Introduction
Obesity (OB) is a risk factor for various metabolic
disorders and diseases, such as type II diabetes, hypertension,
cardiovascular disease and pulmonary diseases (1,2).
Development of metabolic syndrome is highly associated with
childhood OB (3). Metabolic
syndrome is a collection of risk factors associated with the
development of type II diabetes and cardiovascular diseases
(4). Dyslipidemia can lead to
cardiovascular diseases (5) and
studies have documented that obese children tend to become obese
adults (6). OB also elevates the
risk of viral infections and influenza (7), and defects in host defense in
response to mycobacterial, amoeba and fungal infections (8,9). A
decreased inflammatory state observed in obese children with
decreased cytokine production may lead to various complications
associated with OB (10).
Peripheral blood mononuclear cells (PBMCs) are the
sentinels of the innate immune system (11) and include monocytes, natural killer
(NK) cells, and T and B lymphocytes (12). Lipopolysaccharide (LPS) derived
from gram negative bacteria is a danger signal recognized by PBMCs.
Detection of LPS results in transcriptional responses involving the
expression of immunological and inflammation-related genes that
serve to clear the infection (13). A previous study demonstrated that
stimulated PBMCs that are macrophage precursors may contribute to
the secretion of cytokines leading to systemic inflammation
(14). Peripheral blood-related
immunological cells serve as a surveillance body to identify
invading microbes (11). Patients
with asthma suffer more persistent and severe lower respiratory
tract symptoms with decreased gene expression and/or synthesis of
interferon (IFN)-α, IFN-β and IFN-λ in epithelial cells and
alveolar macrophages (15,16). PBMCs isolated from asthmatic
children were reported to secrete less IFN-α following in
vitro single stranded viral exposure (17), due to reduced function of toll-like
receptor (TLR)-7 (18).
Interleukin (IL)-1β, IL-8 and nuclear factor (NF)-κB are
interrelated genes that are highly associated during expression in
LPS intoxication (13). In OB,
increased levels of pro-inflammatory cytokines, namely tumor
necrosis factor-α (TNF-α) and IL-6, were observed, which can lead
to metabolic syndrome (19) and
insulin resistance (20). In
addition, higher expression of TLRs and inflammatory cytokines were
observed in overweight subjects who were susceptible to metabolic
syndrome (21). A positive
correlation was observed between levels of IL-6 and TLR-4 in the
serum and monocytes (21).
Activation of the TLR signaling cascade in PBMCs may lead to the
secretion of a number of cytokines resulting in systemic
inflammation (14).
The present study conducted ex vivo
stimulation of PBMCs with LPS to demonstrate the expression pattern
of inflammatory cytokines in subjects with childhood OB.
Materials and methods
Study design
Children with OB symptoms who admitted to the
Changzhou Central Hospital between January 2014 and April 2015 were
screened for this study. Based on body mass index (BMI) calculated
using the formula: Weight (kg)/height (m)2, lipid
profile (LP) status, plasma marker enzymes [aspartate transaminase
(AST) and alanine transaminase (ALT)] and total white blood cell
count in the range 6,000–8,000 cells/µl, subjects were selected in
the age group between 7 and 9 years in the OB and non-obesity (NOB)
groups. This study included 23 children (12 boys and 11 girls) in
the OB group with an age and gender matched NOB group containing 21
children (11 boys and 10 girls). Their respective BMI was
calculated and those exceeding 95th percentile for children and
teens of the same age and sex based on World Health Organization
were categorized as obese. Informed consent was obtained from all
the subjects and their families for participation in the study and
ethical approval was obtained from the ethical Committee of
Changzhou Central Hospital, (Changzhou, China).
Levels of clinical chemistry
parameters in subjects
Clinical chemistry parameters total cholesterol
(TC), triglycerides (TG), high-density lipoprotein (HDL), total
protein (TP) and albumin were analyzed in a fasting (at least 10 h
after dinner) plasma sample of the subjects, prepared from 4 ml of
10 ml venous blood, with heparin used as an anticoagulant. This was
calculated using biochemistry autoanalyzer kits from Biosystems
S.A. (Barcelona, Spain). according to the manufacturer's protocol
using standard solutions provided with the kits. Levels of very
low-density lipoprotein (VLDL) were calculated using the formula
0.2xTG. Levels of low-density lipoprotein (LDL) were calculated
using Friedewald's formula (22)
as follows: LDL=TC-HDL-(0.2xTG).
Assay of reduced glutathione and
malondialdehyde (MDA) in subjects
Plasma reduced glutathione levels were analyzed
according to the method of Ellman (23) and were expressed as mg/dl. MDA was
determined in plasma by the thiobarbituric acid reaction as
described by Ohkawa et al (24).
Assay of marker enzymes in
subjects
Marker enzymes for tissue damage, namely AST and
ALT, were assayed in the plasma of the subjects using enzymatic
method kits (MAK055 and MAK052, Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) according to the manufacturer's protocols.
Absorbance was determined using a UV–VIS spectrophotometer.
Isolation and culture of PBMCs
Part of the collected whole blood was used
immediately for the isolation of live and active PBMCs. Density
gradient ultracentrifugation techniques (20) were used for the isolation of PBMCs
after treatment with red blood cell lysis buffer (Sigma-Aldrich;
Merck Millipore). Collected samples were centrifuged at room
temperature on Ficoll gradient for 30 min at 300 × g. The
separated mononuclear cell layer was twice washed with
phosphate-buffered saline (PBS) and PBMCs were cultured in
RPMI-1640 medium with 10% fetal bovine serum (Equitech Bio, Inc.,
Guangdong, China), 100 U/ml penicillin and 100 µg/ml streptomycin
in a humidified carbon-dioxide incubator with 5% CO2 at 37°C.
PBMC stimulation and RNA
extraction
Isolated PBMCs were seeded in a 24-well cell culture
plate at a concentration of 105 cells per ml. After 18 h
of seeding, 1 µg/ml LPS (Sigma-Aldrich; Merck Millipore) was added
and cells were incubated for 24 h. After incubation, 50% of the
cells were harvested, and supernatants were collected and stored at
−20°C for cytokine analysis. Cytokine levels were determined by
enzyme-linked immunosorbent assay (ELISA), according to the
manufacturer's instructions. Other cells were treated with TRIzol
reagent (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA,
USA) for the preparation and isolation of total RNA for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
amplification of cytokine gene expression. All the experiments were
conducted in triplicate and pooled samples were used for RT-qPCR
analyses.
Assay of cytokines by ELISA
IL-2 (cat. no. 290063; Eton Bioscience Inc., San
Diego, CA, USA), IFN-γ (cat. no. 290051; Eton Bioscience Inc.) and
TNF-α (cat. no. 430205; BioLegend, Inc., San Diego, CA., USA)
levels were assayed using ELISA kits that were purchased from
Dakewe Biotech Company Ltd. (Beijing, China). Manufacturer's
instructions were followed for all assays and final concentrations
were calculated using a standard plot.
Assay of IFN-α and IL-6 by
RT-qPCR
Total RNA was extracted using an RNA extraction kit
(Roche Diagnostics, Basel, Switzerland) from isolated PBMCs and the
quality was measured spectrometrically and the ratio was found to
be between 1.8 and 2.0 at 260/280 nm. After quantification, 200 ng
isolated total RNA was used to construct cDNA by reverse
transcription (RT) using an RT kit (Roche Diagnostics). DNAse
(Roche Diagnostics) was added for the removal of DNA traces. Using
synthesized cDNA as a template, relative quantification (ΔΔCq)
(25) of target genes was done in
a RT-qPCR using SYBR premix reagents (Applied Biosystems, Thermo
Fisher Scientific, Inc.). All the protocols were followed according
to the manufacturer's instructions. glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as a control gene. Thermocycling
conditions were as follows: 94°C for 5 sec of denaturation; 55°C
for 5 sec for annealing and 60°C for 30 sec for extension at 40
cycles. Primers for IFN-α, IL-6 and GAPDH were purchased from
Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China).
Assay of NO
NO in the biological samples was measured indirectly
by measuring the nitrite level in a spectrophotometer at 540 nm
based on the Griess reaction, according to a previous method
(26). A standard curve was
plotted using various concentrations of potassium nitrite.
Statistical analysis. Statistical analysis
was conducted using one-way analysis of variance followed by
lest-significant difference post-hoc test using SPSS version 14.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
BMI
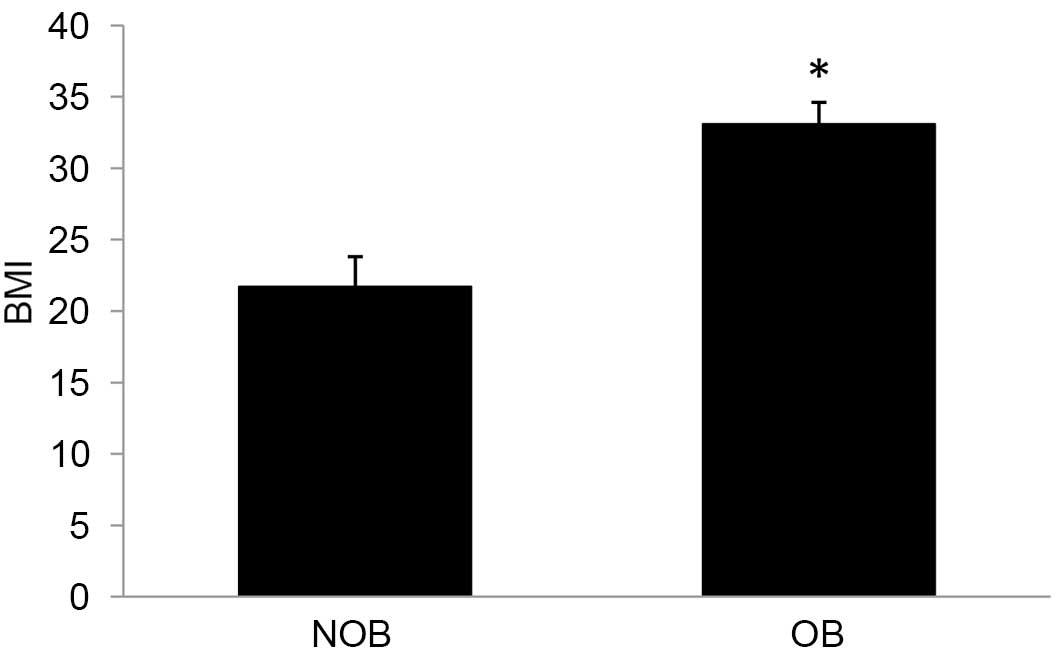
BMI is an index to calculate the severity of
obesity. Subjects are considered obese if BMI>30. A 52.5%
increase in BMI was observed in the OB group (Fig. 1) compared with the matched controls
in the present study.
Plasma levels of clinical chemistry
parameters
Significantly elevated levels of TC, TG, LDL and
VLDL were observed (Table I) and
accounted for 33.0, 58.5, 59.8 and 25.7%, respectively. Conversely,
a 12.95% decrease in the level of HDL was observed in the OB group
compared with the NOB group (Table
I). Similarly, a 10.15% decrease in TP and a 9.94% increase in
albumin levels were observed in the OB group compared with the NOB
group that showed a significant decrease in globulin level in the
OB subjects (Table II). Levels of
plasma lipid peroxidation (LPO) and reduced glutathione (GSH) are
presented in Table III. Levels
of plasma LPO and reduced GSH from LPS exposed cells was quantified
via method described previously (27). An 18.97% increase in LPO and 23.09%
decrease in GSH levels were observed in the OB group compared with
the NOB group. No significant difference was identified in the
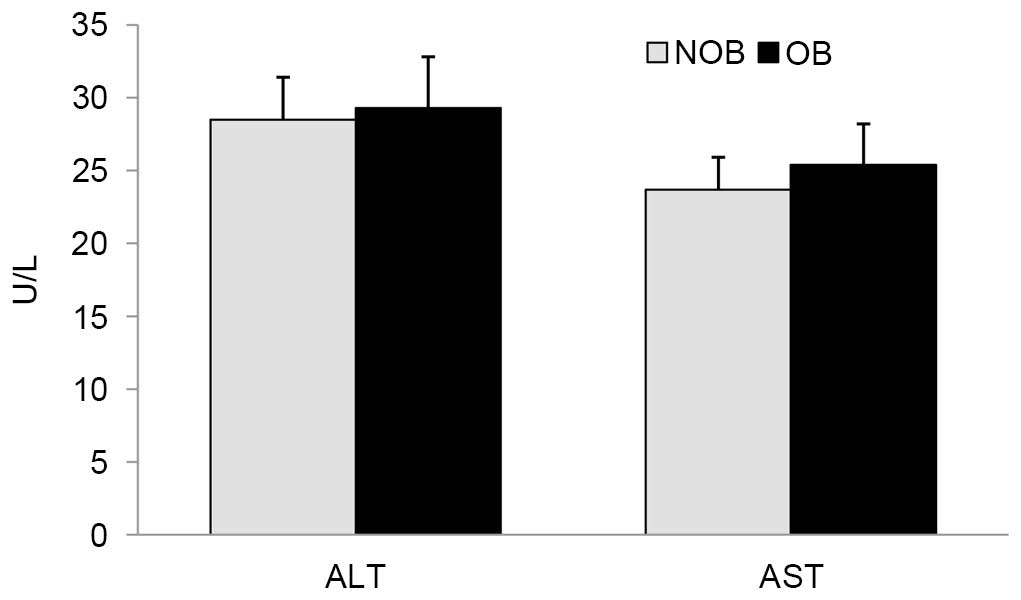
levels of ALT and AST in these groups (Fig. 2).
 | Table I.Levels of lipid profile parameters in
NOB and OB groups. |
Table I.
Levels of lipid profile parameters in
NOB and OB groups.
| Parameter
(mg/dl) | NOB | OB |
|---|
| Total
cholesterol | 131.4±15.3 |
174.8±13.9a |
| Triglycerides | 80.3±9.4 |
127.3±15.4a |
| VLDL | 15.9±1.9 |
25.4±3.1a |
| LDL | 82.6±7.4 |
103.8±10.6a |
| HDL | 52.5±4.3 |
45.7±4.1a |
 | Table II.Levels of total protein, albumin in
NOB and OB groups of children. |
Table II.
Levels of total protein, albumin in
NOB and OB groups of children.
| Parameter
(g/dl) | NOB | OB |
|---|
| Total protein | 7.98±0.64 |
7.17±0.52a |
| Albumin | 3.62±0.26 |
3.98±0.22a |
 | Table III.Levels of plasma LPO and glutathione
in NOB and OB groups of children. |
Table III.
Levels of plasma LPO and glutathione
in NOB and OB groups of children.
| Parameter | NOB | OB |
|---|
| LPO (nM
TBARS/l) | 33.2±2.7 |
39.5±3.4a |
| GSH (mg/dl) | 45.9±6.4 |
35.3±2.8a |
Protein expression of cytokines
Ex vivo stimulation of PBMCs led to secretion of
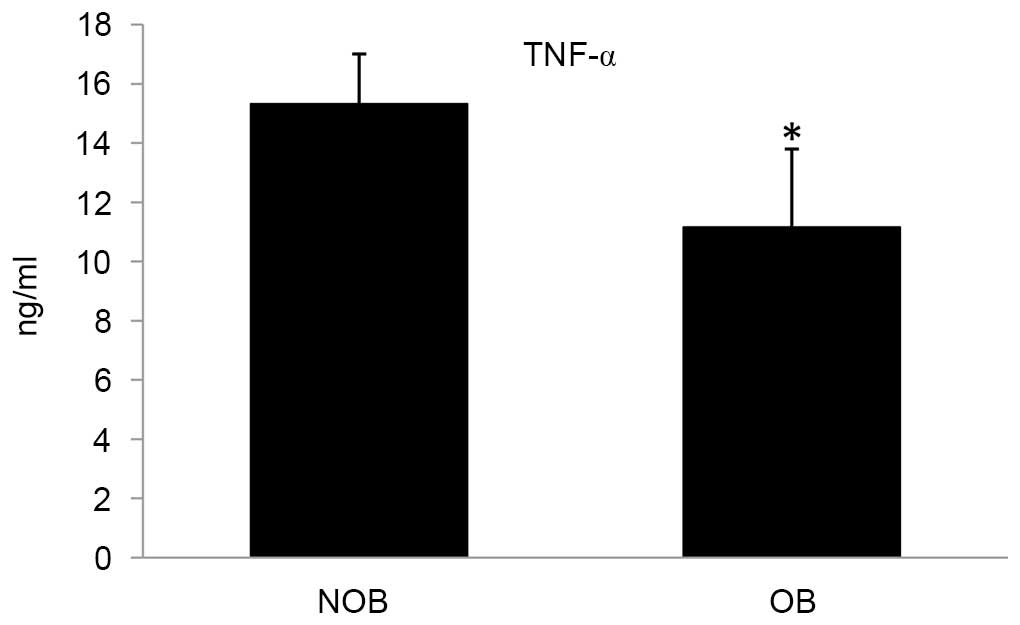
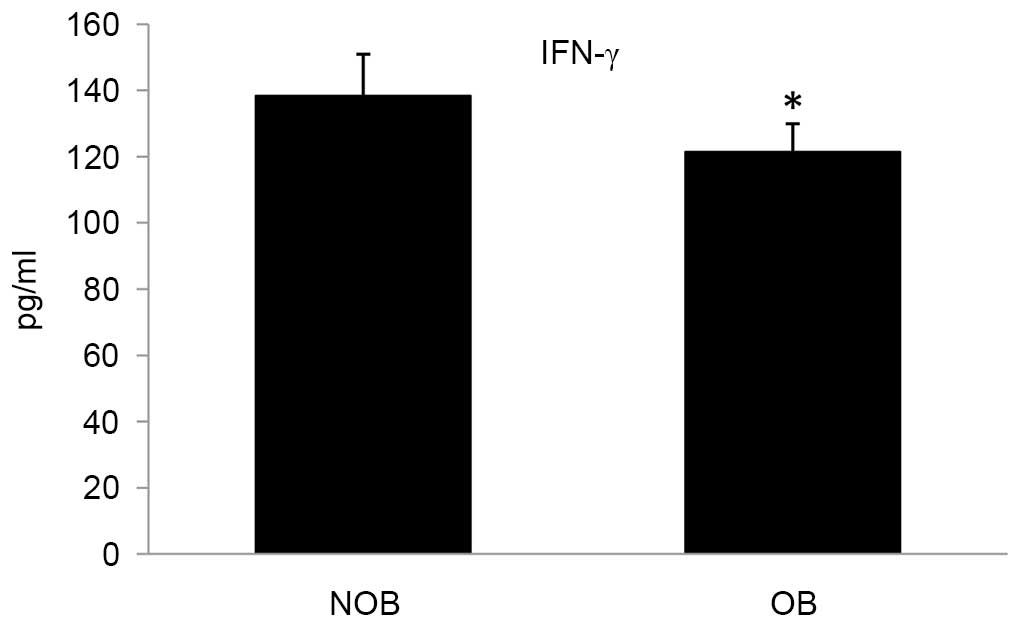
cytokines into the culture medium. In the OB group, the levels of
secretion of the cytokines TNF-α, IL-2 and IFN-γ were significantly
altered compared with that of the NOB group (Figs. 3–5). A 27.15 and 12.28% decrease in the
levels for TNF-α and IFN-γ, respectively; and an increase of 16.75%
was observed in the IL-2 level. Furthermore, a significant decrease
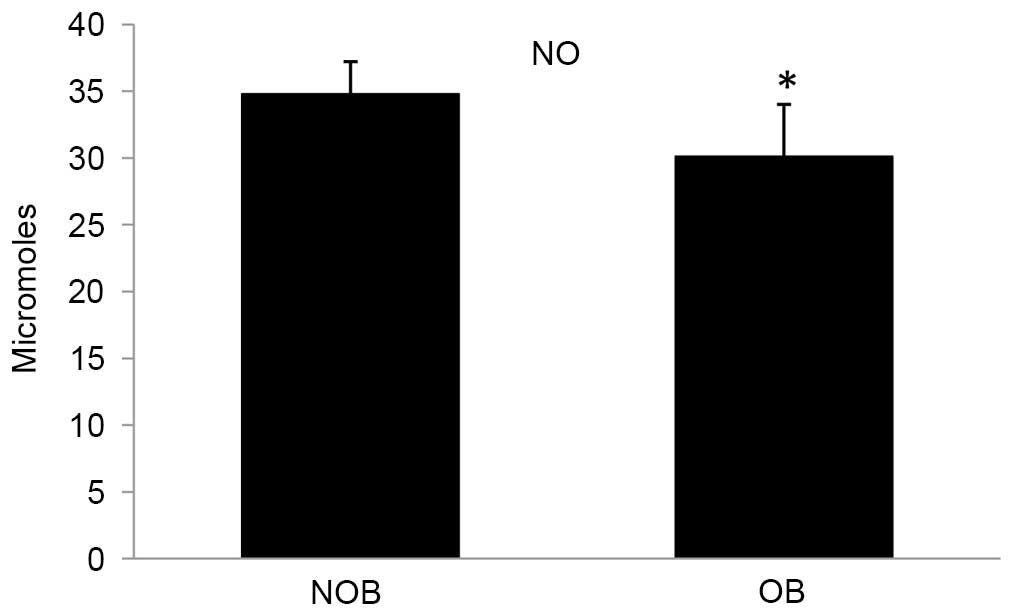
in the NO level (13.51%) was observed in the OB group compared with
the NOB group (Fig. 6).
Cytokine levels detected by
RT-qPCR
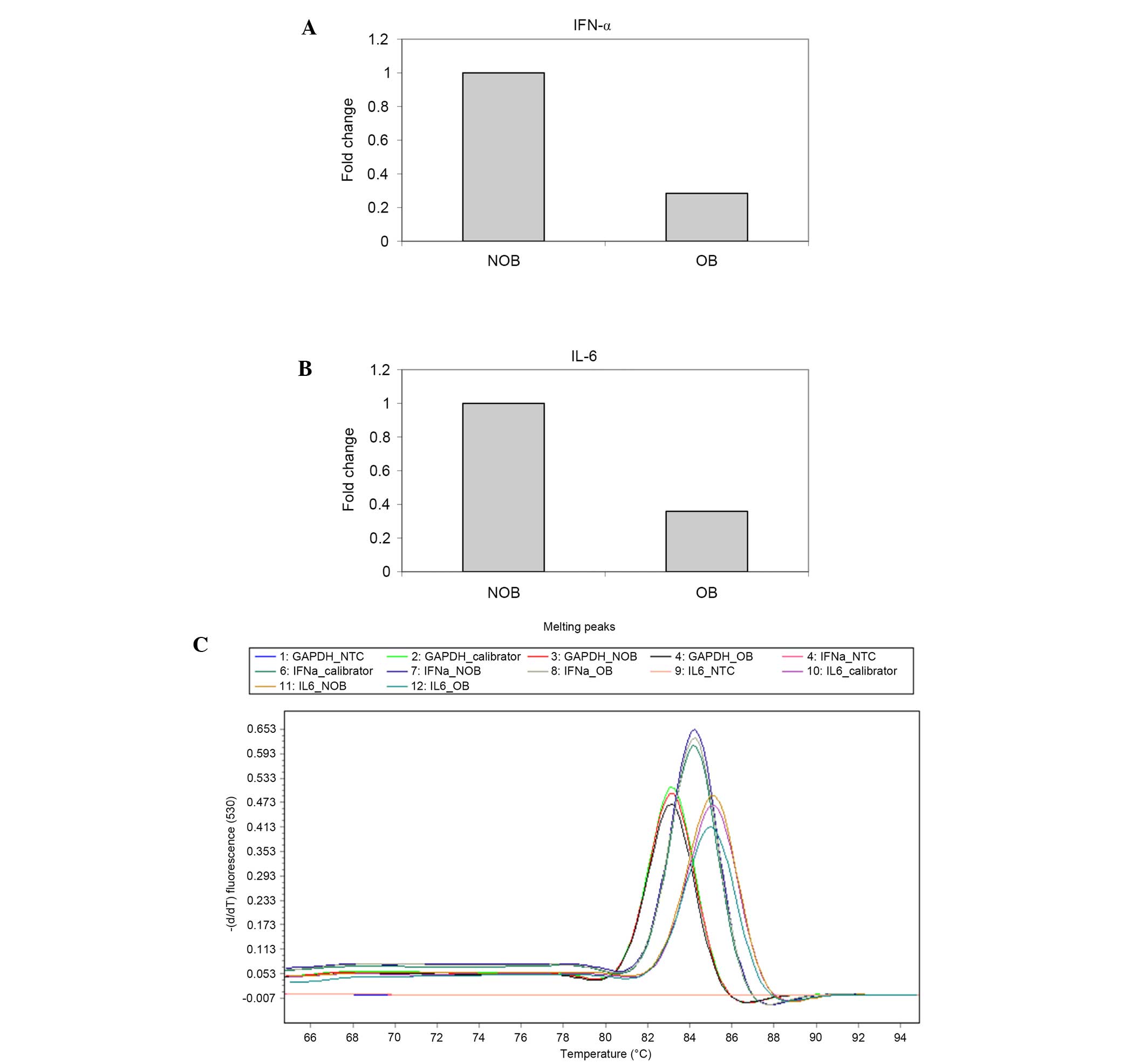
RT-qPCR was conducted to determine the mRNA
expression of IFN-α and IL-6 (Fig.
7A-C) in NOB and OB groups. A 72 and 64% decrease in the
expression levels of IFN-α and IL-6 was observed in the OB group
compared with the NOB group.
Discussion
Oxidative stress was shown to induce the expression
of pro-inflammatory cytokines, such as TNF-α (27). In host tissues, overproduction of
superoxide radicals is associated with diseases, such as cancer and
chronic degenerative diseases (28). Conversely, production of reactive
oxygen species (ROS) in a minimal levels could lead to the
activation of physiological modulations than inducing toxic effects
(29). In the present study, a
compromise in the reducing capacity of the plasma of the subjects,
evidenced by decreased glutathione levels and elevated MDA levels,
depicts the reduced ability of obese subjects to combat
infections.
LPS is a potent stimulant that elicits an innate
immune response (30). LPS-induced
immunological responses mimic the generalized immune response
during sepsis (31). The
LPS-induced immune response during sepsis profoundly reduces the
synthesis of muscle protein in adult animals (32). Septicemia or acute bacterial
intoxication may downregulate gene expression involved in protein
synthesis (33). A decreased level
of total protein in the obese children may underlie the increased
risk of infection, and infection may result in an even greater
decrease in muscle. Furthermore, increased albumin in the plasma
may be interpreted as a decrease in globulins that are directly
involved in immune responses. Due to the decrease in globulins,
such as immunoglobulins, the defense activities of children with OB
could be compromised.
An altered lipid profile with increased total
cholesterol level was observed in the OB group. Increased
cholesterol and decreased HDL-cholesterol (HDL-C) levels could
possibly decrease the level of serum Zn (34). TGs in the serum are positively
correlated with the levels of TNF-α in monocytes (21). In a previous study, an abnormally
low level of Zn was observed in the subjects of non-survivors of
pediatric septic shock (35) and
an extensive downregulation of genes with Zn-related genes
(13). In addition, downregulation
of ~23 Zn-related genes were found in THP-1 cells subjected to LPS
stress (13), which mimics
bacterial infection. Moreover, decreased serum Se and Fe, and
increased serum Cu were observed in obese children (34). From these studies, it is evident
that pediatric OB may modulate Zn-related annotation bearing genes
and possibly favor the chances of infection compared with that in
matched controls.
Neonatal pigs and human infants are similar in
physiology and metabolism (36).
Neonatal pigs exhibit a unique response against acute inflammation
when compared with adults (37).
Inflammatory responses can be mediated by the secretion of
pro-inflammatory cytokines during acute inflammation (38). Administration of LPS or bacterial
peptides or related molecules could upregulate the gene annotations
and its networks associated with inflammation and chemokine-related
biology (13) and is an important
inducer of cytokine secretion (39). Cytokines are important in the
immune response (38). In young
populations, monocytes are the source of circulating cytokines,
such as TNF-α, that are elevated in obese subjects compared with
controls and contribute to co-morbidities (21). TGs in the serum have been
positively correlated with the levels of TNF-α in monocytes
(21). The binding of LPS to its
receptor complex resulted in the activation and nuclear
translocation of interferon regulatory factor-3 and NF-κB,
eventually leading to the expression of TNF-α (40). In the present study, subjects in
the NOB group secreted higher levels of TNF-α compared with
subjects in the OB group in ex vivo experiments. This
suggests compromised sensitivity and signaling of inflammatory
responses in the OB group.
Upregulation of IL-12 and TNF-α can result in
bone-marrow dendritic cell (BMDC) proliferation, initiation of a
CD4+ T cell response and stability of a CD4+
T cell response and a regulation in secretory peptides between DCs
and CD4+ T cells. This in turn increases the secretion
of IL-12 or TNF-α by BMDCs and IFN-γ by CD4+ T cells
leading to immune responses (41).
IFN-α is a soluble cytokine known for its antiviral
and immunotherapeutic properties by regulating a diverse set of
cytokines (42) and linking innate
cell-mediated responses to the adaptive immune response (43). LPS is a natural ligand for the
stimulated secretion of IFN-α. The immunomodulatory effect of IFN-α
against viral infection has been documented in a previous study
(41). Interferons are important
in directing antiviral effects and regulating innate and adaptive
autoimmune systems (44). Alone or
in combination with other cytokines, IFN-α can regulate BMDCs
through direct or indirect pathways. It can also trigger other
cells, such as macrophages and NK cells to secrete more cytokines,
including IFN-α, forming a positive feedback loop (41). IFN-α is an important inducer of the
A3 protein family, cellular DNA cytidine deaminases that function
as inhibitors of viral replication (45). IFN-γ is produced by NK cells and at
later stages by differentiated T cells (46). In the present study, decreased
expression levels of IFN-α in the OB group compared with the NOB
group demonstrated the compromised efficiency to combat viral
infections in obese patients.
IL-2 is a pleiotropic cytokine that acts on T-cells
(47), B-cells (48) and NK cells (49). IL-2 is an important cytokine that
promotes IFN-γ production (50)
and affects Th-1, Th-2, T-reg and Th-17 cell differentiation to
control responsiveness to a range of cytokines after antigen
exposure (51). A significant
increase in IL-2 was identified in the OB group compared with the
NOB group, with the NOB group that confirmed the role of IL-2 in
modulating the related cytokines.
NO is an important physiological messenger molecule
of immunological cells (52). NO
mediates malarial tolerance in endemic populations demonstrated by
increased NO production in children with asymptomatic-malaria
compared with children with severe malaria (53). Exogenous NO supplementation
downregulates the production of TNF-α by polymorphonuclear
leukocytes (PMNs) from patients with type II diabetes following LPS
stimulation and modulation of PMNs response to infection may be
dependent upon NO bioavailability (54). IFN-γ could also modulate NO
production in certain tumors and decreased production of nitrite
could direct the cells to be more prone to apoptosis (55). IFN-γ is a highly potent inducer of
NO in older patients (56). As
more evidence has been published in previous studies (55,56),
it could be concluded that IFN-γ could induce NO production in the
population. In the present study, a decreased level of NO
production could be partially due to the significantly decreased
IFN-γ level in the OB group, which could be correlated from a
decreased response of PBMCs in LPS stimulation (Fig. 5). These results support that
obesity in children could influence the immunological responses
during infection that could lead to life threatening fatal
effects.
In conclusion, the present study has indicated the
clear linkage between status of immune system with obesity and
proper action plan is vital to prevent any mishaps in near
future.
References
|
1
|
Gidding SS, Nehgme R, Heise C, Muscar C,
Linton A and Hassink S: Severe obesity associated with
cardiovascular deconditioning, high prevalence of cardiovascular
risk factors, diabetes mellitus/hyperinsulinemia, and respiratory
compromise. J Pediatr. 144:766–769. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sideleva O, Black K and Dixon AE: Effects
of obesity and weight loss on airway physiology and inflammation in
asthma. Pulm Pharmacol Ther. 26:455–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morrison JA, Friedman LA, Wang P and
Glueck CJ: Metabolic syndrome in childhood predicts adult metabolic
syndrome and type 2 diabetes mellitus 25 to 30 years later. J
Pediatr. 152:201–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanson RL, Imperatore G, Bennett PH and
Knowler WC: Components of the ‘metabolic syndrome’ and incidence of
type 2 diabetes. Diabetes. 51:3120–3127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castelli WP, Garrison MS, Wilson PW,
Abbott RD, Kalousdian S and Kannel WB: Incidence of coronary heart
diseases and lipoprotein cholesterol levels: the Framingham study.
JAMA. 256:2835–2838. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong ND, Bassin S and Deitrick R:
Relationship of blood lipids to anthropometric measures and family
medical history in an ethnically diverse school aged population.
Ethn Dis. 1:351–363. 1991.PubMed/NCBI
|
|
7
|
Akiyama N, Segawa T, Ida H, Mezawa H, Noya
M, Tamez S and Urashima M: Bimodal effects of obesity ratio on
disease duration of respiratory syncytial virus infection in
children. Allergol Int. 60:305–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wieland CW, Florquin S, Chan ED, Leemans
JC, Weijer S, Verbon A, Fantuzzi G and van der Poll T: Pulmonary
Mycobacterium tuberculosis infection in leptin-deficient ob/ob
mice. Int Immunol. 17:1399–1408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo X, Roberts MR, Becker SM, Podd B,
Zhang Y, Chua SC Jr, Myers MG Jr, Duggal P, Houpt ER and Petri WA
Jr: Leptin signaling in intestinal epithelium mediates resistance
to enteric infection by Entamoeba histolytica. Mucosal Immunol.
4:294–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwarzenberg SJ and Sinaiko AR: Obesity
and inflammation in children. Paediatr Respir Rev. 7:239–246. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller SI, Ernst RK and Bader MW: LPS,
TLR4 and infectious disease diversity. Nat Rev Microbiol. 3:36–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luna AL, Acosta-Saavedra LC,
Lopez-Carrillo L, Conde P, Vera E, De Vizcaya-Ruiz A, Bastida M,
Cebrian ME and Calderon-Aranda ES: Arsenic alters monocyte
superoxide anion and nitric oxide production in environmentally
exposed children. Toxicol Appl Pharmacol. 245:244–251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong HR, Odoms K and Sakthivel B:
Divergence of canonical danger signals: The genome-level expression
patterns of human mononuclear cells subjected to heat shock or
lipopolysaccharide. BMC Immunol. 9:242008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dasu MR, Devaraj S, Park S and Jialal I:
Increased toll-like receptor (TLR) activation and TLR ligands in
recently diagnosed type 2 diabetic subjects. Diabetes Care.
33:861–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Contoli M, Message SD, Laza-Stanca V,
Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA,
Parker HL, et al: Role of deficient type III interferon-lambda
production in asthma exacerbations. Nat Med. 12:1023–1026. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wark PA, Johnston SL, Bucchieri F, Powell
R, Puddicombe S, Laza-Stanca V, Holgate ST and Davies DE: Asthmatic
bronchial epithelial cells have a deficient innate immune response
to infection with rhinovirus. J Exp Med. 201:937–947. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gehlhar K, Bilitewski C,
Reinitz-Rademacher K, Rohde G and Bufe A: Impaired virus-induced
interferon-alpha2 release in adult asthmatic patients. Clin Exp
Allergy. 36:331–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roponen M, Yerkovich ST, Hollams E, Sly
PD, Holt PG and Upham JW: Toll-like receptor 7 function is reduced
in adolescents with asthma. Eur Respir J. 35:64–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hivert MF, Sullivan LM, Fox CS, Nathan DM,
D'Agostino RB Sr, Wilson PW and Meigs JB: Associations of
adiponectin, resistin, and tumor necrosis factor-alpha with insulin
resistance. J Clin Endocrinol Metab. 93:3165–3172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardy OT, Kim A, Ciccarelli C, Hayman LL
and Wiecha J: Increased toll-like receptor (TLR) mRNA expression in
monocytes is a feature of metabolic syndrome in adolescents.
Pediatr Obes. 8:e19–e23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Warnick GR, Knopp RH, Fitzpatrick V and
Branson L: Estimating low-density lipoprotein cholesterol by the
Friedewald equation is adequate for classifying patients on the
basis of nationally recommended cutpoints. Clin Chem. 36:15–19.
1990.PubMed/NCBI
|
|
23
|
Ellman GL: Tissue sulfhydryl groups. Arch
Biochem Biophys. 82:70–77. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Ana Biochem. 95:351–358. 1979. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hilbert T, Poth J, Frede S, Klaschik S,
Hoeft A, Baumgarten G and Knuefermann P: Anti-atherogenic effects
of statins: Impact on angiopoietin-2 release from endothelial
cells. Biochem Pharmacol. 86:1452–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakurai T, Kaise T and Matsubara C:
Inorganic and methylated arsenic compounds induce cell death in
murine macrophages via different mechanisms. Chem Res Toxicol.
11:273–283. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goetz ME and Luch A: Reactive species: A
cell damaging rout assisting to chemical carcinogens. Cancer Lett.
266:73–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Webel DM, Finck BN, Baker DH and Johnson
RW: Time course of increased plasma cytokines, cortisol and urea
nitrogen in pigs following intraperitoneal injection of
lipopolysaccharide. J Anim Sci. 75:1514–1520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Agwunobi AO, Reid C, Maycock P, Little RA
and Carlson GL: Insulin resistance and substrate utilization in
human endotoxemia. J Clin Endocrinol Metab. 85:3770–3778. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lang CH, Frost RA, Jefferson LS, Kimball
SR and Vary TC: Endotoxin-induced decrease in muscle protein
synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am
J Physiol Endocrinol Metab. 278:E1133–E1143. 2000.PubMed/NCBI
|
|
33
|
Orellana RA, O'Connor PM, Bush JA,
Suryawan A, Thivierge MC, Nguyen HV, Fiorotto ML and Davis TA:
Modulation of muscle protein synthesis by insulin is maintained
during neonatal endotoxemia. Am J Physiol Endocrinol Metab.
291:E159–E166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azab SF, Saleh SH, Elsaeed WF, Elshafie
MA, Sherief LM and Esh AM: Serum trace elements in obese Egyptian
children: A case-control study. Ital J Pediatr. 40:202014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong HR, Shanley TP, Sakthivel B,
Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman
M, Tofil NM, et al: Genome-level expression profiles in pediatric
septic shock indicate a role for altered zinc homeostasis in poor
outcome. Physiol Genomics. 30:146–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orellana RA, Kimball SR, Nguyen HV, Bush
JA, Suryawan A, Thivierge MC, Jefferson LS and Davis TA: Regulation
of muscle protein synthesis in neonatal pigs during prolonged
endotoxemia. Pediatr Res. 55:442–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yelich MR and Witek-Janusek L: Glucose,
lactate, insulin, and somatostatin responses to endotoxin in
developing rats. Shock. 2:438–444. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borish L and Rosenwasser L: Update on
cytokines. J Allergy Clin Immunol. 97:719–730; quiz 734. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Okeoma CM, Low A, Bailis W, Fan HY,
Peterlin BM and Ross SR: Induction of APOBEC3 in vivo causes
increased restriction of retrovirus infection. J Virol.
83:3486–3495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
May MJ and Ghosh S: Signal transduction
through NF-kappa B. Immunol Today. 19:80–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song Q, Meng Y, Wang Y, Li M, Zhang J, Xin
S, Wang L and Shan F: Maturation inside and outside bone marrow
dendritic cells (BMDCs) modulated by interferon-α (IFN-α). Int
Immunopharmacol. 17:843–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perales C, Beach NM, Gallego I, Soria ME,
Quer J, Esteban JI, Rice C, Domingo E and Sheldon J: Response of
hepatitis C virus to long-term passage in the presence of alpha
interferon: Multiple mutations and a common phenotype. J Virol.
87:7593–7607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lamm D, Brausi M, O'Donnell MA and Witjes
JA: Interferon-alpha in the treatment paradigm for
non-muscle-invasive bladder cancer. Urol Oncol. 32:35.e21–35.e30.
2014. View Article : Google Scholar
|
|
44
|
Perry AK, Chen G, Zheng D, Tang H and
Cheng G: The host type I interferon response to viral and bacterial
infections. Cell Res. 15:407–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Zhang X, Tian C, Wang T, Sarkis
PT, Fang Y, Zheng S, Yu XF and Xu R: Cytidine deaminase APOBEC3B
interacts with heterogeneous nuclear ribonucleoprotein K and
suppresses hepatitis B virus expression. Cell Microbiol.
10:112–121. 2008.PubMed/NCBI
|
|
46
|
Schoenborn JR and Wilson CB: Regulation of
interferon-gamma during innate and adaptive immune responses. Adv
Immunol. 96:41–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rochman Y, Spolski R and Leonard WJ: New
insights into the regulation of T cells by gamma (c) family
cytokines. Nat Rev Immunol. 9:480–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Blackman MA, Tigges MA, Minie ME and
Koshland ME: A model system for peptide hormone action in
differentiation: Interleukin 2 induces a B lymphoma to transcribe
the J chain gene. Cell. 47:609–617. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Henney CS, Kuribayashi K, Kern DE and
Gillis S: Interleukin-2 augments natural killer cell activity.
Nature. 291:335–338. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reem GH and Yeh NH: Interleukin 2
regulates expression of its receptor and synthesis of gamma
interferon by human T lymphocytes. Science. 225:429–430. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: New insights into the complex roles of IL-2 as a broad
regulator of t helper cell differentiation. Current Opinion
Immunology. 23:598–604. 2011. View Article : Google Scholar
|
|
52
|
Bredt DS and Snyder SH: Nitric oxide: A
physiologic messenger molecule. Annu Rev Biochem. 63:175–195. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Anstey NM, Weinberg JB, Hassanali MY,
Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D,
McDonald MI and Granger DL: Nitric oxide in Tanzanian children with
malaria: Inverse relationship between malaria severity and nitric
oxide production/nitric oxide synthase type 2 expression. J Exp
Med. 184:557–567. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Elahi MM and Matata BM: Nitric
oxide-dependent regulation of cytokines release in type-II diabetes
mellitus. ISRN Inflamm. 2013:5310262013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ghosh S, Bandyopadhyay S, Mukherjee K,
Mallick A, Pal S and Mandal C, Bhattacharya DK and Mandal C:
O-acetylation of sialic acids is required for the survival of
lymphoblasts in childhood acute lymphoblastic leukemia (ALL).
Glycoconj J. 24:17–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Faulkner H, Turner J, Kamgno J, Pion SD,
Boussinesq M and Bradley JE: Age- and infection intensity-dependent
cytokine and antibody production in human trichuriasis: The
importance of IgE. J Infect Dis. 185:665–672. 2002. View Article : Google Scholar : PubMed/NCBI
|