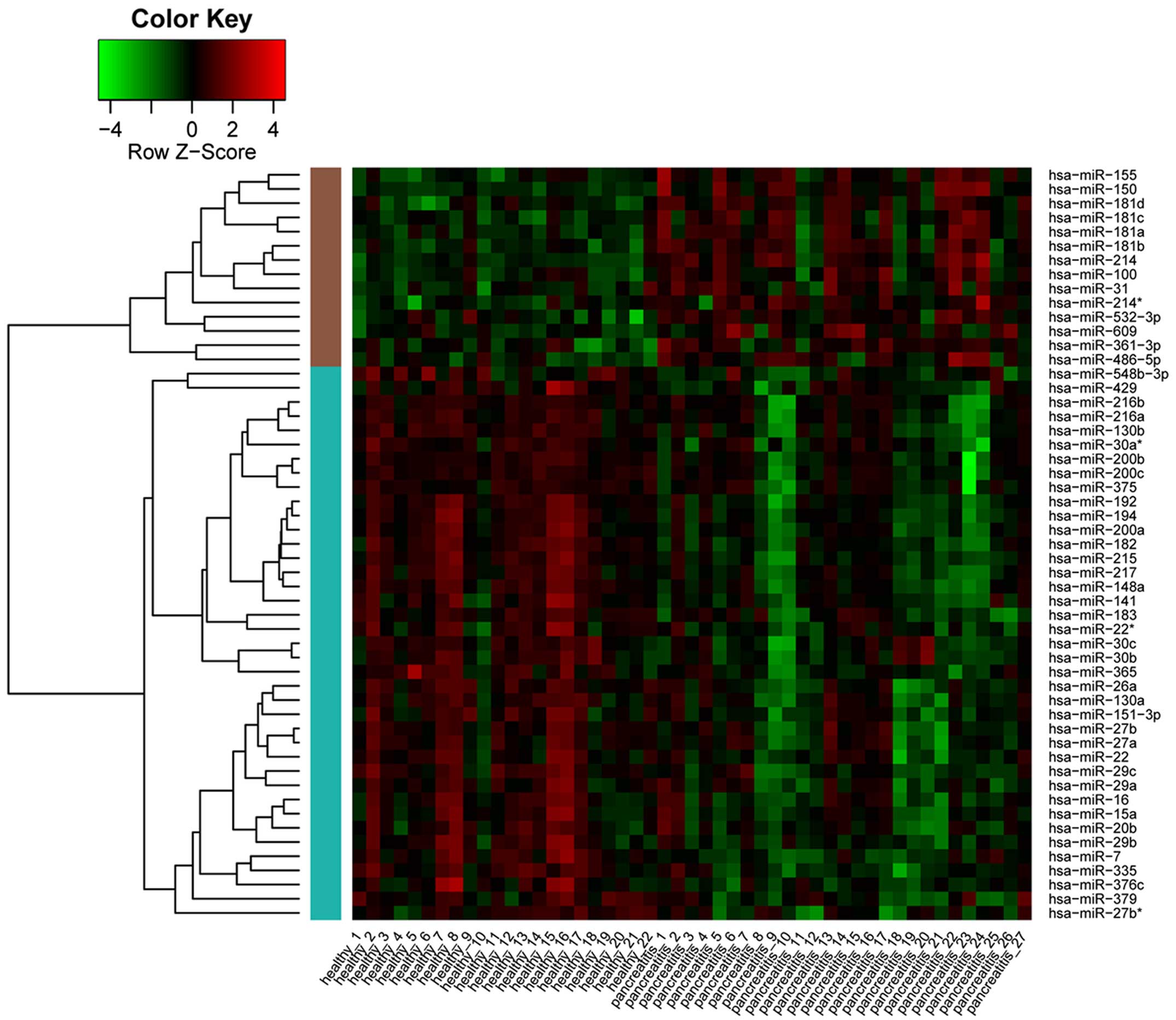
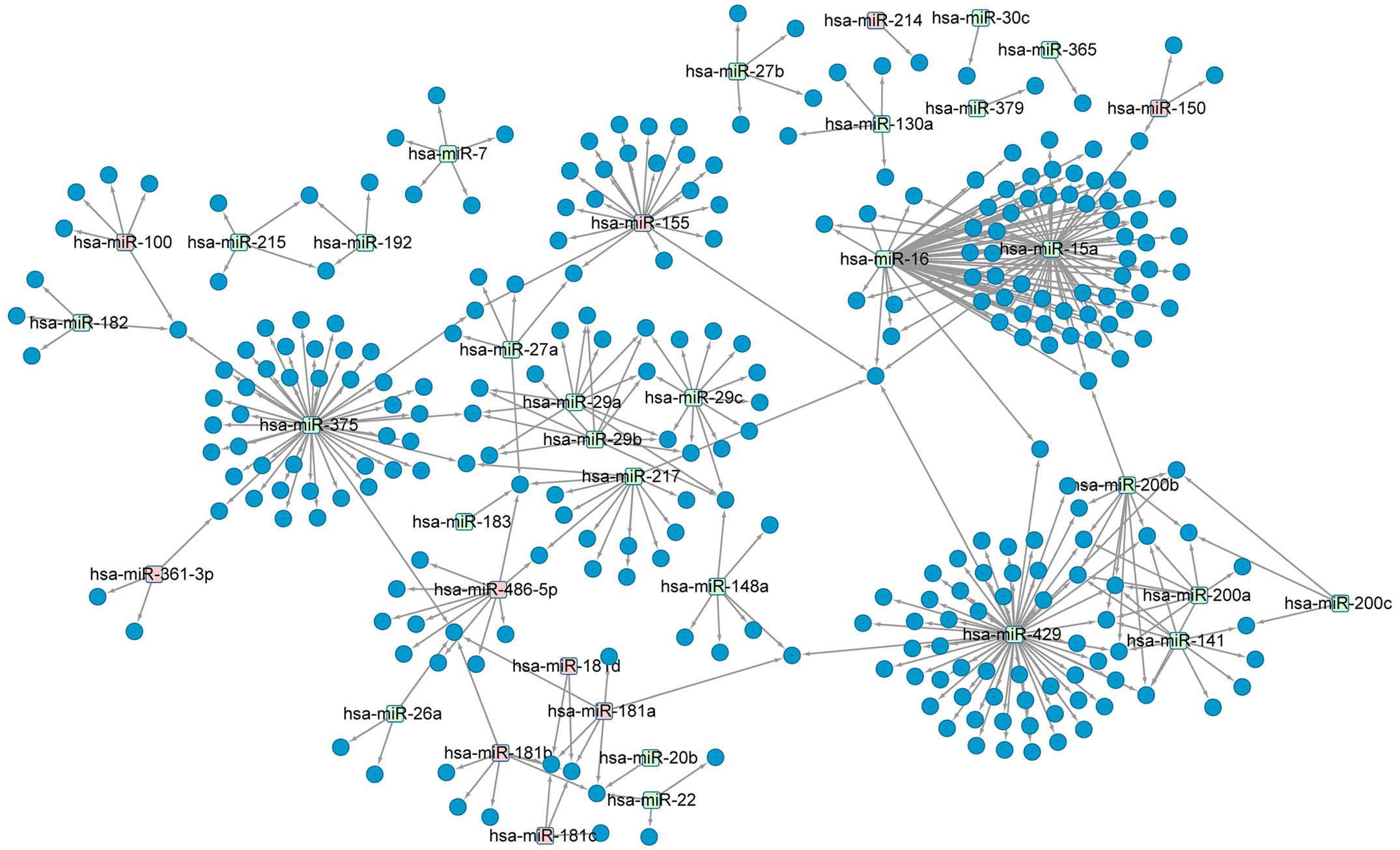
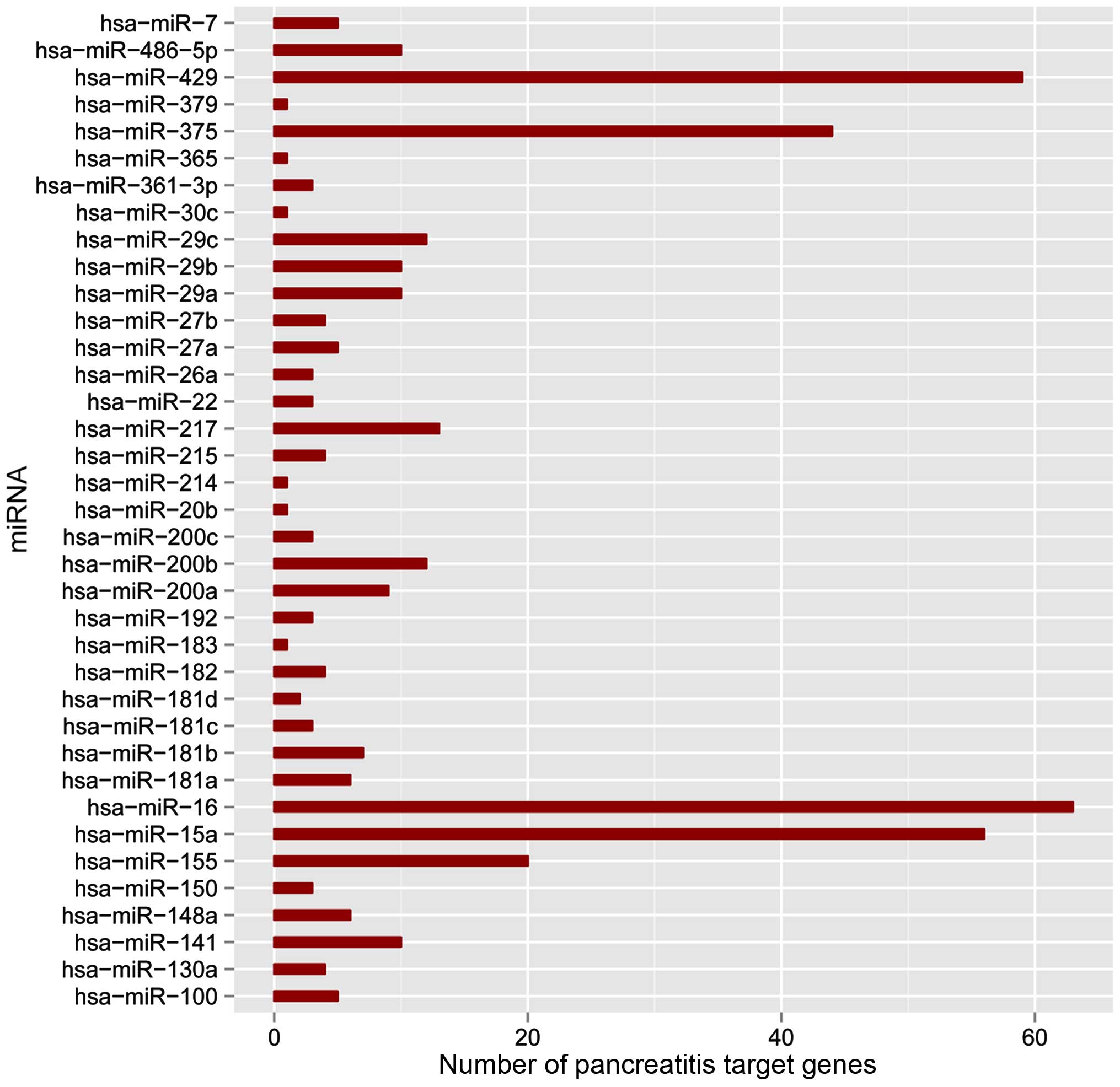
| BP | GO:0010604 | Positive regulation
of macromolecule metabolic process | 80 | MYOD1, PPARA,
HNF1A, PDGFB, BTRC, MITF, PPARG, SNCA, SPI1, FOXO1, TLR4, PDX1,
ZEB1, FOXO3, GLI2, GLI3, IL10, CTNNB1, TGFB2, CCNE1, GATA6, FOXF1,
HMOX1, IFNG, YAP1, MKL1, MYC, IHH, AKT2, IRS2, EGR2, RARG, ESR1,
RB1, PPARGC1A, HMGA1, GTF2H1, ACVR2A, CCND1, EP300, HNF4A, CCND3,
CCND2, JUN, FOXC2, ZFPM2, CLOCK, CSF1, SOX2, SOX9, SRF, WT1, IGF1R,
IL17A, HAND1, AGT, BCL2, POU3F1, RUNX1, DNMT3B, RUNX2, CEBPB, MAFB,
NR4A2, CD276, SMAD3, SMAD2, HDAC4, NOTCH1, SP1, PLK1, ETS1, NOTCH4,
FOXE1, DNMT1, JAK2, TNK2, MTOR, BMP7, KLF4 |
1.20×10−32 |
|
| GO:0009891 | Positive regulation
of biosynthetic process | 67 | PPARA, MYOD1,
HNF1A, PDGFB, PPARG, MITF, SPI1, FOXO1, TLR4, PDX1, ZEB1, FOXO3,
GLI2, GLI3, IL10, CTNNB1, TGFB2, CCNE1, GATA6, FOXF1, HMOX1, IFNG,
YAP1, MKL1, MYC, AKT2, IHH, IRS2, EGR2, RARG, RB1, PPARGC1A, HMGA1,
GTF2H1, EP300, HNF4A, JUN, FOXC2, ZFPM2, CLOCK, SOX2, SOX9, SRF,
WT1, IGF1R, IL17A, HAND1, AGT, PRKAA1, RUNX1, RUNX2, CEBPB, MAFB,
NR4A2, CD276, SMAD3, SMAD2, HDAC4, NOTCH1, SP1, ETS1, NOTCH4,
FOXE1, JAK2, MTOR, BMP7, KLF4 |
9.62×10−28 |
|
| GO:0010557 | Positive regulation
of macromolecule biosynthetic process | 64 | PPARA, MYOD1,
HNF1A, PDGFB, PPARG, MITF, SPI1, FOXO1, TLR4, PDX1, FOXO3, ZEB1,
GLI2, GLI3, IL10, CTNNB1, TGFB2, CCNE1, GATA6, FOXF1, HMOX1, IFNG,
YAP1, MKL1, MYC, AKT2, IHH, IRS2, EGR2, RARG, RB1, PPARGC1A, HMGA1,
GTF2H1, EP300, HNF4A, JUN, FOXC2, ZFPM2, CLOCK, SOX2, SOX9, SRF,
WT1, IGF1R, IL17A, HAND1, RUNX1, RUNX2, CEBPB, MAFB, NR4A2, CD276,
SMAD3, SMAD2, HDAC4, NOTCH1, SP1, ETS1, NOTCH4, FOXE1, MTOR, BMP7,
KLF4 |
8.77×10−27 |
|
| GO:0031328 | Positive regulation
of cellular biosynthetic process | 65 | PPARA, MYOD1,
HNF1A, PDGFB, PPARG, MITF, SPI1, FOXO1, TLR4, PDX1, ZEB1, FOXO3,
GLI2, GLI3, IL10, CTNNB1, CCNE1, GATA6, FOXF1, HMOX1, IFNG, YAP1,
MKL1, MYC, AKT2, IHH, IRS2, EGR2, RARG, RB1, PPARGC1A, HMGA1,
GTF2H1, EP300, HNF4A, JUN, FOXC2, ZFPM2, CLOCK, SOX2, SOX9, SRF,
WT1, IGF1R, IL17A, HAND1, AGT, RUNX1, RUNX2, CEBPB, MAFB, NR4A2,
CD276, SMAD3, SMAD2, HDAC4, NOTCH1, SP1, ETS1, NOTCH4, FOXE1, JAK2,
MTOR, BMP7, KLF4 |
1.79×10−26 |
|
| GO:0010628 | Positive regulation
of gene expression | 60 | PPARA, MYOD1,
HNF1A, PPARG, MITF, SPI1, FOXO1, PDX1, FOXO3, ZEB1, GLI2, GLI3,
IL10, CTNNB1, CCNE1, GATA6, FOXF1, IFNG, YAP1, MKL1, MYC, IHH,
RARG, EGR2, ESR1, RB1, HMGA1, PPARGC1A, GTF2H1, EP300, HNF4A, JUN,
FOXC2, ZFPM2, CLOCK, CSF1, SOX2, SOX9, SRF, WT1, IL17A, HAND1,
POU3F1, RUNX1, RUNX2, DNMT3B, CEBPB, MAFB, NR4A2, SMAD3, SMAD2,
HDAC4, NOTCH1, SP1, ETS1, NOTCH4, FOXE1, DNMT1, BMP7, KLF4 |
3.06×10−26 |
| CC | GO:0031981 | Nuclear lumen | 69 | BACH1, MYOD1,
RNASEL, NBN, HNF1A, CDC14B, EZH2, FOXO1, ZEB1, GLI3, IQGAP1,
CTNNB1, PNN, PRIM1, CCNE1, GATA6, FOXF1, HMOX1, YAP1, MYB, MYC,
TWIST2, ZBTB20, RARG, UBE2I, RB1, PPARGC1A, HMGA1, GTF2H1, CCND1,
EP300, HNF4A, CFL2, JUN, MATR3, CLOCK, ME1, MTDH, MCL1, HOXA11,
SOX2, SOX9, SRF, WT1, HAND1, NPAT, POU3F1, WIPF1, DNMT3B, RUNX2,
DNMT3A, CEBPB, TBX2, MAFB, PHB, NLK, SMAD3, SMAD2, ATM, POLD3,
RPS6KA5, HDAC4, SNAI3, SP1, PLK1, TDG, JAK2, RERE, KLF4 |
1.25×10−14 |
|
| GO:0005667 | Transcription
factor complex | 26 | MYOD1, HNF1A,
HOXA11, SOX2, ZEB1, CTNNB1, GATA6, FOXF1, POU3F1, YAP1, RUNX2,
TWIST2, RARG, TBX2, MAFB, SMAD3, SMAD2, RB1, HMGA1, GTF2H1, SNAI3,
EP300, HNF4A, JUN, KLF4, CLOCK |
3.85×10−14 |
|
| GO:0005654 | Nucleoplasm | 49 | MYOD1, NBN,
HNF1A, EZH2, FOXO1, ZEB1, GLI3, CTNNB1, PNN, PRIM1, CCNE1, GATA6,
FOXF1, YAP1, MYC, TWIST2, RARG, UBE2I, RB1, HMGA1, PPARGC1A,
GTF2H1, CCND1, EP300, HNF4A, JUN, CLOCK, MCL1, HOXA11, SOX2, WT1,
HAND1, NPAT, POU3F1, RUNX2, TBX2, MAFB, PHB, SMAD3, SMAD2, ATM,
RPS6KA5, POLD3, HDAC4, SNAI3, PLK1, TDG, KLF4, RERE |
1.44×10−12 |
|
| GO:0031974 | Membrane-enclosed
lumen | 75 | BACH1, MYOD1,
RNASEL, NBN, HNF1A, PDGFB, CDC14B, EZH2, FOXO1, ZEB1, GLI3, IQGAP1,
CTNNB1, TGFB2, PNN, PRIM1, CCNE1, GATA6, FOXF1, HMOX1, YAP1, MYB,
MYC, TWIST2, ZBTB20, RARG, UBE2I, RB1, PPARGC1A, HMGA1, GTF2H1,
TIMM8A, CCND1, EP300, C1QBP, HNF4A, CFL2, JUN, MATR3, CLOCK, ME1,
MTDH, MCL1, HOXA11, SOX2, SOX9, SRF, WT1, HAND1, NPAT, WIPF1,
POU3F1, DNMT3B, RUNX2, DNMT3A, CEBPB, TBX2, MAFB, PHB, NLK, SMAD3,
SMAD2, SPARC, ATM, POLD3, RPS6KA5, HDAC4, HSP90B1, SNAI3, SP1,
PLK1, TDG, JAK2, RERE, KLF4 |
2.47×10−12 |
|
| GO:0043233 | Organelle
lumen | 74 | BACH1, MYOD1,
RNASEL, NBN, HNF1A, PDGFB, CDC14B, EZH2, FOXO1, ZEB1, GLI3, IQGAP1,
CTNNB1, TGFB2, PNN, PRIM1, CCNE1, GATA6, FOXF1, HMOX1, YAP1, MYB,
MYC, TWIST2, ZBTB20, RARG, UBE2I, RB1, PPARGC1A, HMGA1, GTF2H1,
CCND1, EP300, C1QBP, HNF4A, CFL2, JUN, MATR3, CLOCK, ME1, MTDH,
MCL1, HOXA11, SOX2, SOX9, SRF, WT1, HAND1, NPAT, WIPF1, POU3F1,
DNMT3B, RUNX2, DNMT3A, CEBPB, TBX2, MAFB, PHB, NLK, SMAD3, SMAD2,
SPARC, ATM, POLD3, RPS6KA5, HDAC4, HSP90B1, SNAI3, SP1, PLK1, TDG,
JAK2, RERE, KLF4 |
2.78×10−12 |
| MF | GO:0043565 | Sequence-specific
DNA binding | 51 | BACH1, PPARA,
MYOD1, HNF1A, PPARG, MITF, SPI1, FOXO1, FOXO3, ZEB1, PDX1, GLI2,
GLI3, CTNNB1, GATA6, FOXF1, MYC, RARG, SOX13, ESR1, HMGA1, HNF4A,
JUN, FOXC2, HOXA11, SOX2, SOX9, SRF, WT1, HAND1, HOXA5, POU3F1,
DNMT3B, CREBL2, FOXL1, CEBPB, TBX2, MAFB, MSH2, NR4A2, SMAD3,
SMAD2, HDAC4, NOTCH1, NR1I2, SP1, MEOX2, ETS1, FOXE1, KLF4,
RERE |
2.73×10−18 |
|
| GO:0016563 | Transcription
activator activity | 41 | MYOD1, PPARA,
HNF1A, MITF, PPARG, FOXO1, PDX1, FOXO3, ZEB1, SOX9, GLI2, WT1,
CTNNB1, CCNE1, HAND1, GATA6, FOXF1, BCL2, NPAT, YAP1, POU3F1, MKL1,
MYB, RUNX1, RUNX2, CEBPB, PHB, NR4A2, SMAD3, SMAD2, RB1, HMGA1,
PPARGC1A, HDAC4, NOTCH1, NR1I2, EP300, SP1, HNF4A, FOXC2,
KLF4 |
3.06×10−17 |
|
| GO:0030528 | Transcription
regulator activity | 79 | BACH1, PPARA,
MYOD1, HNF1A, ZNFX1, EZH2, PPARG, MITF, SPI1, FOXO1, PDX1, ZEB1,
FOXO3, GLI2, GLI3, CTNNB1, CCNE1, GATA6, FOXF1, YAP1, MYB, MKL1,
MYC, TWIST2, TWIST1, EGR2, RARG, SOX13, MECP2, ESR1, UBE2I, RB1,
PPARGC1A, HMGA1, GTF2H1, MYCN, EP300, HNF4A, JUN, MAP3K10, FOXC2,
ZFPM2, CLOCK, HOXA11, SOX2, SOX9, SRF, WT1, HAND1, HOXA5, BCL2,
NPAT, POU3F1, RUNX1, DNMT3B, RUNX2, CREBL2, PLAG1, CEBPB, FOXL1,
TBX2, MAFB, JARID2, PHB, NR4A2, SMAD3, SMAD2, HDAC4, NOTCH1, SNAI3,
NR1I2, SP1, MEOX2, DMTF1, ETS1, FOXE1, FABP4, RERE, KLF4 |
3.63×10−16 |
|
| GO:0003700 | Transcription
factor activity | 61 | BACH1, PPARA,
MYOD1, HNF1A, ZNFX1, PPARG, MITF, SPI1, FOXO1, PDX1, FOXO3, ZEB1,
GLI2, GLI3, CTNNB1, GATA6, FOXF1, MYC, TWIST2, RARG, EGR2, SOX13,
ESR1, RB1, HMGA1, MYCN, EP300, HNF4A, JUN, FOXC2, CLOCK, HOXA11,
SOX2, SOX9, SRF, WT1, HAND1, HOXA5, NPAT, POU3F1, RUNX1, RUNX2,
CREBL2, PLAG1, CEBPB, FOXL1, TBX2, MAFB, NR4A2, SMAD3, SMAD2,
SNAI3, NOTCH1, NR1I2, SP1, MEOX2, ETS1, DMTF1, FOXE1, RERE,
KLF4 |
9.84×10−16 |
|
| GO:0008134 | Transcription
factor binding | 35 | MYOD1, NBN,
YWHAZ, MTDH, PPARG, ZEB1, SRF, CTNNB1, CCNE1, HAND1, GATA6, BCL2,
NPAT, YAP1, MKL1, RUNX1, DNMT3B, RARG, MAFB, NLK, MECP2, SMAD3,
SMAD2, RB1, HMGA1, PPARGC1A, HDAC4, EP300, NR1I2, HNF4A, ETS1, JUN,
MAP3K10, DNMT1, ZFPM2 |
5.43×10−10 |