Introduction
It is well known that stress causes considerable
harm to human health and also impairs livestock production. The
hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic
nervous system (SNS) are stimulated by exogenous and endogenous
factors, which may induce stress reactions, thus triggering
compensatory physiological responses (1). After the HPA axis and SNS receive
stimulatory signals, the secretion of glucocorticoids and
catecholamines is increased. Glucocorticoids exhibit feedback
inhibition on HPA function through binding with receptors in the
pituitary gland, hypothalamus and prefrontal cortex (2). Long-term stress may cause depression
and neurological disorders in animals and humans. Behavioral stress
is a major risk factor for several clinical disorders, including
cardiovascular disease, metabolic disease and psychosis.
Furthermore, stress is associated with mental disorders, such as
anxiety and personality disorders. Several studies have
demonstrated that stress can increase the incidence of diseases,
including gastroenteritis (3–5).
However, research on stress and male reproduction is fairly
limited.
The insulin-like growth factor 1 (IGF-1)/phosphatase
and tensin homologue deleted on chromosome 10 (PTEN)/Akt/forkhead
box (FoxO) signal pathway serves important roles in various cells
and tissues (6,7). IGF-1 has a role in signal
transduction by binding to its receptor, and subsequently
regulating cellular processes via phosphorylation (8). Phosphoinositide 3-kinase (PI3K)/Akt
is the key downstream signaling pathway of IGF-1, which regulates
cell proliferation, growth and apoptosis. The Akt family, also
known as protein kinase B, has three members: Akt1, Akt2 and Akt3,
which have high homology and specific expression patterns. Akt1 and
Akt2 are widely expressed in tissues and cells; however, Akt3 is
only expressed in specific tissues and cells (9). PTEN is a tumor suppressor gene with
phosphatase activity, which regulates cell proliferation cycles and
inhibits cell migration. PTEN is the phosphatase of
phosphatidylinositol (3,4,5)-trisphosphate (PIP3), reverses the
transformation of phosphatidylinositol 4,5-bisphosphate to PIP3,
inhibits PI3K phosphorylation, suppresses the activity of Akt and
downstream kinases, arrests the cell cycle at G1 phase,
and negatively regulates cell growth (10). FoxO is one of the most important
target genes of the downstream IGF-I/PTEN/Akt signaling pathway,
and includes FoxO1, FoxO3a, FoxO4 and FoxO6 (11). FoxO is regulated by Akt
phosphorylation (12), following
which its nuclear export is inactivated; therefore, the activation
of apoptotic genes downstream of FoxO is inhibited. During this
process, the nuclear-cytoplasmic trafficking of FoxO is ultimately
involved in the regulation of cell apoptosis (13).
Water immersion and restraint stress (WRS) has been
reported to increase the expression of IGF-1 in rat gastric mucosa
tissue, and regulate cell apoptosis of the gastric mucosa via the
PI3K/Akt signal pathway (14).
IGF-1 is a specific anti-apoptotic factor present in mouse Leydig
cells during embryonic developmental stages (15). IGF-1 also serves an important role
in the proliferation and differentiation of mouse Sertoli cells,
through the PI3K/Akt signal pathway during the adolescent stage
(16,17). In the proliferation and
differentiation of Sertoli cells of 20-day-old mice,
follicle-stimulating hormone (FSH) can activate the PI3K/Akt
signaling pathway. The role of PTEN in spermatogenesis also remains
controversial. Wu et al hypothesized that PTEN may have a
role in the late stage of sperm development (18). Dupont et al demonstrated
that estrogen induced testicular tumors in mice via the PTEN/Akt
signal pathway (19). Similarly,
Kimura et al revealed that PTEN gene knockout could lead to
testicular tumors in mice (20).
The expression levels of PTEN were gestational age-specific in the
fetal rat testes, thus suggesting that PTEN may have a key role in
fetal rat testis development (21). However, Huang et al reported
that PTEN did not serve a role in regulating spermatogenesis in
mice (22). Previous reports have
also focused on the role of FoxO in male reproduction. FoxO1 has a
crucial role in the initiation of spermatogenesis; however, two
other members, FoxO3a and FoxO4, do not appear to serve any roles
(23–26). Therefore, the IGF-1/PTEN/Akt/FoxO
signaling pathway may have an important role in male
reproduction.
In the present study, immunohistochemistry (IHC),
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) staining and quantitative polymerase chain reaction (qPCR)
were conducted to investigate the effects of WRS on male
reproductive function in rats. In addition, the possible roles of
the IGF-1/PTEN/Akt/FoxO signaling pathway were investigated
following WRS.
Materials and methods
Reagents
Antibodies against FoxO1 (cat. no. 9462), FoxO3a
(cat. no. 9467), FoxO4 (cat. no. 9472) and total Akt (cat. no. 9272
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Antibodies against IGF-1 (cat. no. BA0939) were purchased
from Wuhan Boster Biological Technology Co., Ltd. (Wuhan, China).
Antibodies against PTEN (cat. no. sc-9145) were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Avidin-biotin complex
kits were obtained from BioGenex (Fremont, CA, USA) and
3,3′-diaminobenzidine tetrachloride (DAB) was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). SYBR Premix
Ex Taq (cat. no. DRR420A) and PrimeScript™ RT reagent kit with
gDNA Eraser (cat. no. DRR047S) were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). One-step TUNEL Apoptosis
Assay kit (cat. no. C1088) and Caspase 3 Activity Assay kit (cat.
no. C1116) were obtained from Beyotime Institute of Biotechnology
(Haimen, China). All other chemicals were purchased commercially
and were reagent grade.
Animals and sample collection
A total of 50 intact male Sprague-Dawley rats (age,
9–11weeks; weight, 200–220 g) were obtained from Qinglongshan
Experimental Animal Breeding Farm (Nanjing, China) for use in the
present study. Uniform commercial diets used in the present study
were also purchased from Qinglongshan Experimental Animal Breeding
Farm. Rats received regular rat chow and tap water ad
libitum, and were housed individually at 25°C and 65–70%
humidity under a 12-h:12-h light/dark cycle. WRS models were
established as described in our previous study (27). For each time point, six rats were
sacrificed after 0, 3 and 7 h of WRS, whereas others were fed
normally 1 h after WRS and six were sacrificed at several time
points (4, 8 and 15 days) after the end of 7 h WRS. Rats were
anesthetized with ether inhalation and sacrificed by cervical
dislocation. In order to examine protein localization and conduct
TUNEL staining, testicular samples were immediately removed from
anesthetized rats and fixed in 4% (v/v) paraformaldehyde at room
temperature overnight. In addition, testicular samples were stored
in liquid nitrogen for the analysis of gene expression and
caspase-3 activity. All procedures were designed in accordance with
generally accepted ethical standards for animal experimentation and
the guidelines established by the institutional animal care and use
committee of Jiangsu University (Jiangsu, China).
Epididymal sperm reserves
Epididymal sperm morphology was observed as
described previously (28).
Briefly, cauda epididymides were sampled, and gently minced in 2.0
ml phosphate-buffered saline (PBS). The minced material was then
homogenized for 1 min, filtered through a nylon mesh screen, and
the filtrate was brought up to a final volume of 10 ml with PBS.
Epididymal sperm morphology was observed under a high power light
microscope following Giemsa staining (Shanghai Gefan Biotechnology,
Co., Ltd., Shanghai, China) at room temperature. The morphology of
200 sperm per rat was evaluated. The sperm malformation rate
percentage was then calculated using a random selection of one
optical area, in which 200 sperm were consecutively evaluated for
malformation.
Assessment of apoptotic cell
number
The testicular samples were embedded in paraffin wax
and cut into 7-µm sections. The TUNEL staining was performed using
a one-step TUNEL Apoptosis Assay kit, as described previously
(29). Briefly, testicular cells
were counterstained with DAPI (Beyotime Institute of Biotechnology)
to label all nuclear DNA, and fragmented DNA was end-labeled with
fluorescein isothiocyanate-labeled dUTP using terminal transferase.
The sections were then examined under a confocal immunofluorescence
microscope (LSM5 PASCAL; Carl Zeiss, Oberkochen, Germany). Sections
exposed to DNase I, which causes DNA fragmentation, exhibited
intense nuclear staining and were used as positive controls. For
negative controls, dUTP was omitted, resulting in uniformly
negative staining. Ten optical areas, containing 500–1,000 cells,
were counted in each slide under high-power (400x) microscopy and
the number of positive cells per area was counted.
Determination of caspase-3
activity
Testicular samples (n=6 for each treatment) were
rinsed with cold PBS, and homogenized on ice in lysis buffer (3–10
mg/100 µl). Homogenates were transferred to 1.5 ml centrifuge
tubes, lysed on ice for 5 min and centrifuged at 18,000 × g
for 10 min at 4°C. Caspase-3 activity was determined in the lysates
of testicular samples from rats that underwent various treatments
using a caspase-3 activity kit. Briefly, this colorimetric assay is
based on hydrolysis of the substrate peptide, Ac-DEVD-pNA, by
caspase-3. The released moiety (p-nitroaniline) has a high
absorbance at 405 nm. Therefore, the concentration of
p-nitroaniline (µM) released from the substrate is calculated from
the absorbance values at 405 nm, or from a calibration curve
prepared using defined p-nitroaniline solutions (30).
IHC for IGF-1 (1:1,00), PTEN (1:200), total Akt
(1:200) and FoxO1 (1:300) was performed on formalin-fixed,
paraffin-embedded testicular tissue sections using a standard
protocol, as described previously, using DAB and hematoxylin
counterstaining (27,31).
RNA extraction, reverse transcription
and qPCR
Total RNA was extracted from testicular samples
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). RNA concentration and purity
were determined using a spectrophotometer (NanoVue; GE Healthcare,
Piscataway, NJ, USA), and the integrity was examined using 1.2%
agarose gels containing 0.1% ethidium bromide. Total RNA (1 µg)
obtained from each extraction was reverse transcribed in a 20 µl
reaction volume using an RT reagent kit with gDNA Eraser according
to the manufacturer's protocol. The primers (Thermo Fisher
Scientific, Inc.) were designed based on the corresponding gene
sequences (Table I). qPCR was
performed with a 20 µl reaction volume containing 2 µl template
cDNA, 0.4 µl forward/reverse primers, 10 µl 2X SYBR qPCR mix and
0.4 µl ROX reference dye (Takara Bio, Inc., Otsu, Japan). on an ABI
7300 instrument (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR conditions were as follows: 95°C for 10 min,
followed by 45 cycles of denaturation at 95°C for 15 sec and 60°C
for 45 sec. Experiments for the detection of all genes, including
the housekeeping gene hypoxanthine phosphoribosyltransferase 1
(HPRT), were performed in triplicate. The relative
expression levels of the genes tested were calculated using the
2−ΔΔCq method (32).
 | Table I.Primers used for quantitative PCR
analysis. |
Table I.
Primers used for quantitative PCR
analysis.
| Gene and sequence
reference (GenBank no.) | Primer
sequence | Size of PCR product
(bp) | Annealing
temperature (°C) |
|---|
| HPRT
(X62085) |
F:5′-AGTGATGATGAACCAGGTTA-3′ | 556 | 58.0 |
|
|
R:5′-ATTATAGTCAAGGGCATATC-3′ |
|
|
| IGF-1
(BC086374) |
F:5′-TGGTGGACGCTCTTCAGTTC-3′ | 168 | 58.0 |
|
|
R:5′-GCTTCAGCGGAGCACAGTAC-3 |
|
|
| PTEN
(NM031606) |
F:5′-AGCGTGCGGATAATGACAAG-3′ | 151 | 56.0 |
|
|
R:5′-GGATTTGATGGCTCCTCTACTG-3′ |
|
|
| Akt1
(NM033230) |
F:5′-TAGGCATCCCTTCCTTACAG-3′ | 269 | 58.0 |
|
|
R:5′-GCCCGAAGTCCGTTATCT-3′ |
|
|
| Akt2
(NM017093) |
F:5′-GAGCCGAGTCCTACAGAATACC-3′ | 263 | 58.0 |
|
|
R:5′-GGCCATCTTTGTCCAGCATA-3′ |
|
|
| Akt3
(NM031575) |
F:5′-AACGACCAAAGCCAAATACA-3′ | 498 | 58.0 |
|
|
R:5′-CCCCATTAACATATTCCATCAC-3′ |
|
|
| FoxO1
(NM001191846) |
F:5′-CGTCCTCGAACCAGCTCAA-3′ | 292 | 57.4 |
|
|
R:5′-TTGGCGGTGCAAATGAATAG-3′ |
|
|
Testosterone concentration
Rat blood samples were obtained from the heart and
transferred to centrifuge tubes. After 2 h standing at room
temperature, samples were centrifuged at 1,400 × g for 10
min at 4°C to obtain serum. Testosterone concentrations in the
serum were detected using a commercial radioimmunoassay kit (cat.
no. DF00008; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's protocols. The
cross-reaction rates of this antiserum with progesterone, cortisol,
estradiol and dehydroepiandrosterone (Beijing North Institute of
Biological Technology, Beijing, China) were <0.01%. The
intra-coefficients of variation for androgen determination in this
laboratory were <9%. Treatments were performed in triplicate,
and each experiment was repeated at least three times.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to conduct statistical analyses. All experiments were
repeated in triplicate, and representative data is presented. Data
are presented as the mean ± standard error of the mean. Data were
analyzed using one-way analysis of variance and Fisher's protected
least significant difference test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of WRS on sperm malformation
rate
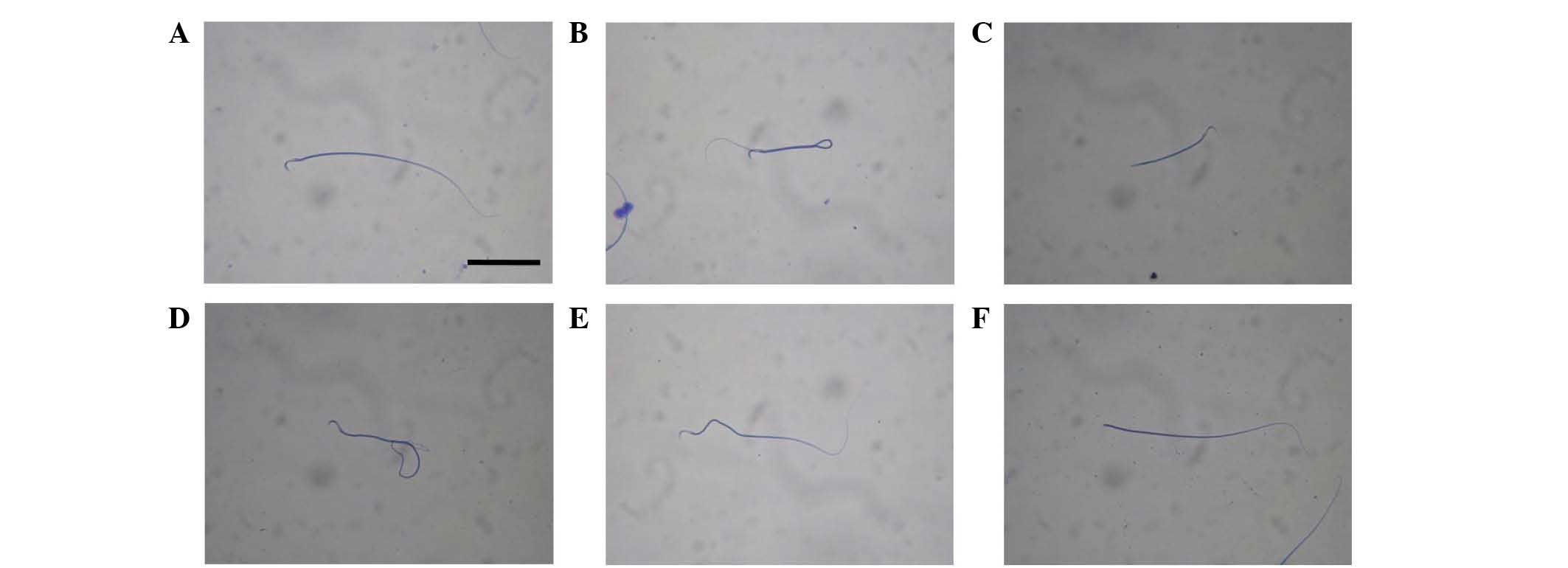
Following WRS, sperm in rat epididymis exhibited
several morphologies, as follows: Normal morphology (control group,
Fig. 1A), malformation in the tail
(7 h group, Fig. 1B; 3 h group,
Fig. 1C; 15 day group, Fig. 1D), malformation in the middle (3 h
group, Fig. 1E), and decapitated
sperm (7 h group, Fig. 1F). In
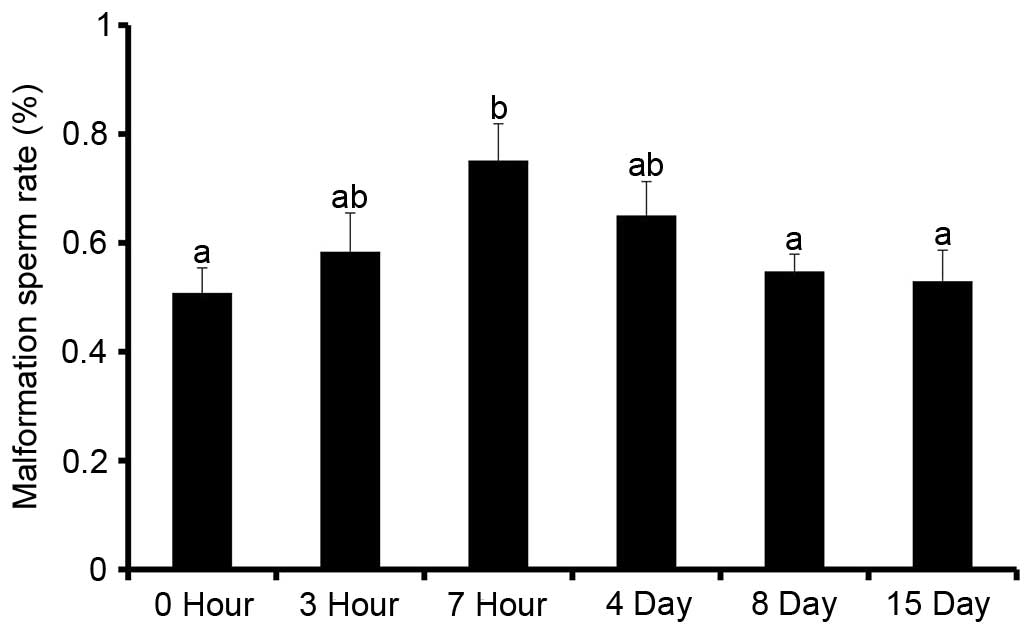
addition, WRS increased the sperm malformation rate in the rat
epididymis (Fig. 2). When WRS was
extended to 7 h, the sperm malformation rate was significantly
higher compared with in the other groups (P<0.05; n=6).
Effects of WRS on serum testosterone
concentrations
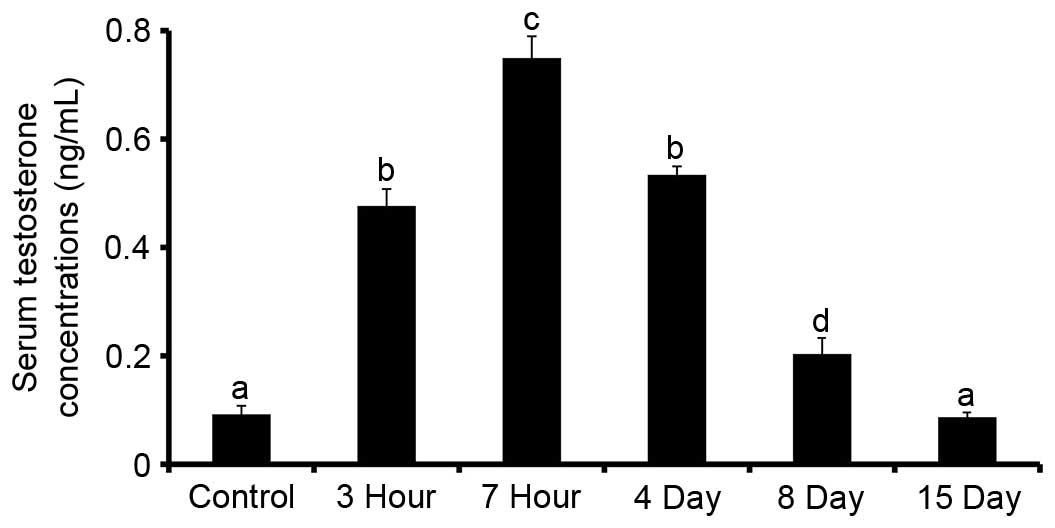
Testosterone concentrations in the sera of rats 3
and 7 h after WRS were increased in a time-dependent manner, as
compared with the control group (P<0.05; n=6). On days 4, 8 and
15 after 7 h WRS, testosterone concentrations were gradually
decreased compared with in the 7 h WRS group (Fig. 3).
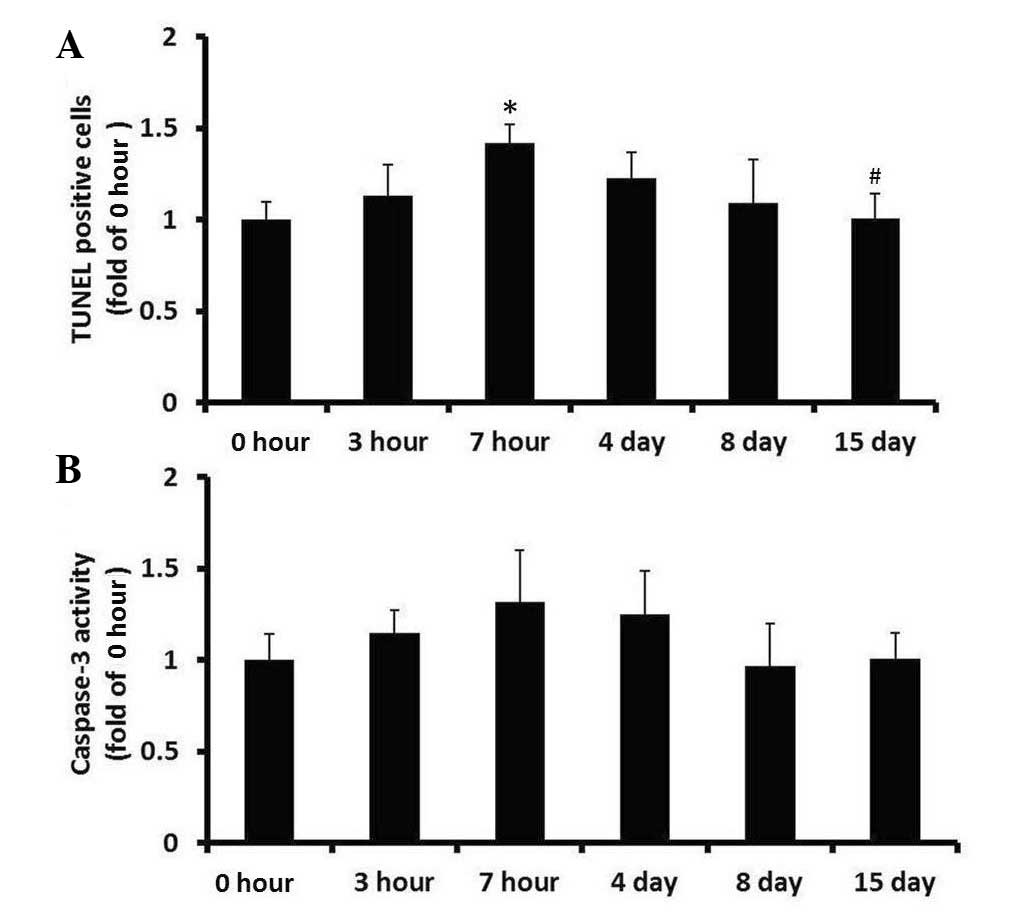
Effects of WRS on apoptosis in rat
testes
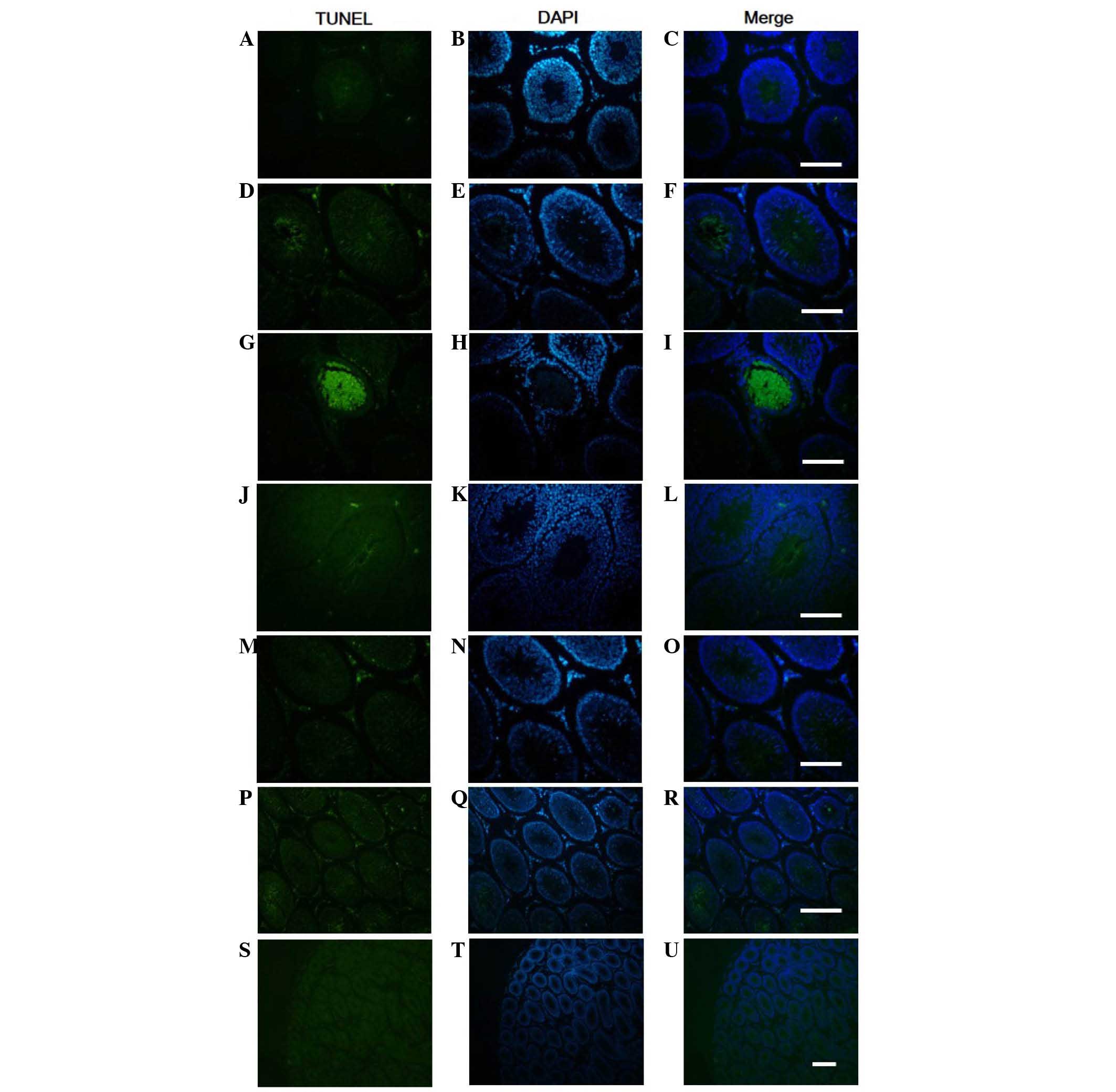
Testis tissue sections were stained using the TUNEL
method, in order to determine the quantity and distribution of
apoptotic cells, and examine nuclear condensation and fragmentation
(Fig. 4). Sections exposed to
DNase I, which causes DNA fragmentation, exhibited intense staining
of all nuclei and were used as positive controls (data not shown).
Sections stained using the described procedure, but without the
terminal deoxynucleotidyl transferase enzyme exhibited no staining
and were used as negative controls (Fig. 4S-U). In the testis of non-WRS rats,
a small amount of labeling was detected, which was predominantly
concentrated in the cells of late generation spermatogonia in the
seminiferous tubules. Scattered TUNEL-positive cells were also
detected in Leydig cells (Fig.
4A-C). Compared with in the control group, the distribution of
TUNEL-positive cells in rat testis following WRS was not markedly
altered (Fig. 4D-R). The number of
TUNEL-positive cells in the rat testes subjected to 7 h WRS was
significantly increased, compared with in the control group
(Fig. 5A; P<0.05; n=6).
Subsequently, from day 4 to 15 after 7 h WRS, the number of
TUNEL-positive cells in the testes decreased gradually, and on day
15, the number of TUNEL-positive cells recovered to normal levels
(Fig. 5A).
To confirm cell apoptosis in the testes of WRS rats,
caspase-3 activity was detected in the testes using a colorimetric
assay. As shown in Fig. 5B,
caspase-3 activity in the testes following WRS was not
significantly different compared with in the control group
(P>0.05).
Immunohistochemical localization of
IGF-1, PTEN, total Akt and FoxO1 in the testes of rats after
WRS
To assess the localization of IGF-1, PTEN, total
Akt, and FoxO1 in rat testes, sections from WRS rat testes were
stained with specific antibodies targeting these proteins (Fig. 6). IGF-1 (3 h group, Fig. 6A; 4 day group, Fig. 6B) and FoxO1 (control group,
Fig. 6E; 7 h group, Fig. 6F) were widely observed in the sperm
cytoplasm during late stage spermatogenesis; FoxO1 was also
expressed in Leydig cell cytoplasm. In addition, PTEN (15 day
group, Fig. 6C) and total Akt (15
day group, Fig. 6D) were localized
in Leydig cells and cytoplasm of spermatogonia. PTEN was also
detected in vascular endothelial cells.
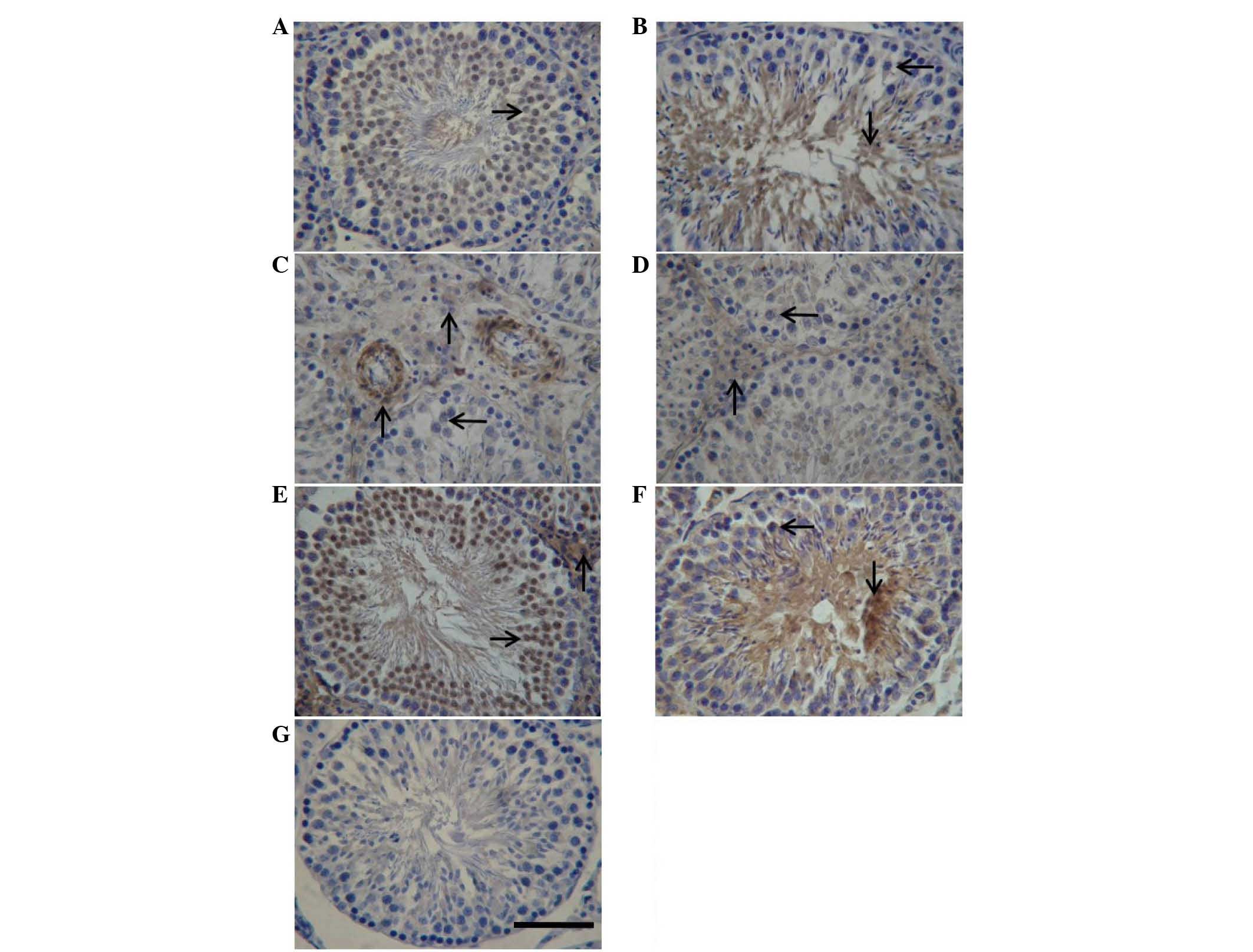 | Figure 6.Immunohistochemical localization of
IGF-1, PTEN, total Akt and FoxO1 in the testes of rats following
water immersion and restraint stress. The immunohistochemical
signals appear brown and the counterstained background appears blue
in color. Immunohistochemical localization of (A and B) IGF-1, (C)
PTEN, (D) total Akt and (E and F) FoxO1. (G) In control sections,
bovine serum albumin was used instead of primary antibody. →,
spermatocyte; ↓, spermatid; ↑, interstitial tissue. Scale bar=50
µm. IGF-1, insulin-like growth factor 1; PTEN, phosphatase and
tensin homolog deleted on chromosome 10; FoxO1, forkhead box
protein O1. |
Relative expression levels of IGF-1,
PTEN, Akt1, Akt2, Akt3 and FoxO1 in rat testes after WRS
The expression levels of selected genes were
analyzed using qPCR. Amplification products were identified through
melting curve profile analysis and were confirmed with gel
electrophoresis and sequencing. The relative transcript of each
target gene was normalized to HPRT (Fig. 7). All selected genes were
transcriptionally active. The mRNA expression levels of
IGF-1, PTEN, FoxO1 and Akt2 were
increased in the testes of rats subjected to WRS; the levels
reached their peak after 7 h of WRS. In the recovery phase, the
expression levels of these genes gradually dropped to normal
levels. In addition, the results indicated that WRS did not affect
Akt1 and Akt3 gene expression in rat testes.
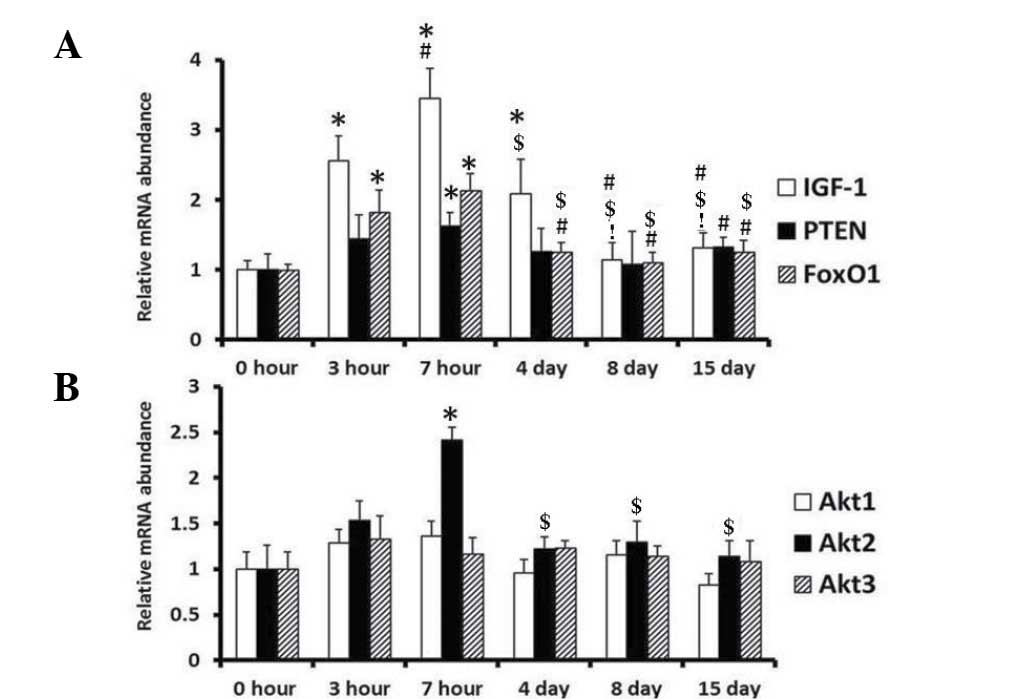 | Figure 7.Relative expression levels of
IGF-1, PTEN, Akt-1, Akt-2, Akt-3
and FoxO1 in the testes of rats after WRS. (A) IGF-1,
PTEN and FoxO1; (B) Akt1, Akt2 and
Akt3 expression. n=6 in each treatment group. *P<0.05 vs.
the 0 h group; #P<0.05 vs. the 3 h group;
$P<0.05 vs. the 7 h group; !P<0.05 vs.
the 4 day group. WRS, water immersion and restraint stress; IGF-1,
insulin-like growth factor 1; PTEN, phosphatase and tensin homolog
deleted on chromosome 10; FoxO1, forkhead box protein O1. |
Discussion
The WRS rat has been widely used as a model of
gastroduodenal mucosal lesions; however, to the best of our
knowledge, the effects of WRS on male reproductive function have
not been reported. In the present study, the WRS rat was used to
investigate the effects of WRS on the reproductive function of
adult male rats. The results indicated that WRS increased sperm
malformation rate and serum testosterone concentrations, thus
suggesting that WRS induced sperm damage in rats. There may be two
reasons by which WRS causes damage: i) When generating the WRS
model, the water temperature was 20±2°C, whereas the room
temperature was 30°C, indicating that the sudden temperature change
may cause sperm damage in rats; ii) environmental stress affects
the HPA axis function, which may increase testosterone
concentration in rats and ultimately affect sperm morphology;
however, the specific mechanisms require further study.
The caspase family serves an important role in
mediating cell apoptosis, and caspase-3 serves as a key execution
molecule. Caspase-3 normally exists in the form of zymogen (32 kD)
in the cytoplasm, which is activated in the early stages of
apoptosis. The activated caspase-3 consists of one large subunit
(17 kD) and two small subunits (12 kD), which recognize related
substrates in the cytoplasm and nucleus, eventually leading to
apoptosis. Creagh et al demonstrated that numerous external
stressors induce apoptosis, then inhibit caspase activity (33). In the present study, the effects of
WRS on apoptosis in the rat testes were investigated by caspase-3
activity assay and the TUNEL method. The results indicated that WRS
increased the number of TUNEL-positive cells, and the
TUNEL-positive cells were predominantly distributed in
spermatoblasts that were in the late stages of spermatogenesis in
the seminiferous tubules; however, caspase-3 activity was not
changed. These results suggested that WRS may induce damage to the
spermatoblast in the late stages of spermatogenesis.
The IGF-1/PTEN/Akt/FoxO signaling pathway serves
critical roles in regulating cell differentiation, migration and
apoptosis in various cells and tissues (34). The present study demonstrated that
WRS induced rat sperm damage. To confirm whether the
IGF-1/PTEN/Akt/FoxO signaling pathway was involved in the
anti-damage mechanism of sperm, the localization and expression
levels of IGF-1, PTEN, total Akt and FoxO proteins were detected.
The results indicated that the IGF-1 protein was widely expressed
in sperm cytoplasm, during late stage spermatogenesis. In addition,
WRS increased the gene expression levels of IGF-1 in rat
testes. Similarly, a previous study reported that WRS could
increase the expression levels of IGF-1 in rat gastric mucosa,
which was able to regulate the downstream PI3K/Akt signaling
pathway via cyclooxygenase-2, and participated in regulation of
gastric mucosal cell apoptosis (35). Colon et al demonstrated that
IGF-1 is a cell-specific anti-apoptotic factor in mouse testes
during embryonic development (36). In the newborn period, IGF-1 served
an important role in sustentacular cell differentiation and
proliferation via the PI3K/Akt signaling pathway in mouse testes
(16,17). Unlike previous studies, mature rats
were used in the present study, thus suggesting that IGF-1 may
serve different roles at different developmental stages of
testicular tissue; this requires further investigation. In
addition, the gene expression levels of IGF-1, which has
been recognized as an anti-apoptotic factor, were elevated after
WRS, thus suggesting that IGF-1 may be involved in anti-injury
mechanisms in sperm.
Several studies have reported that Akt widely
participates in the growth and development of testicular tissue and
serves a critical role in cell differentiation and proliferation
(16,17,36).
However, the role of PTEN in spermatogenesis remains controversial.
Wu et al demonstrated that PTEN may have a role in the late
period of sperm development (18).
Dupont et al (19) treated
mice with exogenous FSH and estrogen, and reported that FSH
controls proliferation and differentiation of Sertoli cells via
stimulation of PTEN activity. Moe-Behrens et al (37) reported estrogen exposure induced
formation of germ cell testicular tumors via the Akt/PTEN signaling
pathway. Similarly, Kimura et al indicated that PTEN gene
knockout caused testiculoma (20).
Furthermore, PTEN gene expression in fetal rat testes has been
shown to be age-specific, thus suggesting that PTEN has an
important role in fetal rat testis development (21). However, Huang et al reported
that during mouse spermatogenesis, PTEN did not have a key role in
spermatogenesis regulation (22).
The present study demonstrated that PTEN and total Akt proteins
were predominantly localized in the cytoplasm of Leydig cells and
spermatogonia, and PTEN was also expressed in vascular endothelial
cells. In addition, the results of a qPCR demonstrated that WRS
increased gene expression levels of PTEN and Akt2 in
rat testes, thus indicating that PTEN and Akt2 may be involved in
the anti-stress mechanism of testes in rats subjected to WRS.
FoxO genes are the most important downstream target
genes of the IGF-1/PTEN/Akt signaling pathway, which participate in
several physiological processes, including cell differentiation,
proliferation, apoptosis, migration and stress resistance (38). Our previous study demonstrated that
FoxO3a and FoxO4 serve major roles in the digestive tract (27,39–41).
A previous study regarding the male reproductive system
demonstrated that FoxO1 served a key role in initiating
spermatogenesis; however, FoxO3a and FoxO4 did not (39). Therefore, the expression and
location of FoxO1 in WRS rat testes were determined. The results
demonstrated that FoxO1 protein was widely expressed in the
cytoplasm of spermatids, which were in the late stages of
spermatogenesis and in testicular interstitial cells, thus implying
that FoxO1 may be involved in spermatogenesis and cell apoptosis
regulation in the late stages of spermatogenesis. Furthermore, WRS
increased FoxO1 gene expression levels in rat testes,
indicating that the FoxO1 gene may participate in the
anti-stress mechanisms of rat testes.
In conclusion, WRS induced sperm injury in rat
testes. These results suggested that the IGF-1/PTEN/Akt/FoxO
signaling pathway may serve an anti-stress role in the testes of
rats subjected to WRS. Future research will study the effect of
IGF-1 on male reproduction and the involvement of the
IGF-1/PTEN/Akt/FoxO signaling pathway.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81300287), the Senior
Talents Scientific Research Foundation of Jiangsu University (grant
no. 12JDG084) and the Nature Science Foundation of Jiangsu
Province, China (grant nos. BK 2011499 and BK20140541).
References
|
1
|
Vazquez-Palacios G and Velazquez-Moctezuma
J: Effect of electric foot shocks, immobilization and
corticosterone administration on the sleep-wake pattern in the rat.
Physiol Behav. 71:23–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marin MT, Cruz FC and Planeta CS: Chronic
restraint or variable stresses differently affect the behavior,
corticosterone secretion and body weight in rats. Physiol Behav.
90:29–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adachi M, Horiuchi G, Ikematsu N, Tanaka
T, Terao J, Satouchi K and Tokumura A: Intragastrically
administered lysophosphatidic acids protect against gastric ulcer
in rats under water-immersion restraint stress. Dig Dis Sci.
56:2252–2261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nie SN, Qian XM, Wu XH, Yang SY, Tang WJ,
Xu BH, Huang F, Lin X, Sun DY, Sun HC and Li ZS: Role of TFF in
healing of stress-induced gastric lesions. World J Gastroenterol.
9:1772–1776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang P, Chang L, Pan CS, Qi YF and Tang
CS: Protective role of metallothionein in stress-induced gastric
ulcer in rats. World J Gastroenterol. 11:2739–2743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castrillon DH, Miao L, Kollipara R, Horner
JW and DePinho RA: Suppression of ovarian follicle activation in
mice by the transcription factor Foxo3a. Science. 301:215–218.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reddy P, Liu L, Adhikari D, Jagarlamudi K,
Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, et al:
Oocyte-specific deletion of Pten causes premature activation of the
primordial follicle pool. Science. 319:611–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carroll PV: Treatment with growth hormone
and insulin-like growth factor-I in critical illness. Best Pract
Res Clin Endocrinol Metab. 15:435–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vara JA Fresno, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leslie N and Downes C: PTEN function: How
normal cells control it and tumour cells lose it. Biochem J.
382:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sengupta A, Molkentin JD, Paik JH, DePinho
RA and Yutzey KE: FoxO transcription factors promote cardiomyocyte
survival upon induction of oxidative stress. J Biol Chem.
286:7468–7478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burgering BM and Kops GJ: Cell cycle and
death control: Long live Forkheads. Trends Biochem Sci. 27:352–360.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watanabe S, Wang X, Hirose M, Kivilioto T,
Osada T, Miwa H, Oide H, Kitamura T, Yoneta T, Seto K and Sato N:
Insulin-like growth factor I plays a role in gastric wound healing:
Evidence using a zinc derivative, polaprezinc, and an in vitro
rabbit wound repair model. Aliment Pharmacol Ther. 12:1131–1138.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colón E, Zaman F, Axelson M, Larsson O,
Carlsson-Skwirut C, Svechnikov KV and Söder O: Insulin-like growth
factor-I is an important antiapoptotic factor for rat leydig cells
during postnatal development. Endocrinology. 148:128–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan SA, Ndjountche L, Pratchard L, Spicer
L and Davis JS: Follicle-stimulating hormone amplifies insulin-like
growth factor I-mediated activation of AKT/protein kinase B
signaling in immature rat Sertoli cells. Endocrinology.
143:2259–2267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tai P, Shiraishi K and Ascoli M:
Activation of the lutropin/choriogonadotropin receptor inhibits
apoptosis of immature Leydig cells in primary culture.
Endocrinology. 150:3766–3773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Dowbenko D, Pisabarro MT,
Dillard-Telm L, Koeppen H and Lasky LA: PTEN 2, a Golgi-associated
testis-specific homologue of the PTEN tumor suppressor lipid
phosphatase. J Biol Chem. 276:21745–21753. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dupont J, Musnier A, Decourtye J, Boulo T,
Lécureuil C, Guillou H, Valet S, Fouchécourt S, Pitetti JL, Nef S,
et al: FSH-stimulated PTEN activity accounts for the lack of FSH
mitogenic effect in prepubertal rat Sertoli cells. Mol Cell
Endocrinol. 315:271–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kimura T, Suzuki A, Fujita Y, Yomogida K,
Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW and Nakano T:
Conditional loss of PTEN leads to testicular teratoma and enhances
embryonic germ cell production. Development. 130:1691–1700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luukko K, Ylikorkala A, Tiainen M and
Mäkelä TP: Expression of LKB1 and PTEN tumor suppressor genes
during mouse embryonic development. Mech Dev. 83:187–190. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Mao X, Boyce T and Zhu GZ:
Dispensable role of PTEN in mouse spermatogenesis. Cell Biol Int.
35:905–908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bagchi G, Zhang Y, Stanley KA and Waxman
DJ: Complex modulation of androgen responsive gene expression by
methoxyacetic acid. Reprod Biol Endocrinol. 9:422011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goertz MJ, Wu Z, Gallardo TD, Hamra FK and
Castrillon DH: Foxo1 is required in mouse spermatogonial stem cells
for their maintenance and the initiation of spermatogenesis. J Clin
Invest. 121:3456–3466. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
John GB, Gallardo TD, Shirley LJ and
Castrillon DH: Foxo3 is a PI3K-dependent molecular switch
controlling the initiation of oocyte growth. Dev Biol. 321:197–204.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
John GB, Shirley LJ, Gallardo TD and
Castrillon DH: Specificity of the requirement for Foxo3 in
primordial follicle activation. Reproduction. 133:855–863. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang P, Zhou Z, Wang H, Wei Q, Zhang L,
Zhou X, Hutz RJ and Shi F: Effect of the IGF-1/PTEN/Akt/FoxO
signaling pathway on the development and healing of water immersion
and restraint stress-induced gastric ulcers in rats. Int J Mol Med.
30:650–658. 2012.PubMed/NCBI
|
|
28
|
Wang H, Huang P, Lie T, Li J, Hutz RJ, Li
K and Shi F: Reproductive toxicity of acrylamide-treated male rats.
Reprod Toxicol. 29:225–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly KJ, Sandoval RM, Dunn KW, Molitoris
BA and Dagher PC: A novel method to determine specificity and
sensitivity of the TUNEL reaction in the quantitation of apoptosis.
Am J Physiol Cell Physiol. 284:C1309–C1318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salimi A, Roudkenar MH, Sadeghi L, Mohseni
A, Seydi E, Pirahmadi N and Pourahmad J: Ellagic acid, a
polyphenolic compound, selectively induces ROS-mediated apoptosis
in cancerous B-lymphocytes of CLL patients by directly targeting
mitochondria. Redox Biol. 6:461–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding W, Wang W, Zhou B, Zhang W, Huang P,
Shi F and Taya K: Formation of primordial follicles and
immunolocalization of PTEN, PKB and FOXO3A proteins in the ovaries
of fetal and neonatal pigs. J Reprod Dev. 56:162–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Creagh EM, Conroy H and Martin SJ:
Caspase-activation pathways in apoptosis and immunity. Immunol Rev.
193:10–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rabinovsky ED: The multifunctional role of
IGF-1 in peripheral nerve regeneration. Neurol Res. 26:204–210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nguyen T, Chai J, Li A, Akahoshi T,
Tanigawa T and Tarnawski AS: Novel roles of local insulin-like
growth factor-1 activation in gastric ulcer healing: Promotes actin
polymerization, cell proliferation, re-epithelialization, and
induces cyclooxygenase-2 in a phosphatidylinositol
3-kinase-dependent manner. Am J Pathol. 170:1219–1228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Colon E, Zaman F, Axelson M, Larsson O,
Carlsson-Skwirut C, Svechnikov KV and Söder O: Insulin-like growth
factor-I is an important antiapoptotic factor for rat leydig cells
during postnatal development. Endocrinology. 148:128–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moe-Behrens GH, Klinger FG, Eskild W,
Grotmol T, Haugen TB and De Felici M: Akt/PTEN signaling mediates
estrogen-dependent proliferation of primordial germ cells in vitro.
Mol Endocrinol. 17:2630–2638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakae J, Biggs WH III, Kitamura T, Cavenee
WK, Wright CV, Arden KC and Accili D: Regulation of insulin action
and pancreatic beta-cell function by mutated alleles of the gene
encoding forkhead transcription factor Foxo1. Nat Genet.
32:245–253. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang P, Zhou ZQ, Huang RH, Zhou B, Wei QW
and Shi FX: Age-dependent expression of forkhead box O proteins in
the duodenum of rats. J Zhejiang Univ Sci B. 12:730–735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou ZQ, Wang T, Pan LM, Huang RH and Shi
FX: FoxO4 is the main forkhead transcriptional factor localized in
the gastrointestinal tracts of pigs. J Zhejiang Univ Sci B.
8:39–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang P, Zhou Z, Zheng M and Shi F: Effect
of the IGF-1/PTEN/Akt/FoxO signaling pathway in the duodenal mucosa
of rats subjected to water immersion and restraint stress. Genet
Mol Res. 11:4775–4788. 2012. View Article : Google Scholar : PubMed/NCBI
|