Introduction
Gene-directed enzyme-prodrug therapy (GDEPT), or
suicide gene therapy, is a promising treatment strategy, which acts
by tumor-targeted delivery of an exogenous genes that may express
an enzyme capable of converting a non-toxic prodrug into an
activated cytotoxic agent, which may then result in apoptosis of
tumor cells (1–3). Various suicide gene therapy systems
have been previously investigated, including the herpes simplex
thymidine kinase/prodrug GCV (HSV1-tk-GCV) and cytosine deaminase
and 5-fluorocytosine (CD/5-FC) (4–6).
Nitroreductase/(5-(aziridin-1-yl)-2,4-dinitrobenzamide)
(NTR/CB1954) is another GDEPT strategy that has been previously
investigated in clinical trials (7). NTR is responsible for the conversion
of CB1954, a weak monofunctional alkylating agent, into
5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, a DNA
inter-strand cross-linking agent that may trigger apoptosis of
cancer cells that express the enzyme NTR. Tumor cells are radiation
resistant when they are in the S phase of the cell cycle. Previous
studies have confirmed that compared with the HSV1-tk-GCV system,
the advantages of the NTR/CB1954 suicide gene system are as
follows: i) Independence from the cell cycle; ii) ability to target
both dividing and growth-arrested cancer cells; and iii) induction
of a potent bystander effect on the cell cycle (8–11).
Radiotherapy has been used due to its efficacy
against various tumor types, including head and neck, lung and
gastrointestinal tumors. Radiotherapy is usually used alone or in
combination with surgery and chemotherapy, and is important for the
successful clinical treatment of patients with cancer. However, the
modality of this treatment is associated with serious side-effects,
including damage to normal tissues, thus requiring restriction of
the doses used. A radiosensitizing agent or system may provide the
opportunity to circumvent these issues (12,13).
Previous studies have confirmed that gene-directed
enzyme prodrug therapy may sensitize tumor cells to the effects of
ionizing radiation, and have demonstrated excellent results,
including enhanced radiosensitivity of numerous tumor cells in
vitro and in vivo (14–17).
These observations have promoted the use of cancer gene therapy to
enhance the effect of ionizing radiation.
The present study investigated whether the
NTR/CB1954 suicide gene system and γ-rays have a combined effect
and whether NTR/CB1954 may enhance the cytotoxic effect of γ-rays
on cervical carcinoma cells in vitro.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) with high
glucose and 10% fetal calf serum (FCS) was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Reverse transcription-polymerase chain reaction (RT-PCR) assay kits
were obtained from Beijing Modern Gold Biotechnology Co., Ltd.
(Beijing, China). The endonucleases and ligase enzymes used for
molecular cloning technology were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Lipofectamine 2000, G418,
CB1954 and MTT were purchased from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany).
Plasmid vector construction
The nfsB sequence was amplified by PCR from
the Escherichia coli k12 genome using the following primers:
5′-CGGGATCCATGGATATCATTTCTGTCG-3′ and
5′-CGGAATTCTTACACTTCGGTTAAGGTG-3′. This sequence contained
BamHI and EcoRI sites for insertion into the pcDNA3
plasmid. Following endonuclease digestion and DNA sequencing, the
successful insertion of the nfsB gene was confirmed and was
re-cloned into the pcDNA3 plasmid. The following the following
thermocycling conditions: 94°C for 3 min, 94°C for 30 sec, 55°C for
30 sec, 72°C for 1 min and 72°C for 5 min, 35 cycles. The resulting
plasmid was termed pcDNA3-nfsB.
Cell culture and transfection
HeLa cells were maintained in DMEM, supplemented
with 10% FCS, 100 U/ml penicillin and 100 mg/ml streptomycin and
were incubated at 37°C in a 5% CO2 atmosphere. All of
the cultures were demonstrated to be free of mycoplasma. The HeLa
cells were transfected with the pcDNA3 plasmid and
pcDNA3-nfsB with G418 selection at a concentration of 400
µg/ml. Following 1 month of selection, several independent clones
were selected and the NTR mRNA and protein expression levels were
determined. Subsequently, the concentration of G418 was reduced to
200 µg/ml.
RT-PCR and sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Total RNA was extracted from the
pcDAN3-nfsB-HeLa, pcDNA3-HeLa and non-transfected control
HeLa cells using TRIzol reagent. RT was performed according to the
manufacturer's protocol (Transgen Biotech Co., Ltd. (Beijing,
China). The reaction mixture was placed in a 42°C water bath for 50
min, the temperature was increased to 70°C for 15 min to inactivate
TransScript RT and synthesise cDNA. For nfsB, the following
primers were used: Sense, 5′-ATGGACGATGTCTGGCTGAA-3′ and antisense
5′-AACGCTGTGATGACCTACCG-3′. Endogenously expressed human β-actin
mRNA was used as an internal control with the following primers:
Sense 5′-GGCATCCTCACCCTGAAGTA-3′ and antisense
5′-GGGGTGTTGAAGGTCTCAAA-3′. A DNA product of 208 base pairs (bp)
was then amplified. A total of 500 ng cDNA were used. The following
the following thermocycling conditions: 94°C for 3 min, 94°C for 30
sec, 55°C for 30 sec, 72°C for 1 min and 72°C for 5 min, 35 cycles.
The PCR products of nfsB and β-actin were separated by
electrophoresis on 1.5% agarose gel. The DNA bands were visualized
and analyzed by staining with ethidium bromide. The proteins were
collected from the three cell lines and SDS-PAGE was performed with
a 10% acrylamide separating gel and 4% acrylamide, 10 µg protein
were loaded per lane. The samples were prepared in a Tris-glycine
buffer with 1% SDS at pH 8.8. Electrophoresis was conducted at a
current of 10 mA for 5 h in electrophoretic Tris-glycine buffer
with 0.1% SDS. Following electrophoresis, the gel sheets were
stained for proteins with 0.25% coomassie brilliant blue-R250 and
then were destained with 10% acetic acid and 20% methanol. Next,
the protein bands were visualized and analyzed.
Cell growth curve and the
determination of the cell survival fraction and survival curves
following transfection of HeLa cells
The pcDNA3-nfsB-HeLa, pcDNA3-HeLa and HeLa
cells were incubated for 96 h and counted every 12 h. The number of
cells was determined using trypan blue staining. Subsequently, a
cell growth curve was generated and the differences between the
three cell lines were compared. pcDNA3-nfsB-HeLa,
pcDNA3-HeLa and HeLa cells of different concentrations were
irradiated with different doses of γ-rays. The cells were
subsequently incubated for 12 days and the survival fraction of the
cells was detected by a colony-formation assay. The cell survival
curve was generated using SPSS software, version 15.0 (SPSS, Inc.,
Chicago, IL, USA) and Microsoft Excel (Microsoft Corporation,
Redmond, WA, USA).
Analysis of apoptosis by Hoechst
33258/propidium iodide (PI) fluorescent vital staining
Hoechst 33258/PI fluorescent vital stain was used to
quantitatively determine the percentage of apoptotic cells.
Briefly, the cells were washed with PBS, and Hoechst 33258 (15
µg/ml) was added. Following a 50 min incubation at ambient
temperature, PI (10 µg/ml) was added, followed by another
incubation for 10 min. The samples were analyzed using fluorescence
microscopy. The nuclei of apoptotic cells appeared bright
fluorescent blue, whereas the nuclei of necrotic cells were bright
fluorescent red. The nuclei of normal cells appeared only weakly
fluorescent blue (Fig. 1). This
method was used to analyze the effects of the NTR/CB1954 suicide
gene system in combination with γ-ray treatment on HeLa cells.
Bradford assay (Leagene Biotech Co., Ltd., Beijing, China) was used
for quantification.
Hypodiploid HeLa cell formation
detected by flow cytometry following NTR/CB1954 treatment
The three cell lines were plated at a density of
1.0×106 cells/well in a 6-well plate. Following
incubation with CB1954 at various concentrations (0, 12.5, 25, 37.5
and 50 µmol/l) for 36 h, the cells were collected, washed twice
with ice-cold PBS (pH 7.4), fixed with 70% alcohol for a minimum of
18 h and then stained with PI (50 µg/ml) in the presence of 20
µg/ml RNAse A for a minimum of 30 min prior to flow cytometric
analysis. The data were analyzed with ModFit version 3.2 (Verity
Software House, Inc., Topsham, ME, USA) and CellQuest version 7.5.3
(BD Biosciences, Franklin Lakes, NJ, USA).
Detection of the combined effect of
γ-rays and the NTR/CB1954 suicide gene system via Hoechst 33258/PI
fluorescent vital staining
pcDNA3-nfsB-HeLa cells in the exponential
growth phase were incubated for 24 h at 37°C and CB1954 was added
at concentrations of 0, 12.5 and 25 µmol/l. After 24 h the cells
were irradiated at doses of 0, 2, 4 and 6 Gy with Co60
γ-rays. Subsequently, the cells were incubated at 37°C with an
atmosphere of 5% CO2 for 48 h. Detection of apoptosis
was performed using Hoechst/PI fluorescent vital staining.
Determination of cell survival via
colony-formation assay following treatment with NTR/CB1954 combined
with γ-rays
Cells were cultured in 25 cm2 flasks.
Single suspension was obtained, the cells were diluted to
1×104/ml and 1×103/ml. Next, 0.2, 0.4, 0.6,
0.8 and 1 ml from the concentration of 1×103/ml, and a
cell suspension of 0.3, 0.8 and 1 ml from the concentration of
1×104/ml, all of which were cultured in 50
cm2 flasks, and the number of cells were 200, 400, 600,
800, 1,000, 5,000, 8,000 and 10,000 from low to high. Following the
addition of CB1954 at concentrations of 0, 12.5 or 25 µmol/l, the
cells were irradiated at doses of 0, 0.5, 1, 1.5, 2, 4, 6 and 8 Gy
with Co60 γ-rays, according to the number of cells from
low to high. Next, the cells were incubated at 37°C with 5%
CO2 atmosphere for 2–15 days. Following this, the medium
was removed, the cell culture plate was washed with PBS, and the
cells were fixed in methanol for 15 min. The colonies were then
stained with a solution of crystal violet for 20 min. Colonies that
were visible to the naked eye were counted and the cell survival
fraction (SF) was calculated using the following formulas: Plating
efficiency (PE) = cell colonies formed / cells inoculated and SF =
cell colonies formed / cells innoculated × PE.
Statistical analysis
Differences between treatment groups were determined
using Student's t-test and one-way analysis of variance.
Statistical analysis was performed using Statistics Analysis System
version 8.0 (SAS Institute, Inc., Cary, NC, USA). Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
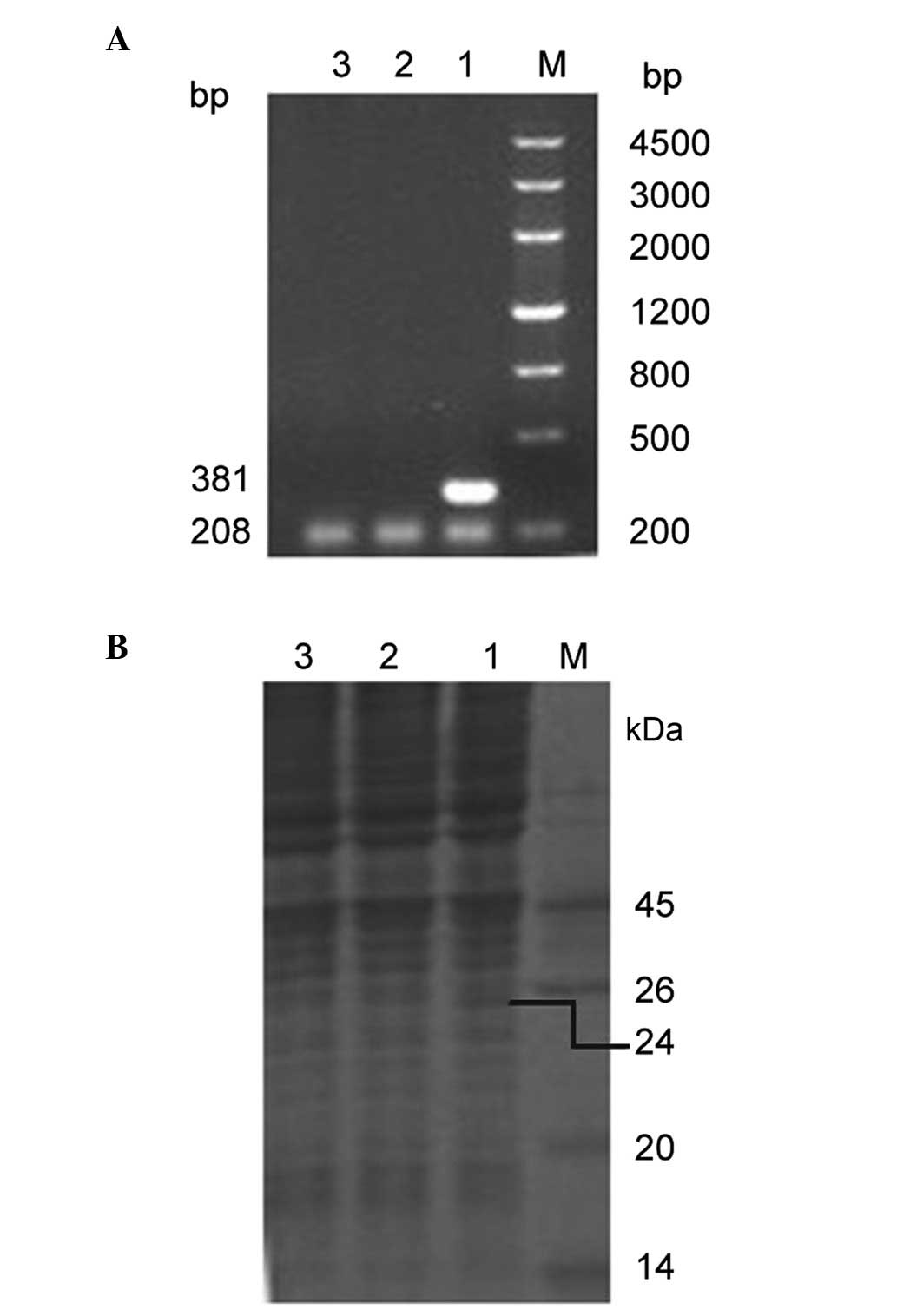
NTR mRNA expression in HeLa cells
NTR mRNA expression level was determined using
RT-PCR. In the pcDNA3-nfsB-HeLa cells, a 381 bp DNA fragment
of nfsB was amplified, whereas no mRNA was detected in
either the control pcDNA3-HeLa cells or in the non-transfected HeLa
cells. A 208 bp human β-actin mRNA fragment, which was used
as an internal control, was amplified in the three different cell
lines (Fig. 2A). NTR protein
expression level was determined using SDS-PAGE. In the
pcDNA3-nfsB-HeLa cells, a 24 kDa protein was identified,
which coincided with the size of NTR. However, in the control
pcDNA3-HeLa cells and in the non-transfected HeLa cells, no
corresponding protein band was detected (Fig. 2B). These observations indicated
that NTR was stably and correctly expressed in the transfected HeLa
cells.
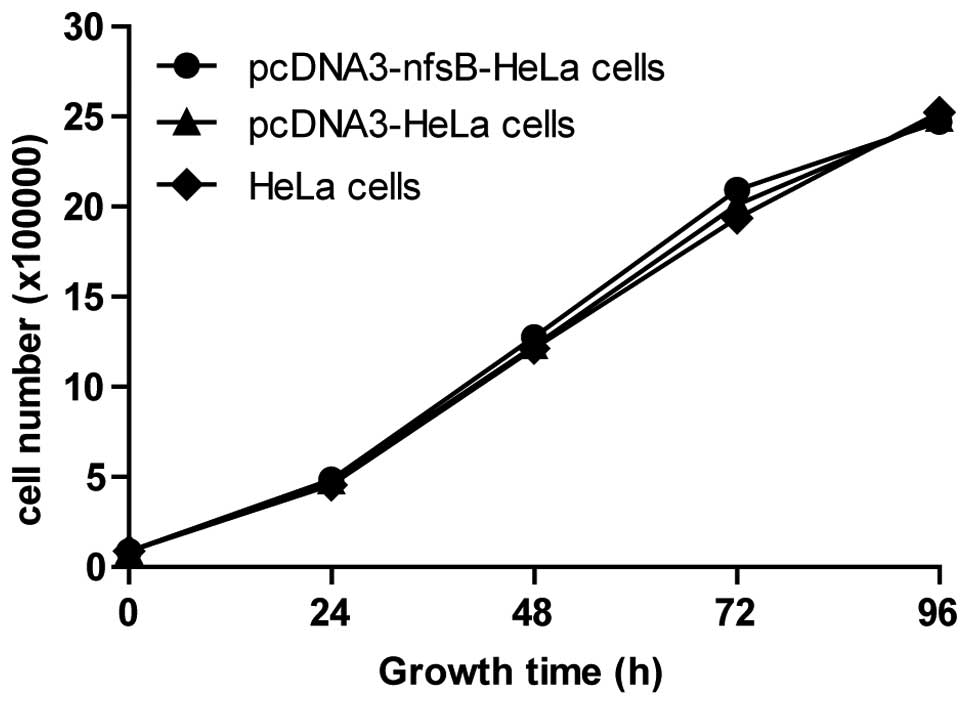
Transfection of the nfsB gene had no
effect on cell growth or on the reaction of HeLa cells to γ-ray
irradiation
The cell counting method was used following the
transfection with the nfsB gene to observe the growth and
proliferation of the three groups of cells. No significant
differences were observed between the pcDNA3-nfsB-HeLa cells
and the control cells (Fig. 3),
which indicated that the nfsB gene did not affect the growth
and proliferation of HeLa cells. Additionally, the apoptotic
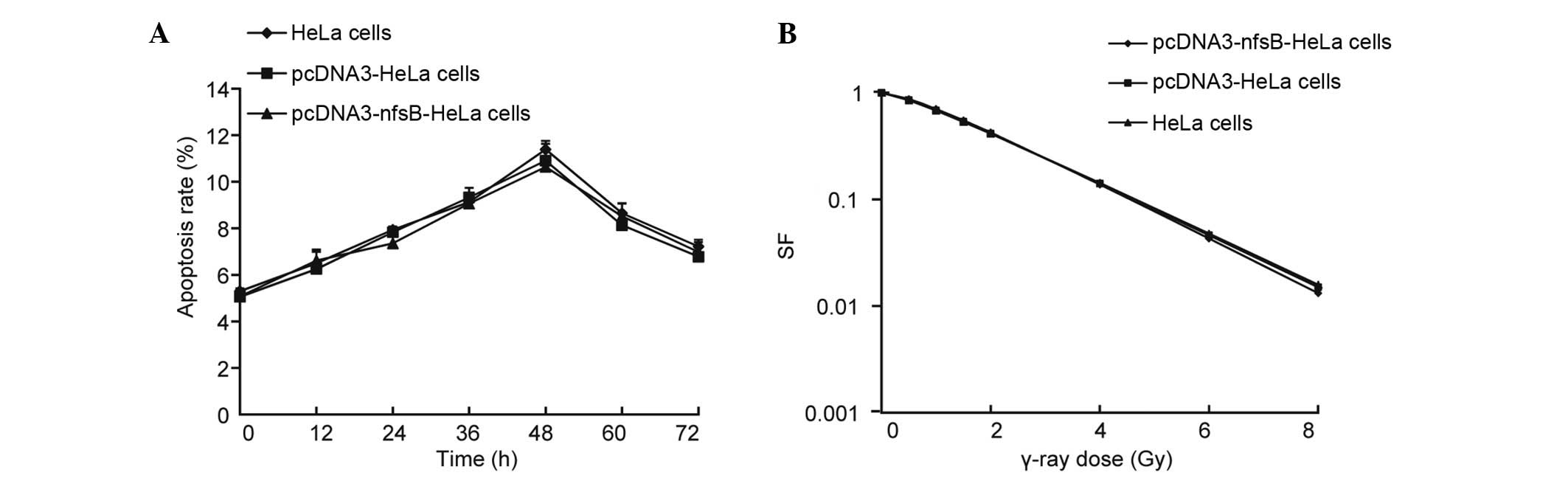
activity of the pcDNA3-nfsB-HeLa, pcDNA3-HeLa and
non-transfected HeLa cells was examined following γ-ray irradiation
(6 Gy) using Hoechst 33258/PI fluorescent vital staining. No
significant differences were observed among the three different
groups of cells in terms of apoptotic rate over time (0–72 h)
(Fig. 4A). The cell survival
fraction in the three groups of cells was also quantified.
Following treatment with various doses of γ-ray irradiation, the
cells were incubated for 12 days. No significant differences were
identified among the three groups of cells in terms of the cell
survival fraction (Fig. 4B). These
observations demonstrated that transfection with the nfsB
gene alone did not have an influence on the reaction of the cells
to γ-rays.
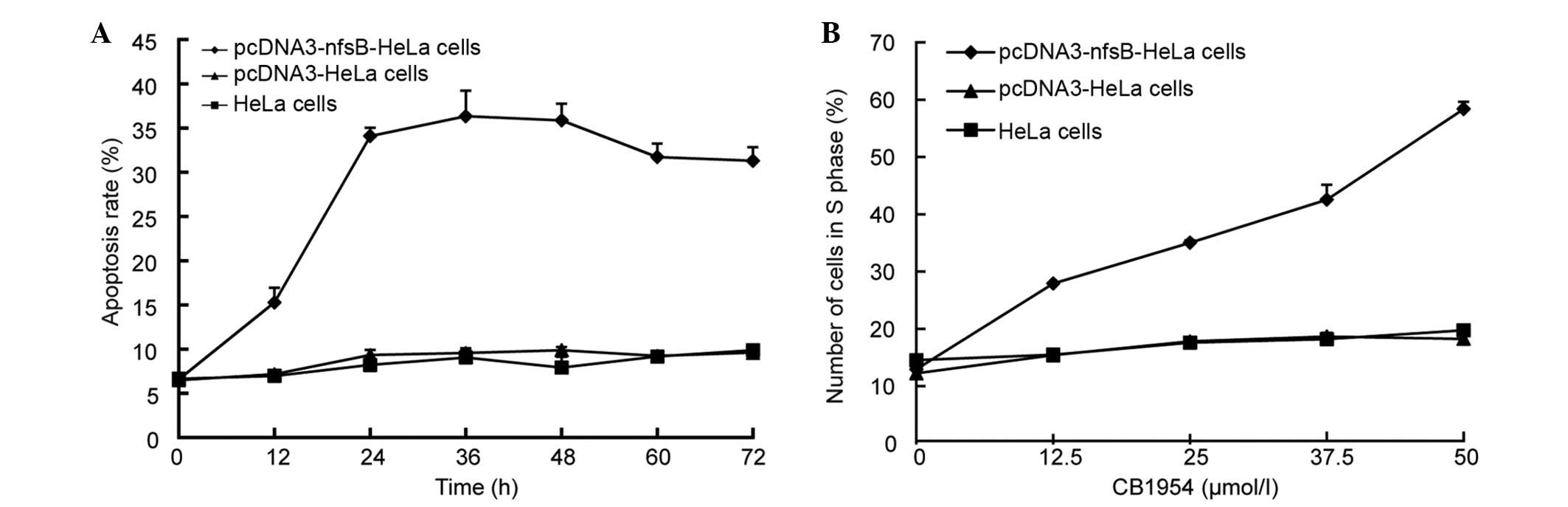
CB1954 increased the cytotoxicity in
NTR-expressing cells and led to an extended S phase
The rate of apoptosis of pcDNA3-nfsB-HeLa,
pcDNA3-HeLa and HeLa cells treated with CB1954 was determined using
Hoechst 33258/PI fluorescent staining. Apoptosis was observed in
the pcDNA3-nfsB-HeLa cells 12 h following treatment with
CB1954 (Fig. 5A). No apoptosis was
observed in the pcDNA3-HeLa cells or in the wild-type HeLa cells.
The rate of apoptosis of the pcDNA3-nfsB-HeLa cells peaked
at 36 h of treatment (Fig. 5A).
Additionally, an MTT assay and flow cytometry were used to
determine cell viability and apoptotic rates, with the observations
consistent with the previous experiments (data not shown). The
pcDNA3-nfsB-HeLa, pcDNA3-HeLa and non-transfected HeLa cells
were treated with various concentrations of CB1954 (0, 12.5, 25 and
50 µmol/l) for 36 h. Flow cytometry was then used to analyze the
number of cells in the different cell cycle phases, and the results
demonstrated that the NTR/CB1954 suicide gene system primarily
affected HeLa cells in S phase. When the concentration of CB1954
was 50 µmol/l, the percentage of cells in S phase was 70.51%,
whereas no clear alterations were observed in the control cell
groups, the pcDNA3-HeLa and HeLa cells (Fig. 5B).
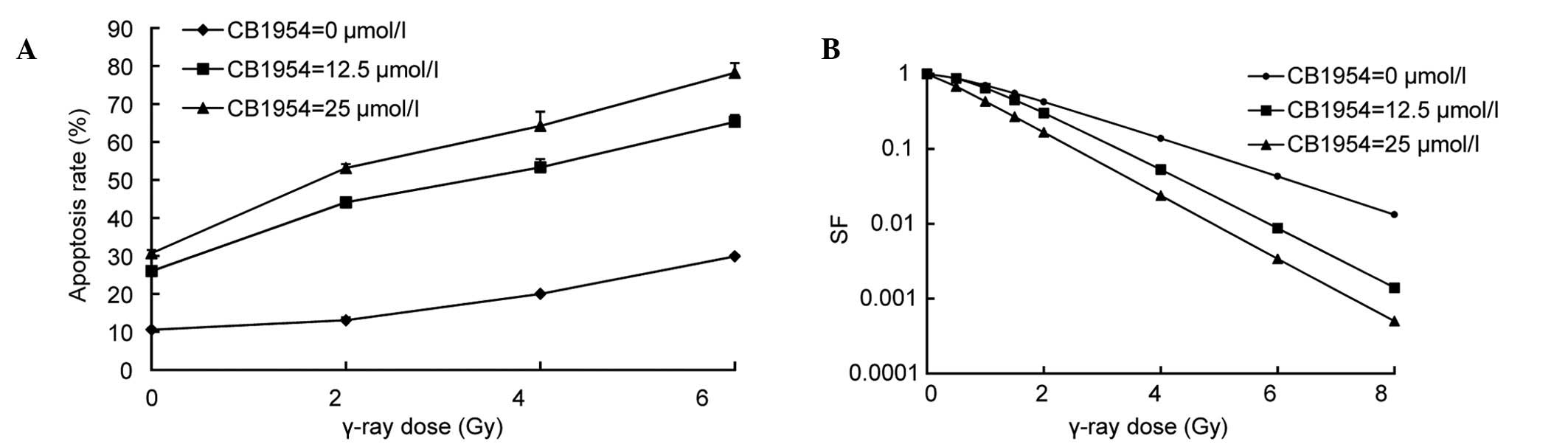
CB1954 combined with γ-ray irradiation
increased cytotoxicity and reduced the cell survival fraction in
pcDNA-nfsB-HeLa cells
pcDNA-nfsB-HeLa cells were treated with
CB1954 at various concentrations (0, 12.5 and 25 µmol/l) for 16 h
prior to γ-ray irradiation (0, 2, 4 and 6 Gy), and the rate of
apoptosis was determined using Hoechst 33258/PI fluorescent vital
staining. The cytotoxicity was proportional to the radiation dose
in pcDNA-nfsB-HeLa cells and the CB1954 concentration used.
Cytotoxicity increased with the combination of radiation and CB1954
at 12.5 and 25 µmol/l. For example, when the concentration of
CB1954 was 12.5 µmol/l, the apoptotic rate of
pcDNA3-nfsB-HeLa cells was 12.92% and at a dose of γ-ray
irradiation of 6 Gy, the rate of apoptosis was 12.84%. However,
with the combination of 12.5 µmol/l of CB1954 and 6 Gy of γ-ray
irradiation, the rate of apoptosis rate increased to 39.9%, which
was increased compared with irradiation or CB1954 alone for
pcDNA3-nfsB-HeLa cells (Fig.
6A). These observations demonstrated that the cytotoxic effect
on HeLa cells was due to the interaction between the suicide gene
system NTR/CB1954 and radiation, and was not a simple additive
effect. In addition, the present study determined the cell survival
fraction with a colony-formation assay. The cells were treated with
CB1954 at various concentrations (0, 12.5 and 25 µmol/l) for 16 h
prior to the delivery of various doses of γ-rays (0, 0.5, 1, 1.5,
2, 4, 6 and 8 Gy). Next, the cell survival fraction was then
determined using a colony-formation assay. Following treatment with
12.5 and 25 µmol/l of CB1954, reduced cell survival fraction was
evident with the combination of radiation and CB1954. SER was
obtained from the cell survival curve. At concentrations of 12.5
and 25 µmol/l CB1954 radiosensitivity ratios were 1.54 and 1.66,
respectively (Fig. 6B). These
results indicated that the NTR/CB1954 suicide gene system may
significantly enhance the sensitivity of HeLa cells to
radiation.
Discussion
The GDEPT treatment approach may sensitize tumor
cells to ionizing radiation. Previous studies determined that the
HSV1-tk-GCV and CD/5-FC suicide gene therapy systems may improve
the sensitivity of tumor cells to radiotherapy, and these systems
have demonstrated considerable advantages both in vitro and
in vivo (5,18). The NTR/CB1954 gene therapy system
is another form of GDEPT and demonstrated an effective tumor
reducing treatment on various cells in vivo and in
vitro (19,20). The NTR/CB1954 system has been
previously investigated in clinical trials. The present study aimed
to investigate this system and determine whether it may be used as
a potential radiosensitizing gene therapy.
The present study cloned the nfsB gene from
the E. coli K12 genome using PCR. This gene was then cloned
into the eukaryotic expression vector pcDNA3 to obtain the
pcDNA3-nfsB vector. Following the transfection of HeLa
cells, RT-PCR and SDS-PAGE were performed to determined that the
DNA fragment and protein size were consistent with those of the
nfsB gene and NTR. The transfection was deemed successful as
the fragment was missing in the control groups (HeLa and
pcDNA3-HeLa cells). The present study demonstrated that the cloned
nfsB gene was correct and that the eukaryotic expression
vector pcDNA3-nfsB was stable and functional, which laid a
foundation for the observation of the effects of NTR/CB1954 suicide
gene therapy in the subsequent experiments.
The growth and proliferation of HeLa cells following
the transfection were determined. No significant differences were
identified between the pcDNA3-nfsB-HeLa cells and the
control groups (pcDNA3-HeLa and HeLa cells). Therefore, the
nfsB gene itself did not affect the cytotoxicity of
NTR/CB1954 and γ-rays. Following the transfection, the rate of
apoptosis of the three groups of HeLa cells following γ-ray
irradiation was quantified and it was confirmed that transfection
of the nfsB gene did not affect the fraction of surviving
cells. Therefore, these observations indicated that the HeLa cell
line and the nfsB gene were suitable for the present
experiments, ensuring the potential for further cytotoxicity
research.
The cytotoxic effects of NTR/CB1954 were also
evaluated and it was confirmed that the NTR/CB1954 suicide gene
system exerted a specific cytotoxic effect on HeLa cells. CB1954
exerted selective cytotoxicity against pcDNA3-nfsB-HeLa
cells compared with the controls (pcDNA3-HeLa cells and HeLa
cells). The present study determined that the mechanism of
cytotoxicity was primarily via apoptosis; however, the peak effect
of cytotoxicity was observed after 36 h, which was sooner compared
with other suicide gene systems, such as the HSV/TK suicide gene
therapy system (21). Cell cycle
analysis of pcDNA3-HeLa cells was performed following treatment
with CB1954. The results indicated that the NTR/CB1954 suicide gene
system led to S-phase arrest. This was not in agreement with a
previous study performed by White et al (18). White et al (18) concluded that the NTR/CB1954 gene
therapy system acted independently of the cell cycle, which was the
greatest advantage of this system compared with other types of
GDEPT (18). However, following
multiple replications of the experiments in the present study, the
results were still in contradiction with that of White et al
(18). This may be due to the cell
line that was used; therefore, it may be useful for future studies
to use different cell lines to verify this.
The present study examined the combined effect of
the NTR-CB1954 gene system and γ-ray irradiation in
pcDNA3-nfsB-HeLa cells. Increased cell apoptosis was
observed when CB1954 treatment was combined with γ-ray radiation in
pcDNA3-nfsB-HeLa cells. Subsequent experiments were
performed to demonstrate the reduced level of surviving cells in
the pcDNA3-nfsB-HeLa cell group that received CB1954 and
γ-ray radiation compared with cells that only received CB1954 or
γ-ray radiation. Based on the survival curve, the SER was 1.54 and
1.56 when the concentrations of CB1954 were 12.5 and 25 µmol/l.
Therefore, this indicated that NTR/CB1954 may enhance the
radiosensitivity of HeLa cells and that this was due to a
synergistic effect as opposed to an additive effect.
The current study demonstrated that the NTR/CB1954
suicide gene system led to S phase arrest in HeLa cells. As cells
in S phase are resistant to radiation, the S phase arrest may be
the result of DNA synthesis inhibition, which was not reduced when
a combination of NTR/CB1954 and γ-ray radiation was used. However,
the result of this combination was cooperative. This may be due to
the fact that the cells were treated when they were in the S phase,
and they became damaged by NTR/CB1954, which resulted in S phase
arrest. It is possible that the cells remained in the S phase in
order to undergo repairs of either lethal or sublethal damage as
the purpose was to enter the next phase of the cell cycle to enable
the cells to proliferate and grow. However, at this time point, as
the cells were exposed to γ-ray radiation they became lethally
injured whilst in the S phase. Therefore, S-phase arrest may be due
to the combined effect of the NTR/CB1954 and the γ-ray radiation.
However, additional studies are required to test this
hypothesis.
The use of suicide genes in combination with
radiation therapy is currently underway and whilst progress has
been achieved, it may take a considerable amount of time for this
to become a widely used treatment. Efforts should be focused on the
identification of a potential means for the development of
strategies that aim to use GDEPT to enhance radiotherapy in
clinical treatments.
Acknowledgements
The present study was supported by the Shandong
Province Science and Technology Development Projects (grant nos.
2011GGH21820 and 2011GGH21837).
References
|
1
|
Carruthers KH, Metzger G, During MJ,
Muravlev A, Wang C and Kocak E: Gene-directed enzyme prodrug
therapy for localized chemotherapeutics in allograft and xenograft
tumor models. Cancer Gene Ther. 21:434–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zawilska JB, Wojcieszak J and Olejniczak
AB: Prodrugs: A challenge for the drug development. Pharmacol Rep.
65:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Both GW: Gene-directed enzyme prodrug
therapy for cancer: A glimpse into the future? Discov Med.
8:97–103. 2009.PubMed/NCBI
|
|
4
|
Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y
and Pu PY: The anti-glioma effect of suicide gene therapy using
BMSC expressing HSV/TK combined with overexpression of Cx43 in
glioma cells. Cancer Gene Ther. 17:192–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi M, Valdes G, Hiraoka K, Inagaki
A, Kamijima S, Micewicz E, Gruber HE, Robbins JM, Jolly DJ, McBride
WH, et al: Radiosensitization of gliomas by intracellular
generation of 5-fluorouracil potentiates prodrug activator gene
therapy with a retroviral replicating vector. Cancer Gene Ther.
21:405–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wester HJ: Nuclear imaging probes: From
bench to bedside. Clin Cancer Res. 13:3470–3481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel P, Young JG, Mautner V, Ashdown D,
Bonney S, Pineda RG, Collins SI, Searle PF, Hull D, Peers E, et al:
A phase I/II clinical trial in localized prostate cancer of an
adenovirus expressing nitroreductase with CB1954 [correction of
CB1984]. Mol Ther. 17:1292–1299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhaumik S, Sekar TV, Depuy J, Klimash J
and Paulmurugan R: Noninvasive optical imaging of nitroreductase
gene-directed enzyme prodrug therapy system in living animals. Gene
Ther. 19:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dachs GU, Hunt MA, Syddall S, Singleton DC
and Patterson AV: Bystander or no bystander for gene directed
enzyme prodrug therapy. Molecules. 14:4517–4545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmer DH, Milner AE, Kerr DJ and Young
LS: Mechanism of cell death induced by the novel enzyme-prodrug
combination, nitroreductase/CB1954, and identification of synergism
with 5-fluorouracil. Br J Cancer. 89:944–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anlezark GM, Vaughan T, Fashola-Stone E,
Michael NP, Murdoch H, Sims MA, Stubbs S, Wigley S and Minton NP:
Bacillus amyloliquefaciens orthologue of Bacillus subtilis ywrO
encodes a nitroreductase enzyme which activates the prodrug CB
1954. Microbiology. 148:297–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin C, Chen X, Bai Q, Davis MR and Fang Y:
Factors associated with radiosensitivity of cervical cancer.
Anticancer Res. 34:4649–4656. 2014.PubMed/NCBI
|
|
13
|
Harrington KJ and Nutting CM: Interactions
between ionizing radiation and drugs in head and neck cancer: How
can we maximize the therapeutic index? Curr Opin Investig Drugs.
3:807–811. 2002.PubMed/NCBI
|
|
14
|
Qu L, Wang Y, Gong L, Zhu J, Gong R and Si
J: Suicide gene therapy for hepatocellular carcinoma cells by
survivin promoter-driven expression of the herpes simplex virus
thymidine kinase gene. Oncol Rep. 29:1435–1440. 2013.PubMed/NCBI
|
|
15
|
Xiong T, Li Y, Ni F and Zhang F:
Monitoring of bystander effect of herpes simplex virus thymidine
kinase/acyclovir system using fluorescence resonance energy
transfer technique. J Biomed Nanotechnol. 8:74–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alerie K, Brust D, Farnsworth J, Amir C,
Taher MM, Hershey C and Feden J: Improved radiosensitization of rat
glioma cells with adenovirus-expressed mutant herpes simplex
virus-thymidine kinase in combination with acyclovir. Cancer Gene
Ther. 7:879–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anello R, Cohen S, Atkinson G and Hall SJ:
Adenovirus mediated cytosine deaminase gene transduction and
5-fluorocytosine therapy sensitises mouse prostate cancer cells to
irradiation. J Urol. 164:2173–2177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White CL, Menghistu T, Twigger KR, Searle
PF, Bhide SA, Vile RG, Melcher AA, Pandha HS and Harrington KJ:
Escherichia coli nitroreductase plus CB1954 enhances the effect of
radiotherapy in vitro and in vivo. Gene Ther. 15:424–433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cobb LM, Connors TA, Elson LA, Khan AH,
Mitchley BC, Ross WC and Whisson ME:
2,4-Dinitro-5-ethyleneiminobenzamide (CB 1954): A potent and
selective inhibitor of growth of the Walker carcinoma 256. Biochem
Pharmacol. 18:1519–1527. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung-Faye G, Palmer D, Anderson D, Clark
J, Downes M, Baddeley J, Hussain S, Murray PI, Searle P, Seymour L,
et al: Virus-directed, enzyme prodrug therapy with nitroimidazole
reductase: A phase I and pharmacokinetic study of its prodrug,
CB1954. Clin Cancer Res. 7:2662–2668. 2001.PubMed/NCBI
|
|
21
|
Sekar TV, Foygel K, Ilovich O and
Paulmurugan R: Noninvasive theranostic imaging of
HSV1-sr39TK-NTR/GCV-CB1954 dual-prodrug therapy in metastatic lung
lesions of MDA-MB-231 triple negative breast cancer in mice.
Theranostics. 4:460–474. 2014. View Article : Google Scholar : PubMed/NCBI
|