cytokines
[(A) TNF-α, (B) IL-1β and (C) IL-8] was observed in the 25%
seawater treatment NR8383 cells, however the addition of siNFAT5
treatment inhibited these effects. Similar results were obtained
when the transcription of NF-κB-dependent genes [(D) TNF-α, (E)
MCP-1 and (F) IκBα] was measured. *P<0.05, **P<0.01,
***P<0.001 vs. the control group; #P<0.05,
##P<0.01 vs. 25% seawater treatment group. si, small
interfering; NFAT5, nuclear factor of activated t cells 5; NF-κB,
nuclear factor κB; TNF-α, tumor necrosis factor α; MCP-1, monocyte
chemoattractant protein 1; IκBα, inhibitor of κB; IL-1β,
interleukin 1β; N, control. |
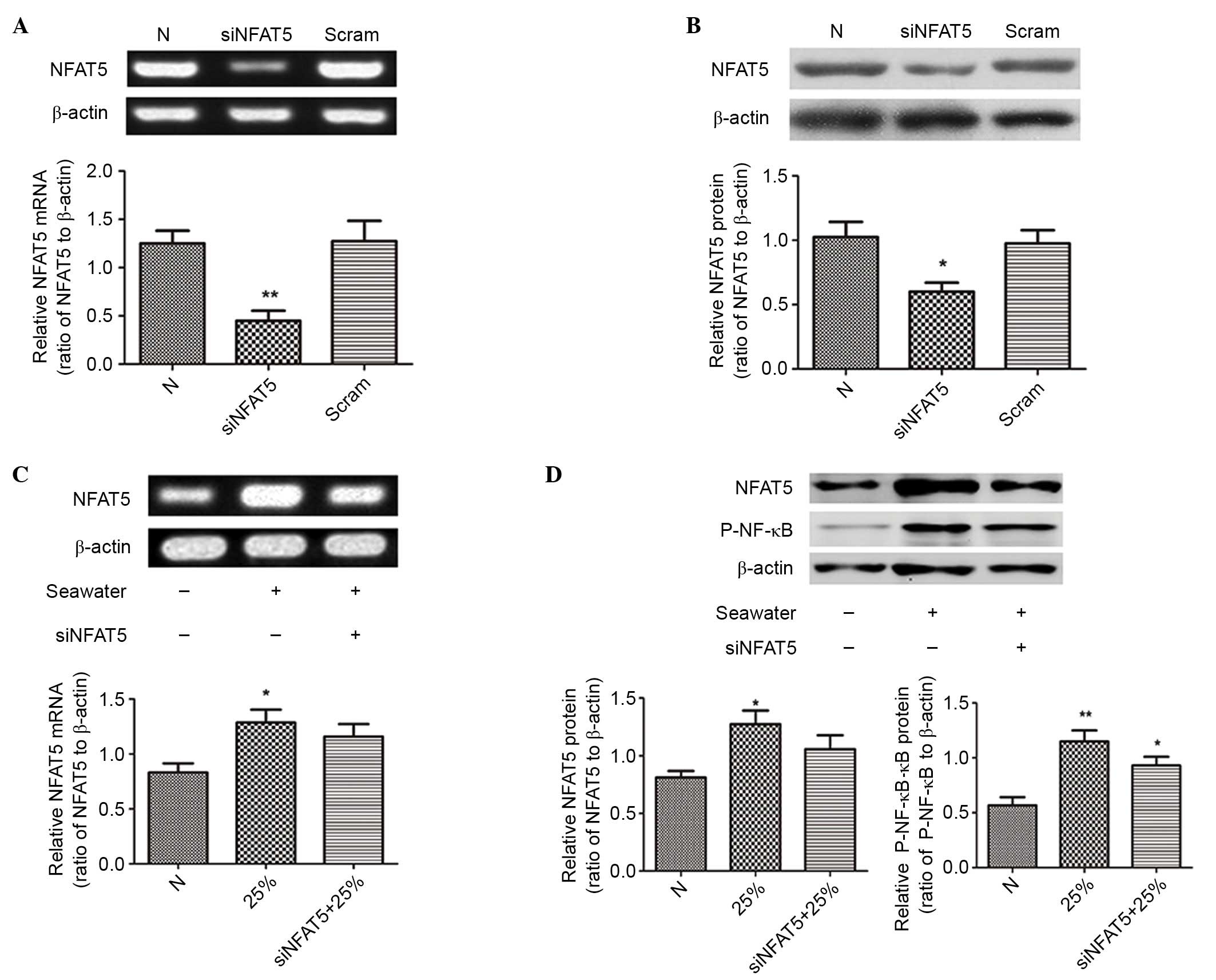
siNFAT5 reduced the expression of
NFAT5 and the activation of NF-κB in NR8383 cells
The siRNA of NFAT5 (siNFAT5) was used to
downregulate NFAT5 expression in NR8383 cells. Results demonstrated
that siNFAT5 significantly reduced the mRNA and protein expression
levels of NFAT5 induced by 25% of seawater exposure in NR8383
cells, and the negative control sequences had no effect (P<0.05;
Fig. 7). In addition, the
activation of NF-κB was significantly blocked, as presented in
Figs. 7D and 6D-F.
siNFAT5 reduced the levels of
inflammatory cytokines in NR8383 cells
Different concentrations of seawater markedly
increased the levels of TNF-α, IL-1β and IL-8 in the NR8383 cell
supernatants, while siNFAT5 markedly reduced the content of TNF-α,
IL-1β and IL-8 induced by seawater exposure (Fig. 6).
Discussion
In the present study, it was demonstrated that NFAT5
serves an important pathophysiological role in seawater
aspiration-induced ALI. Seawater aspiration impaired arterial blood
gas in a short time with a significant reduction in the partial
pressure of O2 and an increase in that of
CO2. In addition, clear pulmonary edema and vascular
leakage were induced. Furthermore, seawater exposure induced the
mRNA and protein expression of NFAT5 and the activation of NF-κB in
lung tissues and NR8383 cells. Using the siRNA of NFAT5, the mRNA
and protein expression levels of NFAT5 and the activation of NF-κB
were markedly reduced, accompanied by the reduction of certain
inflammatory cytokines.
Seawater is a hyperosmolar fluid and its NaCl
concentration is 3–3.5%, approximately 3-fold of that of
physiological saline (18), which,
when drawn into lung tissues, may induce serious complications,
characterized by infiltration of inflammatory cells and changes to
the permeability of the alveolar wall (19). However, the precise mechanisms of
seawater aspiration-induced ALI remain unclear. Previous studies
have demonstrated that the NF-κB pathway serves a key role in the
pathogenesis of ALI/ARDS. Stimulated with lipopolysaccharides,
NF-κB is activated by phosphorylation, enters the nucleus and
regulates the expression of inflammatory cytokines, therefore, the
control of NF-κB activation is crucial for the treatment of
inflammation (20,21).
NFAT5 is the most recently described member of the
Rel family of transcription factors, which includes NF-κB and
NFAT1-4, which serve central roles in inducible gene expression
during the immune response (22).
NFAT5 was initially described to drive osmoprotective gene
expression in renal medullary cells, which are routinely exposed to
high extracellular osmolalities. It has been previously reported
that local or systemic hyperosmolality is evident during the course
of various inflammatory disorders, accordingly, in mononuclear
cells and epithelial cells, NFAT5 stimulates the expression of
various pro-inflammatory cytokines during elevated ambient tonicity
(14). Thus, it is hypothesized
that NFAT5 serves a significant role in the initiation and
progression of the inflammatory disease process (23). However, whether NFAT5 participates
in the development of seawater aspiration-induced ALI and what the
role of NFAT5 is in this process remain to be fully elucidated.
It has been reported that NFAT5 can participate in
the adaption to hypertonicity by enhancing NF-κB activity (16). High levels of NFAT5 expression can
enhance the NF-κB activation (16), and low levels of NFAT5 expression
can reduce NF-κB activation, although it has no effect on p65
nuclear translocation (16). Based
on these previous data, it was suggested that the stimulatory
effect derived from hypertonicity on NF-κB is dependent, at least
in part, on NFAT5 expression. The results additionally demonstrated
that NFAT5 was responsible for the inflammatory responses resulting
from seawater aspiration through affecting the activity of NF-κB.
Subsequent to seawater exposure, the mRNA and protein expression
levels of NFAT5 both in lung tissues and in NR8383 cells were
increased, accompanied by the activation of NF-κB and the
aggregation of inflammatory cytokines. Using NFAT5 siRNA, it was
identified that inhibition of NFAT5 reduced seawater
aspiration-induced activation of NF-κB and inflammatory
cytokines.
In conclusion, the results of the present study
suggest that NFAT5 serves an important pathophysiological role in
seawater aspiration-induced ALI. Seawater inhalation increases the
mRNA and protein expression levels of NFAT5 and the activation of
P-NF-κB, lung tissues and in NR8383 cells. With the addition of the
siRNA of NFAT5, the mRNA and protein expression of NFAT5 and the
activation of NF-κB were markedly reduced, accompanied by the
reduction of inflammatory cytokines. Although the mechanisms of
NFAT5 on seawater aspiration-induced ALI require further
investigation, the present study partially explained the importance
of NFAT5 for ALI/ARDS.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81270124, 81270328,
81372129 and 30901752).
References
|
1
|
Costa EL, Schettino IA and Schettino GP:
The lung in sepsis: Guilty or innocent? Endocr Metab Immune Disord
Drug Targets. 6:213–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frutos-Vivar F, Ferguson ND and Esteban A:
Epidemiology of acute lung injury and acute respiratory distress
syndrome. Semin Respir Crit Care Med. 27:327–336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salomez F and Vincent JL: Drowning: A
review of epidemiology, pathophysiology, treatment and prevention.
Resuscitation. 63:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez-Rodriguez C, Aramburu J, Rakeman AS
and Rao A: NFAT5, a constitutively nuclear NFAT protein that does
not cooperate with Fos and Jun. Proc Natl Acad Sci USA.
96:7214–7219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lam AK, Ko BC, Tam S, Morris R, Yang JY,
Chung SK and Chung SS: Osmotic response element-binding protein
(OREBP) is an essential regulator of the urine concentrating
mechanism. J Biol Chem. 279:48048–48054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lopez-Rodriguez C, Antos CL, Shelton JM,
Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao
A and Olson EN: Loss of NFAT5 results in renal atrophy and lack of
tonicity-responsive gene expression. Proc Natl Acad Sci USA.
101:2392–2397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Na KY, Woo SK, Lee SD and Kwon HM:
Silencing of TonEBP/NFAT5 transcriptional activator by RNA
interference. J Am Soc Nephrol. 14:283–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woo SK, Lee SD, Na KY, Park WK and Kwon
HM: TonEBP/NFAT5 stimulates transcription of HSP70 in response to
hypertonicity. Mol Cell Biol. 22:5753–5760. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko BC, Turck CW, Lee KW, Yang Y and Chung
SS: Purification, identification, and characterization of an
osmotic response element binding protein. Biochem Biophys Res
Commun. 270:52–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyakawa H, Woo SK, Dahl SC, Handler JS
and Kwon HM: Tonicity-responsive enhancer binding protein, a
rel-like protein that stimulates transcription in response to
hypertonicity. Proc Natl Acad Sci USA. 96:2538–2542. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferraris JD, Williams CK, Persaud P, Zhang
Z, Chen Y and Burg MB: Activity of the TonEBP/OREBP transactivation
domain varies directly with extracellular NaCl concentration. Proc
Natl Acad Sci USA. 99:739–744. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakayama Y, Peng T, Sands JM and Bagnasco
SM: The TonE/TonEBP pathway mediates tonicity-responsive regulation
of UT-A urea transporter expression. J Biol Chem. 275:38275–38280.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neuhofer W: Role of NFAT5 in inflammatory
disorders associated with osmotic stress. Curr Genomics.
11:584–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu W, Dong M, Bo L, Li C, Liu Q, Li Y, Ma
L, Xie Y, Fu E, Mu D, et al: Epigallocatechin-3-gallate ameliorates
seawater aspiration-induced acute lung injury via regulating
inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats.
Mediators Inflamm. 2014:6125932014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roth I, Leroy V, Kwon HM, Martin PY,
Féraille E and Hasler U: Osmoprotective transcription factor
NFAT5/TonEBP modulates nuclear factor-kappaB activity. Mol Biol
Cell. 21:3459–3474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Bo L, Liu Q, Liu W, Chen X, Xu D and
Jin F: Activation of TRPV1-dependent calcium oscillation
exacerbates seawater inhalation-induced acute lung injury. Mol Med
Rep. 13:1989–1998. 2016.PubMed/NCBI
|
|
18
|
Suresh R, Kupfer Y and Tessler S: Acute
respiratory distress syndrome. N Engl J Med. 343:660–661. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gregorakos L, Markou N, Psalida V,
Kanakaki M, Alexopoulou A, Sotiriou E, Damianos A and Myrianthefs
P: Near-drowning: Clinical course of lung injury in adults. Lung.
187:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abraham E: NF-kappaB activation. Crit Care
Med. 28:(Suppl 4). N100–N104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blackwell TS and Christman JW: The role of
nuclear factor-kappa B in cytokine gene regulation. Am J Respir
Cell Mol Biol. 17:3–9. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aramburu J, Drews-Elger K, Estrada-Gelonch
A, Minguillón J, Morancho B, Santiago V and López-Rodriguez C:
Regulation of the hypertonic stress response and other cellular
functions by the Rel-like transcription factor NFAT5. Biochem
Pharmacol. 72:1597–1604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halterman JA, Kwon HM and Wamhoff BR:
Tonicity-independent regulation of the osmosensitive transcription
factor TonEBP (NFAT5). Am J Physiol Cell Physiol. 302:C1–C8. 2012.
View Article : Google Scholar : PubMed/NCBI
|