Introduction
Osteoporosis is a key public health issue that
affects millions of people worldwide, and predominantly occurs in
postmenopausal women (1,2). It is a chronic progressive disease
characterized by porous bones and the microarchitectural
deterioration of bones (3). Over
the last 20 years, various pharmacological agents, including
alendronate, risedronate, estrogen and glucocorticoids, have been
used for the prevention and treatment of osteoporosis (4–6). The
primary aim of pharmacological therapy is to reduce the risk of
fractures that may occur as a result of osteoporosis.
Estrogen deficiency is one of the main risk factors
for osteoporosis, and has been associated with the enhanced
production of reactive oxygen species (ROS) (7). Excessive levels of ROS (oxidative
stress) have been demonstrated to be an important contributing
factor in the etiology of various degenerative diseases, including
atherosclerosis, osteoporosis and cancer, where the levels of
markers associated with oxidative stress are markedly increased
(8). At the cellular level,
oxidant-induced injury confers a wide range of responses, including
cell proliferation, differentiation arrest and apoptosis, through
the activation of nuclear factor-κB (NF-κB), p53, c-Jun N-terminal
kinase and extracellular signal-related kinase (ERK) signaling
pathways (9). Previous studies
have demonstrated that a strong correlation exists between
oxidative stress and the pathogenesis of osteoporosis (10,11).
Oxidative stress induced by hydrogen peroxide
(H2O2) inhibits the differentiation of mouse
MC3T3-E1 osteoblast precursor cells and M2-10B4 bone marrow cells
(12,13). In addition, aged osteoporotic women
have been demonstrated to exhibit a marked reduction in plasma
antioxidant levels (14), and a
biochemical association between increased oxidative stress and
reduced bone mineral density was observed in aged women and men
(15). Therefore, ROS may be
considered as a target for the prevention of bone density loss, and
may be used as a potential candidate for the treatment of
osteoporosis.
Morindacitrifolia (M. citrifolia), also known
as noni, is a tree in the Rubiaceae coffee family indigenous to the
Hawaiian and Tahitian islands, and anthraquinones, flavonoids,
iridoids and oligosaccharides have been isolated from M.
citrifolia (16). The roots of
M. officinalis, which are known to possess similar
pharmacological effects to M. citrifolia, have been widely
used in traditional Asian medicine to treat rheumatoid arthritis
and diabetes (17). Monotropein is
the active compound isolated from M. officinalis, and its
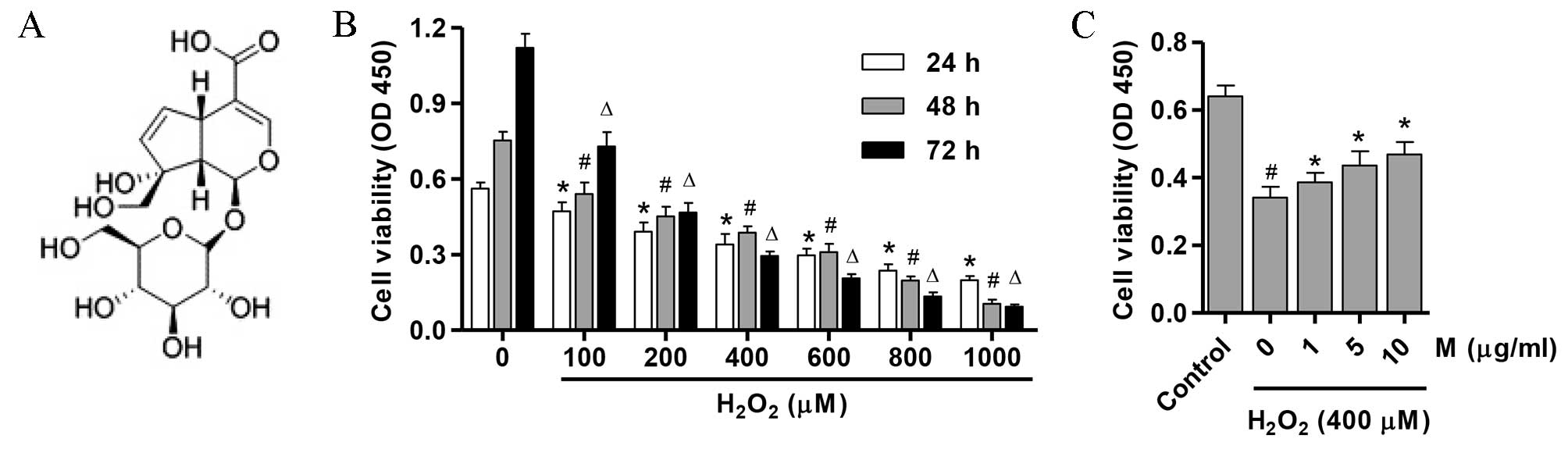
molecular structure is shown in Fig.
1A. Previous studies demonstrated the anti-inflammatory effects
of monotropein in rats with carrageenan-induced edema, in addition
to in RAW 264.7 macrophages (17,18).
However, there is no direct evidence to correlate the
antiosteoporotic effects of monotropein with its antioxidant
effects, and the associated molecular mechanisms remain
unclear.
In the present study, the effects of monotropein on
osteoblast viability and differentiation, and the generation of ROS
in osteoblasts in response to 400 µM H2O2
were investigated. The results demonstrated that monotropein
promoted cell differentiation and protected osteoblasts from
H2O2-induced oxidative damage by inhibiting
the expression of apoptosis-associated markers and the activation
of the NF-κB signaling pathway.
Materials and methods
Cell culture
The current study was approved by the Ethics
Committee of the Xiaoshan Traditional Chinese Medical Hospital
(Hangzhou, China). A total of 30 male Sprague-Dawley rats (age, 3
days; weight, 180 g), purchased from the Experimental Animal Center
of the Xiaoshan Traditional Chinese Medical Hospital (Hangzhou,
China), were housed in the animal facility in individual cages at
25°C and 60–70% humidity with 12 h light/dark cycles and free
access to food and water. The rats were anesthetized by
intraperitoneal injection of 3% sodium pentobarbital (40 mg/kg;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Primary
osteoblasts were prepared according to the methods described
previously (19). Osteoblasts were
isolated from the calvarias of newborn rats. Briefly, five
calvarias were minced and incubated with 0.25% trypsin (Gibco;
Thermo Fisher Scientific, Waltham, MA, USA) for 10 min at 37°C, and
4 mg/ml collagenase I (0.4%; National Biochemicals Corporation,
Twinsburg, OH, USA) for 90 min at 37°C three consecutive times.
Cells isolated from the last four to six digests were cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) containing 10% fetal bovine serum
(FBS; Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) and antibiotics (300,000 U/l
penicillin/streptomycin; Invitrogen; Thermo Fisher Scientific,
Inc.). After reaching 80–90% confluence, the cells were removed
from each flask and pooled together to produce a single osteoblast
culture. Osteoblasts were subsequently collected by centrifugation
(1,000 × g/min for 10 min) at 4°C and resuspended in DMEM
containing 10% FBS and 300,000 U/l penicillin/streptomycin.
H2O2
treatment
Osteoblasts were harvested and randomly divided into
the following 5 groups: The untreated control group; the
H2O2-treated group; and three monotropein
plus H2O2-treated groups, which were treated
with 1, 5 and 10 µg/ml monotropein, respectively. Osteoblasts in
the H2O2 group were incubated for 24 h in
DMEM containing 400 µM H2O2. In the
monotropein plus H2O2-treated groups, the
cells were pre-incubated with the various concentrations of
monotropein for 24 h, prior to incubation with 400 µM
H2O2 for 24 h.
Monotropein
Monotropein (98% purity) was isolated from M.
officinalis (Nanjing Zelang Medical Technological Co., Ltd.,
Nanjing, China). It was dissolved in 150 µl of dimethylsulfoxide
(DMSO) and diluted to the desired concentrations prior to
utilization, with the final concentration of DMSO maintained below
0.5%.
Cell viability assay
Osteoblast viability was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as described previously (20). In brief, cells (5×104
cells/ml) were first seeded in 96-well culture plates and treated
with or without H2O2, in the absence or
presence of monotropein (1, 5 or 10 µg/ml) for 24, 48 and 72 h.
Cell viability was subsequently evaluated using an MTT Cell
Proliferation assay kit (cat. no. 4890-025-K; Wuhan Amyjet
Scientific Co., Ltd., Wuhan, China). The absorbance was measured at
490 nm with an automated Bio-Rad 550 microtiter plate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Analysis of alkaline phosphatase (ALP)
activity
Osteoblasts (1×104 cells/well) were
seeded and cultured in DMEM containing 10% FBS for 4 days, prior to
treatment of the cells with or without H2O2
and in the absence or presence of monotropein (1, 5 and 10 µg/ml)
for a further 2 days. ALP activity was measured at the end of the
treatment period using p-nitrophenylphosphate as a substrate in
0.05 M 2-amino-2-methylpropanol and 2 mM MgCl2 (pH
10.5), according to the methods described previously (21). The amount of p-nitrophenol released
was estimated by measuring the absorbance at 410 nm. The total
protein concentration was determined using the Bradford protein
assay as described previously (22).
Mitochondrial membrane potential
(MMP)
Rhodamine-123 dye (Sigma Aldrich; Merck Millipore)
was used to measure alterations in osteoblast MMP levels. Cells
(1×104 cells/well) were seeded in a 24-well plate.
Following treatment with or without H2O2, in
the absence or presence of monotropein (1, 5 and 10 µg/ml) for 24
h, cells were washed with PBS, incubated with Rho-123 (10 mg/ml)
and subsequently subjected to flow cytometry analysis using the BD
Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA;
excitation wavelength, 480 nm; emission wavelength, 525 nm).
Detection of ROS
Detection of ROS was performed using flow cytometric
analysis as described previously (23). In brief, osteoblasts
(1×104 cells/well) were first seeded in a 24-well plate.
Following treatment with or without H2O2, in
the absence or presence of monotropein (1, 5 and 10 µg/ml) for 24
h, cells were washed with PBS, resuspended in complete medium and
incubated with 0.5 µM dihydrorhodamine 123 (Sigma Aldrich; Merck
Millipore) for 30 min at 37°C. ROS fluorescence intensity was
determined by flow cytometric analysis, with excitation at 490 nm
and emission at 520 nm.
Western blot analysis
Cells were seeded at a density of 1×105
cells/well in 6-well plates, incubated overnight and then treated
with or without H2O2 in the absence or
presence of monotropein (1, 5 and 10 µg/ml) for 24 h. Cells were
lysed using radioimmunoprecipitation buffer supplemented with
protease inhibitor (Beyotime Institute of Biotechnology, Shanghai,
China). The protein concentration was estimated using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). Cell
protein lysates (50 µg) were separated on 10% sodium dodecyl
sulfate-polyacrylamide gels and electroblotted onto a
polyvinylidene fluoride membrane (Roche Diagnostics GmbH, Mannheim,
Germany). Membranes were blocked in fat-free milk overnight at 4°C.
Membranes were then incubated with the following primary antibodies
for 2 h at 25°C: Polyclonal rabbit anti-caspase-3 (dilution, 1:200;
cat. no. ab2302; Abcam, Cambridge, MA, USA); anti-caspase-9
(dilution, 1:500; cat. no. ab69514; Abcam); anti-cyclooxygenase-2
(COX-2; dilution, 1:500; cat. no. ab15191; Abcam); anti-inducible
nitric oxide synthase (iNOS; dilution, 1:800; cat. no. ab3523;
Abcam); anti-NF-κB p65 (dilution, 1:1,000; cat. no. ab16502;
Abcam); and monoclonal mouse anti-sirtuin 1 (SIRT1; dilution,
1:800; cat. no. ab110304; Abcam). Mouse anti-histone protein 3
(dilution, 1:1,000; cat. no. ab1220; Abcam) or anti-GAPDH
(dilution, 1:1,000; cat. no. ab8245; Abcam) monoclonal antibodies
were used as loading controls. After washing, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
(cat. no. A0208) or goat anti-mouse IgG (cat. no. A0216) secondary
antibodies (dilution, 1:1,000; Beyotime Institute of Biotechnology)
at 37°C for 1 h. The blots were visualized using enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA) and signal
intensity was determined using ImageJ software (version 1.46;
National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The protein levels of rat tumor necrosis factor-a
(TNF-α), interleukin (IL)-1β, IL-6 and macrophage-colony
stimulating factor (M-CSF) in osteoblasts were determined using
Quantikine murine-specific sandwich ELISA kits (cat. nos. RTA00,
RLB00, R6000B and MMC00, respectively; R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's instructions.
Absorbance was read at 570 nm using an EL301 Microwell Strip Reader
(Omega Bio-Tek, Inc., Norcross, GA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between groups were analyzed using a two-tailed
Student's t-test. The SPSS statistical software program
(version, 13.0; SPSS, Inc., Chicago, IL, USA) was used for
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of monotropein on the viability
of osteoblasts
To determine whether H2O2 may
exhibit cytotoxic effects on osteoblasts in vitro, the
effect of H2O2 exposure on osteoblast
viability was determined using an MTT assay. Osteoblasts were
treated with 0–1,000 µM H2O2 for 24, 48 and
72 h. As presented in Fig. 1B,
treatment with >400 µM H2O2 significantly
reduced cell viability in a dose and time-dependent manner
(P=0.001). Therefore, 400 µM H2O2 was used in
all subsequent experiments. As presented in Fig. 1C, 1, 5 and 10 µg/ml monotropein
significantly inhibited the H2O2-induced
suppression in osteoblast viability (P=0.011; 13.2±1.63, 27.9±2.65
and 37.5±2.32% viability increase compared with osteoblasts treated
with H2O2 alone, respectively).
Effect of monotropein on the
differentiation of osteoblasts
ALP activation is the earliest marker of osteoblast
differentiation (24). In
addition, the M-CSF cytokine is constitutively expressed during the
growth phase of osteoblasts (25).
As presented in Fig. 2A, a
significant reduction in ALP activity was observed following
incubation of osteoblasts with >200 µM
H2O2 (P<0.05). Notably, pretreatment of
cells with monotropein (1, 5 and 10 µg/ml) for 24 h significantly
attenuated the H2O2-mediated downregulation
of ALP activity (21.7±2.23, 34.2±2.02 and 45.1±1.35% activity
increase, compared with osteoblasts treated with
H2O2 alone, respectively; 1 µg/ml, P=0.005; 5
µg/ml, P=0.0002; 10 µg/ml, P=1.16×10−5; Fig. 2B). In addition, pretreatment with
1, 5 and 10 µg/ml monotropein significantly increased M-CSF
expression compared with H2O2-only treated
osteoblasts (27.1±1.83, 46.7±1.52 and 61.4±1.35%, respectively; 1
µg/ml, P=0.0003; 5 µg/ml, P=2.78×10−5; 10 µg/ml,
P=1.21×10−5; Fig.
2C).
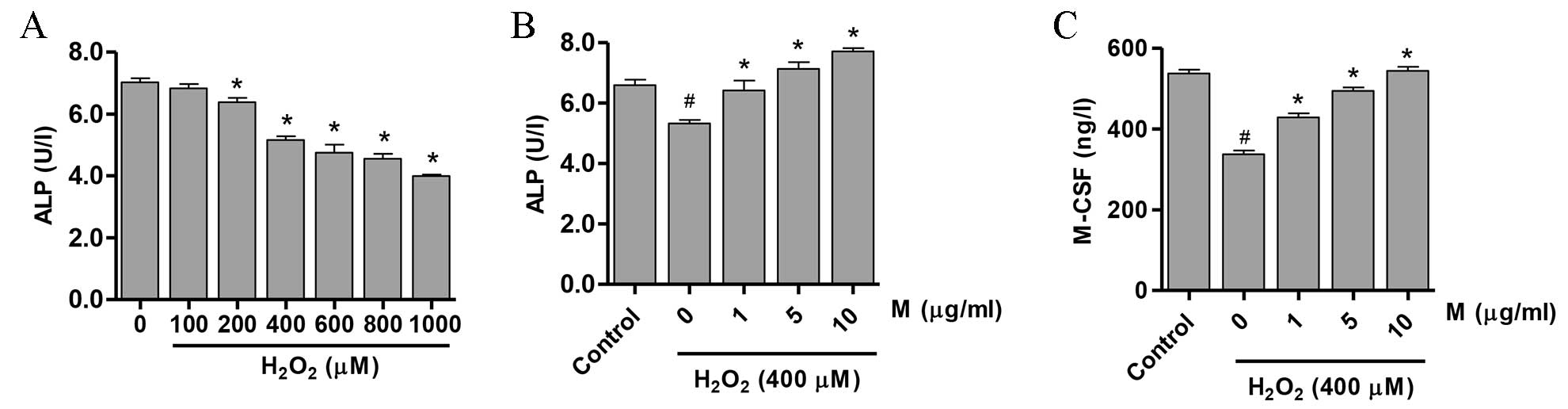 | Figure 2.M induces the differentiation of
H2O2-treated osteoblasts. ALP activity in
osteoblasts following treatment with (A) H2O2
(0–1000 µM) for 24 h demonstrating that ALP activity was reduced in
a dose-dependent manner, and (B) H2O2 (400
µM) following pretreatment with 0, 1, 5 or 10 µg/ml M for 24 h. (C)
M-CSF expression in osteoblasts treated with 0, 1, 5 or 10 µg/ml M
for 24 h prior to exposure to 400 µM H2O2.
Data are presented as the mean ± standard deviation.
#P<0.05, vs. control group; *P<0.05, vs. 0 µM M
group. M, monotropein; ALP, alkaline phosphatase; M-CSF,
macrophage-colony stimulating factor. |
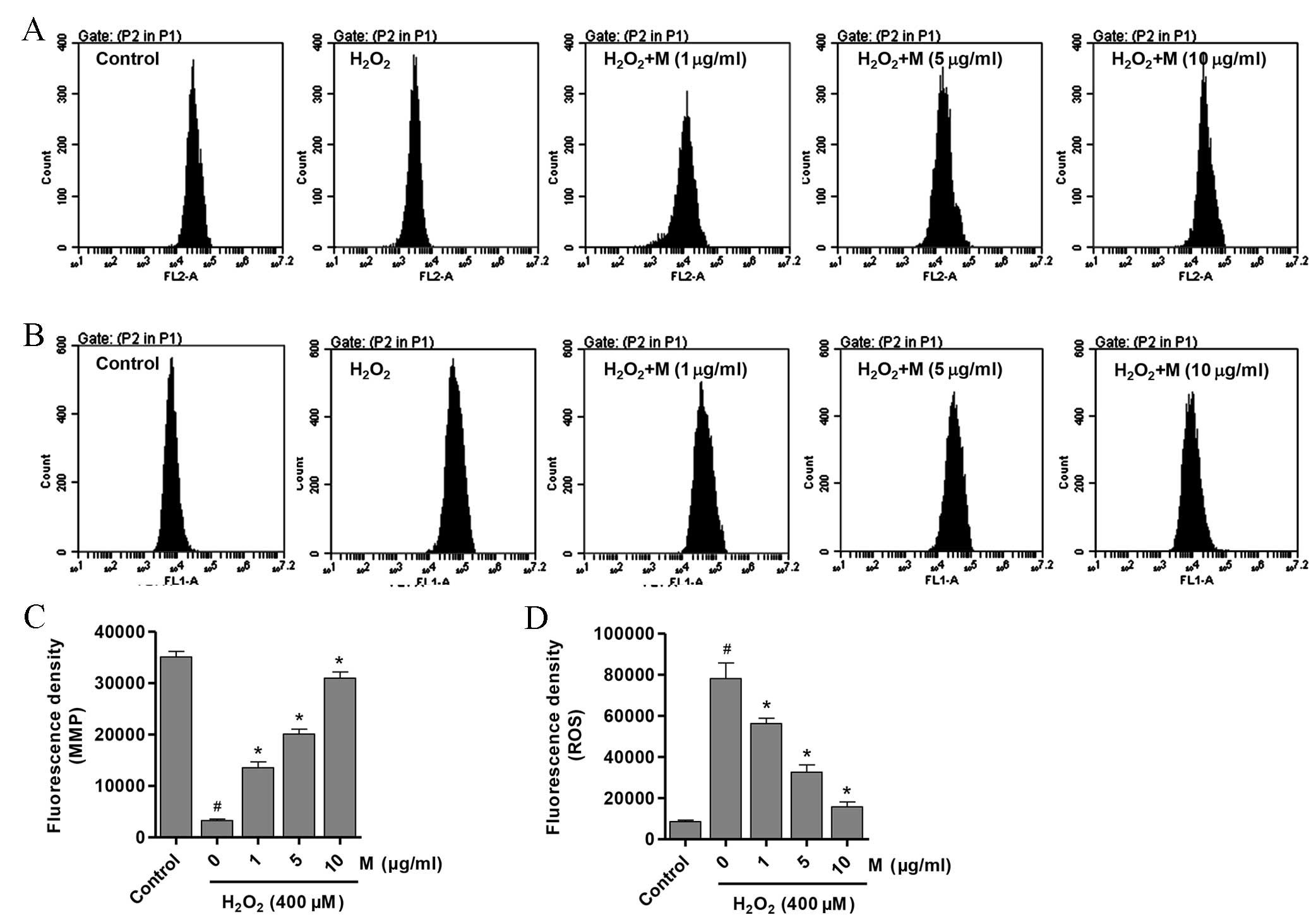
Effect of monotropein on MMP and ROS
levels in H2O2-induced osteoblasts
Destruction of the MMP is the initial process of
mitochondrial-induced apoptosis (26). To elucidate the possible mechanisms
by which monotropein prevented the
H2O2-induced decrease in cell viability and
ALP activity in osteoblasts, the MMP and intracellular ROS levels
in H2O2-treated osteoblasts with or without
monotropein pretreatment were investigated. The MMP level in
H2O2-induced osteoblasts was significantly
decreased compared with that of the untreated control osteoblasts
(Fig. 3A and C;
P=9.98×10−7). However, osteoblasts pretreated with
monotropein (1, 5 and 10 µg/ml) exhibited a significant
dose-dependent increase in MMP levels (Fig. 3A and C; 1 µg/ml,
P=9.59×10−5; 5 µg/ml, P=7.86×10−6; 10 µg/ml,
P=2.72×10−6). MMP levels were increased by 3.1, 5.1 and
8.4-fold following pretreatment with 1, 5 and 10 µg/ml monotropein,
respectively, when compared with that of
H2O2-only treated osteoblasts. Similarly, ROS
generation in monotropein-treated osteoblasts was significantly
reduced in a dose-dependent manner when compared with
H2O2-only treated controls (Fig. 3B and D; 1 µg/ml, P=0.0095; 5 µg/ml,
P=0.0007; 10 µg/ml, P=0.0002). ROS levels were reduced by
27.9±1.26, 58.2±2.16 and 79.7±1.51% following pretreatment with 1,
5 and 10 µg/ml monotropein, compared with
H2O2-only treated osteoblasts.
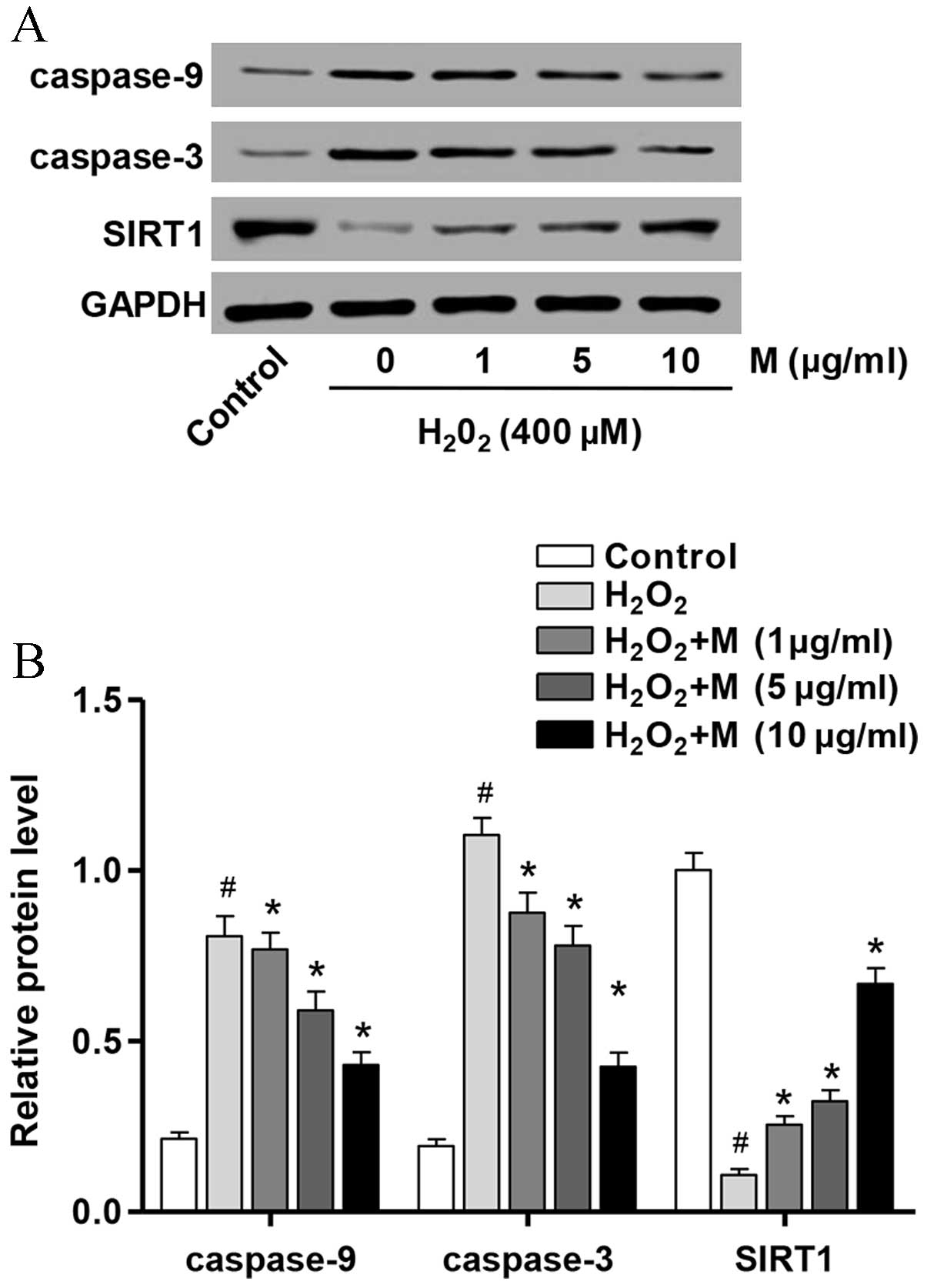
Effect of monotropein on the
expression of apoptosis-associated proteins
In order to investigate the mechanisms underlying
the anti-apoptotic effects of monotropein in
H2O2-induced osteoblasts, the protein
expression levels of apoptosis-associated molecules were determined
by western blot analysis. As presented in Fig. 4, the protein expression levels of
caspase-3 (P=7.58×10−5) and caspase-9
(P=8.57×10−6) were significantly increased following
H2O2 treatment for 24 h compared with
untreated controls, whereas SIRT1 (P=8.14×10−6) protein
expression was significantly reduced. However, pretreatment of
osteoblasts with monotropein (1, 5 and 10 µg/ml) for 24 h
significantly attenuated the H2O2-induced
upregulation of caspase-3 (1 µg/ml, P=0.007; 5 µg/ml, P=0.0018; 10
µg/ml, P=5.77×10−5) and caspase-9 (1 µg/ml, P=0.0489; 5
µg/ml, P=0.009; 10 µg/ml, P=0.0007) protein expression levels and
the H2O2-induced downregulation in SIRT1 (1
µg/ml, P=0.001; 5 µg/ml, P=0.0005; 10 µg/ml,
P=3.89×10−5) protein expression (Fig. 4).
Effect of monotropein on NF-κB p65,
iNOS and COX-2 expression levels
In order to determine whether signaling pathways
downstream of NF-κB p65 were affected by monotropein treatment, the
protein expression levels of NF-κB, iNOS and COX-2 in osteoblasts
following pretreatment with 0, 1, 5 or 10 µg/ml monotropein and
exposure to H2O2 were examined. As presented
in Fig. 5A and B, the protein
expression levels of NF-κB p65 (P=1.35×10−5), iNOS
(P=9.76×10−5) and COX-2 (P=7.89×10−6) were
significantly increased in H2O2-induced
osteoblasts compared with untreated controls. Following monotropein
treatment, osteoblasts exhibited a significant reduction in the
protein expression levels of NF-κB p65 (1 µg/ml, P=0.001; 5 µg/ml,
P=0.0002; 10 µg/ml, P=5.53×10−5), iNOS (1 µg/ml,
P=0.0482; 5 µg/ml, P=0.0162; 10 µg/ml, P=0.0023) and COX-2 (1
µg/ml, P=0.0084; 5 µg/ml, P=0.0017; 10 µg/ml, P=0.0001) compared
with H2O2-only-treated osteoblasts (Fig. 5A and B). These data suggest that
H2O2 may induce osteoblast injury through
activating NF-κB and increasing the expression of downstream
signaling pathways involving iNOS and COX-2.
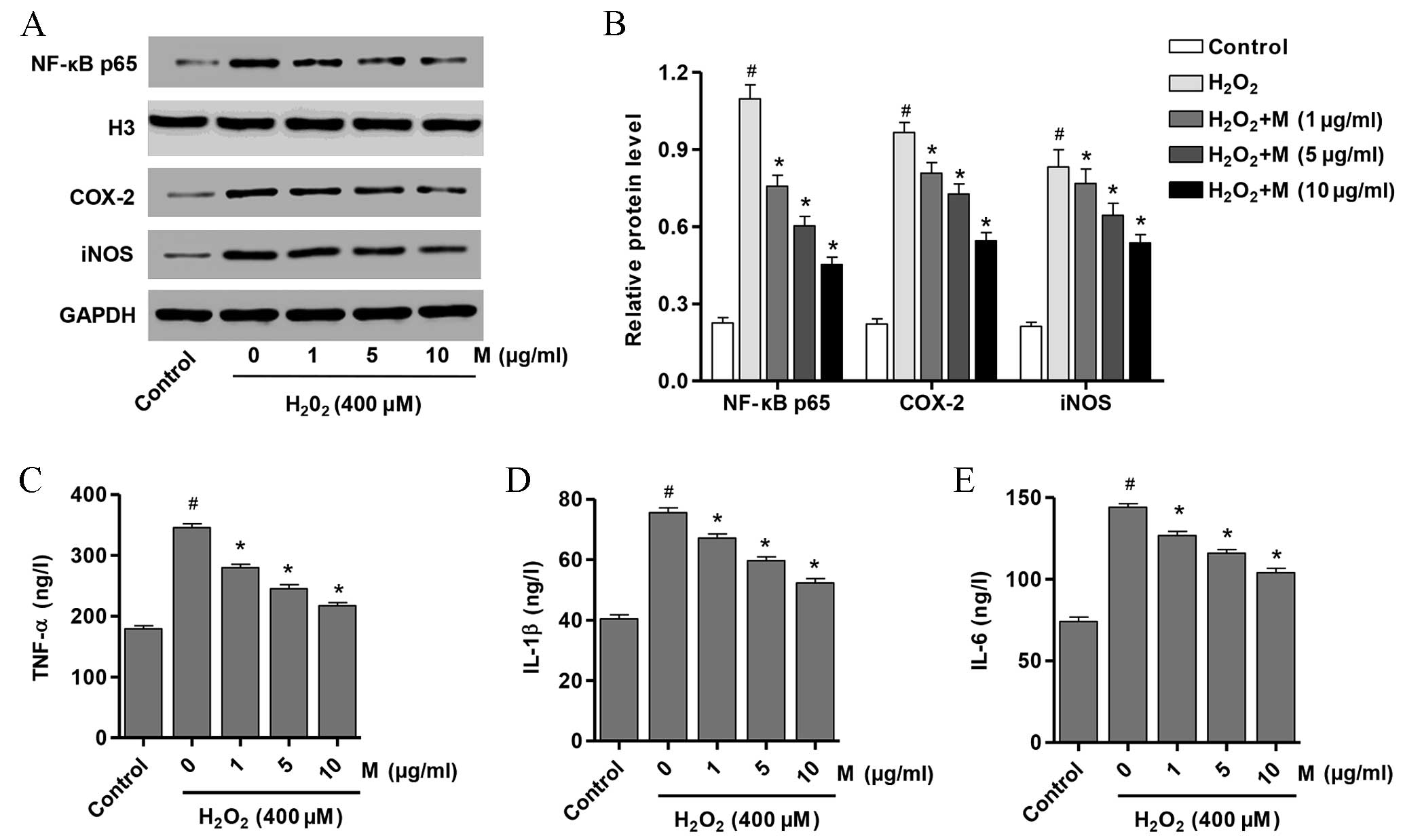 | Figure 5.M suppresses the production of
proinflammatory cytokines from H2O2-induced
osteoblasts. (A) Western blot analysis and (B) quantification of
the protein expression levels of NF-κB p65, COX-2 and iNOS in
osteoblasts pretreated with M (0, 1, 5 and 10 µg/ml) for 24 h prior
to treatment with H2O2 (400 µM)
Quantification of (C) TNF-α, (D) IL-1β and (E) IL-6 protein
expression levels in the same samples as determined by
enzyme-linked immunosorbent assay. Data are presented as the mean ±
standard deviation. #P<0.05 vs. untreated controls;
*P<0.05 vs. the H2O2 only-treated group.
M, monotropein; NF-κB p65, nuclear factorkB p65; COX-2,
cyclooxygenase 2; iNOS, inducible nitric oxide synthase; TNF-α,
tumor necrosis factor α; IL, interleukin; ELISA, enzyme-linked
immunosorbent assay. |
Effect of monotropein on the protein
expression levels of pro-inflammatory mediators
In order to determine whether inflammation was
induced by H2O2, the protein expression
levels of TNF-α, IL-1β and IL-6 in osteoblasts following incubation
with H2O2 and in the presence or absence of
monotropein were determined. As presented in Fig. 5C-E, the protein expression levels
of TNF-α (P=3.59×10−6), IL-1β (P=9.19×10−6)
and IL-6 (P=3.73×10−6) were significantly increased in
H2O2-induced osteoblasts compared with
untreated controls. Following monotropein treatment, osteoblasts
exhibited a significant reduction in TNF-α (1 µg/ml, P=0.0002; 5
µg/ml, P=4.64×10−5; 10 mg/ml, P=9.79×10−6),
IL-1β (1 µg/ml, P=0.0024; 5 µg/ml, P=0.0002; 10 µg/ml,
P=4.76×10−5) and IL-6 (1 µg/ml, P=0.0009; 5 µg/ml,
P=9.96×10−5; 10 µg/ml, P=3.48×10−5) protein
expression levels compared with H2O2-only
treated osteoblasts (Fig. 5C-E).
These data suggest that H2O2 induces
osteoblast injury through stimulating inflammatory responses and
increasing the expression of proinflammatory mediators, including
TNF-α, IL-1β and IL-6.
Discussion
ROS is known to contribute to the pathogenesis of a
number of diseases, such as osteoporosis (27). H2O2 is one of
the major sources of ROS, which disperses across cell membranes and
generates highly reactive hydroxyl radicals that cause various
types of oxidative damage by attacking cellular components
(28).
H2O2-induced apoptosis and inflammation have
been reported to occur in several types of cells including
mesenchymal stem cells, cardiomyocytes and alveolar epithelial
cells (29–31). In the present study, the effect of
different concentrations of H2O2 on
osteoblast viability was investigated. Treatment with
H2O2 for 24 h significantly repressed the
viability of osteoblasts at doses ranging from 100 to 1,000 µM when
compared with untreated controls, which indicates that
H2O2 may inhibit the viability of
osteoblasts. In a previous study, pretreatment with curculigoside,
one of the main bioactive phenolic compounds isolated from the
rhizome of Curculigoorchioides Gaertn, markedly protected
against the H2O2-induced inhibition of
osteoblast viability (32).
Consistent with these observations, pretreatment of osteoblasts
with 1–10 µg/ml monotropein for 24 h in the present study,
significantly suppressed cell injury following exposure to 400 µM
H2O2. Taking these results into account, 400
µM H2O2 was considered to be sufficient for
the induction of oxidative stress, and 1–10 µg/ml monotropein was
selected to examine the effects of monotropein on osteoblast
function.
ALP is widely expressed in various organs, including
the liver, kidney, placenta and bone (33,34).
ALP serves an important role in bone formation and remodeling
through promoting mineralization of the matrix (35). Previous studies observed
H2O2-induced suppression of osteoblast
differentiation in bone marrow stem cells and MC3T3-E1 cells
(36,37). M-CSF, also known as CSF1, is
released by osteoblasts and is involved in the proliferation,
differentiation and survival of bone marrow progenitor cells
(38,39). In the present study, ALP activity
and M-CSF release was observed to be significantly suppressed in
H2O2-induced osteoblasts; the levels of which
recovered following monotropein treatment. These observations
suggest that monotropein may promote osteoblast
differentiation.
Previous studies have demonstrated that activation
of ERK is important for H2O2-induced
apoptosis in cardiomyocytes, endothelial cells and osteoblasts
(40–42). H2O2 treatment
increased Bax expression and led to hyperpolarization of the
mitochondrial membrane potential in MC3T3-E1 mouse osteoblastic
cells (33). This effect was
prevented by treating cells with an inhibitor of the ERK upstream
kinase mitogen activated protein kinase kinase1/2 (PD98059)
(43). Consistent with these
observations, the results presented in the current study
demonstrated that monotropein could significantly reverse the
H2O2-induced reduction in MMP levels and the
H2O2-induced increase in ROS production. In
addition, the protein expression levels of apoptotic markers in
H2O2-induced osteoblasts were investigated,
and the results suggested that the proapoptotic genes, caspase-3
and caspase-9, were significantly increased and the anti-apoptotic
gene SIRT1 was significantly reduced. Notably, treatment with
monotropein significantly reversed the effects of
H2O2 on the expression of
apoptosis-associated proteins in osteoblasts.
NF-κB has been demonstrated to participate in the
regulation of cell survival genes, and mediate the expression of
proinflammatory cytokines, including COX-2, iNOS, TNF-α, IL-1β and
IL-6 (44,45). In the present study, the protein
expression level of nuclear NF-κB p65 was examined, in order to
determine the activity of NF-κB. The results demonstrated that the
protein expression levels of nuclear NF-κB p65, COX-2, iNOS, TNF-α,
IL-1β and IL-6 were significantly increased in
H2O2-induced osteoblasts. In addition, the
expression of these proinflammatory factors was attenuated by
pretreatment of cells with monotropein, which suggests that
monotropein presents a possible approach for the treatment of
various inflammatory diseases.
In conclusion, the results of the present study
demonstrate that monotropein suppresses the functional impairment
of osteoblasts as a result of H2O2-induced
oxidative stress, and its antioxidant properties may be responsible
for these antioxidative effects. Furthermore, the observations of
the present study indicate that the protective effects of
monotropein may be mediated by the inhibition of
apoptosis-associated markers and the activation of the NF-κB
pathway. These results provide a novel insight into the protective
effects of monotropein in osteoblasts via reducing ROS generation,
and suggest that monotropein may be a potential therapeutic agent
for the treatment of osteoporosis.
Acknowledgements
This study was funded by Xiaoshan Science and
Technology Bureau Funds (grant no. 2013304).
References
|
1
|
Reginster JY and Burlet N: Osteoporosis: A
still increasing prevalence. Bone. 38(2): Suppl 1. S4–S9. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landfeldt E, Ström O, Robbins S and
Borgström F: Adherence to treatment of primary osteoporosis and its
association to fractures-the Swedish Adherence Register Analysis
(SARA). Osteoporosis Int. 23:433–443. 2012. View Article : Google Scholar
|
|
3
|
Burghardt AJ, Kazakia GJ, Sode M, de Papp
AE, Link TM and Majumdar S: A longitudinal HR-pQCT study of
alendronate treatment in postmenopausal women with low bone
density: Relations among density, cortical and trabecular
microarchitecture, biomechanics and bone turnover. J Bone Miner
Res. 25:2558–2571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kothawala P, Badamgarav E, Ryu S, Miller
RM and Halbert RJ: Systematic review and meta-analysis of
real-world adherence to drug therapy for osteoporosis. Mayo Clin
Proc. 82:1493–1501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cramer J, Gold D, Silverman S and Lewiecki
E: A systematic review of persistence and compliance with
bisphosphonates for osteoporosis. Osteoporosis Int. 18:1023–1031.
2007. View Article : Google Scholar
|
|
6
|
Siris ES, Harris ST, Rosen CJ, Barr CE,
Arvesen JN, Abbott TA and Silverman S: Adherence to bisphosphonate
therapy and fracture rates in osteoporotic women: Relationship to
vertebral and nonvertebral fractures from 2 US claims databases.
Mayo Clin Proc. 81:1013–1022. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lean JM, Jagger CJ, Kirstein B, Fuller K
and Chambers TJ: Hydrogen peroxide is essential for
estrogen-deficiency bone loss and osteoclast formation.
Endocrinology. 146:728–735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cencioni C, Spallotta F, Martelli F,
Valente S, Mai A, Zeiher AM and Gaetano C: Oxidative stress and
epigenetic regulation in ageing and age-related diseases. Int JMol
Sci. 14:17643–17663. 2013. View Article : Google Scholar
|
|
9
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manolagas SC: From estrogen-centric to
aging and oxidative stress: A revised perspective of the
pathogenesis of osteoporosis. Endocr Rev. 31:266–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sánchez-Rodríguez MA, Ruiz-Ramos M,
Correa-Muñoz E and Mendoza-Núñez VM: Oxidative stress as a risk
factor for osteoporosis in elderly Mexicans as characterized by
antioxidant enzymes. BMC Musculoskelet Disord. 8:1242007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mody N, Parhami F, Sarafian TA and Demer
LL: Oxidative stress modulates osteoblastic differentiation of
vascular and bone cells. Free Radical Biol Med. 31:509–519. 2001.
View Article : Google Scholar
|
|
13
|
Lee DH, Lim BS, Lee YK and Yang HC:
Effects of hydrogen peroxide (H2O2) on alkaline phosphatase
activity and matrix mineralization of odontoblast and osteoblast
cell lines. Cell Biol Toxicol. 22:39–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maggio D, Barabani M, Pierandrei M,
Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R and Cherubini
A: Marked decrease in plasma antioxidants in aged osteoporotic
women: Results of a cross-sectional study. J Clin Endocri Metab.
88:1523–1527. 2003. View Article : Google Scholar
|
|
15
|
Lee YJ, Hong JY, Kim SC, Joo JK, Na YJ and
Lee KS: The association between oxidative stress and bone mineral
density according to menopausal status of Korean women. Obstet Gyn
Sci. 58:46–52. 2015. View Article : Google Scholar
|
|
16
|
Ho CT and Zheng QY: Quality management of
nutraceuticals. Am Chem Soc. 8032002.
|
|
17
|
Choi J, Lee K, Choi MY, Nam JH, Jung HJ,
Park SK and Park HJ: Antinociceptive anti-inflammatory effect of
monotropein isolated from the root of Morinda officinalis. Biol
Pharm Bull. 28:1915–1918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin JS, Yun KJ, Chung KS, Seo KH, Park
HJ, Cho YW, Baek NI, Jang D and Lee KT: Monotropein isolated from
the roots of Morinda officinalis ameliorates proinflammatory
mediators in RAW 264.7 macrophages and dextran sulfate sodium
(DSS)-induced colitis via NF-κB inactivation. Food Chem Toxicol.
53:263–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishizuya T, Yokose S, Hori M, Noda T, Suda
T, Yoshiki S and Yamaguchi A: Parathyroid hormone exerts disparate
effects on osteoblast differentiation depending on exposure time in
rat osteoblastic cells. J Clin Invest. 99:2961–2970. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YK, Hong YJ, Wei M, Wu Y, Huang ZQ,
Chen RZ and Chen HZ: Curculigoside attenuates human umbilical vein
endothelial cell injury induced by H2O2. J Ethnopharmacol.
132:233–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amer J, Goldfarb A and Fibach E: Flow
cytometric measurement of reactive oxygen species production by
normal and thalassaemic red blood cells. Eur J Haematol. 70:84–90.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horii A, Wang X, Gelain F and Zhang S:
Biological designer self-assembling peptide nanofiber scaffolds
significantly enhance osteoblast proliferation, differentiation and
3-D migration. PLoS One. 2:e1902007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mancino AT, Klimberg VS, Yamamoto M,
Manolagas SC and Abe E: Breast cancer increases osteoclastogenesis
by secreting M-CSF and upregulating RANKL in stromal cells. J Surg
Res. 100:18–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cabiscol E, Tamarit J and Ros J: Oxidative
stress in bacteria and protein damage by reactive oxygen species.
Int Microbiol. 3:3–8. 2000.PubMed/NCBI
|
|
29
|
Cremers NA, Lundvig D, van Dalen S,
Schelbergen RF, van Lent PL, Szarek WA, Regan RF, Carels CE and
Wagener FA: Curcumin-induced heme oxygenase-1 expression prevents
H2O2-induced cell death in wild type and heme oxygenase-2 knockout
adipose-derived mesenchymal stem cells. Int J Mol Sci.
15:17974–17999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li K, Yang B and Zhao C: Transforming
growth factor-β-activated kinase 1 enhances H2O2-induced apoptosis
independently of reactive oxygen species in cardiomyocytes. J
Cardiovasc Med (Hagerstown). 15:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei L, Yamaguchi H, Takeuchi R, Matsumoto
H and Shibutani K: Propofol reduces hydrogen peroxide-induced
apoptosis through down-regulating bim expression in alveolar
epithelial cells. Int J Oral Med Sci. 11:274–279. 2013. View Article : Google Scholar
|
|
32
|
Wang Y, Zhao L, Wang Y, Xu J, Nie Y, Guo
Y, Tong Y, Qin L and Zhang Q: Curculigoside isolated from Curculigo
orchioides prevents hydrogen peroxide-induced dysfunction and
oxidative damage in calvarial osteoblasts. Acta Bioch Bioph Sin
(Shanghai). 44:431–441. 2012. View Article : Google Scholar
|
|
33
|
Ðokić-Lišanin M, Pantović V, Jovanović Z,
Samardžić G and Jurišić V: Values of alkaline phosphathase and
their isoenzyme profiles in patients with cancer in respect to bone
and liver metastasis. Arch Oncol. 21:14–16. 2013. View Article : Google Scholar
|
|
34
|
Peters E, Heemskerk S, Masereeuw R and
Pickkers P: Alkaline phosphatase: A possible treatment for
sepsis-associated acute kidney injury in critically ill patients.
Am J Kidney Dis. 63:1038–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sargeant TD, Aparicio C, Goldberger JE,
Cui H and Stupp SI: Mineralization of peptide amphiphile nanofibers
and its effect on the differentiation of human mesenchymal stem
cells. Acta Biomater. 8:2456–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan
G, Cui W, Luo ZP, Pei M, Yang H and He F: Melatonin reverses H2 O2
-induced premature senescence in mesenchymal stem cells via the
SIRT1-dependent pathway. J Pineal Res. 59:190–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu C, Xu D, Wang CY, Jin Y, Liu Q, Meng Q,
Liu KX, Sun HJ and Liu MZ: Alpha-lipoic acid promotes osteoblastic
formation in H2O2-treated MC3T3-E1 cells and prevents bone loss in
ovariectomized rats. J Cell Physiol. 230:2184–2201. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hume DA and MacDonald KP: Therapeutic
applications of macrophage Colony-Stimulating Factor-1 (CSF-1) and
antagonists of CSF-1 receptor (CSF-1R) signaling. Blood.
119:1810–1820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gow DJ, Garceau V, Kapetanovic R, Sester
DP, Fici GJ, Shelly JA, Wilson TL and Hume DA: Cloning and
expression of porcine Colony Stimulating Factor-1 (CSF-1) and
Colony Stimulating Factor-1 Receptor (CSF-1R) and analysis of the
species specificity of stimulation by CSF-1 and Interleukin 34.
Cytokine. 60:793–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun B, Sun GB, Xiao J, Chen RC, Wang X, Wu
Y, Cao L, Yang ZH and Sun XB: Isorhamnetin inhibits
H2O2-induced activation of the intrinsic
apoptotic pathway in H9c2 cardiomyocytes through scavenging
reactive oxygen species and ERK inactivation. J Cell Biochem.
113:473–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Polidoro L, Properzi G, Marampon F,
Gravina GL, Festuccia C, Di Cesare E, Scarsella L, Ciccarelli C,
Zani BM and Ferri C: Vitamin D protects human endothelial cells
from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation.
J Cardiovasc Transl Res. 6:221–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang D, Yang M, Guo B, Cao J, Yang L, Guo
X, Li Y and Gao Z: Zinc inhibits H(2)O(2)-induced MC3T3-E1 cells
apoptosis via MAPK and PI3K/AKT pathways. BiolTrace Elem Res.
148:420–429. 2012. View Article : Google Scholar
|
|
43
|
Park BG, Yoo CI, Kim HT, Kwon CH and Kim
YK: Role of mitogen-activated protein kinases in hydrogen
peroxide-induced cell death in osteoblastic cells. Toxicology.
215:115–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peng C, Perera PK, Li YM, Fang WR, Liu LF
and Li FW: Anti-inflammatory effects of Clematis chinensis Osbeck
extract(AR-6) may be associated with NF-κB, TNF-α and COX-2 in
collagen-induced arthritis in rat. Rheumatol Int. 32:3119–3125.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li M, Zhang L, Cai RL, Gao Y and Qi Y:
Lipid-soluble extracts from Salvia miltiorrhiza inhibit production
of LPS-induced inflammatory mediators via NF-κB modulation in RAW
264.7 cells and perform antiinflammatory effects in vivo. Phytother
Res. 26:1195–1204. 2012. View Article : Google Scholar : PubMed/NCBI
|