Introduction
Cerebral venous sinus thrombosis (CVST) is a rare
cerebrovascular disorder representing between 0.5 and 3% of all
strokes, which predominantly affects younger people (1,2).
Patients with CVST develop venous infarcts in ~50% of cases, often
resulting in a spectrum of clinical manifestations, including
headache, hemiparesis, seizures and intracranial hypertension with
papilledema (3). Although modern
imaging techniques, in combination with improved treatment of CVST,
have significantly improved survival and clinical outcomes in
recent decades, the pathogenesis of CVST remains poorly understood
and effective treatment strategies remain to be elucidated. The use
of heparin and oral anticoagulants is based on the rationale of
reversing the causal thrombotic process and preventing
complications. However, due to the presence of a hemorrhagic
element in 40% of CSVT cases, the administration of anticoagulant
treatment remains controversial (4). One previous study suggested that
early anticoagulation may be beneficial, although it was associated
with an increased risk of symptomatic brain hemorrhage (5). Therefore, a safer alternative therapy
is required to provide marked benefits to CVST patients.
Thrombomodulin (TM) is a glycoprotein present in the
membranes of endothelial cells, which regulates coagulation via
effects on thrombin. TM acts as a cofactor for the
thrombin-catalyzed activation of protein C, enhancing the reaction
rate >1,000-fold, switching thrombin from a procoagulant to an
anticoagulant enzyme (6,7), and exerts anti-inflammatory effects
(8). A previous study demonstrated
that Solulin, a recombinant soluble analog of human TM, facilitated
recanalization and reduced stroke volume following middle cerebral
artery occlusion (9), and
decreased the expression levels of the proinflammatory cytokines,
tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in the
infarct, compared with control animals (10). These studies revealed a protective
effect of Solulin against ischemic brain damage, suggesting that
soluble TM exerts anticoagulant and anti-inflammatory effects
following stroke. However, whether recombinant human soluble
(rhs)-TM has a neuroprotective effect in the CVST model remains to
be elucidated.
In the present study, the expression levels of
proinflammatory cytokines and apoptosis genes in the infarcted
segments of CVST rat brains were analyzed following rhs-TM
treatment, to assess its neuroprotective effect and reveal any
underlying molecular mechanisms.
Materials and methods
Animal preparation
The present study was approved by the ethics
committee of Fuzhou General Hospital (Fuzhou, China), and all
animal experiments were performed in accordance with the
institutional guidelines on the care and use of experimental
animals. A total of 54 male Sprague-Dawley rats (weight, 240–260 g;
age, 6 weeks old), obtained from the Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China), were used in the present study.
Rats were housed in standard conditions at 26–28°C, with 10 h
light/14 h dim light cycles and access to food and water ad
libitum. Of the 54 rats, 36 were randomly assigned to six
subgroups (n=6 rats/group) and one group was sacrificed by
intraperitoneal injection of 30 mg/100 g body weight chloral
hydrate at 0, 6, 12, 24, 48 and 72 h following the induction of
thrombus. The remaining 18 rats were divided into three groups (n=6
rats/group): Sham, CVST, and rhs-TM. The rhs-TM group was injected
with 1 mg/kg rhs-TM (dissolved in saline; Asahi Kasei Pharma Corp.,
Tokyo, Japan) through the tail vein 10 min prior to CVST. The CVST
and the sham groups received normal saline.
Surgical preparation and CVST
model
In all experiment groups, rats were anesthetized
with an intraperitoneal injection 30 mg/100 g body weight chloral
hydrate. To induce venous sinus thrombosis, a 1.5×10 mm cranial
window was made to expose the superior sagittal sinus. A strip of
filter paper soaked with 40% ferric chloride was applied to the
exposed cranial window for 5 min, whereas the sham group received
filter paper soaked with 0.9% saline. Subsequently, the ferric
chloride strip was removed and the field flushed with 0.9% saline;
the removed bone strip was replaced, sealed with bone cement and
the skin sutured.
Neurological evaluation
Neurological evaluations were performed in a blinded
manner. Rats were subjected to neurological evaluation prior to
surgery and at 24 h following CVST using a score of 0–3, where 0=no
observable neurological deficit (normal), 1=failure to extend left
forepaw on lifting whole body by tail (mild), 2=circling to
contralateral side (moderate) and 3=no spontaneous motor activity
(severe) (11).
2,3,5-triphenyltetrazolium chloride
(TTC) staining and stroke volume
Rats were sacrificed with an intraperitoneal
injection of 30 mg/100 g body weight chloral hydrate 24 h following
CVST. Brains were removed, cut into 2-mm-thick coronal sections,
stained with 1% TTC in phosphate-buffered saline for 20 min at 37°C
and fixed in 10% paraformaldehyde solution for 10 min. The sections
were analyzed with the image analysis software Image J version 1.44
(National Institutes of Health, Bethesda, MD, USA). For all 6
slices, the brain infarct volume was calculated as the product of
the average slice thickness (2-mm) and the sum of the infarction
area.
Brain water content
The water content of the brain was determined by the
dry-wet weight method. The brains were removed following sacrifice
by chloral hydrate administration and the cerebellum, pons and
olfactory bulbs from each brain were removed. Each cerebrum was
weighed to determine its wet weight. The brains were subsequently
placed into an 110°C thermal oven for 24 h, and weighed to
determine their dry weight. Water content was calculated using the
following formula: Water content = [(wet weight - dry weight) / wet
weight] × 100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the penumbra regions of
the infarcted hemispheres using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc. Waltham, MA, USA),
according to the manufacturer's instructions. cDNA was generated
with the High-Capacity cDNA RT kit (Roche Diagnostics GmbH,
Mannheim, Germany). The mRNA expression levels of high mobility
group box 1 (HMGB1), receptor for advanced glycation end products
(RAGE), TNF-α, IL-1β, IL-6, caspase-3, B-cell lymphoma-2 (Bcl-2)
and Bcl-2 associated X (Bax) were analyzed by qPCR using a SYBR
Green PCR Master Mix kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in conjunction with an ABI-Prism 7300 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Thermal
cycling parameters consisted of an initial enzyme activation step
at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C
for 15 sec, annealing at 60°C for 30 sec and amplification at 72°C
for 30 sec. The concentrations of the target genes were calculated
by comparing quantification cycle (Cq) values in each sample with
Cq values of the internal standard curve (12). Melting curve analysis and gel
electrophoresis evaluation of RT-qPCR products was routinely
performed to determine the specificity of the reaction. The mRNA
expressions levels were normalized to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNA levels. The following primers were used:
Forward, 5-CGA GTC CGT GTC TAC CAG ATT-3′ and reverse,
5′-GCGGCTGGAATGGAAACTGAA-3′ for RAGE; forward,
5′-GCTCCATAGAGACAGCGCCGGG-3′ and reverse,
5′-CCTCAGCGAGGCACAGAGTCGC-3′ for HMGB1; forward,
5′-CTCCCAGAAAAGCAAGCAAC-3′ and reverse, 5′-CGAGCAGGAATGAGAAGAGG-3′
for TNF-α; forward, 5′-CTGTGACTCGTGGGATGATG-3′ and reverse,
5′-GGGATTTTGTCGTTGCTTGT-3′ for IL-1β; forward,
5′-ACAGTGCATCATCGCTGTTC-3′ and reverse, 5′-CCGGAGAGGAGACTTCACAG-3′
for IL-6; forward, 5′-CTGGACTGCGGTATTGAG-3′ and reverse,
5′-GGAACATCGGATTTGATT-3′ for caspase-3; forward,
5′-ATACCTGGGCCACAAGTGAG-3′ and reverse, 5′-TGATTTGACCATTTGCCTGA-3′
for Bcl-2; forward, 5′-CGAGCTGATCAGACATCA-3′ and reverse,
5′-CTCAGCCCATCTTCTTCCAG-3′ for Bax; and forward,
5′-TCGGAGTCAACGGATTTGG-3′ and reverse, 5′-CATGGTGGATCATGGA-3′ for
GAPDH.
Western blot analysis
Rats were sacrificed with an intraperitoneal
injection of 30 mg/100 g body weight chloral hydrate and penumbra
regions of the infarcted hemispheres were lysed in
radioimmunoprecipitation assay buffer (EMD Millipore, Billerica,
MA, USA). The concentration of the protein extracts was determined
by the Bradford assay (Bradford Protein assay kit; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). A total of 40 µg protein
was loaded onto gels, separated by 8–15% SDS-PAGE and transferred
onto polyvinylidene difluoride membranes. Membranes were blocked
with 5% non-fat milk in Tris-buffered saline containing 0.1%
Tween-20, and subsequently probed with primary antibodies overnight
at 4°C. The following antibodies were used: Monoclonal rabbit
anti-HMGB1 (cat. no. ab92310; dilution, 1:1,000; Abcam, Cambridge,
MA, USA), monoclonal rabbit anti-RAGE (cat. no. ab172473; dilution,
1:500; Abcam), polyclonal rabbit anti-TNF-α (cat. no. ab6671;
dilution, 1:500; Abcam), polyclonal rabbit anti-IL-1β (cat. no.
ab9722; dilution, 1:500; Abcam), monoclonal mouse anti-IL-6 (cat.
no. ab9324; dilution, 1:500; Abcam), polyclonal rabbit
anti-caspase-3 (cat. no. ab4051; dilution, 1:500; Abcam),
polyclonal rabbit anti-Bcl-2 (cat. no. ab59348; dilution, 1:500;
Abcam) and polyclonal rabbit anti-Bax (cat. no. ab59348; dilution,
1:500; Abcam). The membranes were washed three times with PBS-Tween
20, before they were incubated with horseradish
peroxidase-conjugated rabbit anti-mouse or goat anti-rabbit IgG
secondary antibodies (H+L; cat. nos. ab6728 and ab6721,
respectively; dilution, 1:2,000; Abcam) for 1 h at room
temperature. The blots were developed using the SuperSignal™ West
Pico Chemiluminescent Substrate (cat. no. 34077; Invitrogen; Thermo
Fisher Scientific, Inc.). Band intensities were normalized to
β-actin and measured using spot densitometry analysis with Image J
software (version 1.44, National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed in SPSS software version 13.0
(SPSS, Inc., Chicago, IL, USA). Intergroup differences were
analyzed using paired Student's t-test and multiple comparisons
among various groups were conducted by analyses of variance with a
post-hoc Tukey test. P<0.05 was considered to indicate a
statistically significant difference.
Results
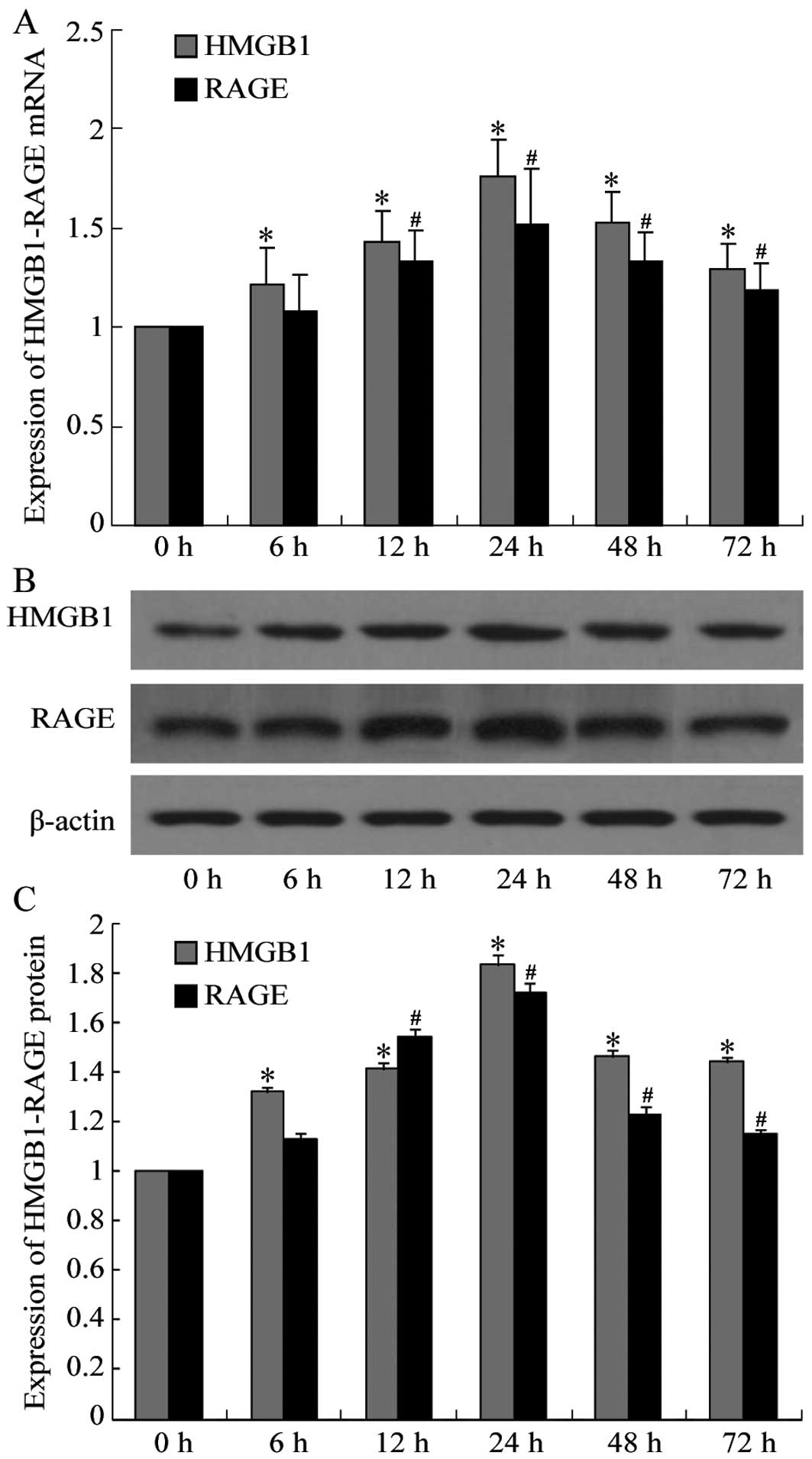
Expression levels of HMGB1-RAGE
increased in infarcted segments of CVST rat brains
The mRNA and protein expression levels of HMGB1-RAGE
in the infarcted segments of CVST rat brains were analyzed by
RT-qPCR and western blot analysis, respectively. HMGB1 mRNA
expression levels were upregulated in the infarcted segments of rat
brains 6 h following CVST, and RAGE mRNA expression levels were
upregulated 12 h following CVST. mRNA expression levels of the two
peaked at 24 h and gradually declined, although significant
differences compared with the control group were observed even at
72 h following CVST (P<0.001; Fig.
1A). Western blot analysis demonstrated that HMGB1 and RAGE
protein expression levels were consistent with the RT-qPCR data
(Fig. 1B and C). The time point of
24 h was therefore selected for subsequent analyses.
rhs-TM may protect against
neurological deficits
In the sham group no neurological deficits were
observed. The neurological deficit score 24 h following surgery was
1.49±0.38 in the CVST group and 1.02±0.29 in the rhs-TM group
(P=0.036; Fig. 2A). This suggests
that rhs-TM may protect against neurological deficits.
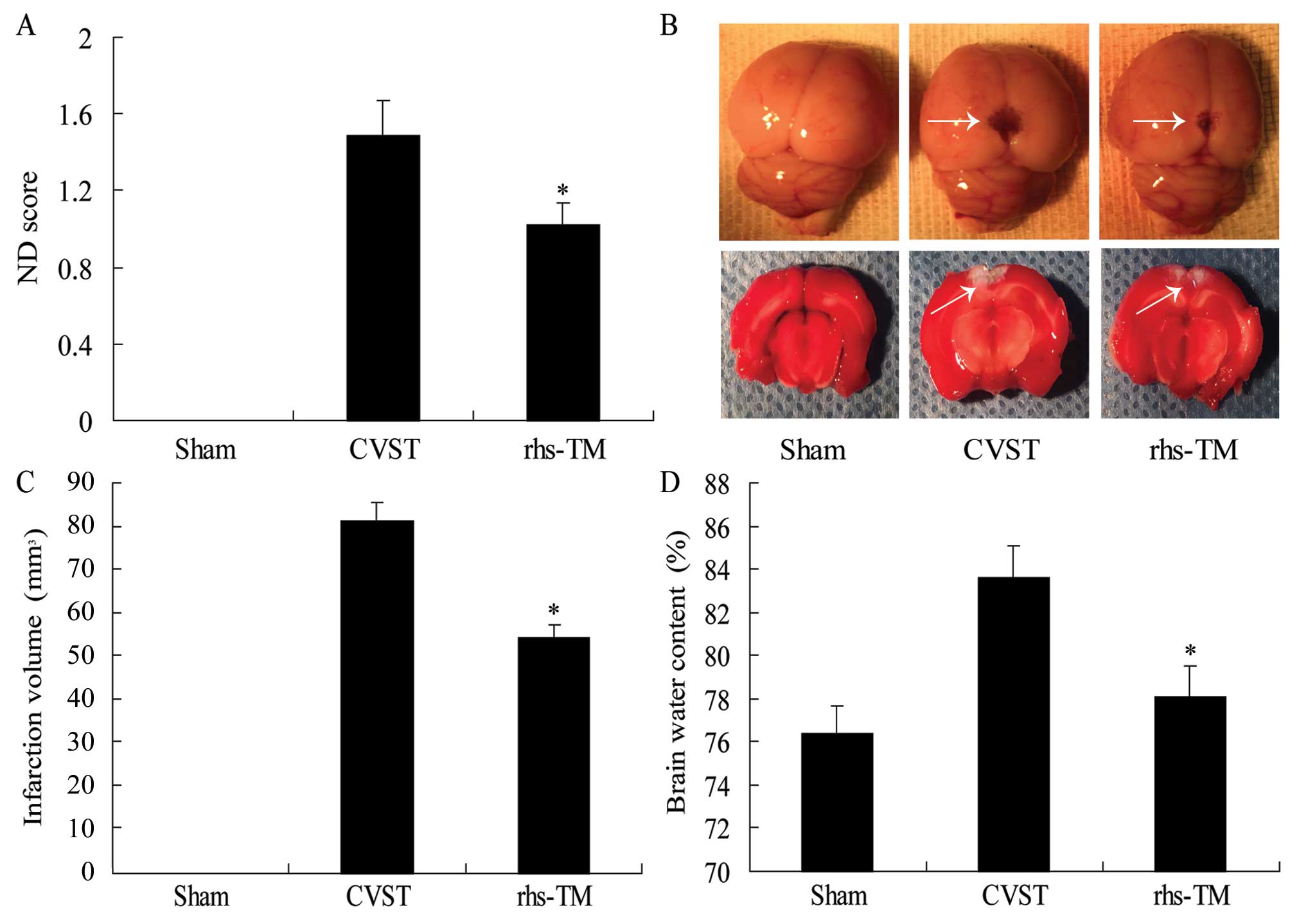 | Figure 2.Effect of rhs-TM on ND score,
infarction volume and brain water content following CVST. (A) ND
score was decreased in the rhs-TM group compared with the CVST
alone group, 24 h following surgery. Sham group animals had no
observable neurological deficits. (B) Photographs (magnification,
×100) of unstained (top panel) and TTC-stained rat brains (bottom
panel). Arrows indicate infarcted regions. (C) Quantification of
infarction volume from TTC staining demonstrated that the
infarction volume was decreased in the rhs-TM group compared with
the CVST alone group, 24 h following surgery. (D) Brain water
content was decreased in the rhs-TM group compared with the CVST
alone group. Data are expressed as the mean ± standard deviation of
three independent experiments. *P<0.05 vs. the CVST group.
rhs-TM, recombinant human soluble thrombomodulin; ND, neurological
deficit; CVST, cerebral venous sinus thrombosis; TTC,
2,3,5-triphenyltetrazolium chloride. |
rhs-TM reduced infarction volume
Brain infarctions were identified by TTC staining of
the brain slices, revealing a bilateral infarction in the CVST
group (Fig. 2B). The brain
infarction volume was 83.3±4.6 mm3 24 h following CVST
alone, compared with 65.3±3.8 mm3 in the rhs-TM group
(P<0.001; Fig. 2C). No brain
lesions were observed in the sham group.
rhs-TM reduced brain water
content
The water content of rat brains was 76.3±0.15% in
the sham group, 80.3±0.34% in the CVST group, and 78.21±0.24% in
the rhs-TM group. This difference between the CVST and rhs-TM
groups was significant (P<0.001; Fig. 2D).
rhs-TM downregulated the mRNA and
protein expression levels of HMGB1-RAGE and their downstream
effectors in infarcted segments of CVST rat brain
mRNA and protein expression levels of HMGB1-RAGE and
their downstream effectors, TNF-α, IL-1β and IL-6 were assessed by
RT-qPCR and western blot analysis, respectively. A significant
downregulation of HMGB1-RAGE, TNF-α, IL-1β and IL-6 mRNA (Fig. 3) and protein (Fig. 4) expression levels was detected in
infarcted segments of the brains of rats treated with rhs-TM
compared with CVST alone (P<0.001). In addition, expression
levels of apoptosis-associated genes and proteins (caspase-3, Bcl-2
and Bax) in infarcted segments were affected by rhs-TM treatment;
however, the differences were not statistically significant when
compared with the sham group (caspase-3 mRNA, P=0.052; Bcl-2 mRNA,
P=0.192; Bax mRNA, P=0.077; caspase-3 protein, P=0.213; Bcl-2
protein, P=0.06; Bax protein, P=0.176).
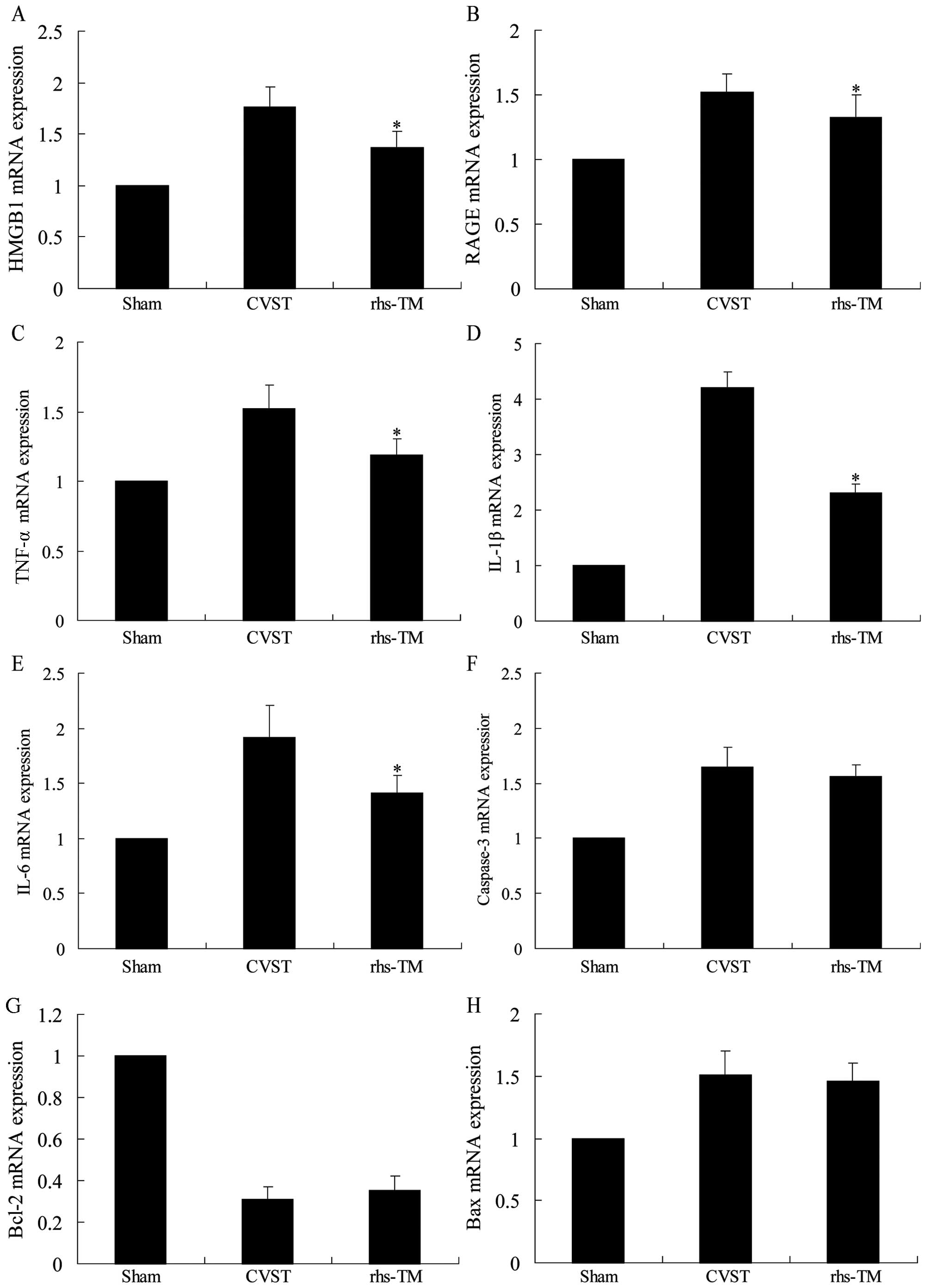 | Figure 3.Effect of rhs-TM on mRNA expression
levels following CVST. The mRNA expression levels of (A) HMGB1, (B)
RAGE, (C) TNF-α, (D) IL-1β and (E) IL-6 were significantly
downregulated in infarcted segments of the brains of rats treated
with rhs-TM compared with CVST alone (P<0.05). The mRNA
expression levels of apoptosis-associated (F) caspase-3, (G) Bcl-2
and (H) Bax in infarcted segments of the brains of rats treated
with rhs-TM were not significantly different to CVST alone. Data
are expressed as the mean ± standard deviation of three independent
experiments. *P<0.05 vs. the CVST alone group. rhs-TM,
recombinant human soluble thrombomodulin; CVST, cerebral venous
sinus thrombosis; HMGB1, high mobility group box 1; RAGE, receptor
for advanced glycation endproducts; TNF-α, tumor necrosis factor-α;
IL, interleukin; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2 associated
X. |
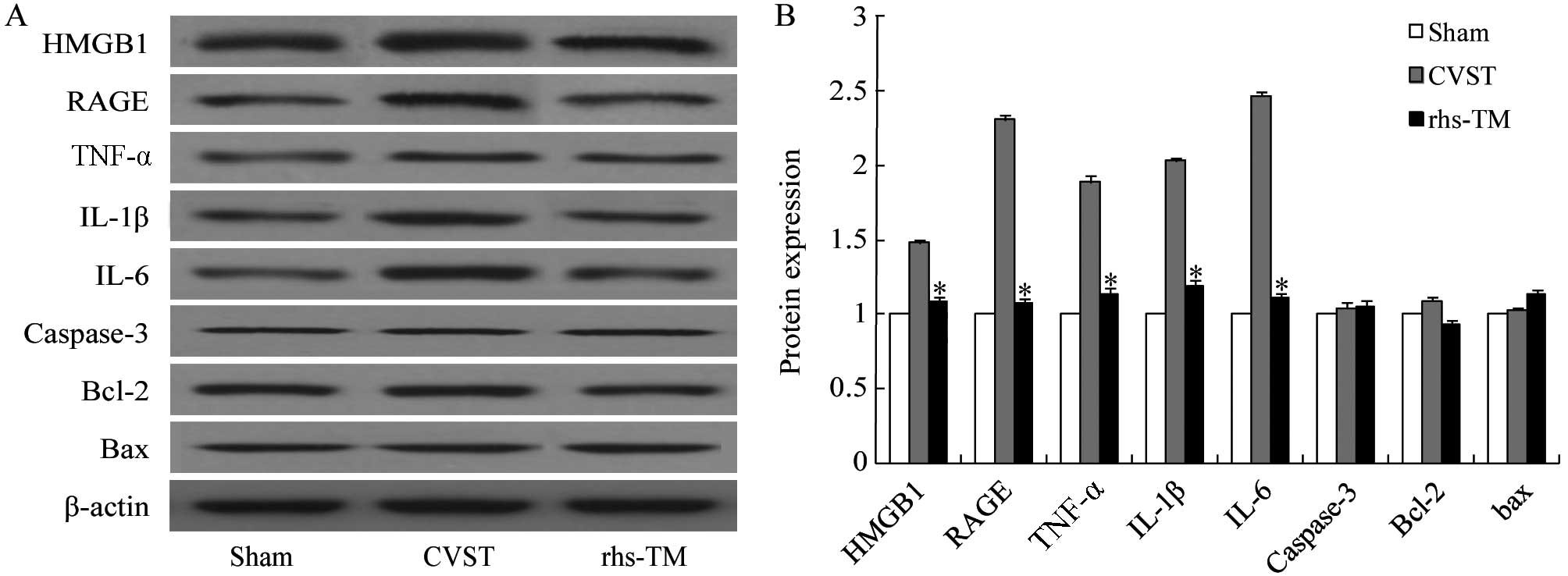 | Figure 4.Effect of rhs-TM on protein expression
levels following CVST. HMGB1, RAGE, TNF-α, IL-1β, IL-6, caspase-3,
Bcl-2 and Bax protein expression levels were (A) detected and (B)
quantified by western blot analysis. The protein expression levels
of HMGB1, RAGE, TNF-α, IL-1β and IL-6 were significantly
downregulated in infarcted segments of the brains of rats treated
with rhs-TM compared with CVST alone (P<0.05). The protein
expression levels of apoptosis-associated caspase-3, Bcl-2 and Bax
in infarcted segments of the brains of rats treated with rhs-TM
were not significantly different to CVST alone. Data are expressed
as the mean ± standard deviation of three independent experiments.
*P<0.05 vs. CVST alone. rhs-TM, recombinant human soluble
thrombomodulin; CVST, cerebral venous sinus thrombosis; HMGB1, high
mobility group box 1; RAGE, receptor for advanced glycation
endproducts; TNF-α, tumor necrosis factor-α; IL, interleukin;
Bcl-2, B-cell lymphoma-2; Bax, Bcl-2 associated X. |
Discussion
CVST is a rare cerebrovascular disease with various
causes. Puerperium, oral hormonal contraception and coagulation
disorders remain the most frequently identified risk factors.
However, the etiology remains unknown in ~15% of cases (1,12).
CVST is associated with protein C deficiency. Protein C interacts
with the thrombin-TM complex and the endothelial protein C receptor
(EPCR), transforming into activated protein C, which may inactivate
factors Va and VIIIa with protein S assistance (13). TM is a membrane protein expressed
by endothelial cells, including in arteries, veins, capillaries and
lymphatic vessels, and in other cell types, including astrocytes,
keratinocytes and neutrophils (14). Previous studies verified that TM
was part of the anticoagulant protein C system; however it has
become clear that, in addition, TM provides anti-inflammatory
protection independently of activated protein C, with or without
activation of thrombin-activated fibrinolysis inhibitor (15).
Recombinant TM contains all the extracellular
domains (rTMD123, also known as ART-123 or Recomodulin) and has
been approved for clinical use in the treatment of disseminated
intravascular coagulation in Japan (16). Previous studies have demonstrated
that recombinant soluble TM protects against tissue damage and
functional deterioration following ischemia in various organs. It
may protect against ischemic brain damage via decreasing the
expression levels of the proinflammatory cytokines TNF, IL-1β and
IL-6 in the infarcted regions following ischemic stroke (10). Su et al (9) demonstrated Solulin partially restored
blood flow following arterial occlusion and reduced ischemia with
no overt indications of bleeding experienced with other
anticoagulants. Ryang et al (10) demonstrated that Solulin decreased
the infarct volume in an artery occlusion model of stroke. In
addition, recombinant soluble TM reduced proinflammatory mediators,
inhibited macrophage recruitment and suppressed HMGB1-RAGE
signaling, to protect against abdominal aortic aneurysm development
(17). Javanmard et al
(18) analyzed the concentrations
of soluble TM and soluble EPCR, using ELISA, of 19 CVST patients
without protein C or protein S deficiency and 53 healthy controls.
The results indicated that the plasma soluble TM level in CVST
patients was reduced compared with controls and the adjusted odds
ratio for CVST associated with low (<10th percentile) levels of
soluble TM was 2.3 (95% confidence interval: 1.29–20.08; P=0.012),
following adjustment for confounding factors.
HMGB1 is a chromatin-binding protein, which may act
as mediator in stroke and other inflammatory diseases (19,20).
Previous studies have demonstrated that HMGB1 binding to RAGE and
Toll-like receptors contributes to inflammation, affecting the
activation of the immune system following stroke (21,22).
The lectin-like domain of TM sequesters HMGB1 protein to prevent it
from engaging RAGE, which may sustain chronic inflammatory
responses and result in tissue damage (23,24).
In addition, TM facilitates the proteolytic cleavage of HMGB1 by
thrombin (25). In the present
study, HMGB1-RAGE mRNA and protein levels were demonstrated to be
upregulated in the infarcted segments of rat brains following CVST.
rhs-TM administration inhibited neurological deficits and decreased
infarction volume, and resulted in a downregulation of the
expression levels of HMGB1-RAGE in the penumbra. In addition, the
expression levels of the proinflammatory cytokines, TNF-α, IL-1β
and IL-6 were decreased. Although a study by Yang et al
(26) demonstrated that apoptosis
is crucial during CVST development, in the present study rhs-TM
administration did not alter the expression levels of the
apoptosis-associated caspase-3, Bcl-2 and Bax in the infarcted
regions of brains of rats subjected to CVST. These results indicate
that the mechanism underlying the protection of the brains of CVST
rats by rhs-TM involves the inhibition of inflammation by blocking
HMGB1 binding to RAGE, and not the regulation of apoptosis.
In conclusion, although the pathogenesis of CVST
remains to be fully elucidated, the results of the present study
suggest that the inflammatory response is critical in CVST. rhs-TM
reduced infarct volume in a model of CVST, potentially via the
inhibition of inflammation. rhs-TM may therefore be a novel
potential strategy for the treatment of CVST; however, further
studies are required to confirm that rhs-TM does not increase the
risk of bleeding in this model.
References
|
1
|
Bousser MG and Ferro JM: Cerebral venous
thrombosis: An update. Lancet Neurol. 6:162–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruiz-Sandoval JL, Chiquete E,
Bañuelos-Becerra LJ, Torres-Anguiano C, González-Padilla C, Arauz
A, León-Jiménez C, Murillo-Bonilla LM, Villarreal-Careaga J,
Barinagarrementería F, et al: Cerebral venous thrombosis in a
Mexican multicenter registry of acute cerebrovascular disease: The
RENAMEVASC study. J Stroke Cerebrovasc Dis. 21:395–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schaller B and Graf R: Cerebral venous
infarction: The pathophysiological concept. Cerebrovasc Dis.
18:179–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guenther G and Arauz A: Cerebral venous
thrombosis: A diagnostic and treatment update. Neurologia.
26:488–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camerlingo M, Salvi P, Belloni G, Gamba T,
Cesana BM and Mamoli A: Intravenous heparin started within the
first 3 hours after onset of symptoms as a treatment for acute
nonlacunar hemispheric cerebral infarctions. Stroke. 36:2415–2420.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esmon CT and Owen WG: Identification of an
endothelial cell cofactor for thrombin-catalyzed activation of
protein C. Proc Natl Acad Sci USA. 78:2249–2252. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lane DA, Philippou H and Huntington JA:
Directing thrombin. Blood. 106:2605–2612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conway EM, Van de Wouwer M, Pollefeyt S,
Jurk K, Van Aken H, De Vriese A, Weitz JI, Weiler H, Hellings PW,
Schaeffer P, et al: The lectin-like domain of thrombomodulin
confers protection from neutrophil-mediated tissue damage by
suppressing adhesion molecule expression via nuclear factor kappaB
and mitogen-activated protein kinase pathways. J Exp Med.
196:565–577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su EJ, Geyer M, Wahl M, Mann K, Ginsburg
D, Brohmann H, Petersen KU and Lawrence DA: The thrombomodulin
analog Solulin promotes reperfusion and reduces infarct volume in a
thrombotic stroke model. J Thromb Haemost. 9:1174–1182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryang YM, Dang J, Kipp M, Petersen KU,
Fahlenkamp AV, Gempt J, Wesp D, Rossaint R, Beyer C and Coburn M:
Solulin reduces infarct volume and regulates gene-expression in
transient middle cerebral artery occlusion in rats. BMC Neurosci.
12:1132011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Z, Huang PL, Panahian N, Dalkara T,
Fishman MC and Moskowitz MA: Effect of cerebral ischemia in mice
deficient in neuronal nitric oxide synthase. Science.
265:1883–1885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weimar C: Diagnosis and treatment of
cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep.
14:4172014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esmon CT: The protein C pathway. Chest.
124:(Suppl 3). 26S–32S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wenzel J, Assmann JC and Schwaninger M:
Thrombomodulin-a new target for treating stroke at the crossroad of
coagulation and inflammation. Curr Med Chem. 21:2025–2034. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van de Wouwer M, Plaisance S, De Vriese A,
Waelkens E, Collen D, Persson J, Daha MR and Conway EM: The
lectin-like domain of thrombomodulin interferes with complement
activation and protects against arthritis. J Thromb Haemost.
4:1813–1824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito T and Maruyama I: Thrombomodulin:
Protectorate God of the vasculature in thrombosis and infiammation.
J Thromb Haemost. 9:(Suppl 1). S168–S173. 2011. View Article : Google Scholar
|
|
17
|
Lai CH, Shi GY, Lee FT, Kuo CH, Cheng TL,
Chang BI, Ma CY, Hsu FC, Yang YJ and Wu HL: Recombinant hman
thrombomodulin suppresses experimental abdominal aortic aneurysms
induced by calcium chloride in mice. Ann Surg. 258:1103–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Javanmard SH, Shahsavarzadeh T and
Saadatnia M: Soluble thrombomodulin and endothelial cell protein C
receptor levels in patients with cerebral venous and sinus
thrombosis. Eur Neurol. 70:156–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrassy M, Volz HC, Igwe JC, Funke B,
Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK,
et al: High-mobility group box-1 in ischemia-reperfusion injury of
the heart. Circulation. 117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abeyama K, Stern DM, Ito Y, Kawahara K,
Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, et
al: The N-terminal domain of thrombomodulin sequesters
high-mobility group-B1 protein, a novel antiinflammatory mechanism.
J Clin Invest. 115:1267–1274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsung A, Klune JR, Zhang X, Jeyabalan G,
Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR and Billiar TR:
HMGB1 release induced by liver ischemia involves Toll-like receptor
4 dependent reactive oxygen species production and calcium-mediated
signaling. J Exp Med. 204:2913–2923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rauvala H and Rouhiainen A: Physiological
and pathophysiological outcomes of the interactions of HMGB1 with
cell surface receptors. Biochim Biophys Acta. 1799:164–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The multiligand receptor RAGE as a progression factor amplifying
immune and inflammatory responses. J Clin Invest. 108:949–955.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito T, Kawahara K, Okamoto K, Yamada S,
Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T and
Maruyama I: Proteolytic cleavage of high mobility group box 1
protein by thrombin-thrombomodulin complexes. Arterioscler Thromb
Vasc Biol. 28:1825–1830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Meng Z, Zhang C, Zhang P and Wang
Q: Establishing a new rat model of central venous sinus thrombosis
and analyzing its pathophysiological and apoptotic changes. J
Neurosci Methods. 203:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|