Introduction
The intestinal epithelium is frequently exposed to
various pathogens and toxic compounds. Therefore, an efficient and
powerful immune system is required (1). The intestinal tract of Drosophila
melanogaster is structurally and functionally similar to that
of mammals (2). Signaling
mechanisms that control epithelial regeneration, innate immunity
and inflammation are evolutionarily conserved. Drosophila is
a simple and powerful model that is frequently used to investigate
the signaling events of intestinal homeostasis. The
Drosophila midgut maintains a balance between immune
suppression against indigenous intestinal flora and a robust immune
response against invading microbes (3). The generation of antimicrobial
peptides (AMPs) and production of reactive oxygen species (ROS) are
two important microbicidal systems that may inhibit infection by
external pathogens (4,5). The local production of AMPs is
important for the inducible defense mechanisms of intestinal
immunity, which are triggered by the immune deficiency pathway
(6,7). The production of ROS by the NADPH
oxidase and dual oxidase 1 is a complementary mechanism for host
defense against pathogens (7). ROS
are able to disrupt DNA, RNA and proteins of external pathogens,
and of degrading lipids in their cell membrane. Furthermore, ROS
may stimulate intestinal stem cell (ISC) proliferation (8). However, excessive levels of ROS may
lead to cytotoxicity, cancer, degenerative diseases of aging, and
damage the host intestinal epithelial cells. Therefore, a balance
between the production and removal of ROS is essential for host
health (5). ISCs equivalent to
those in the mammalian intestine have been identified in the
Drosophila midgut (9). In
order to maintain intestinal homeostasis, the rate of stem cell
division is increased in response to stressful conditions, which
may be induced by pathogens, toxic compounds or aging (10,11).
Dysfunctional intestinal cell turnover may lead to compromised
tissue integrity or cancer (12).
Additionally, various toxic compounds, such as sodium dodecyl
sulfate (SDS), dextran sulfate sodium (DSS) and paraquat, may
affect intestinal homeostasis by inducing a stress response that
may lead to damage and apoptosis of epithelial cells (8,13).
Medicinal plants are an important source of
potentially therapeutic chemical substances. C. sativus L.
is a traditional medicine, also termed as saffron, that consists of
crocin, croetin, safranal and picrocrocin (14). It has been previously used in
traditional Chinese, Korean and Japanese medicine to treat spasms,
bronchospasm, liver disease, pain, insomnia and digestive ailments.
It has also been used as a stimulant and for supportive treatment
of cancer, including lung cancer (15). Previous studies have identified the
anti-inflammatory (16),
anti-nociceptive (17),
antimicrobial (18), antioxidant
(19) and immunomodulatory effects
of C. sativus L. extract with high efficacy and low toxicity
(20). However, the protective
effect of C. sativus L. against intestinal damage, and the
underlying mechanism of action, remain to be elucidated.
The present study investigated the protective effect
of C. sativus L. extract against intestinal damage by using
Drosophila as a model system to analyze the lifespan and
intestinal homeostasis following the ingestion of toxic compounds.
It was demonstrated that C. sativus L. may significantly
increase the lifespan of Drosophila. In addition, aqueous
extracts of C. sativus L. may significantly increase the
survival rate of Drosophila following treatment with SDS,
DSS and paraquat. Normal adult intestinal morphology was observed
in the C. sativus L.-fed group following exposure to SDS.
This may be due to decreased levels of ROS and reduced epithelial
cell death. These findings may improve the understanding of
clinical researchers on the complex function of C. sativus
L. in intestinal disorders. Further investigation is required to
determine which components of C. sativus L. exhibit the
protective effects observed in the present study.
Materials and methods
Drosophila strains
Wild-type w1118 flies
(Drosophila melanogaster) were obtained from the Bloomington
Drosophila Stock Centre (Bloomington, IN, USA), and reared on a
standard cornmeal-yeast medium at 25°C and 60% humidity under a
12/12-h light/dark cycle.
Aqueous extracts of C. sativus L. and
growth medium of Drosophila
The dried stigmas of C. sativus L. were
obtained from the Qinghai-Tibet plateau of China in 2014 and were
identified by Professor Xiuhua Wang (Northeast Forestry University,
China). Aqueous extracts were obtained as previously described
(21). C. sativus L. (2 g)
were immersed in 100 ml deionized water overnight at 25°C. The
aqueous extraction was boiled for 3 h, and the extraction process
was repeated twice. Total extracts were mixed and concentrated to
50 ml. Drosophila that were fed a standard cornmeal-yeast
medium were used as the control group, and those fed the standard
medium containing 1% extract of C. sativus L. were used as
the experimental group.
Quantitative analysis of Crocin I
Crocin I (Chengdu Must Bio-Technology Co., Ltd.,
Chengdu, China) was used as a reference standard for the quality
control of the C. sativus L. extracts. High-performance
liquid chromatography (HPLC) analysis was performed as previously
described (22). Briefly, the HPLC
system (LC-20AT, Shimadzu Corporation, Kyoto, Japan) consisted of a
quaternary pump, C18 gravity column (Alltima C18; 250×4.6 mm, 5 µm)
and HPD-20A detector. The mobile phase was 15% methanol in water at
a flow rate of 1.0 ml/min and detector wavelength of 275 nm.
Lifespan analysis
The lifespan of the Drosophila was determined
at 25°C and 60% humidity with a 12/12-h light/dark cycle. Newly
enclosed adult flies were collected within a 24-h period, and the
flies were allowed to mate for 48 h. A total of 30 adult flies were
cultured and transferred to new vials containing fresh food every
2–3 days. The surviving flies were counted every day, and each
experiment was repeated three times.
Survival experiments
The 3-to 5-day old adult flies (15 males and 15
females) were starved for 2 h prior to being transferred to a vial
containing 5 layers of filter paper hydrated with 5% sucrose (w/v)
with toxic compounds, including 0.6% SDS (w/v; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany), 6 mM paraquat (Sigma-Aldrich; Merck
Millipore) or 4% DSS (w/v; MP Biomedicals, LLC, Santa Ana, CA,
USA). Sucrose (5%) solution with no additives was used as the
positive control for all experiments. Filter papers were changed
every day, and the survival rate was monitored daily, as mentioned
above.
7-Amino-actinomycin D (7-AAD)
staining
Adult Drosophila were orally exposed to 0.6% SDS and
incubated at 25°C for 96 h. A total of 10–15 dissected fly
intestines were stained with 7-AAD (5 µg/ml in PBS; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at
room temperature, fixed for 30 min in PBS with 4% paraformaldehyde,
and then stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10
min, as previously described (21). The intestines were mounted using
mounting medium [70% glycerol in PBS containing 2.5%
1,4-diazabicyclo[2,2,2]octane (DABCO); Sigma-Aldrich; Merck
Millipore), and images were captured using an Axioskop 2 Plus
microscope (Carl Zeiss AG, Oberkochen, Germany). The presented data
are from three independent experiments.
ROS detection
Adult females were orally exposed to 1% SDS and
incubated at 25°C for 48 h. A total of 10–15 intestines were
dissected in ice-cold PBS and incubated in dihydroethidium (DHE; 5
µM in PBS; Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min
at room temperature, then rinsed 4 times in PBS for 5 min each,
fixed in PBS with 4% paraformaldehyde for 20 min, and then stained
with DAPI for 10 min. Intestines were then rinsed 4 times with PBS
and mounted using mounting medium (70% glycerol in PBS containing
2.5% DABCO; Sigma-Aldrich, Merck Millipore), then analyzed with an
Axioskop 2 Plus microscope (Carl Zeiss AG). The data presented are
from three independent experiments.
Statistical analysis
Statistical analysis was performed using a
two-tailed unpaired Student's t-test with Prism 6 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.001 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± standard error.
Results
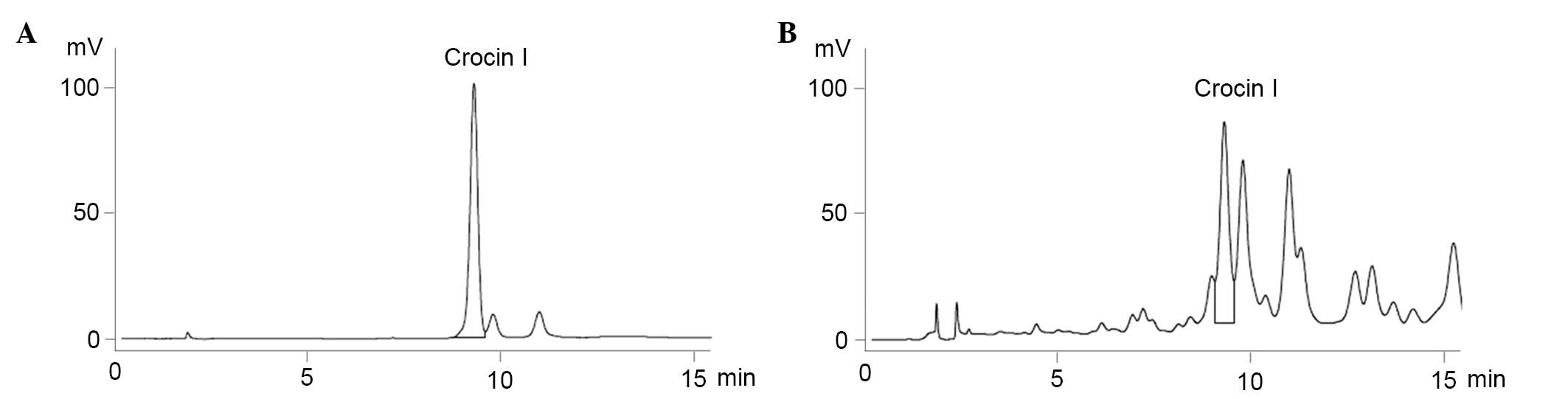
Identification of Crocin I in C.
sativus L. aqueous extracts using HPLC
HPLC analysis was used for the identification and
quantification of Crocin I in the previously collected C.
sativus L. aqueous extracts. The chromatogram of the standard
was eluted at a retention time of 9.3 min, and it was determined
that the C. sativus L. aqueous extracts that were collected
previously contained 0.181% Crocin I (Fig. 1)
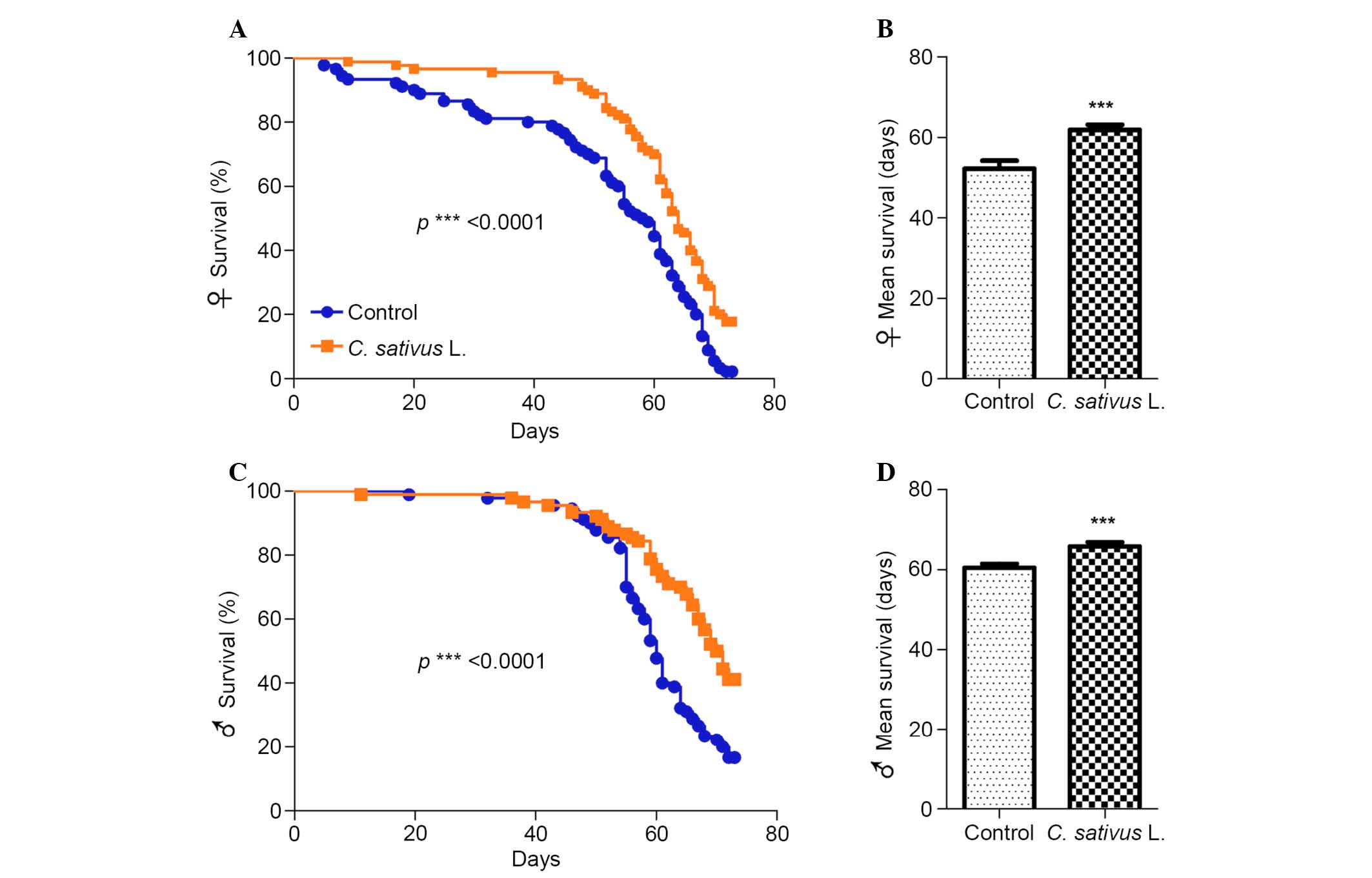
C. sativus L. extends the lifespan of
adult flies
Aging is a multifaceted process associated with a
gradual decline in physiological function, which leads to serious
diseases, including cancer and diabetes (23). A previous study demonstrated that
various traditional plants and their extracts contain high levels
of phytochemicals, which are capable of extending survival and
preventing or delaying age-associated diseases (24). Drosophila fed with the
standard medium with 1% extract of C. sativus L. were used
as the experimental group (Fig.
2). It was determined that significant increases in the maximum
lifespan of female and male flies occurred in the group fed with
the C. sativus L. extract compared with the control group
(P<0.0001; Fig. 2A and C). In
addition, the mean lifespan increased by 18.3% in females and 8.8%
in males (Fig. 2B and D). These
findings suggest that C. sativus L. extract may promote
longevity and increase the mean lifespan, and that the magnitude of
this increase may be associated with gender.
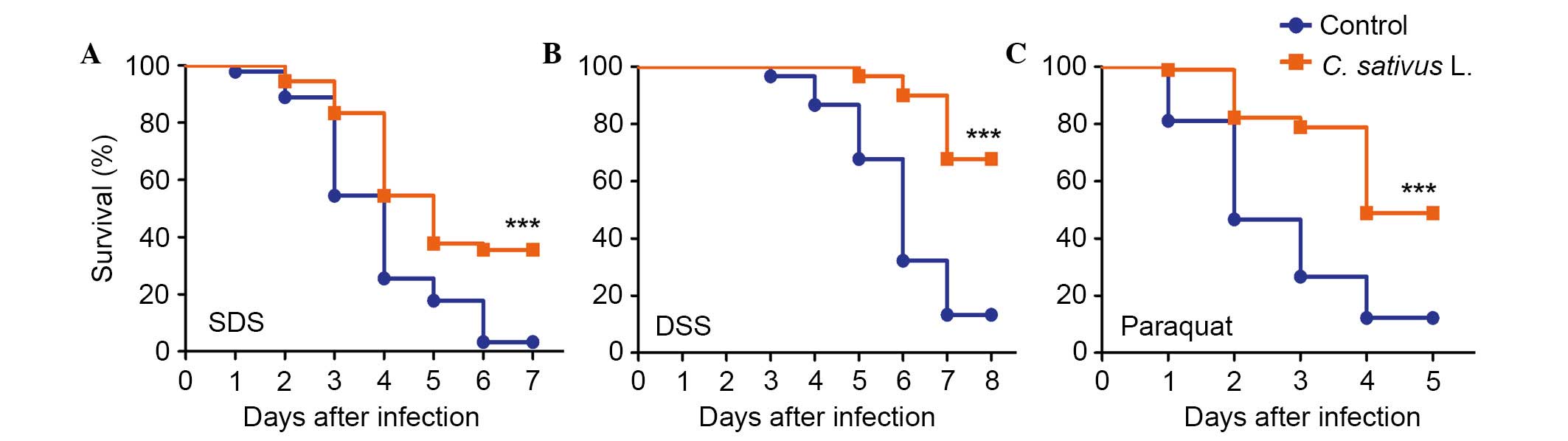
C. sativus L. extract increases the
survival rate following the ingestion of toxic compounds
The intestinal epithelium is susceptible to pathogen
damage, oxidative stress and toxic compounds. In order to determine
the protective effect of C. sativus L. extract, adult flies
from both culture conditions (the control and experimental groups)
were orally treated with the inflammatory reagent, SDS or DSS.
These chemicals may interfere with the normal function of the
intestinal barrier and stimulate local and systemic inflammation.
This may result in tissue damage and melanotic phenotypes in the
adult Drosophila intestine (8,10).
As presented in Fig. 3A and B,
survival rates were significantly increased in the C.
sativus L. feeding groups compared with the control group,
following exposure to SDS or DSS for 6–7 days (P<0.001). The
survival rate in the treatment groups fed with C. sativus L.
extract were 35.5 and 67.7% for SDS and DSS, respectively, which
were significantly higher compared with the survival rates of the
control group (3.3 and 13.2%, respectively). Following ingestion of
an ROS-producing agent, paraquat, for 4 days, the C. sativus
L. feeding groups exhibited significantly higher survival rates
(48.9%) compared with the control group (P<0.001; Fig. 3C). This survival rate was >36.7%
compared with the control group (12.2%; Fig. 3C). These findings indicated that
C. sativus L. extract may increase the Drosophila
survival rate following oral infection with SDS, DSS and paraquat.
Therefore, C. sativus L. may contribute to the resistance of
infection due to pro-inflammatory reagents.
C. sativus L. extract protects the
adult intestine against SDS-induced epithelial cell damage
The ingestion of toxic compounds, such as SDS, has
been identified to induce epithelial cell damage (8). To determine whether the reduced
survival rate of adult flies resulted from increased cell death in
response to SDS treatment, adult flies in the control and C.
sativus L. feeding groups were treated with 0.6% SDS for 96 h.
Subsequently cell viability of intestinal epithelial cells was
detected using 7-AAD staining, which is a type of nucleic acid dye
that is capable of passing through the plasma membrane and
combining with the DNA of the dead cells. An increase in the number
of dead cells was observed in the control group, whereas only a few
dead cells were detected in the experimental group (Fig. 4A).
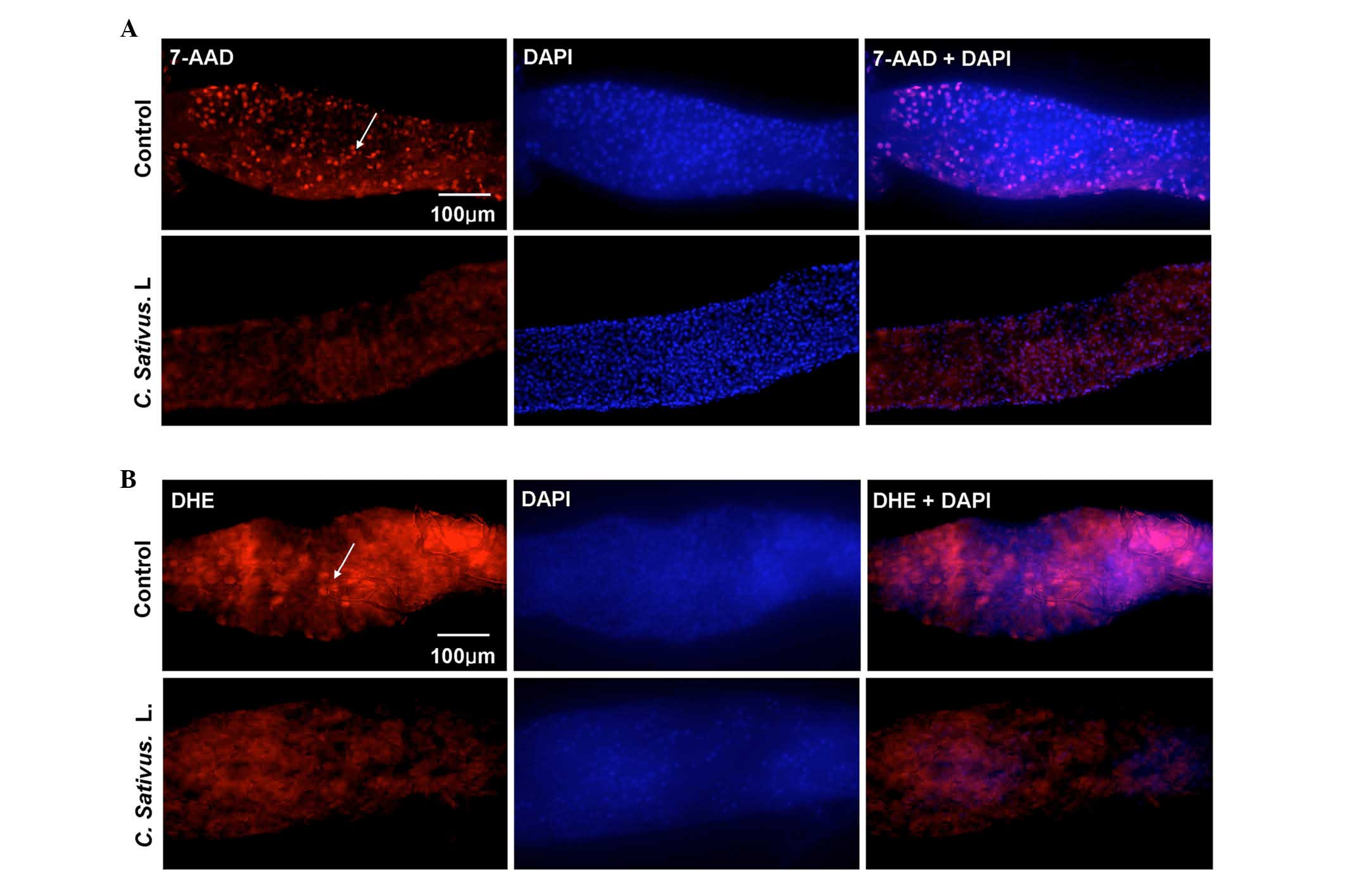 | Figure 4.C. sativus L. extracts protect
against SDS-induced epithelial cell damage. (A) The 7-AAD staining
of mid guts of female flies following ingestion of SDS (0.6%, w/v)
for 96 h. (B) DHE staining of mid guts of female flies following
ingestion of SDS (1%, w/v) for 48 h. The control contained standard
cornmeal-yeast medium, whereas the C. sativus L. group had a
standard medium containing 1% C. sativus L. extracts (w/v).
The results represent at least three independent experiments.
7-AAD, dead cells (red); DHE, reactive oxygen species levels (red);
DAPI, nucleus (blue). 7-AAD, 7-amino-actinomycin D; DHE,
dihydroethidium; DAPI, 4′,6-diamidino-2-phenylindole. |
Various stresses may lead to the production of
excessive ROS, which may then cause oxidative damage, and
ultimately cell death. This depends on the maintenance of an
equilibrium between ROS production and scavenging. Bandegi et
al (25) have proposed that
the C. sativus L. extract and its active constituent, Crocin
I, may prevent chronic stress-induced oxidative stress damage to
the brain, liver and kidneys in rats (25). The present study determined whether
treatment with C. sativus L. eliminated the excessive ROS
levels in the Drosophila intestine. DHE, a redox sensitive
dye that intercalates into cellular DNA when oxidized (26), was used to quantify ROS levels.
Adult flies were exposed to SDS for 48 h. DHE fluorescence was
reduced in the posterior midgut of the C. sativus L.-fed
group compared with the control (Fig.
4B). These findings demonstrated that C. sativus L.
extract may maintain the host redox homeostasis following SDS
exposure, therefore protecting against damage of intestinal
epithelial cells.
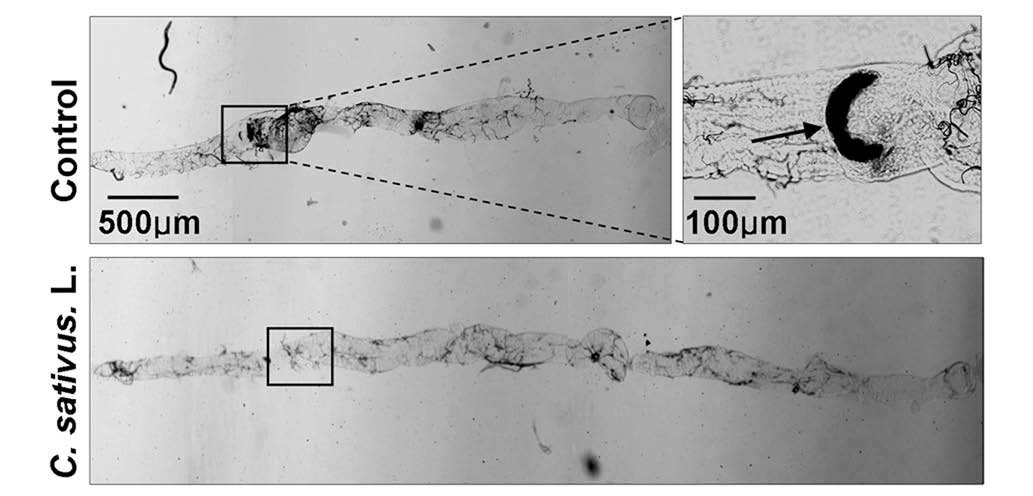
C. sativus L. extract maintains
Drosophila intestinal morphology following SDS ingestion
A previous study revealed that SDS may induce
melanotic tumors and morphological changes in the Drosophila
intestine (21). Following
treatment with 0.6% SDS for 4 days, the intestinal length of the
control group was observably shorter than the C. sativus
L.-fed group. Additionally, melanotic tumors were observed in the
posterior midgut of the control group. No melanotic masses were
observed in the C. sativus L.-fed group (Fig. 5). These findings indicated that
increased epithelial cell death may induce morphological changes in
the adult Drosophila intestine.
Discussion
The intestinal epithelium in the majority of animals
undergoes rapid regeneration under homeostatic conditions and in
response to tissue damage (3). The
generation of ROS and local production of AMPs are two
complementary effector mechanisms of controlling pathogen infection
in the Drosophila intestine (4). In addition, nutritional status and
tissue damage may influence stem cell turnover rates. The loss of
intestinal homeostasis is critical for inflammatory diseases of the
intestine, and may influence overall health and lifespan (27).
C. sativus L., also termed saffron, is a
small perennial plant from the Iridaceae family, which is widely
cultivated, and its stigma is used medicinally. Drosophila
flies frequently inhabit decaying and fermenting matter; therefore,
they are exposed to numerous bacteria, fungi and viruses, which
they must defend themselves against. The simplicity of the
structure and the multipotency of the Drosophila posterior
midgut make it a suitable model for investigating adult epithelial
cell homeostasis and regeneration. The present study investigated
the protective effect of C. sativus L in intestinal immunity
using Drosophila as a model system to analyze lifespan and
intestinal homeostasis following the ingestion of toxic compounds.
Significant increases in the survival rate of Drosophila
following SDS, DSS and paraquat treatment were observed in the
C. sativus L. feeding group compared with the control group.
This may be due to the elimination of excess ROS by treatment with
C. sativus L., therefore decreasing epithelial cell death
and improving intestinal morphology. In order for intestinal
homeostasis to be maintained, the rate of stem cell division is
increased in response to intestinal damage (8). However, 1% C. sativus L.
extract did not significantly change the proliferation of stem
cells (data not shown). Therefore, the mechanism by which C.
sativus L. protects the intestine may be due to a decrease in
epithelial cell death.
Aerobic metabolism, with the concomitant generation
of ROS, remains the most widely accepted cause of aging. The
present study determined higher survival rates in the C.
sativus L. feeding group following treatment with ROS-producing
agents, such as paraquat. Furthermore, the maximum and mean
lifespan was significantly increased in female and male adult flies
(Fig. 2). These findings indicated
that C. sativus L. extracts may eliminate ROS in the
Drosophila intestine, thus protecting adult flies. A
previous study determined that C. sativus L. and its active
constituent, Corcin I, may exert a protective effect against
chronic stress-induced oxidative damage of the brain, liver and
kidneys in rats (23).
Furthermore, safranal (an organic compound isolated from saffron)
may be effective in protecting the susceptible brains of aged rats
from oxidative damage by increasing antioxidant defenses (28). Further investigation is required to
determine the active components that exert protective effects on
the Drosophila intestine.
In conclusion, the findings of the present study
revealed the preventive effect of C. sativus L. on
Drosophila intestinal damage, and its ability to increase
lifespan. A possible mechanism to account for these findings may be
that the active constituent exerts anti-inflammatory and
anti-oxidant effects.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. J1210053 and
31270923) and University Student Innovation Program of Northeast
Forestry University (grant no. 201410225019).
References
|
1
|
Macpherson AJ and Harris NL: Interactions
between commensal intestinal bacteria and the immune system. Nat
Rev Immunol. 4:478–485. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casali A and Batlle E: Intestinal stem
cells in mammals and Drosophila. Cell Stem Cell. 4:124–127. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buchon N, Broderick NA, Poidevin M,
Pradervand S and Lemaitre B: Drosophila intestinal response to
bacterial infection: Activation of host defense and stem cell
proliferation. Cell Host Microbe. 5:200–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ha EM, Oh CT, Bae YS and Lee WJ: A direct
role for dual oxidase in Drosophila gut immunity. Science.
310:847–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW,
Jang IH, Brey PT and Lee WJ: An antioxidant system required for
host protection against gut infection in Drosophila. Dev Cell.
8:125–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liehl P, Blight M, Vodovar N, Boccard F
and Lemaitre B: Prevalence of local immune response against oral
infection in a Drosophila/Pseudomonas infection model. PLoS Pathog.
2:e562006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryu JH, Ha EM, Oh CT, Seol JH, Brey PT,
Jin I, Lee DG, Kim J, Lee D and Lee WJ: An essential complementary
role of NF-kappaB pathway to microbicidal oxidants in Drosophila
gut immunity. EMBO J. 25:3693–3701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buchon N, Broderick NA, Chakrabarti S and
Lemaitre B: Invasive and indigenous microbiota impact intestinal
stem cell activity through multiple pathways in Drosophila. Genes
Dev. 23:2333–2244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chatterjee M and Ip YT: Pathogenic
stimulation of intestinal stem cell response in Drosophila. J Cell
Physiol. 220:664–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amcheslavsky A, Jiang J and Ip YT: Tissue
damage-induced intestinal stem cell division in Drosophila. Cell
Stem Cell. 4:49–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biteau B, Hochmuth CE and Jasper H: JNK
activity in somatic stem cells causes loss of tissue homeostasis in
the aging Drosophila gut. Cell Stem Cell. 3:442–455. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang H, Patel PH, Kohlmaier A, Grenley
MO, McEwen DG and Edgar BA: Cytokine/Jak/Stat signaling mediates
regeneration and homeostasis in the Drosophila midgut. Cell.
137:1343–1345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seisenbacher G, Hafen E and Stocker H:
MK2-dependent p38b signalling protects Drosophila hindgut
enterocytes against JNK-induced apoptosis under chronic stress.
PLoS Genet. 7:e10021682011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bathaie SZ, Farajzade A and Hoshyar R: A
review of the chemistry and uses of crocins and crocetin, the
carotenoid natural dyes in saffron, with particular emphasis on
applications as colorants including their use as biological stains.
Biotech Histochem. 89:401–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt M, Betti G and Hensel A: Saffron
in phytotherapy: Pharmacology and clinical uses. Wien Med
Wochenschr. 157:315–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poma A, Fontecchio G, Carlucci G and
Chichiriccò G: Anti-inflammatory properties of drugs from saffron
crocus. Antiinflamm Antiallergy Agents Med Chem. 11:37–51. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosseinzadeh H and Younesi HM:
Antinociceptive and anti-inflammatory effects of Crocus sativus L.
stigma and petal extracts in mice. BMC Pharmacol. 2:72002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sales E Hamid, Motamedi Sedeh F and
Rajabifar S: Effects of gamma irradiation and silver nano particles
on microbiological characteristics of saffron, using hurdle
technology. Indian J Microbiol. 52:66–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghadrdoost B, Vafaei AA, Rashidy-Pour A,
Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR and
Pahlvan S: Protective effects of saffron extract and its active
constituent crocin against oxidative stress and spatial learning
and memory deficits induced by chronic stress in rats. Eur J
Pharmacol. 667:222–2229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kianbakht S and Ghazavi A:
Immunomodulatory effects of saffron: A randomized double-blind
placebo-controlled clinical trial. Phytother Res. 25:1801–1805.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Luo Q and Jin LH: Acanthopanax
senticosus extracts have a protective effect on Drosophila gut
immunity. J Ethnopharmacol. 146:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao Y, Li Y and Yao N: Simultaneous
determination of salidroside and tyrosol in extracts of Rhodiola L.
by microwave assisted extraction and high-performance liquid
chromatography. J Pharm Biomed Anal. 45:510–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fontana L, Partridge L and Longo VD:
Extending healthy life span-from yeast to humans. Science.
328:321–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boyd O, Weng P, Sun X, Alberico T, Laslo
M, Obenland DM, Kern B and Zou S: Nectarine promotes longevity in
Drosophila melanogaster. Free Radic Biol Med. 50:1669–1678. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bandegi AR, Rashidy-Pour A, Vafaei AA and
Ghadrdoost B: Protective effects of Crocus sativus L. extract and
Crocin against chronic-stress induced oxidative damage of brain,
liver and kidneys in rats. Adv Pharm Bull. 4:(Suppl 2). S493–S499.
2014.
|
|
26
|
Hochmuth CE, Biteau B, Bohmann D and
Jasper H: Redox regulation by Keap1 and Nrf2 controls intestinal
stem cell proliferation in Drosophila. Cell Stem Cell. 8:188–199.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Brien LE, Soliman SS, Li X and Bilder D:
Altered modes of stem cell division drive adaptive intestinal
growth. Cell. 147:603–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samarghandian S, Azimi-Nezhad M and Samini
F: Preventive effect of safranal against oxidative damage in aged
male rat brain. Exp Anim. 64:65–71. 2015. View Article : Google Scholar : PubMed/NCBI
|