Introduction
Vasculature in the body occurs by the arrangement of
different types of cells throughout the development process and
later during growth into adulthood. Endothelial cells form the
luminal side of a blood vessel while pericytes or mural cells
contribute to the formation of the outer surface. As the vessel
size increases, smooth muscle cells and elastic and collagen fibers
along with fibroblasts take part in structural organization
(1). Blood vessel formation can be
of two types, angiogenesis and vasculogenesis. Angiogenesis is a
process of proliferation and sprouting of pre-formed blood vessels,
whereas vasculogenesis is due to the de novo genesis of
vessels from circulating progenitor cells and stem cells, mostly
derived from bone marrow, in the blood (2). Angiogenesis is of particular
significance in tumor growth and progression while vasculogenesis
is important for the recovery of cardiac muscle following ischemia,
as it can help restore heart muscle function after heart failure
(3). There are different types of
adult stem cells in the bone marrow types of stem cells, and these
include hematopoietic stem cells (HSC) or hematopoietic progenitor
cells (HPC), which are CD34+ cells and mesenchymal stem
cells (MSC). In various animal models of ischemia, these stem cells
have been shown to be effective in improving vessel formation and
in restoring heart muscle function in vivo (4). MSC from bone marrow can differentiate
into endothelial progenitor cells (EPCs), which are known to
express hematopoietic markers, CD34 or CD133 and also vascular
endothelial growth factor (VEGF) receptor 2.
Although different types of stem cells have been
shown to have the potential to contribute to the generation of
vasculature, disease conditions are likely to affect the ability
and also availability of the stem cells and progenitor cells
(5). Thus, factors that lead to
diabetes and/or cardiovascular complications have been found to
reduce the vascularization ability of the progenitor cells
(6). Several advances have been
made in stem cell therapy and considering that patient-derived stem
cells may not have the necessary potential to differentiate to form
vasculature, there is a need to develop methods for generating
large quantity of the EPCs so that they can be used for therapeutic
purposes.
In the present study, we developed methods to
differentiate human and canine bone marrow MSC in to EPCs and to
vascular endothelial cells, which were stable for extended period
(30 generations) in culture and could form vessel-like structures
in vitro and thus have the potential to be used in
vivo and for therapeutic purposes.
Materials and methods
Ficoll stock solution was purchased from Amersham
Pharmacia Biotech (Piscataway, NJ, USA); heparin was purchased from
the Shanghai Biochemical Pharmaceutical Factory (Shanghai, China);
trypsin was purchased from Sino-American Biotechnology Co., Ltd.
(Shanghai, China); Dulbecco's modified Eagle's medium (DMEM) was
purchased from Gibco (Grand Island, NY, USA); and vVEGF, β-FGF were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA). ECGF
was prepared by our research laboratory. Sources of antibodies were
as follows: Sheep anti-rabbit monoclonal rhodamine antibody
(dilution 1:5,000, Promega, Madison, USA; cat no.: AS1270);
FITC-labeled mouse monoclonal CD133 antibody (1:50; Cell Signaling
Technology, Danvers, MA, USA; cat no.: 60577), mouse monoclonal
CD31 antibody (1:50; Cell Signaling Technology, Danvers, MA, USA;
cat no.: 3568); mouse monoclonal CD34 antibody (1:50; Cell
Signaling Technology, Danvers, MA, USA; cat no.: 79253S); rabbit
polyclonal VEGF antibody (dilution: 1:200, Abcam, Cambridge, MA,
USA; cat no.: ab2349); and goat polyclonal factor VIII antibody
(dilution: 1:200, Abcam, Cambridge, MA, USA; cat no.: ab139391);
and CD34 magnetic separation kit (Miltenyi Biotec, Bergisch
Gladbach, Germany). All the procedures involving human bone marrow
collection and preparation of bone marrow cells and animal tissues
were approved by the Ethics Committee of Shanghai Jiaotong
University School of Medicine. Signed written informed consent was
obtained from the participants before the study. The study was also
approved by the Animal Ethics Committee of Shanghai Jiaotong
University Animal Center.
Isolation, culture, purification and
passage of MSCs
Bone marrows were obtained from 6 patients (4 males
and 2 females; 45–72 years) who were free of any types of cancer or
bone metastases, admitted for thoracic surgery at the Shanghai
Chest Hospital. Bone marrows were also obtained from 5 mongrel dogs
of both genders under surgery after pentobarbital anesthesia. The
bone marrow cavity was washed with 50 ml phosphate-buffered saline
(PBS), containing 50 µg/ml heparin, to collect bone marrow cell
suspension.
Primary culture of MSCs
Gelatin-coated culture bottles were added with 8 ml
of DMEM containing 20% fetal bovine serum, 10 ng/ml ECGF, 10 ng/ml
VEGF, 10 µml heparin, 1 ng/ml bFGF and other cell growth factors at
37°C and inoculated with bone marrow cell preparation, and
incubated in 5% CO2, 95% O2 atmosphere at
37°C. Cell attachment was monitored every day and the culture
medium was changed after 2 days. The culture dishes were agitated
gently to detach the red blood cells and other cells that did not
adhere and removed these cells by washing in PBS 2–3 times. Several
multiple clones of cells were formed and were continued to culture
under the same conditions with fresh culture medium. Primary cells
showed ~80% confluency after 4–7 days, and were subcultured.
Isolation of MSCs and phenotypic
characterization by flow cytometry
The cultured primary cells from bone marrow isolates
were harvested after 3rd-4th generation, by washing with PBS once,
followed by RPMI-1640 + 0.02 U collagenase + 100 U/ml DNAse for 45
min. The resulting cell suspension was filtered through 30-µm
filter, washed with PBS, and centrifuged at 1,500 × g for 10 min.
The pelleted cells were suspended in 5 ml PBS and mixed with 5 ml
Ficoll, and centrifuged at 200 × g for 25 min, to separate
karyocytes, which were further washed with PBS + EDTA twice, and
counted. The cells were further processed for the separation and
quantification of CD34+ cells using MACS cell separation
kit as per the manufacturer's instructions, followed by flow
cytometry. The CD34+ cells (1×106) were
suspended in M199 culture medium (HyClone, Logan, UT, USA) and
cultured in the same medium. The phenotype of the MSCs was
identified with flow cytometry after labeling the cells
(1×106) in 100 µl, with FITC-conjugated monoclonal
antibodies for CD31, CD34 or rhodamine-conjugated CD133 antibody
(1:100 dilution), with corresponding isotype controls. After
incubation in the dark at 4°C for 30 min, 1 ml of PBS+1% FBS+0.1%
NaN3 was added and centrifuged at 800 × g for 5 min, and
the supernatant was decanted. After washing twice in 3 ml of PBS+1%
FBS+0.1% NaN3, the cells were processed for flow
cytometry.
Immunofluorescent microscopy
Immunofluorescence phenotype analysis was performed
on the third, fifth and seventh generation MSCs (the earliest
primary continuous culture for 7 days). The cells were grown on
gelatin-coated cover-slips in 6-well culture plates. At 60%
confluency, the cells were washed with PBS 3 times, and fixed with
acetone for 15 min, followed by washing in PBS twice. Then the
coverslips were left in 3% H2O2 at room
temperature for 15 min, with agitation and washed with PBS twice.
After blocking with sheep serum for 30 min, and washing in PBS,
primary antibodies (Ki67, anti-human factor VIII, anti-VEGF, CD31,
CD34, CD133) were added (1:100 dilution) and incubated in
humidified box at 37°C for 1 h, followed by washing in PBS, twice.
FITC-labeled second antibody (1:200 dilution) was then added and
incubated for another 1 h, and then washed with PBS twice. Double
labeling was carried out by incubating the coverslips with two
antibodies. The coverslips were then observed under a fluorescent
microscope (IX70, Olympus, Tokyo, Japan).
Growth and ultra structure of the
MSCs
Cell proliferation was monitored with the 5th
generation of cells by cell count every day. Growth and
morphological characteristics of live cells were observed under
inverted microscope. Transmission electron microscopic examination
of human and canine EPCs was done under normal operation, and human
EPCs were also examined by scanning electron microscopy (JEM-F200,
Japan Electron Optics Laboratory Co., Ltd., Tokyo, Japan).
Results
Purification and cell culture of bone
marrow-derived EPCs
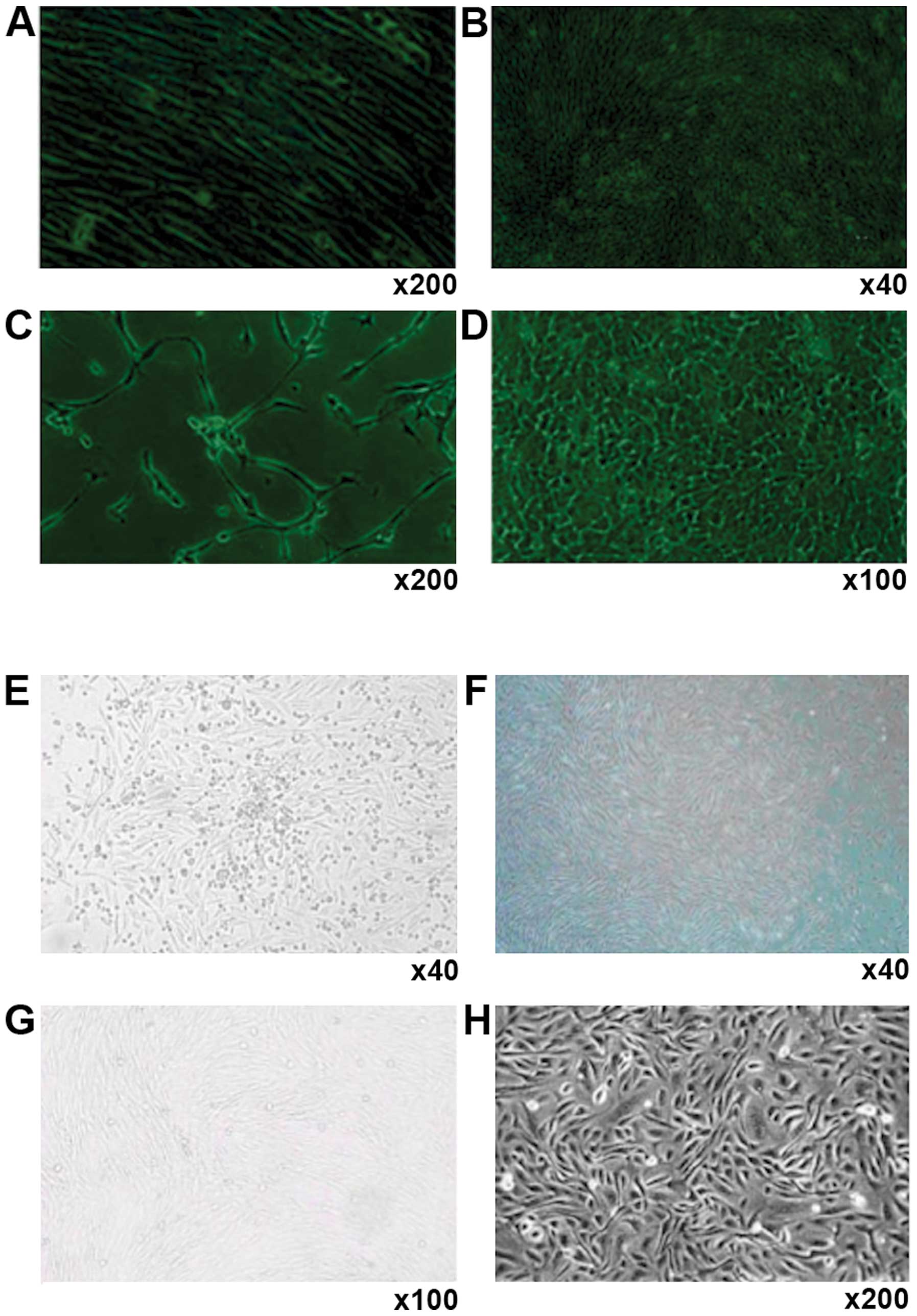
Human (Fig. 1 A-D)
and canine bone marrow stromal cells (Fig. 1E-H) showed similar changes towards
EPCs during cell culture. After adhering to the dish surface for 24
h, cell colonies formed, with the adherent cells extending into
spindle shape, and small cytoplasm and there was a small quantity
of hemocytes (Fig. 1A and G). One
week later, the spindle cells dominated in the culture dish, with
large nucleus, and these cells grew in parallel arrangement and
there were greatly reduced number of hemocytes (Fig. 1B and F). At this stage, the cells
grew vigorously and could be passaged twice each week at a ratio of
1:3. The cells began to develop into short spindle shape (Fig. 1C and G). The cells also displayed a
marked contact growth inhibition. After a month of continuous
culture, cell morphology was stable even after 30 generations, and
the cells were arranged in pebble shape and showed no aging-related
phenomena or detachment (Fig. 1D and
H).
Phenotype of the bone marrow-derived
cells
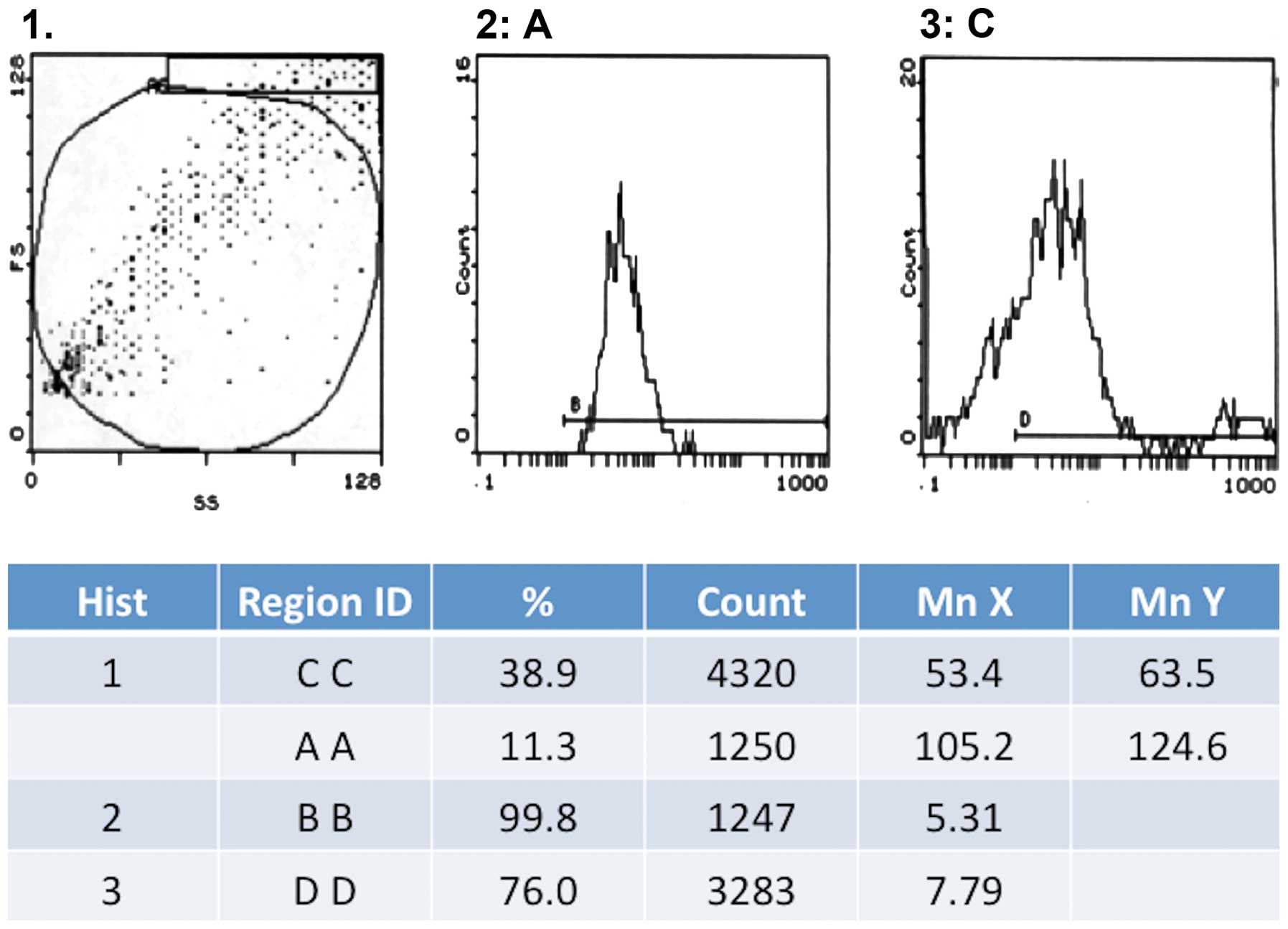
We noted a gradual decline in the expression of CD31
in human- and canine-derived cells, during their multiplication.
Thus, in the 5th generation CD31 expression was observed in 31.1%
cells, which decreased to 18.6% in the 9th generation and was
completely absent by 15th generation. On the other hand, the
expression of factor VIII was strongly positive in the 15th
generation with 76% of cells showing a positive expression
(Fig. 2). We did not observe any
expression of Ki67 during the whole cell culture period.
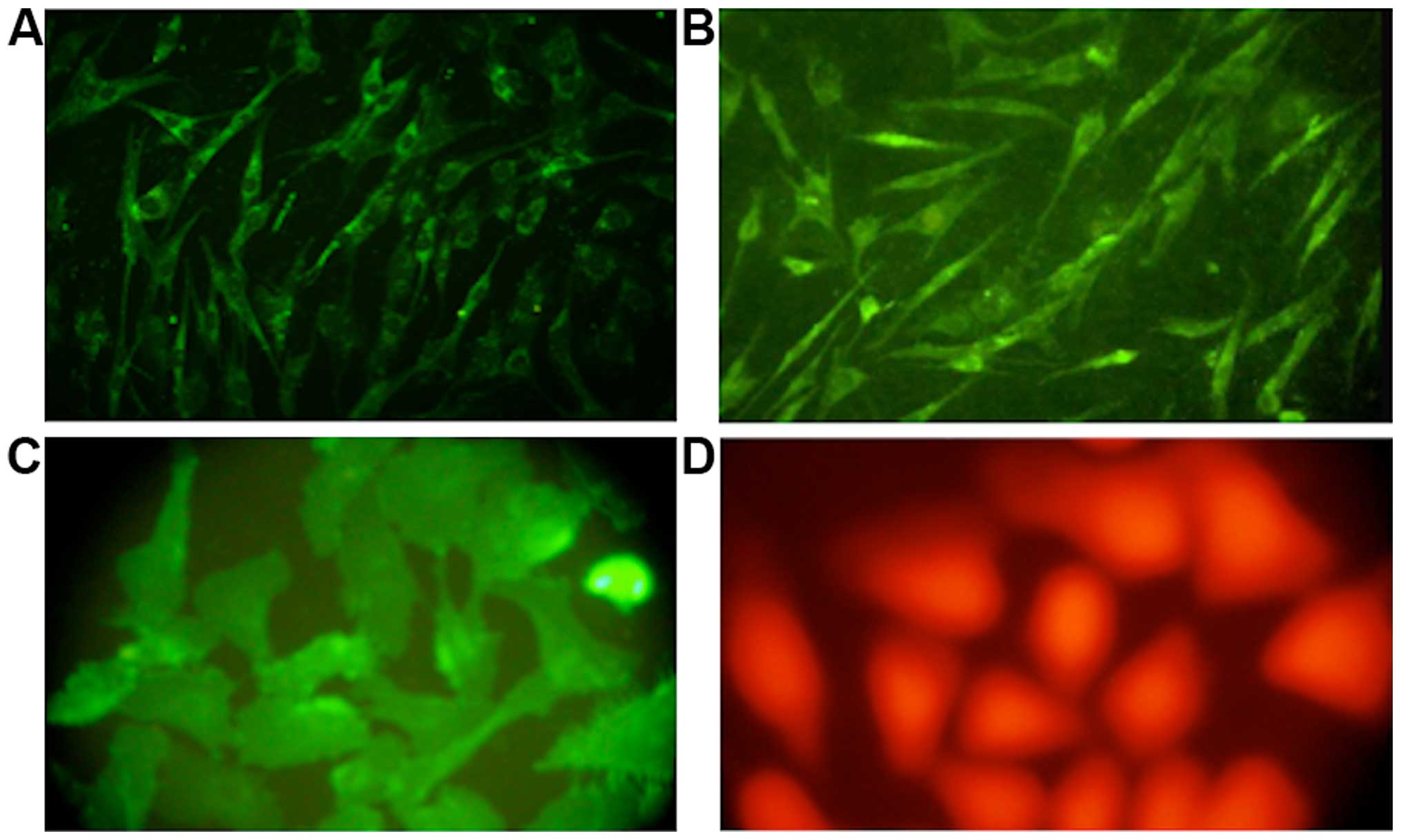
Immunofluorescence
Immunohistochemical examination of cells after
staining with specific antibodies revealed that only a few
mononuclear cells at the beginning of the cell culture expressed
VEGF, while almost 35% of the cells showed positive factor VIII
staining. As the culture progressed, the number of cells showing a
positive expression of CD133 and VEGF gradually increased. By the
6th and 7th generation, factor VIII showed a strong positive
expression in almost 99 and 95% of the cells from human and canine
bone marrow, respectively (Fig.
3).
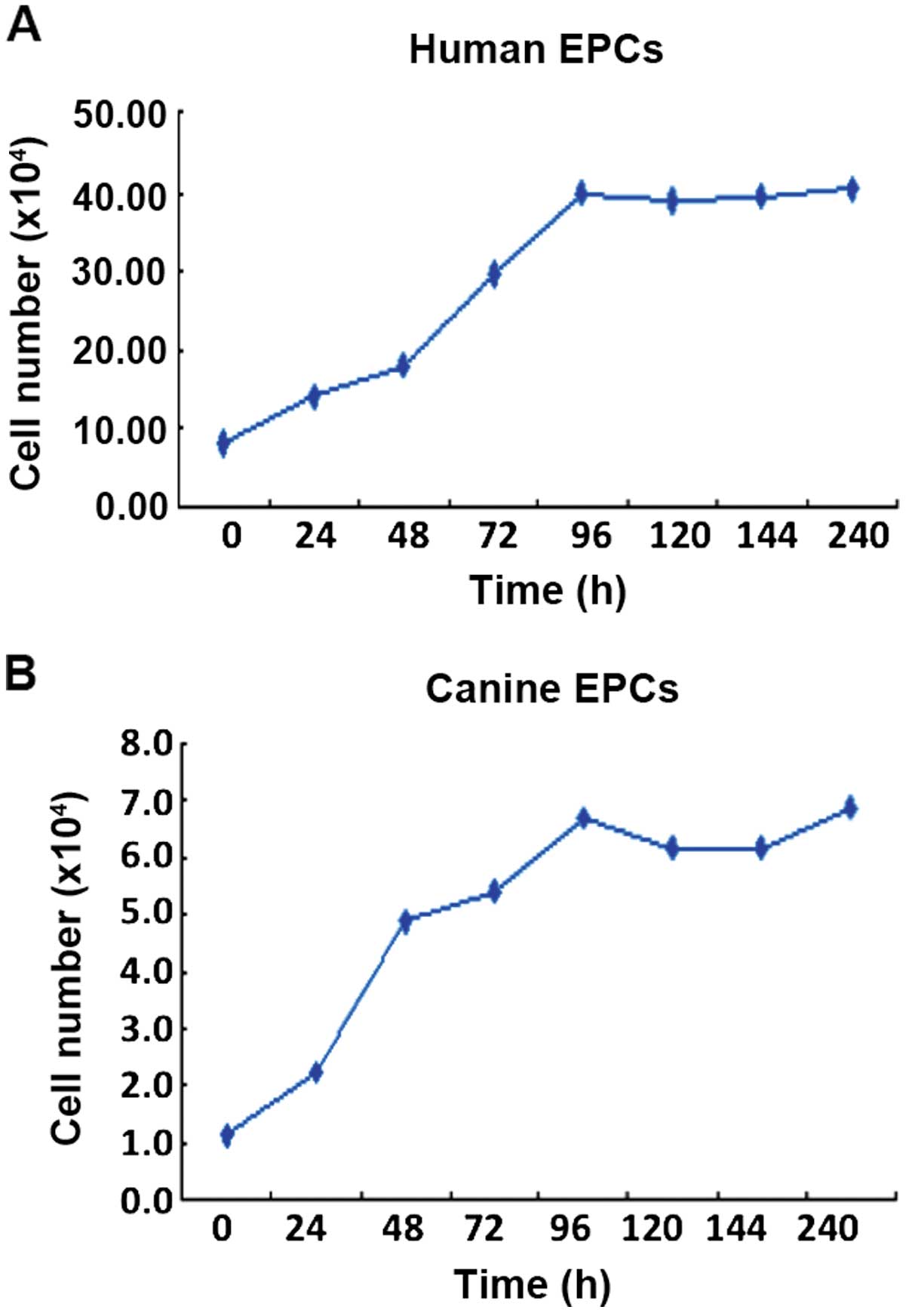
Growth curve and ultrastructure of the
EPCs
EPCs from humans and canines were counted every day
of the culture for five days, and from the growth curve, cell
doubling time was calculated. This doubling time for human EPCs was
30.5 h (Fig. 4A; y=6.13+0.328x;
correlation coefficient, r=0.977) and for canine EPCs it was 13.4 h
(Fig. 4B; y=1.196+0.06x;
correlation coefficient, r=0.978).
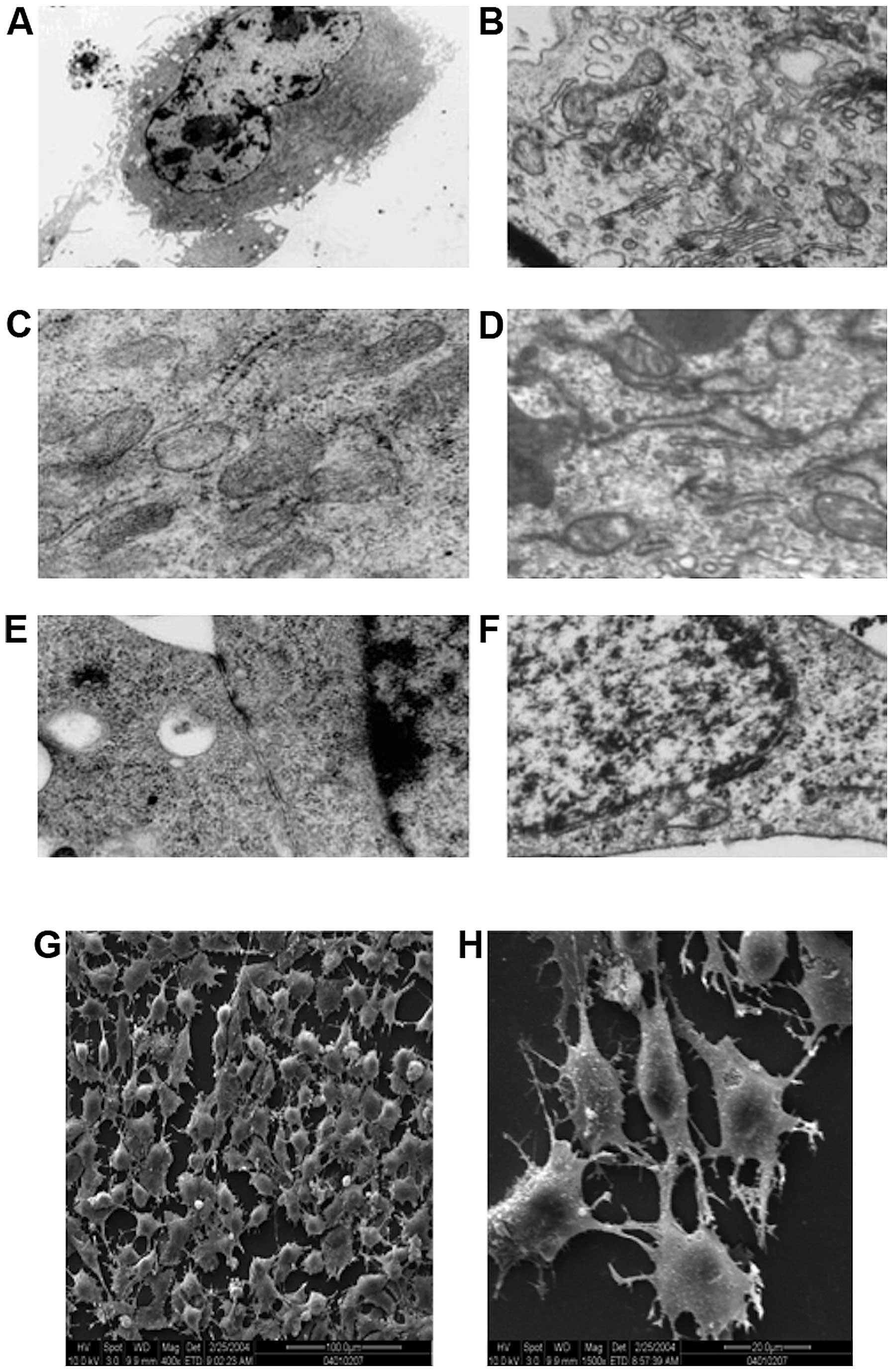
Transmission electron microscopic examination of the
ultrastructure of human-derived EPCs (Fig. 5A, C and E) and canine-derived EPC
(Fig. 5B, D and F) revealed
hyperchromatism on the periphery of cell nucleus, and that the cell
nuclei were large and elliptical (Fig.
5A and F). It was also noted that the cytoplasm contained
abundant cellular organelles, such as mitochondria, rough
endoplasmic reticulum and Golgi complex, which indicated that these
cells had strong protein synthesis capability, thus being able to
secrete many growth factors to maintain their own growth and
differentiation (Fig. 5C and D).
Gap junction of some adjacent cell membranes were observed in
human-derived cells (Fig. 5E). Gap
junctions can strengthen the connection of the adjacent cells, but
more importantly, these act as a communication structures of the
cell, for information transfer between cells and are related to
cell secretion, proliferation and differentiation. While in the
canine-derived cells, Weibel-Palade bodies (Fig. 5B) with endothelial cell
characteristics were observed in the endochylema, these bodies were
encapsulated by monifilm structure and contained medium electron
dense materials. Canaliculus separated by electron dense materials
were identified within certain bodies. These different shapes
probably represent different storage stages of factor von
Willebrand.
Scanning electron microscopic observation revealed
that the cells on the processed slides were found to be rounded or
quasi-circular (Fig. 5G), with
short and thick microvilli protruding on the cell surface, and
cytoplasm extended and protruding towards the two poles (Fig. 5H). This indicated that cells were
quite active metabolically and functionally and had strong
secretion capacity, and could absorb large quantity of nutrients to
support growth and proliferation.
Discussion
Vascular development is a regulated process that
consists of the proliferation and migration of adjacent vascular
endothelial cells (angiogenesis), and the differentiation of EPCs
into mesodermal precursor cells (vasculogenesis) (7–9).
EPCs and HPC originate from the blood islands in the extraembryonic
mesoderm in the embryonic stage (10). EPCs and HPCs have many common cell
surface antigens, such as EGFR-2 (mouse Flk-1), Tie-2, and CD34
(11). Therefore, it is speculated
that these cell types originate from the same precursor stem cell,
the hematopoietic cells (12).
In vitro, embryonic bodies differentiate into hematopoietic
and vascular endothelial cells (13). After birth, EPCs, HSCs and MSC
reside together in the bone marrow and are released from the bone
marrow into the peripheral blood circulation (12).
Examination of cell surface markers and biological
characteristics of vascular EPCs in the present study, revealed
that EPCs are a subgroup of CD34+ cells. These EPCs
after purification and subsequent cell culture with added VEGF and
other growth factors may be induced into endothelial cells.
Therefore, there is an absolute dependence on VEGFR-2 expression,
which acted as the surface receptor for mediating the VEGF signals,
in the early stage and during different stages of differentiation
of vascular endothelial cells.
The findings of the present study support the
hypothesis that a large scale preparation of EPCs that are capable
of differentiating into vascular endothelial cells, can be prepared
by continuous culture of cells from bone marrow-derived stromal
cells. Such a supply of the EPCs can be of great value for various
therapeutic applications.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (contract grant no.
81271304).
References
|
1
|
Spyridopoulos I and Andres V: Control of
vascular smooth muscle and endothelial cell proliferation and its
implication in cardiovascular disease. Front Biosci. 3:d269–d287.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn GO and Brown JM: Role of endothelial
progenitors and other bone marrow-derived cells in the development
of the tumor vasculature. Angiogenesis. 12:159–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimmeler S: Regulation of bone
marrow-derived vascular progenitor cell mobilization and
maintenance. Arterioscler Thromb Vasc Biol. 30:1088–1093. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walter DH, Haendeler J, Reinhold J,
Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R,
Arenzana-Seisdesdos F, et al: Impaired CXCR4 signaling contributes
to the reduced neovascularization capacity of endothelial
progenitor cells from patients with coronary artery disease. Circ
Res. 97:1142–1151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi DJ, Jamieson CH and Weissman IL:
Stem cells and the pathways to aging and cancer. Cell. 132:681–696.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimmeler S and Leri A: Aging and disease
as modifiers of efficacy of cell therapy. Circ Res. 102:1319–1330.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eguchi M, Masuda H and Asahara T:
Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp
Nephrol. 11:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murasawa S and Asahara T: Endothelial
progenitor cells for vasculogenesis. Physiology (Bethesda).
20:36–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
LaRue AC, Lansford R and Drake CJ:
Circulating blood island-derived cells contribute to vasculogenesis
in the embryo proper. Dev Biol. 262:162–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Case J, Mead LE, Bessler WK, Prater D,
White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS and
Ingram DA: Human
CD34+AC133+VEGFR-2+ cells are not
endothelial progenitor cells but distinct, primitive hematopoietic
progenitors. Exp Hematol. 35:1109–1118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khakoo AY and Finkel T: Endothelial
progenitor cells. Annu Rev Med. 56:79–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi K, Kennedy M, Kazarov A,
Papadimitriou JC and Keller G: A common precursor for hematopoietic
and endothelial cells. Development. 125:725–732. 1998.PubMed/NCBI
|