Introduction
Pancreatic cancer, one of the most aggressive types
of human malignancy, has a poor prognosis with a five-year survival
rate of <5% worldwide (1). It
is characterized by rapid disease progression without specific
symptoms, thus, early diagnosis and curative treatment are almost
impossible (1,2). The standard treatment strategies for
pancreatic cancer include surgery, which is restricted to early
disease stages, radiation and/or gemcitabine-based chemotherapies.
However, pancreatic cancer is among the most intrinsically
resistant types of malignancy to radiation and chemotherapy,
resulting in an overall survival rate of <7%. Therefore,
scientists and oncologists are attempting to identify novel and
more efficient anti-pancreatic cancer agents or adjuvants (3–5). A
previous report showed that ~90% of patients succumb to the disease
within 1 year following diagnosis, and the five-year survival rate
is <5% worldwide (6). The
incidence of pancreatic cancer has been increasing in previous
years, demonstrating the importance of investigating its
pathogenesis. Alterations in gene and protein expression levels,
and the activation of signaling pathways are associated with the
occurrence and progression of pancreatic cancer (7).
Proprotein convertases (PCs) are a family of
enzymes, which are responsible for the activation of numerous
protein precursors. At present, nine PCs have been identified,
namely, furin, paired basic amino acid cleaving enzyme 4 (PACE4),
PC1/3, PC2, PC4, PC5/6, PC7, PCSK9 and SKI-1/S1P (8). PACE4 is considered to be important in
the development and progression of cancer. It activates several
biologically relevant substrates; and a number have been shown to
be significantly involvement in tissue homeostasis and cancer
growth (9,10). Among these are numerous
metalloproteinases, growth factors, growth factor receptors and
adhesion molecules, which are directly associated with tumor
development (11–14). PACE4 is expressed at low levels in
several mammalian tissues and has been demonstrated to be
upregulated in certain tumor cell lines, including murine squamous
cell carcinoma (15). In addition,
mice overexpressing PACE4 have been found to exhibit tumors with
increased growth rates (16).
Previously, two independent studies demonstrated the
overexpression of PACE4 mRNA in prostate cancer tissues (17,18).
This overexpression was correlated with higher circulating protein
levels in certain patients (18).
However, the possible role of PACE4 in apoptosis and the potential
molecular mechanisms of pancreatic cancer remain to be elucidated.
In the present study, molecular silencing with small interfering
(si)RNA was used to knock down endogenously expressed PACE4 in the
Panc-1 cell line, following which cell proliferation and the
apoptotic response was examined.
Materials and methods
Reagents and antibodies
The following polyclonal antibodies were purchased
from Proteintech Group, Inc. (Wuhan, China): Rabbit anti-human
cleaved caspase-3 (19677-1-AP; 1:3,000), rabbit B cell lymphoma
(Bcl)-2 (12789-1-AP; 1:3,000), rabbit Bcl-2-associated X protein
(Bax; 50599-2-Ig; 1:3,000), rabbit cytochtome c oxidase
subunit IV and X-linked inhibitor of apoptosis protein (XIAP;
10037-1-Ig; 1:2,000) antibodies, and the mouse anti-GAPDH
(MM-0163-P; 1:1,000) antibody. Polyclonal rabbit anti-human PACE4
(AB151562; 1:3,000) and cytochrome c (ab154476;
1:3,000)antibodies were purchased from Abcam (Cambridge, UK).
Polyclonal rabbit anti-human phosphorylated-Akt (p-Akt; 13038;
1:2,000) and Akt (4685; 1:3,000) antibodies were purchased from
Cell Signaling Technologies, Inc. (Danvers, MA, USA). Anti-rabbit
(15134-1-AP) and anti-mouse (30000-0-AP) IgG-horseradish peroxidase
antibodies were purchased from Proteintech Group, Inc. A Cell
Counting Kit-8 (CCK-8) and Hoechst 33258 were purchased from
Beyotime Institute of Biotechnology (Haimen, China). Other reagents
were of analytical grade.
Cell culture and small interfering RNA
transfection
Human pancreatic cancer Panc-1 cells were obtained
from American Type Culture Collection (Manassas, VA, USA). The
cells were routinely grown in Dulbecco's modified Eagle's medium
(DMEM; GE Healthcare Life Sciences, Beijing, China) containing 10%
fetal bovine serum (FBS; GE Healthcare Life Sciences), 100 U/ml
penicillin (Sigma-Aldrich, St. Louis, MO, USA) and 100 µg/ml
streptomycin (Sigma-Aldrich) at 37°C in a humidified atmosphere of
5% CO2. The cultures were replaced every 2–3 days and the cultures
were divided into two at 80% confluence.
The Panc-1 cells were transfected with 100 nM of
PACE4 siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat.
no. sc-45482; Genbank ID for PACE4, NM_174936) or control siRNA
(scrambled siRNA, a universal negative control; Sangon Biotech Co.,
Ltd., Shanghai, China) with GeneSilencer siRNA transfection reagent
(Genlantis, San Diego, CA, USA) at 37°C, according to the
manufacturer's protocol. At 48 h post-transfection, the efficiency
of siRNA-mediated PACE4 knockdown was determined using Western blot
analysis.
Cell proliferation assay
The Panc-1 cells were plated in each well of a
96-well plate at a density of 5×103 cells/well in the
culture medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
at 37°C. Following 24 h of incubation, the cells were transfected
with PACE4 siRNA or control siRNA, as described above, for 12, 24,
36 and 48 h, followed by the addition of 10 µl CCK-8 solution at
37°C. The cells were then incubated for 3 h at 37°C. Absorbance was
measured at 450 nm using a spectrophotometer (NanoDrop ND-1000;
Thermo Fisher Scientific, Inc.).
Morphological analysis following
Hoechst 33258 staining
The Panc-1 cells were seeded in 24-well plates
(6×104 cells per well) overnight, and transfected with
PACE4 siRNA or control siRNA for 48 h. The cells were then fixed
and stained with Hoechst 33258 (Invitrogen; Thermo Fisher
Scientific, Inc.). The apoptotic cells were visualized under a
fluorescence microscope (M165 FC; Leica Microsystems GmbH, Wetzlar,
Germany).
Detection of caspase-3/7 protein
activity
The activities of caspase-3/7 were measured using a
colorimetric method, according to the manufacturer's protocol,
using a Caspase-glo 3/7 Assay kit (G8093; Promega Corporation,
Madison, WI, USA). Briefly, 2×104 Panc-1 cells were
seeded in 96-well plates and, after 24 h, the cells were
transfected with PACE4 siRNA or control siRNA for another 48 h.
Subsequently, the lysates (lysed using Sigma-Aldrich lysis buffer)
of the Panc-1 cells were mixed with equilibrated caspase-glo 3/7
reagents for 1 h at room temperature. Luminescence was measured
using a GloMax 96 luminometer (Promega Corporation).
Preparation of mitochondria and
cytosol
A mitochondria/cytosol kit (C3601; Beyotime
Institute of Biotechnology, Beijing, China) was used to isolate the
mitochondria and cytosol, according to the manufacture's protocol.
Following transfection, as described above, the cells
(5×107 cells) were collected by centrifugation at 600 ×
g for 5 min at 4°C, washed twice with ice-cold phosphate-buffered
saline and then resuspended in 500 µl isolation buffer (Tiangen
Biotech Co., Ltd., Beijing, China) containing protease inhibitors
for 10 min on ice. The cells were then mechanically homogenized
using a Dunce grinder (ATX810; Tiangen Biotech Co., Ltd.). The
unbroken cells, debris and nuclei were discarded by centrifugation
at 800 × g for 10 min at 4°C. The supernatants were centrifuged at
12,000 × g for 15 min at 4°C. The supernatant of the cytosol was
collected and the pellet fraction mitochondria was dissolved in 50
µl lysis buffer (Sigma-Aldrich).
Western blot analysis
The Panc-1 cells were transfected, as described
above, and were then lysed using radioimmunoprecipitation assay
buffer (Sigma-Aldrich). A Bicinchoninic Acid Protein Assay kit
(NEP045-2; Beijing Dingguo Changsheng Biotechnology Co., Ltd.,
Beijing, China) was used to measure the protein concentrations. The
total protein (0.2 µg) was loaded onto 12% SDS-PAGE gels (Tiangen
Biotech Co., Ltd.) and transferred onto polyvinylidene fluoride
membranes (Sigma-Aldrich). The membranes were probed overnight at
4°C with the indicated primary antibodies in Tris-buffered saline
with Tween-20 (Tiangen Biotech Co., Ltd.), containing 1% bovine
serum albumin (w/v; Gibco; Thermo Fisher Scientific, Inc.). The
blots were then incubated for 1 h with anti-rabbit or mouse
secondary antibodies at 37°C. The immune complexes were detected
using an ECL Detection kit (32132; Thermo Fisher Scientific, Inc.)
and quantified using a scanning densitometer (SD4; Tobias
Associates, Inc., Ivyland, PA, USA) with molecular analysis
software (Quantity One 1-D Analysis software; version 4.6.9;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). Quantification of
band density was additionally performed using Quantity One 1-D
Analysis software with normalization to the GAPDH signal. The
expression levels of the proteins of interest in the various
treatment groups were expressed relative to those under non-treated
conditions.
Statistical analysis
All data are presented as the mean ± standard
deviation, and were analyzed using Student's t-test and one-way
analysis of variance to determine the levels of significance.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS/Windows
11.0 software (SPSS Inc., Chicago, IL, USA).
Results
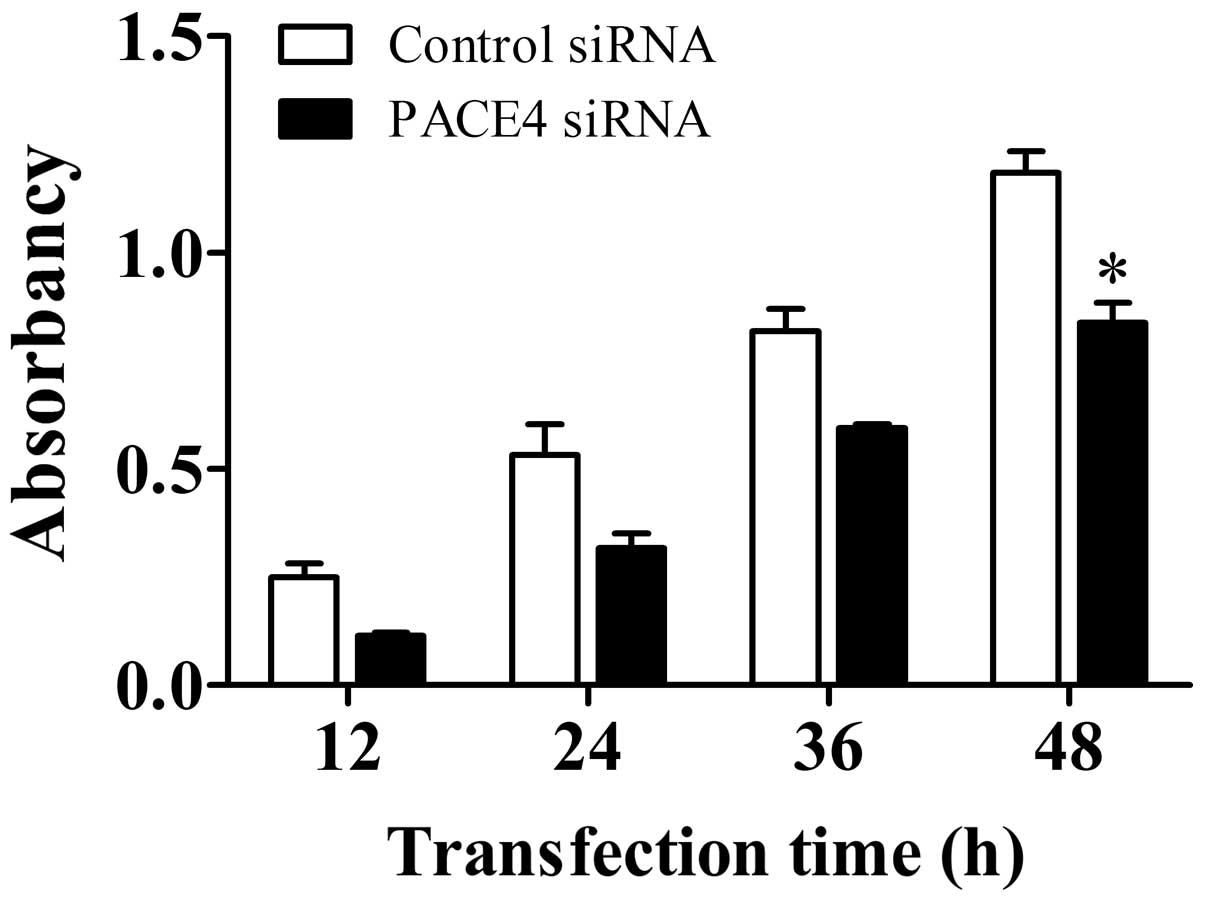
PACE4 reduces Panc-1 cell
proliferation
Panc-1 cell proliferation was examined using a CCK-8
assay following transfection of the cells with PACE4 siRNA or
control siRNA. As shown in Fig. 1,
PACE4 siRNA inhibited cell proliferation, with a significant
reduction observed 48 h post-transfection, compared with that in
the control siRNA-treated group (P<0.05). Thus, these data
indicated that PACE4 may affect cellular proliferation in Panc-1
cells.
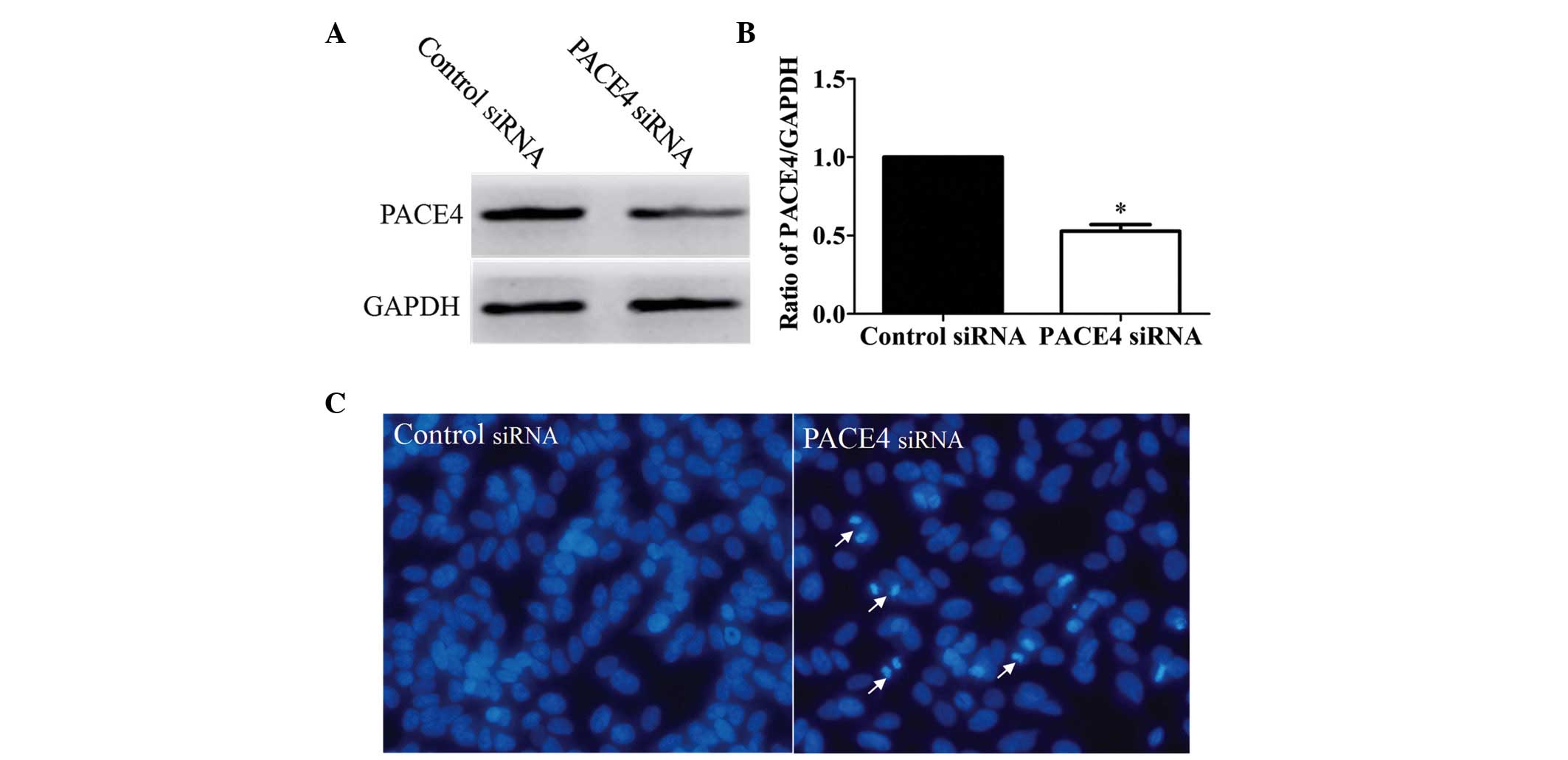
PACE4 siRNA induces the apoptosis of
Panc-1 cells
In the present study, the Panc-1 cells were
transfected with PACE4 siRNA. The results of the Western blot
analysis indicated that the protein expression of PACE4 was
inhibited in the PACE4 siRNA-transfected group, compared with the
control siRNA-transfected group (Fig.
2A and B). In order to evaluate whether the proliferation
inhibition induced by PACE4 siRNA in the Panc-1 cells was
associated with apoptosis, the present study examined the
morphological changes in the cells using Hoechst 33258 staining.
The Panc-1 cells were transfected with PACE4 siRNA for 48 h, and
the apoptotic morphological changes were observed and compared with
the appearances in the control group. In the control siRNA group,
the nuclei of the Panc-1 cells were round and homogeneously stained
(Fig. 2C). However, the PACE4
siRNA-transfected cells exhibited evident apoptotic
characteristics, including cell shrinkage and membrane integrity
loss or deformation, nuclear fragmentation and chromatin compaction
of a late apoptotic appearance. Together, these data indicated that
PACE4 siRNA induced apoptosis in the Panc-1 cells.
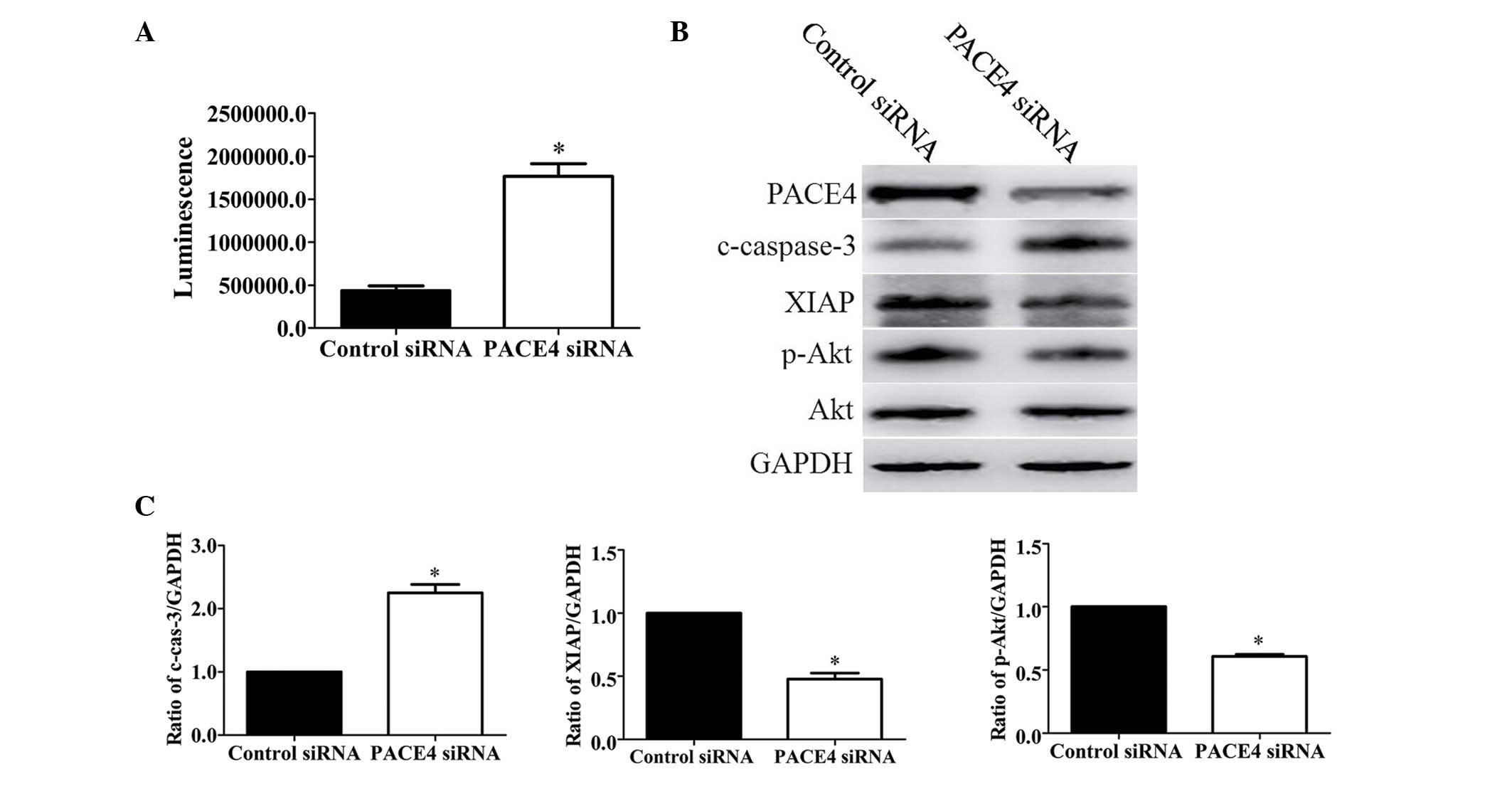
PACE4 siRNA induces apoptosis via a
caspase-dependent pathway
Caspase-3 is a critical executioner of apoptosis,
and its activation is essential for DNA fragmentation and a number
of the typical biochemical and morphological changes observed in
cells undergoing apoptosis. Therefore, to evaluate whether PACE4
siRNA-induced apoptosis is involved in the activation of
caspase-3/7, the present study investigated the activities of
caspase-3/7 through measuring the bioluminescence intensities. The
activities of caspase-3/7 were significantly activated following
PACE4 siRNA transfection (Fig.
3A).
To further assess the role of PACE4 in Panc-1 cell
apoptosis, the present study evaluated the expression levels of
apoptosis-associated proteins. These included pro-apoptotic cleaved
caspase-3 (c-caspase-3), anti-apoptotic XIAP and p-Akt. The results
of the Western blot analysis and subsequent statistical analysis
indicated that PACE4 siRNA increased the levels of c-caspase-3 by
~2.2-fold (P<0.05). By contrast, the levels of XIAP and p-Akt
were decreased by ~53% (P<0.05) and ~40% (P<0.05),
respectively, following PACE4 siRNA transfection (Fig. 3B and C).
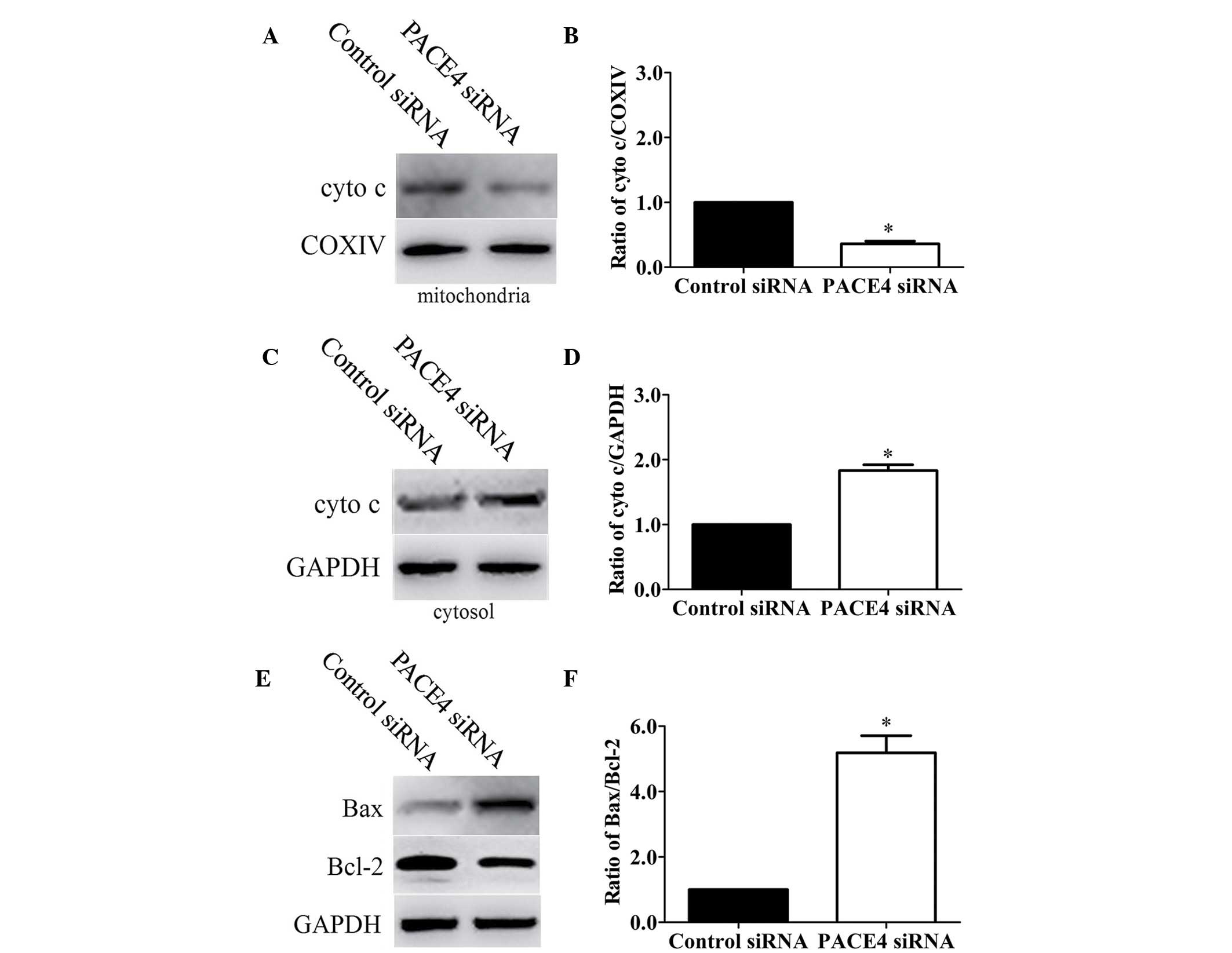
PACE4 siRNA induces apoptosis via the
mitochondrial signaling pathway
In order to further understand the molecular
mechanisms by which PACE4 siRNA exerts pro-apoptotic effects, the
present study examined the protein expression of mediators in the
mitochondrial signaling pathway. Initially, the present study
determined whether PACE4 siRNA stimulated the release of cytochrome
c into the cytosolic fraction in Panc-1 cells. As expected,
cytochrome c was redistributed following PACE4 siRNA
transfection. The level of cytochrome c in the mitochondria
was significantly decreased by 64% (Fig. 4A and B; P<0.05).
Correspondingly, the levels of cytochrome c in the cytosol
were increased by 1.83-fold (Fig. 4C
and D; P<0.05).
As the Bcl-2 family of proteins are critical in
regulating the release of cytochrome c, the present study
subsequently investigated the possible involvement of Bax and Bcl-2
in the process of PACE4 siRNA-mediated Panc-1 cell apoptosis. As
shown in Fig. 4E and F, the level
of Bax was significantly increased and the level of Bcl-2 was
markedly decreased in the PACE4 siRNA-transfected cells, compared
with the control cells. Statistical analysis showed that PACE4
siRNA increased the ratio of Bax/Bcl-2 by ~5.5-fold
(P<0.05).
Discussion
PACE4 has already been highlighted for its potential
role in several types of neoplasia, including oral tongue carcinoma
(19), hepatocellular carcinoma
(20), glioma (21), skin cancer (16,22)
and prostate cancer (17). Whereas
previous studies have predominantly examined PACE4 overexpression,
the present study focused on gene silencing as a predictive
approach to define potential therapeutic benefits. In the present
study, the effect of PACE4 siRNA on apoptosis in a cellular model
of pancreatic cancer was investigated. The results indicated that
PACE4 siRNA inhibited the proliferation of Panc-1 cells. Based on
the results of Hoechst 33258 staining, measurement of caspase-3/7
activities and Western blot analysis, it was concludes that PACE4
siRNA induced apoptosis in the Panc-1 cells. Thus, the present
study provided the first evidence, to the best of our knowledge,
that PACE4 has an anti-apoptotic effect in pancreatic cancer
cells.
As a primary executioner caspase in the majority of
pathways of the caspase protein family, the activation of caspase-3
often results in the irreversible commitment of a cell to
apoptosis. Therefore, the activation of caspase-3 is considered a
reliable marker for cells undergoing apoptosis (23). The present study found that the
activity of caspase-3/7 was significantly activated following PACE4
siRNA transfection (Fig. 3A). An
effective strategy for destroying cancer cells is to induce cell
apoptosis. XIAP, a member of the inhibitor of apoptosis protein
family, contributes to the apoptosis resistance of cancer cells
(24,25). Akt is a promoter of cell
proliferation and survival, and has been found to be overexpressed
in tumor formation (26). Thus,
the present study investigated whether these apoptosis-associated
proteins were involved in PACE4 siRNA-induced apoptosis. The
results of this investigation confirmed the role of PACE4 in the
apoptosis of Panc-1 cells, based on the following lines of
evidence: PACE4 siRNA increased the apoptosis of cells by
regulating the expression levels of the apoptosis-associated
factors c-caspase-3, XIAP and p-Akt (Fig. 3B and C). The inactivation of XIAP
and p-Akt by PACE4 siRNA may prevent the development and
progression of cancer.
The Bcl-2 family of proteins are important in the
apoptosis of cancer cell apoptosis (27,28).
The Bcl-2 family can primarily regulate mitochondrial membrane
permeabilization (29). The
Bax/Bcl-2 ratio is usually regarded as a criterion for apoptosis
(30). The results from the
present study demonstrated that the level of cytochrome c in
the mitochondria was significantly decreased (Fig. 4A and B), whereas that in cytosol
was increased (Fig. 4C and D). In
addition, PACE4 siRNA increased the levels of Bax and decreased the
level of Bcl-2, leading to changes in the ratio of Bax/Bcl-2
(Fig. 4E and F). These results
indicated that PACE4 siRNA had an effect on mitochondrial membrane
stability. This was evidenced by the increased Bax/Bcl-2 ratio and
the release of cytochrome c into the cytoplasm. Taken
together, these data demonstrated that PACE4 siRNA may exert its
anti-tumor activity through the mitochondrial signaling pathway
(intrinsic pathway) in pancreatic cancer cells.
In conclusion, the results of the present study
suggested PACE4 siRNA possesses anti-proliferation and
apoptosis-inducing properties in human pancreatic cancer Panc-1
cells. The PACE4 siRNA-induced apoptosis of Panc-1 cells may be
mediated through the mitochondria pathway. These results support
the potential of PACE4 to be developed as a promising agent for the
treatment of pancreatic cancer.
References
|
1
|
Feng J, Ma T, Ge Z, Lin J, Ding W, Chen H,
Zhu W, Zhou S and Tan Y: PKM2 gene regulates the behavior of
pancreatic cancer cells via mitogen-activated protein kinase
pathways. Mol Med Rep. 11:2111–2117. 2015.PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim J, Kim YC, Fang C, Russell RC, Kim JH,
Fan W, Liu R, Zhong Q and Guan KL: Differential regulation of
distinct Vps34 complexes by AMPK in nutrient stress and autophagy.
Cell. 152:290–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong L, Yang B, Xu M, Cheng B, Tang X,
Zheng P, Jing Y and Wu GJ: Bortezomib-induced apoptosis in cultured
pancreatic cancer cells is associated with ceramide production.
Cancer Chemother Pharmacol. 73:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Min H, Xu M, Chen ZR, Zhou JD, Huang M,
Zheng K and Zou XP: Bortezomib induces protective autophagy through
AMP-activated protein kinase activation in cultured pancreatic and
colorectal cancer cells. Cancer Chemother Pharmacol. 74:167–176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin C, Yao L, Long J, Fu DL, Yu XJ, Yang
F, Ni QX and Xu J: Effect of multiple-phase regional intra-arterial
infusion chemotherapy on patients with resectable pancreatic head
adenocarcinoma. Chin Med J (Engl). 122:284–290. 2009.PubMed/NCBI
|
|
7
|
Preis M and Korc M: Signaling pathways in
pancreatic cancer. Crit Rev Eukaryot Gene Expr. 21:115–129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seidah NG and Prat A: The biology and
therapeutic targeting of the proprotein convertases. Nat Rev Drug
Discov. 11:367–383. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuji A, Sakurai K, Kiyokage E, Yamazaki
T, Koide S, Toida K, Ishimura K and Matsuda Y: Secretory proprotein
convertases PACE4 and PC6A are heparin-binding proteins which are
localized in the extracellular matrix. Potential role of PACE4 in
the activation of proproteins in the extracellular matrix. Biochim
Biophys Acta. 1645:95–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuasa K, Masuda T, Yoshikawa C, Nagahama
M, Matsuda Y and Tsuji A: Subtilisin-like proprotein convertase
PACE4 is required for skeletal muscle differentiation. J Biochem.
146:407–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato H, Kinoshita T, Takino T, Nakayama K
and Seiki M: Activation of a recombinant membrane type 1-matrix
metalloproteinase (MT1-MMP) by furin and its interaction with
tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett.
393:101–104. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yana I and Weiss SJ: Regulation of
membrane type-1 matrix metalloproteinase activation by proprotein
convertases. Mol Biol Cell. 11:2387–2401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dubois CM, Blanchette F, Laprise MH, Leduc
R, Grondin F and Seidah NG: Evidence that furin is an authentic
transforming growth factor-beta1-converting enzyme. Am J Pathol.
158:305–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khatib AM, Siegfried G, Prat A, Luis J,
Chrétien M, Metrakos P and Seidah NG: Inhibition of proprotein
convertases is associated with loss of growth and tumorigenicity of
HT-29 human colon carcinoma cells: Importance of insulin-like
growth factor-1 (IGF-1) receptor processing in IGF-1-mediated
functions. J Biol Chem. 276:30686–30693. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hubbard FC, Goodrow TL, Liu SC, Brilliant
MH, Basset P, Mains RE and Klein-Szanto AJ: Expression of PACE4 in
chemically induced carcinomas is associated with spindle cell tumor
conversion and increased invasive ability. Cancer Res.
57:5226–5231. 1997.PubMed/NCBI
|
|
16
|
Mahloogi H, Bassi DE and Klein-Szanto AJ:
Malignant conversion of non-tumorigenic murine skin keratinocytes
overexpressing PACE4. Carcinogenesis. 23:565–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D'Anjou F, Routhier S, Perreault JP, Latil
A, Bonnel D, Foumier I, Salzet M and Day R: Molecular validation of
PACE4 as a target in prostate cancer. Transl Oncol. 4:157–172.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klee EW, Bondar OP, Goodmanson MK, Dyer
RB, Erdogan S, Bergstralh EJ, Bergen HR III, Sebo TJ and Klee GG:
Candidate serum biomarkers for prostate adenocarcinoma identified
by mRNA differences in prostate tissue and verified with protein
measurements in tissue and blood. Clin Chem. 58:599–609. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Estilo CL, O-charoenrat P, Talbot S, Socci
ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y,
Boyle JO, et al: Oral tongue cancer gene expression profiling:
Identification of novel potential prognosticators by
oligonucleotide microarray analysis. BMC Cancer. 9:112009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurokawa Y, Matoba R, Nakamori S, Takemasa
I, Nagano H, Dono K, Umeshita K, Sakon M, Monden M and Kato K:
PCR-array gene expression profiling of hepatocellular carcinoma. J
Exp Clin Cancer Res. 23:135–141. 2004.PubMed/NCBI
|
|
21
|
Delic S, Lottmann N, Jetschke K,
Reifenberger G and Riemenschneider MJ: Identification and
functional validation of CDH11, PCSK6 and SH3GL3 as novel glioma
invasion-associated candidate genes. Neuropathol Appl Neurobiol.
38:201–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bassi DE, De Cicco R Lopez, Cenna J,
Litwin S, Cukierman E and Klein-Szanto AJ: PACE4 expression in
mouse basal keratinocytes results in basement membrane disruption
and acceleration of tumor progression. Cancer Res. 65:7310–7319.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
LaCasse EC, Mahoney DJ, Cheung HH,
Plenchette S, Baird S and Korneluk RG: IAP-targeted therapies for
cancer. Oncogene. 27:6252–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boonyarat C, Yenjai C, Vajragupta O and
Waiwut P: Heptaphylline induces apoptosis in human colon
adenocarcinoma cells through bid and Akt/NF-kB (p65) pathways.
Asian Pac J Cancer Prev. 15:10483–10487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Serrano ML, Sánchez-Gómez M, Bravo MM,
Yakar S and LeRoith D: Differential expression of IGF-I and insulin
receptor isoforms in HPV positive and negative human cervical
cancer cell lines. Horm Metab Res. 40:661–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leber B, Lin J and Andrews DW: Still
embedded together binding to membranes regulates Bcl-2 protein
interactions. Oncogene. 29:5221–5230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014.PubMed/NCBI
|