Introduction
The human immunodeficiency virus (HIV) is a
lentivirus that results in acquired immune deficiency syndrome
(AIDS). HIV infects critical cells of the human immune system,
including T cells (specifically CD4+ T cells),
macrophages and dendritic cells, and reduces the numbers of
CD4+ T cells. Once the numbers of CD4+ T
cells fall below a certain threshold, cell-mediated immunity is
lost, increasing susceptibility to life-threatening opportunistic
infections. Without treatment, the average survival time of
patients following HIV infection is estimated to be 9–11 years. The
first case of AIDS was observed in 1981 in the USA. Two years
later, Luc Montagnier and his group at Institute Pasteur and the
group of Robert Gallo at the National Institutes of Health
independently discovered the AIDS-causing virus (1,2).
This virus was identified and named as HIV (−1) in 1986 (3). In the same year, Montagnier's group
discovered a novel type of HIV in West Africa and named it HIV-2
(4). HIV-2 takes longer to cause
AIDS and has a low prevalence rate. Following the first discovery,
the number of AIDS cases has steadily increased with 35 million
people infected worldwide by 2013. In 2013, 2.1 million people were
newly-infected with HIV and 1.5 million patients lost their lives
to AIDS (5).
With the increase in the incidence of AIDS, numerous
research groups have attempted to discover and develop novel
therapeutic agents for its treatment. Since the approval of
zidovudine (trade name, azidothymidine) as an HIV drug by the USA
government in 1987, numerous novel therapeutic agents have been
developed. However, the use of these medicines has been terminated
due to adverse effects, including a lack of oral availability,
cardiac disturbances and drug-resistance. Currently, to minimize
resistance and delay the progression of HIV infection to AIDS,
multiple drugs acting on different viral targets are typically used
in combination. However, anti-retroviral therapy has its caveats
and access to such treatment remains a concern around the world,
particularly in developing countries. In the developing world,
treatment for opportunistic infections is not readily available and
thus, low-cost treatments are a necessity.
As a result of this, natural bioactive compounds
have been investigated as sources of next-generation anti-HIV
therapeutics, which should have greater effectiveness, fewer
adverse effects and reduced costs. Marine life may become one of
the leading sources of anti-HIV natural products. Marine organisms
produce numerous novel substances due to the unique, demanding and
aggressive environment in which they exist. Recently, a there has
been a focus on developing marine-derived anti-HIV agents. Various
studies have reported that marine peptides may be used as anti-HIV
agents in functional foods or neutraceuticals and pharmaceuticals,
due to their therapeutic potential for the treatment or prevention
of infectious diseases (6–10).
The present study revealed that an Alaska pollack
hydroxyproline-containing collagen peptide (APHCP) inhibited HIV-1
infection in an MT-4 human T cell line.
Materials and methods
Materials
Alaska pollack skins were obtained from a local
fisheries company and stored at −20°C. Alaska pollack skin gelatin
was extracted in our laboratory. Alaska pollack skins were first
washed in ice water containing 0.3 M Ca(OH)2 to remove
flesh and other impurities and then washed again with water,
neutralized with 0.15 M acetic acid. Gelatin was extracted with
distilled water at 45°C for 4 h at the skin/water ratio of 1:5
(w/v). The extract was filtered through two layers of cheese
clothes and evaporated at 70°C to remove ~70% of water. The
filtrate was dried in a 50°C hot-air oven for 12 h. The resulting
Alaska pollack gelatin was stored in desiccators for further use.
APHCP peptide was prepared as described by Kim et al
(11). Briefly, Alaska pollack
skin gelatin (1% w/v) was hydrolyzed with Pronase E (enzyme to
substrate ratio, 1:50) at pH 8.0 and 50°C for 12 h. APHCP peptide
was purified by consecutive chromatographic methods including gel
filtration on a Sephadex G-25 column, ion-exchange chromatography
on a SP-Sephadex C-25 column, and high-performance liquid
chromatography on an octadecyl-silica column. The molecular mass
and amino acid sequences of the purified peptides were determined
using quadrupole time-of-flight (Q-TOF) mass spectroscopy and
N-terminal Edman sequencing analysis, respectively. APHCP is
composed of 10 amino acid residues containing a Gly residue at the
C-terminus and the repeating motif Gly-Pro-Hyp (787 Da). MTT was
purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Dimethyl sulfoxide (DMSO) was obtained from Amresco, LLC (Solon,
OH, USA). Specific antibodies against p24 and β-actin for western
blot analysis were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA) and Merck Millipore (Darmstadt,
Germany).
Cells and viruses
MT-4, H9/HIV-1IIIB and
H9/HIV-2ROD cell lines were obtained from the National
Institutes of Health AIDS Reagent Program (Germantown, MD, USA).
All cell lines were cultured in RPMI-1640 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.), 50 µg/ml streptomycin and 50 U/ml penicillin in 5%
CO2 at 37°C. Cells were passaged every 2–4 days and
maintained at a cell density of 5×105-1×106
cells/ml. HIV-1IIIB and HIV-2ROD viral
particles were obtained from the supernatants of
H9/HIV-1IIIB and H9/HIV-2ROD cell lines,
respectively. The viruses were stored at −80°C until use. Viral
titer was determined by measuring p24 production on infection in
MT-4 cells, and expressed as 50% tissue culture infective dose
(TCID50).
Cell viability assay
The 50% cytotoxic concentration of APHCP was
determined by MTT assay. MT-4 cells were seeded in a 96-well plate
at 2×104 cells/well in RPMI-1640 medium containing 10%
FBS. A total of 24 h later, cells were treated with 0–0.75 mg/ml
APHCP and incubated for 24 h at 37°C. Fresh RPMI-1640 medium
containing 10% FBS was added to each well 24 h later. After 84 h,
20 µl MTT solution (final concentration, 0.5 mg/ml) was added to
each well and the plate was incubated for 4 h at 37°C. DMSO (200
µl) was added to dissolve the purple formazan. The quantity of
formazan was determined by measuring the absorbance at a wavelength
of 595 nm using a FilterMax F5 microplate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA). Cell viability was determined
and compared with untreated MT-4 cells.
HIV-1 lytic effect
To determine the anti-HIV activity of APHCP on
HIV-infected MT-4 cells, an MTT assay was performed. MT-4 cells
were washed and resuspended in fresh RPMI-1640 medium, and seeded
in duplicate in a 96-well plate at a density of 2×104
cells/well. A total of 24 h later, stock virus of
HIV-1IIIB and HIV-2ROD were added to each
well at 50 TCID50 with different dilutions of APHCP. The
plate was incubated for 84 h at 37°C with 5% CO2. Cell
viability was determined by an MTT assay as described previously
(12).
p24 antigen assay
MT-4 cells at a density of 2×104 were
seeded in a 96-well plate. After 1 day, MT-4 cells were treated
with APHCP and infected with 50 TCID50 of
HIV-1IIIB and HIV-2ROD. The plate was
incubated for 84 h. The supernatant was harvested by centrifugation
at 200 × g for 5 min at 4°C. To determine the quantity of
HIV, a Lenti-X™ p24 Rapid Titer kit (Clontech Laboratories, Inc.,
Mountainview, CA, USA) was used according to the manufacturer's
protocol.
Reverse transcriptase (RT) activity
assay
The activity of HIV-1 RT in the virus supernatant
was determined using an RT assay kit (Roche Diagnostics GmbH,
Mannheim, Germany) according to the manufacturer's protocol.
Briefly, a reaction mixture containing
poly(A)xoligo(dT)15 was added to the virus supernatant
and incubated for 4 h at 37°C. Subsequently, anti-Digoxigenin-POD
and 2,7′-azinobis[3-ethylbenzothiazolone-6-sulfonic acid]
diammonium salt (200 µl) were added stepwise. The virus supernatant
was incubated at room temperature until the color development was
sufficient for detection. The absorbance of the virus supernatant
was measured at a wavelength of 405 nm using a microplate
reader.
Syncytia formation analysis
The inhibitory effect of APHCH on syncytia formation
was determined by visualization under a light microscope. MT-4
cells were seeded in a 96-well plate at a density of
2×104 cells/well. After 24 h, the cells were infected
with 10 µl stock supernatant of HIV-1IIIB virus diluted
at 50 TCID50 in the absence or presence of APHCP. The
plate was incubated for 3 days and the syncytia formation was
observed by microscopy using an inverted light microscope [Zeiss
LSM 510 confocal imaging system (Carl Zeiss, Oberkochen,
Germany)].
Western blot analysis
MT-4 cells were seeded at a density of
2×104 cells/well in 80 µl fresh medium and incubated for
1 day at 37°C with 5% CO2. MT-4 cells were infected with
HIV-1IIIB stock supernatant in the absence or presence
of APHCP. After 84 h, cells were pelleted at 200 × g for 5
min at 4°C and separated from the supernatant. MT-4 cell pellets
were harvested and solubilized in 2X SDS sample buffer containing
100 mM dithiothreitol, 100 mM Tris-HCl, 0.4% bromophenol blue, and
20% glycerol. The virus lysates (25 µl) were loaded onto an 10%
SDS-PAGE gel. Separated proteins were transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked for 1 h with 1% bovine serum
albumin (Sigma-Aldrich; Merck Millipore) in 10 mM Tris-HCl, 150 mM
NaCl (pH 7.5) containing 0.1% Tween-20, and incubated with primary
antibodies against p24 (cat. no. MAB7360; R&D Systems; dilution
ratio 1:200) and β-actin (cat. no. MAB1501; Merck Millipore;
dilution ratio 1:1,000) for 1 h. The membranes were then washed and
incubated for 30 min with the goat anti-mouse IgG secondary
antibody conjugated to alkaline phosphatase (cat. no. sc-2008;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA; dilution ratio
1:1,000). The protein bands were detected by colorimetric reaction
using 5-bromo-4-chloro-3′-indolyphosphate/nitro-blue tetrazolium
alkaline phosphatase substrate (Sigma-Aldrich; Merck
Millipore).
Statistical analysis
Data were analyzed using InStat statistics software
(GraphPad 6.0 Software, Inc., La Jolla, CA, USA). Statistical
comparisons were performed using one-way analysis of variance
followed by Bonferroni post-hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
A collagen peptide derived from Alaska
pollack skin
A peptide derived from the skin of Alaska pollack
was prepared and purified from protease E hydrolysates of Alaska
pollack skin as described by Kim et al (11). The accurate molecular mass and
amino acid sequence of the peptide as ascertained by Q-TOF mass
spectroscopy and N-terminal Edman sequencing analysis was 787 Da
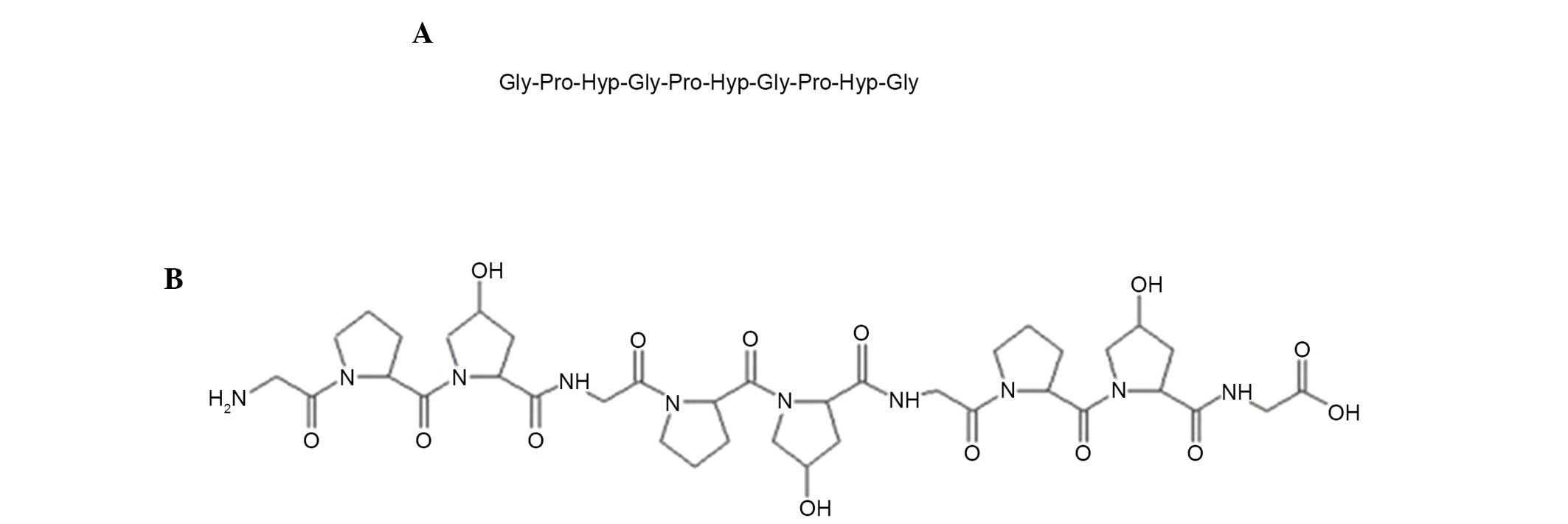
and Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly, respectively (Fig. 1). The amino acid sequence of this
peptide was similar to that reported by Kim et al (11), except for having three or six
additional amino acid residues [Gly-Pro-Hyp(−Gly-Pro-Hyp)] at the
C-terminus. A database search of non-redundant protein sequences
using the Basic Local Alignment Search Tool (blast.ncbi.nlm.nih.gov/Blast.cgi) revealed that the
amino acid sequence of this peptide is the most typical sequence
present in collagens. The present study named this peptide APHCP
(Alaska pollack skin hydroxyproline-containing collagen
peptide).
Cytotoxicity and anti-HIV-1 activity
of APHCP
The cytotoxicity of APHCP in MT-4 human T cells was
assessed. Cells were treated with 0–0.75 mg/ml APHCP for 84 h and
the viability of MT-4 cells was measured by an MTT assay. APHCP did
not affect the viability of MT-4 cells at concentrations <0.75
mg/ml (954 µM; Fig. 2A).
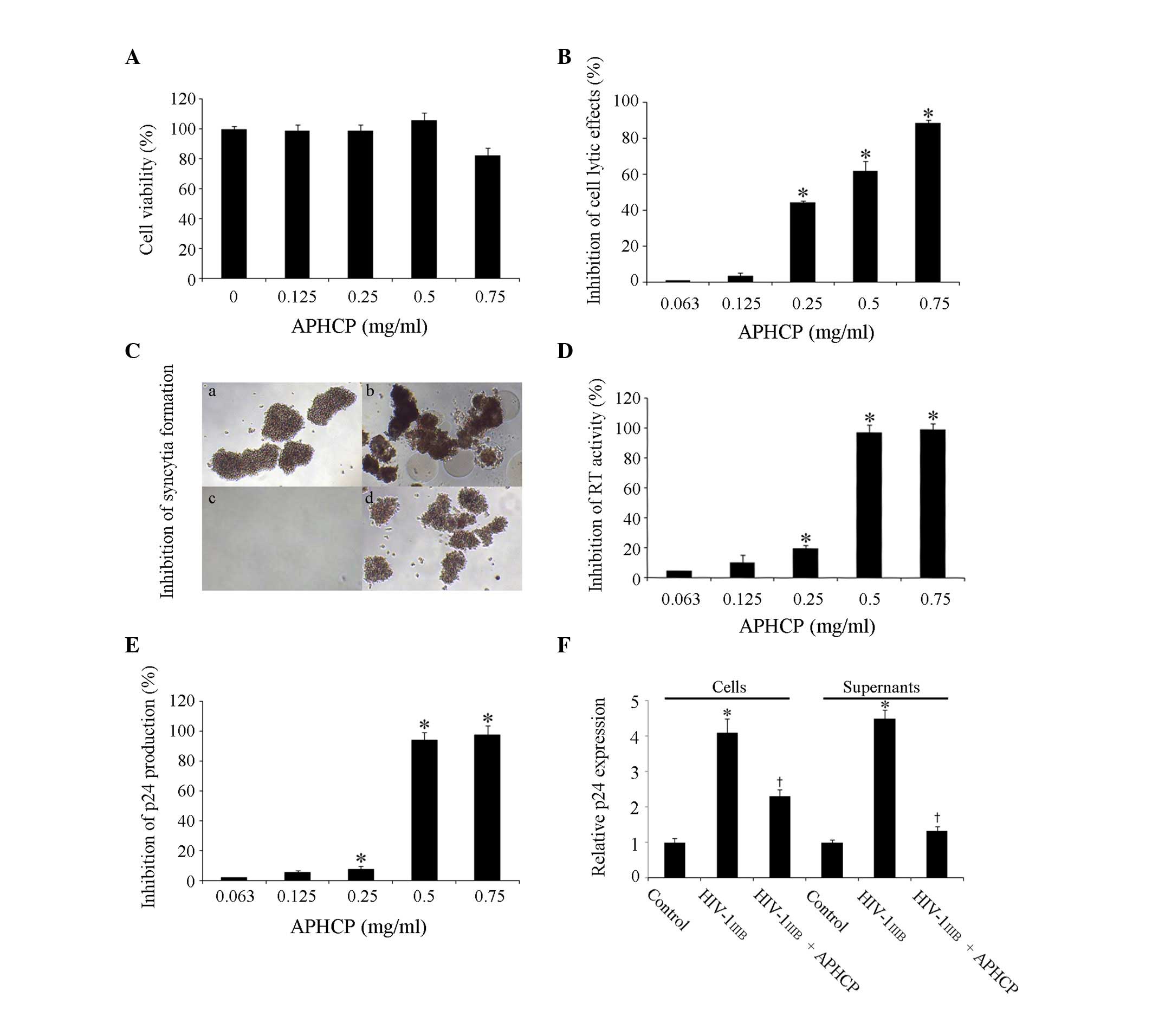 | Figure 2.Cytotoxity and anti-HIV-1 activity of
APHCP. (A) Effect of APHCP on the viability of MT-4 cells, as
measured by an MTT assay. APHCP did not affect the viability of
MT-4 cells at concentrations less than 0.75 mg/ml. Data are
presented as the mean ± standard deviation (n=3). (B) Effect of
APHCP on HIV-1IIIB-induced cell lysis, as measured by an
MTT assay. Data are expressed as the percentage inhibition compared
with HIV-1IIIB-infected untreated control cells. Data
are presented as the mean ± standard deviation (n=3). *P<0.05
compared with HIV-1IIIB-infected untreated cells. (C)
Images of HIV-1IIIB-infected MT-4 cells. Syncytia
formation was detected using an inverted light microscope (original
magnification, ×100). Representative images from 3 independent
experiments are presented. (a) Uninfected cell control, (b)
HIV-1IIIB-infected cells, (c) HIV-1IIIB virus
and APHCP, and (d) HIV-1IIIB infected cells with APHCP.
(d) has less syncytia formation than in (b) or was similar to the
uninfected in (a). (D) Effect of APHCP on RT activity. Data are
expressed as the percentage inhibition compared with
HIV-1IIIB-infected untreated control cells. Data are
presented as the mean ± standard deviation (n=3). *P<0.05
compared with HIV-1IIIB-infected untreated cells. (E)
Effect of APHCP on p24 production by HIV-1IIIB-infected
MT-4 cells. Data are expressed as the percentage inhibition
compared with HIV-1IIIB-infected untreated control
cells. Data are presented as the mean ± standard deviation (n=3).
*P<0.05 compared with HIV-1 IIIB-infected untreated
cells. (F) Intracellular and secreted p24 protein expression levels
were detected by western blot analysis. Data are presented as the
mean ± standard deviation (n=3). *P<0.05 compared with
HIV-1IIIB-infected untreated control cells.
†P<0.05 compared with HIV-1IIIB. APCHP
treatment inhibited the lytic effects, syncytia formation, RT
activity and p24 production of HIV-1 IIIB-infected MT-4
cells, in a dose-dependent manner. HIV, human immunodeficiency
virus; APHCP, Alaska pollack skin hydroxyproline-containing
collagen peptide; RT, reverse transcriptase. |
The inhibitory effect of APHCP on HIV-1 infection in
MT-4 cells was investigated. The protective effect of APHCP on
HIV-1IIIB-induced cell lysis was examined using an MTT
assay. Cell lysis by HIV-1IIIB infection was decreased
following treatment with APHCP (Fig.
2B). The half-maximal effective concentration (EC50)
of APHCP against anti-HIV-1IIIB infection was assessed
to be 0.403 mg/ml (459 µM). In addition, the effect of APHCP on
HIV-1-induced syncytia formation was examined. Syncytia formation
between HIV-infected cells and uninfected cells is a feature of HIV
infection, and the fused cells are destroyed within a few days.
Furthermore, the fusion of HIV-infected cells and uninfected cells
is a critical step during the entry stage of HIV. APHCP-treated
HIV-1IIIB-infected MT-4 cells behaved similarly to
uninfected control cells, whereas HIV-1IIIB-infected
MT-4 cells fused with adjacent cells and formed syncytia. This
suggested that APHCP inhibits HIV-1-induced syncytia formation by
interfering with the fusion between HIV or HIV-infected cells and
target cells (Fig. 2C). The
inhibitory activity of APHCP on HIV-1IIIB infection was
further studied by determining its effect on HIV-1 RT activity. The
RT activity in infected host cells converts viral RNA to DNA, a
critical stage of HIV-1 replication. APHCP treatment inhibited
HIV-1IIIB-induced RT activation in MT-4 cells in a
dose-dependent manner (Fig. 2D).
At a concentration of 0.5 mg/ml APHCP, the RT activity in
HIV-1IIIB infected cells was inhibited by ~97% compared
with the untreated control. The EC50 was assessed to be
0.327 mg/ml (374 µM). In addition, the anti-HIV-1 activity of APHCP
was examined by quantifying p24 protein production. As p24 is a
lentiviral capsid protein and is indispensable for subsequent
reproduction of HIV in HIV-infected cells, it is an important
indicator of viral replication in infected cells. As presented in
Fig. 2E, p24 production was
suppressed by >90% in cells treated with 0.5 mg/ml APHCP
compared with untreated cells. The EC50 of APHCP for
HIV-1IIIB-induced p24 production was calculated to be
0.356 mg/ml (405 µM). The inhibitory effect of APHCP on p24
production was confirmed by western blot analysis of cell lysates
and culture supernatants using an anti-p24 antibody. APHCP
treatment of cells decreased the HIV-1-induced p24 protein
expression levels markedly in cells and supernatant (Fig. 2F). This inhibitory effect of APHCP
on p24 protein production was similar to the results of
HIV-1IIIB-induced RT activity assay, indicating that the
inhibition of HIV RT activity correlates well with that of
subsequent HIV-1 replication activity. These results indicated that
APHCP inhibits HIV-1-induced cytopathic effects by suppressing the
fusion between HIV-infected and target cells, as well as HIV-1 RT
activity and p24 production.
Specificity of APHCP for anti-HIV-1
activity
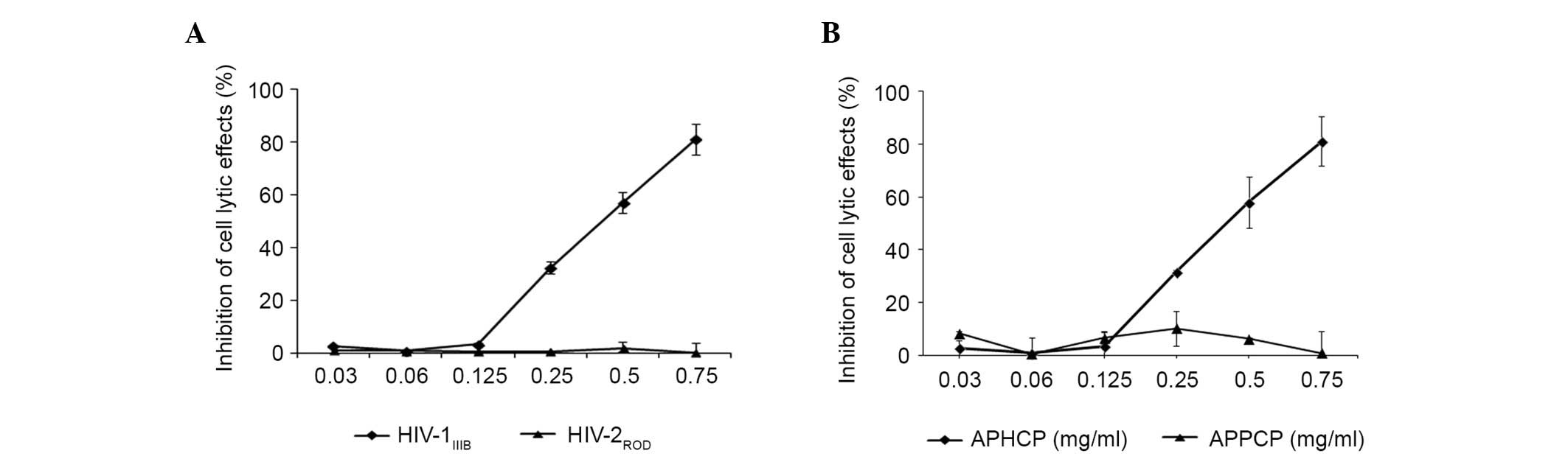
The specificity of the inhibitory effect of APHCP on
HIV-1 infection was examined. As presented in Fig. 3A, APHCP did not inhibit
HIV-2ROD-infected MT-4 cell lysis. This result suggested
that the anti-HIV activity of APHCP is specific to HIV-1. To
examine the essential residues responsible for the anti-HIV-1
activity of APHCP, an Alaska pollack skin proline-containing
collagen peptide (APPCP) was synthesized in which the
hydroxyproline residues in APHCP were replaced by prolines. APHCP
is composed of ten amino acid residues:
Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly. Various studies have
reported that the tripeptide (Gly-Pro-Hyp)n repeat is
the primary component of collagen degradation products and that it
adopts a triple-helical structure (13–16).
The hydroxyproline residues in the peptide contribute to the
stability and biological activity of the collagen triple helix
(13–16). The anti-HIV-1 activity of APPCP was
examined by measuring HIV-1IIIB-induced lytic effects.
APPCP did not demonstrate anti-HIV-1 activity (Fig. 3B), indicating that that hydroxyl
group of hydroxyprolines is required for the anti-HIV-1 activity of
APHCP.
Discussion
Currently, no cure or preventive vaccine is
available for HIV/AIDS. A primary anti-HIV therapy combines the use
of at least three antiretroviral drugs to achieve maximum
suppression of the HIV virus and prevent the progression of HIV.
Combination antiretroviral therapy has improved treatment; however,
this treatment is lifelong, has serious adverse effects and causes
viral resistance. Therefore, the identification of novel
antiretroviral drugs with unique underlying mechanisms of action is
required. In the present study, the marine peptide APHCP was
assessed for its potential to provide effective treatment and
prevention of HIV.
HIV infection has been demonstrated to cause
collagen deposition (17–19). The virus induces an imbalance
between matrix metalloproteinases (MMPs) and endogenous tissue
inhibitors of MMPs, leading to remodeling of the extracellular
matrix and HIV-associated pathology (20). The collagen triple peptide of
Gly-Pro-Hyp may interfere with the binding of proMMP2 to fibrillar
collagen, promoting the release and reducing activation of
collagen-sequestered proMMP-2, which is associated with the
resolution of liver fibrosis via fibrotic matrix-sequestered
gelatinases (21,22). Therefore, HIV infection-induced
activation of collagen-sequestered proMMP2 may result in
accelerated collagen resolution, tissue damage and the collapse of
immune system. Hydroxyproline-containing triple collagen peptides,
including APHCP, may prevent binding of proMMP2 to native collagen
and promote the release of proMMP2 bound to fibrillar collagen,
resulting in reduced MMP2 activation, collagen stabilization and
immune cell homeostasis consistent with anti-HIV activity.
Therefore, the structural and functional role of the collagen
triple peptide may be important for the anti-HIV activity of APHCP
in MT-4 cells. Further studies on the effect of APHCP on collagen
remodeling are required to fully elucidate the underlying
mechanisms of the anti-HIV activity of APHCP. This may provide a
basis for further investigation into the use of the marine peptide
APHCP as a potential therapeutic agent for the treatment of
HIV/AIDS.
In conclusion, the results of the present study
revealed that APHCP is non-cytotoxic at concentrations that inhibit
HIV-1-induced cell lysis, syncytia formation, RT activity and viral
p24 antigen production. APHCP is a novel, natural peptide with
potent HIV-1 inhibitory activity and may be a potential therapeutic
agent for the treatment of HIV-1.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology (grant
nos. 2014-047149 and 2015-018506).
References
|
1
|
Gallo RC, Sarin PS, Gelmann EP,
Robert-Guroff M, Richardson E, Kalyanaraman VS, Mann D, Sidhu GD,
Stahl RE, Zolla-Pazner S, et al: Isolation of human T-cell leukemia
virus in acquired immune deficiency syndrome (AIDS). Science.
220:865–867. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barré-Sinoussi F, Chermann JC, Rey F,
Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C,
Vézinet-Brun F, Rouzioux C, et al: Isolation of a T-lymphotropic
retrovirus from a patient at risk for acquired immune deficiency
syndrome (AIDS). Science. 220:868–871. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aldrich R and Wotherspoon G: Who's Who in
Gay and Lesbian History: From Antiquity to the Mid-Twentieth
Century Routledge. 5282001.
|
|
4
|
Clavel F, Mansinho K, Chamaret S, Guetard
D, Favier V, Nina J, Santos-Ferreira MO, Champalimaud JL and
Montaqnier L: Human immunodeficiency virus type 2 infection
associated with AIDS in West Africa. N Engl J Med. 316:1180–1185.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasedde S, Kapogiannis BG, McClure C and
Luo C: Executive summary: Opportunities for action and impact to
address HIV and AIDS in adolescents. J Acquir Immune Defic Syndr.
66:(Suppl 2). S139–S143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee TG and Maruyama S: Isolation of HIV-1
protease-inhibiting peptides from thermolysin hydrolysate of oyster
proteins. Biochem Biophys Res Commun. 253:604–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plaza A, Bifulco G, Keffer JL, Lloyd JR,
Baker HL and Bewley CA: Celebesides A-C and theopapuamides B-D,
depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J
Org Chem. 74:504–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plaza A, Gustchina E, Baker HL, Kelly M
and Bewley CA: Mirabamides A-D, depsipeptides from the sponge
Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J Nat Prod.
70:1753–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zampella A, Sepe V, Luciano P, Bellotta F,
Monti MC, D'Auria MV, Jepsen T, Petek S, Adeline MT, Laprévôte O,
et al: Homophymine A, an anti-HIV cyclodepsipeptide from the sponge
Homophymia sp. J Org Chem. 73:5319–5327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oku N, Gustafson KR, Cartner LK, Wilson
JA, Shigematsu N, Hess S, Pannell LK, Boyd MR and McMahon JB:
Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New
Guinea marine sponge Neamphius huxleyi. J Nat Prod. 67:1407–1411.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SK, Kim YT, Byun HG, Nam KS, Joo DS
and Shahidi F: Isolation and characterization of antioxidative
peptides from gelatin hydrolysate of Alaska pollack skin. J Agric
Food Chem. 49:1984–1989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Artan M, Li Y, Karadeniz F, Lee SH, Kim MM
and Kim SK: Anti-HIV-1 activity of phloroglucinol derivative,
6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem. 16:7921–7926.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doig AJ: Statistical thermodynamics of the
collagen triple-helix/coil transition. Free energies for amino acid
substitutions within the triple-helix. J Phys Chem B.
112:15029–15033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai N, Wang XJ and Etzkorn FA: The effect
of a trans-locked Gly-Pro alkene isostere on collagen triple helix
stability. J Am Chem Soc. 130:5396–5397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raman SS, Vijayaraj R, Parthasarathi R and
Subramanian V: Helix forming tendency of valine substituted
poly-alanine: A molecular dynamics investigation. J Phys Chem B.
112:9100–9104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Squeglia F, Bachert B, De Simone A,
Lukomski S and Berisio R: The crystal structure of the
streptococcal collagen-like protein 2 globular domain from invasive
M3-type group A Streptococcus shows significant similarity to
immunomodulatory HIV protein gp41. J Biol Chem. 289:5122–5133.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Estes JD, Haase AT and Schacker TW: The
role of collagen deposition in depleting CD4+ T cells
and limiting reconstitution in HIV-1 and SIV infections through
damage to the secondary lymphoid organ niche. Semin Immunol.
20:181–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diaz A, Alós L, León A, Mozos A, Caballero
M, Martinez A, Plana M, Gallart T, Gil C, Leal M, et al: Factors
associated with collagen deposition in lymphoid tissue in long-term
treated HIV-infected patients. AIDS. 24:2029–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kusko RL, Banerjee C, Long KK, Darcy A,
Otis J, Sebastiani P, Melov S, Tarnopolsky M, Bhasin S and Montano
M: Premature expression of a muscle fibrosis axis in chronic HIV
infection. Skelet Muscle. 2:102012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mastroianni CM and Liuzzi GM: Matrix
metalloproteinase dysregulation in HIV infection: Implications for
therapeutic strategies. Trends Mol Med. 13:449–459. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruehl M, Muche M, Freise C, Erben U,
Neumann U, Schuppan D, Popov Y, Dieterich W, Zeitz M, Farndale RW
and Somasundaram R: Hydroxyproline-containing collagen analogs
trigger the release and activation of collagen-sequestered proMMP-2
by competition with prodomain-derived peptide P33-42. Fibrogenesis
Tissue Repair. 4:12011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freise C, Ruehl M, Erben U, Farndale RW,
Somasundaram R and Heimesaat MM: The synthetic
hydroxyproline-containing collagen analogue (Gly-Pro-Hyp)10
promotes enzymatic activity of matrixmetalloproteinase-2 in vitro.
Eur J Microbiol Immunol (Bp). 2:186–191. 2012. View Article : Google Scholar : PubMed/NCBI
|