Introduction
Renal cell carcinoma (RCC) is one of the most
commonly detected urological tumors, which comprises ~3% of
detected human malignancies (1).
During the last 30 years, a marked increase has been observed in
the incidence and mortality rate of RCC (1). Histologically, there are several
subtypes of RCC, and the associated genetic and biological features
determine the success of the chosen treatment (2). In ~30% of patients RCC is detected at
its metastatic stage, making treatment difficult (3). Despite advances in chemotherapy,
which led to the development of multikinase inhibitors, the
survival rate of patients with RCC remains low (4,5).
Enhanced Jagged1 and Notch1 expression has been reported to be
associated with a worse prognosis in patients with carcinoma
(6).
Blister beetles constitute an important member of
the Meloidae family of the Coleoptera order, and are of traditional
medicinal importance (7).
Phytochemical analysis of blister beetles has led to the isolation
of several phytochemicals, including cantharidin, which is a
terpenoid molecule. Cantharidin is used in Chinese medicine and has
been shown to exert a wide range of biological activities (8). Treatment with cantharidin has been
reported to induce cell cycle arrest and promote apoptosis in
various types of carcinoma cell lines (9), including hepatoma (10), colon (11), bladder (12), breast (13), oral buccal, and leukemia cells
(14). The present study aimed to
investigate the effects of cantharidin on the inhibition of cell
proliferation, cell cycle arrest and induction of apoptosis in RCC
cell lines. Furthermore, its effects on Notch1 and Jagged1
expression in RCC tissues were investigated. The results of the
present study revealed a significant reduction in cell viability,
and an induction of cell cycle arrest at G2/M phase and
apoptosis in ACHN and Caki-1 RCC cells.
Materials and methods
Reagents and chemicals
Cantharidin, propidium iodide (PI) and dimethyl
sulfoxide (DMSO) were purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). RPMI-1640 medium, 10% fetal bovine
serum (FBS) and 100 U/ml penicillin and 100 µg/ml streptomycin were
obtained from Merck Millipore.
Cell lines and culture
ACHN and Caki-1 RCC cell lines were obtained from
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in RPMI-1640 medium supplemented with 10% FBS and
penicillin-streptomycin at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air.
Cell viability assay
The effects of cantharidin on the viability of RCC
cells were determined using the CellTiter 96® AQueous
One Solution Cell Proliferation (MTS) assay (Promega Corporation,
Madison, WI, USA). Briefly, the cells were dispersed at a density
of 5×105 cells/well onto 96-well microtiter plates in
100 µl culture medium and incubated for 48 h at 37°C. Next, the
cells were treated with cantharidin (0, 5, 10, 15, 20, 25 µM) fro
24 h, and the cells were incubated for an additional 24 h at 37°C
or with 20 µM cantharidin for 0, 12, 24, 48 and 72 h at 37°C. The
control cultures (received 0 µM cantharidin) were treated with DMSO
only for 24 h. An EnVision Multilabel Plate Reader (PerkinElmer,
Inc., Waltham, MA, USA) was used to measure absorbance at 465 nm.
All experiments were carried out three times and the results are
presented as the mean ± standard deviation.
Flow cytometric analysis
Apoptosis of RCC cells was determined by flow
cytometry using Annexin V binding and PI staining. Following
incubation with 20 µM cantharidin or DMSO (control) at 37°C for 48
h, the cells were washed twice with ice-cold phosphate-buffered
saline (PBS). The cells were subsequently double-stained with
fluorescein isothiocyanate (FITC)-conjugated Annexin V (Trevigen,
Inc., Gaithersburg, MD, USA) and PI (Sigma-Aldrich; Merck
Millipore) for 30 min at room temperature in the dark. A 488-nm
laser coupled to a cell sorter (FACSCalibur; BD Biosciences, San
Jose, CA, USA) was used for flow cytometric analysis an
microscopy.
Cell cycle analysis
ACHN and Caki-1 RCC cells were seeded at a density
of 5×105 cells/well onto 96-well cell culture plates
(Corning Inc., New York, NY, USA). The cells were incubated with
various concentrations of 20 µM cantharidin or DMSO (control) for
48 h at 37°C. After 48 h of incubation, the cells were trypsinized,
washed with PBS and resuspended into single cell suspension. Then,
the single cell suspension was fixed in 70% ethanol at −20°C for 24
h. The cells were washed with PBS and incubated in 100 µg/ml PI
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 200 µg/ml
RNase (Sigma-Aldrich; Merck Millipore) for 30 min in the dark. Flow
cytometry (BD FACSArray; BD Biosciences, Franklin Lakes, NJ, USA)
was used to determine cell cycle distribution following
incubation.
Tissue samples
The tumor renal tissue samples were obtained from
patients with RCC during an operation at the Department of Urology,
Ningbo Urology and Nephrology Hospital (Ningbo, China). The renal
tissue samples were obtained from 10 patients between the age of
32–49 (6 males and 4 females). Immediately after extraction, the
RCC tissue samples were frozen and stored in liquid nitrogen until
further analysis. The present study was approved by the ethical
committee of Ningbo Urology and Nephrology Hospital. Written
consent was obtained from all of the patients.
Western blot analysis
The non-cantharidin-treated neoplastic renal tissue
and 20 µM cantharidin-treated renal cell carcinoma tissue grind
broken fully in liquid nitrogen, and then treated with lysis buffer
containing 150 mM NaCl, 10 mM Tris-HCl (pH 7.9), 0.5% Triton X-100,
0.6% NP-40, and the following protease inhibitors: 1 mg/ml
leupeptin, 1 mg/ml pepstatin A and 2 mg/ml aprotinin. Sonication of
the lysates for 15 sec was followed by centrifugation at 12,000 × g
for 45 min at 4°C. The detergent compatible protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for
determination of protein content. Subsequently, the proteins (50
µg) were separated by 10% sodium dodecyl sulphate-polyacrylamide
gel electrophoresis and were transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). The membranes were
incubated with blocking buffer (PBS containing 7.5% non-fat dry
milk, 2% bovine serum albumin and 0.1% Tween) for 2 h at room
temperature, and were then incubated with anti-Notch1 (cat. no.
3680; 1:1,000; Cell Signaling Technology, Inc.), anti-GAPDH (cat.
no. 2118; 1:1,000; Cell Signaling Technology, Inc.) and
anti-Jagged1 (cat. no. ab109536; 1:1,000; Abcam, Cambridge, UK) at
4°C overnight followed by washing with PBS containing 0.1%
Tween-20. Subsequently, the membranes were incubated with
peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no.
BA1055; Boster Biological Technology, Ltd., Wuhan, China) at room
temperature for 1 h. After washing with PBS, the membranes were
developed using an enhanced chemiluminescence detection system
(Amersham; GE Healthcare Life Sciences, Uppsala, Sweden).
Immunohistochemistry
Paraffin-embedded RCC tissues were sliced into 2 µM
sections. The sections were deparaffinized in xylene, followed by
rehydration in gradient alcohol, and were treated with hydrogen
peroxide. Following boiling with citrate buffer (pH 6.0) for 20
min, the tissues were incubated for 1 h at room temperature with
goat serum (Invitorgen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and were washed with PBS. The primary antibody was added
for incubation at room temperature for 30 min and then maintained
overnight at 4°C. The primary antibodies were anti-Notch1 (1:400),
anti-GAPDH (1:800) and anti-Jagged1 (1:300) and the slices were
incubated overnight at 4°C. The secondary antibody was goat
anti-rabbit IgG H&L (cat. no. ab109536; 1:5,000; Abcam) and the
slices were maintained at room temperature for 30 min. The slides
were then stained with 3,3′-diaminobenzidine and were
counterstained with hematoxylin & eosin after being washed with
PBS. The stained slices were photographed using fluorescence
inverted microscope (BSF-60, Shanghai Batuo Instrument Co., Ltd.,
Shanghai, China).
Statistical analysis
All experiments were performed in triplicate and the
results are presented as the mean ± standard deviation. One-way
analysis of variance was used for statistical analysis followed by
Tukey's Honest Significant Difference test as a post-hoc
comparison. SPSS version 18 (SPSS, Inc., Chicago, IL, USA) was used
for analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
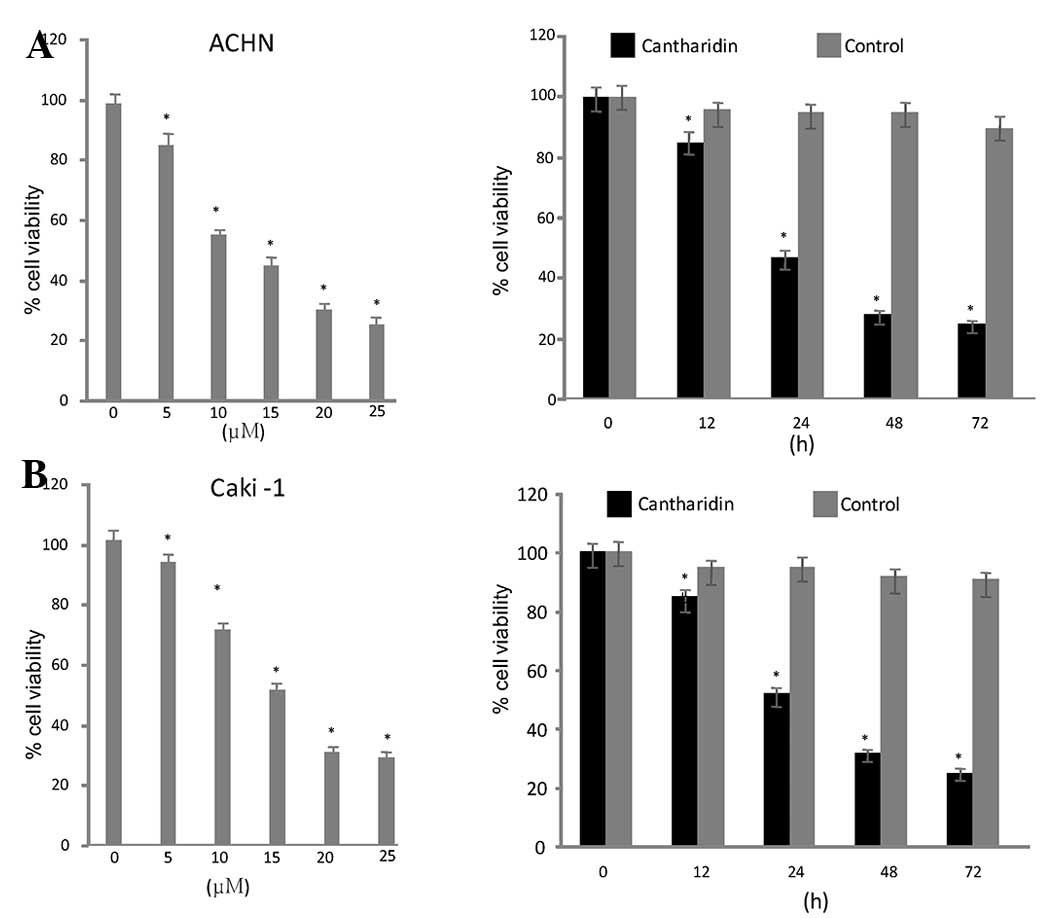
Cantharidin inhibits growth of RCC
cells
Exposure of the RCC cell lines to a range of
concentrations of cantharidin (5–25 µg/ml) for various durations
resulted in a dose- and time-dependent reduction in cell viability.
ACHN and Caki-1 cells exhibited a significant reduction in
viability after 48 h treatment with 20 µM cantharidin. Cell
viability was reduced to 26 and 32% in ACHN and Caki-1 cells
respectively, after 48 h (Fig.
1).
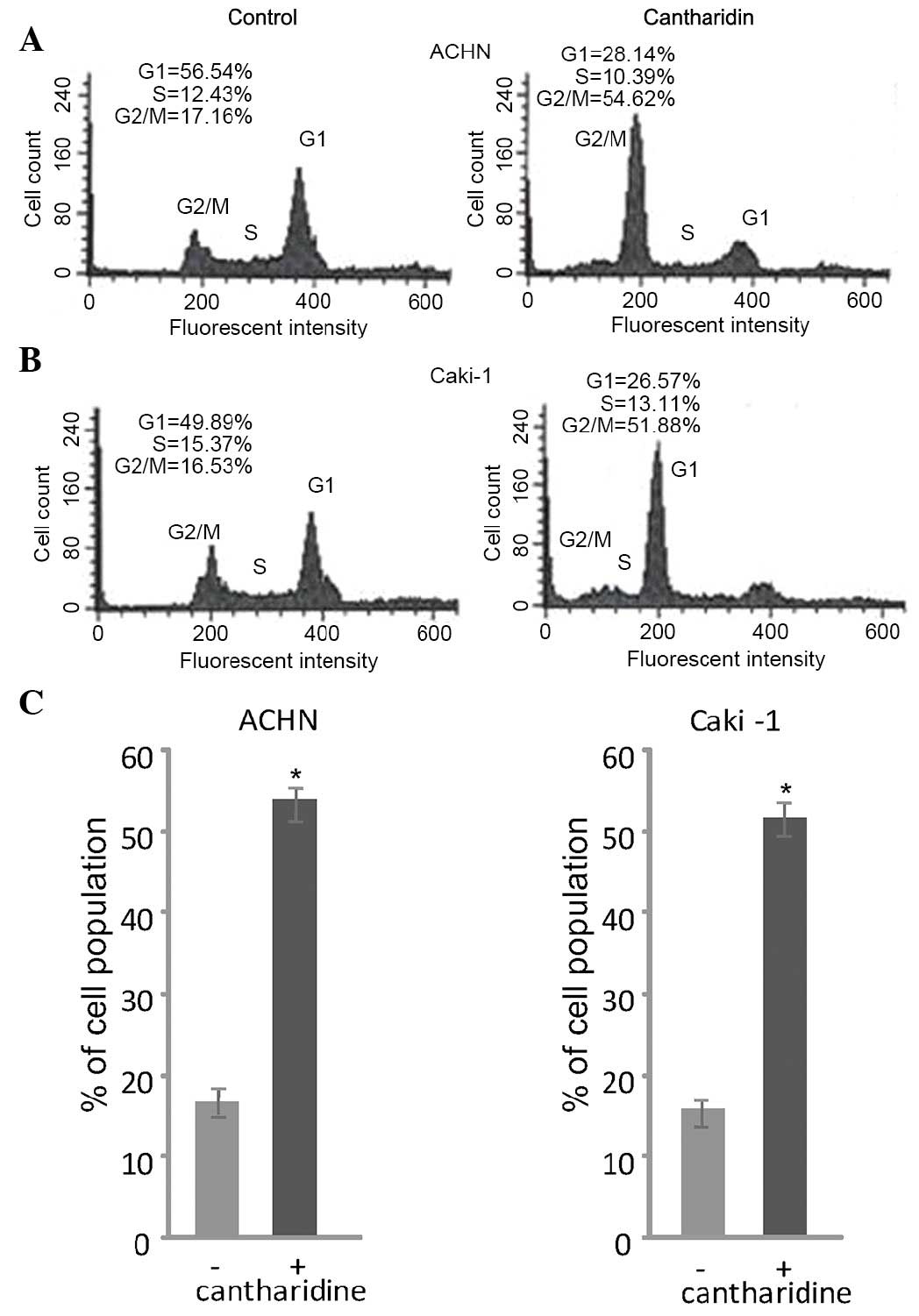
Cantharidin treatment induces a cell
cycle arrest at G2/M phase
Flow cytometry revealed that treatment of RCC cells
with cantharidin induced a cell cycle arrest at G2/M phase.
Treatment of ACHN and Caki-1 RCC cells with 20 µM cantharidin
induced a marked increase in the population of cells in
G2/M phase after 48 h. In cantharidin-treated ACHN cells
the proportion of cells in G2/M phase was 54.62%, as
compared with 17.16% in the control group. Similarly, cantharidin
treatment enhanced the proportion of Caki-1 cells in
G2/M phase to 51.887%, as compared with 16.53% in the
control group (Fig. 2).
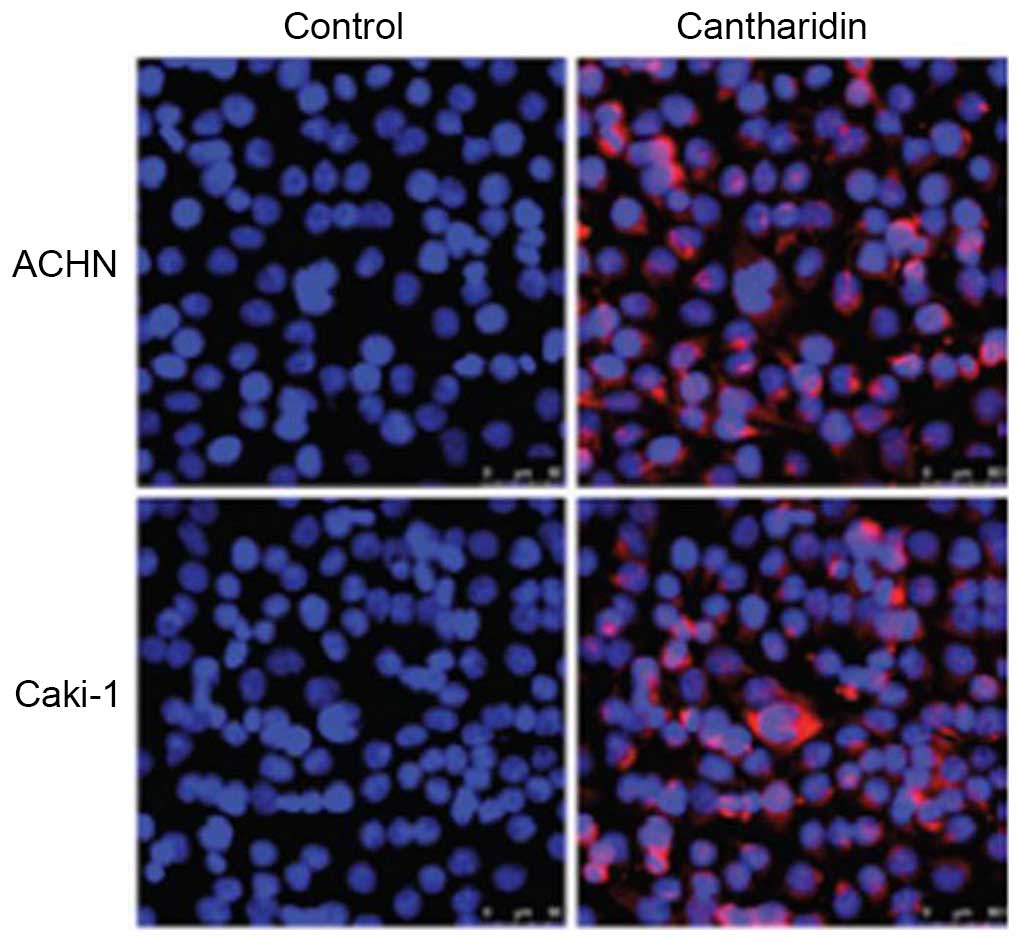
Effects of cantharidin on
apoptosis
In order to investigate the effects of cantharidin
(20 µM) on apoptosis of RCC cells, flow cytometry using Annexin
V-FITC/PI double-staining was performed. Condensation of chromatin
and fragmentation of nuclear material was detected in ACHN and
Caki-1 cells following a 48 h exposure to cantharidin (Fig. 3). The percentage of apoptotic ACHN
cells in the cantharidin-treated group was 57.23%, as compared with
2.27% in the control. The percentage of apoptotic
cantharidin-treated Caki-1 cells was 62.34%, as compared with 3.06%
in the control group.
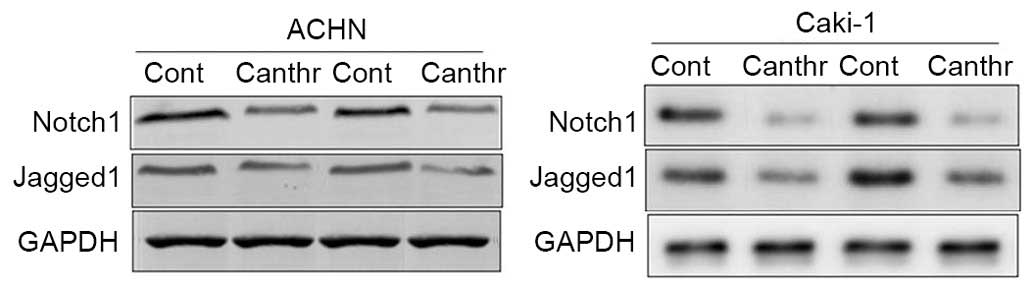
Notch1 and Jagged1 expression in renal
carcinoma tissues
Notch1 and Jagged1 expression was detected in RCC
tissues, as determined by western blot analysis. The results
demonstrated that Notch1 and Jagged1 expression was increased in
all 12 tissues collected from patients with RCC (Fig. 4; representative blots from two
patients are presented).
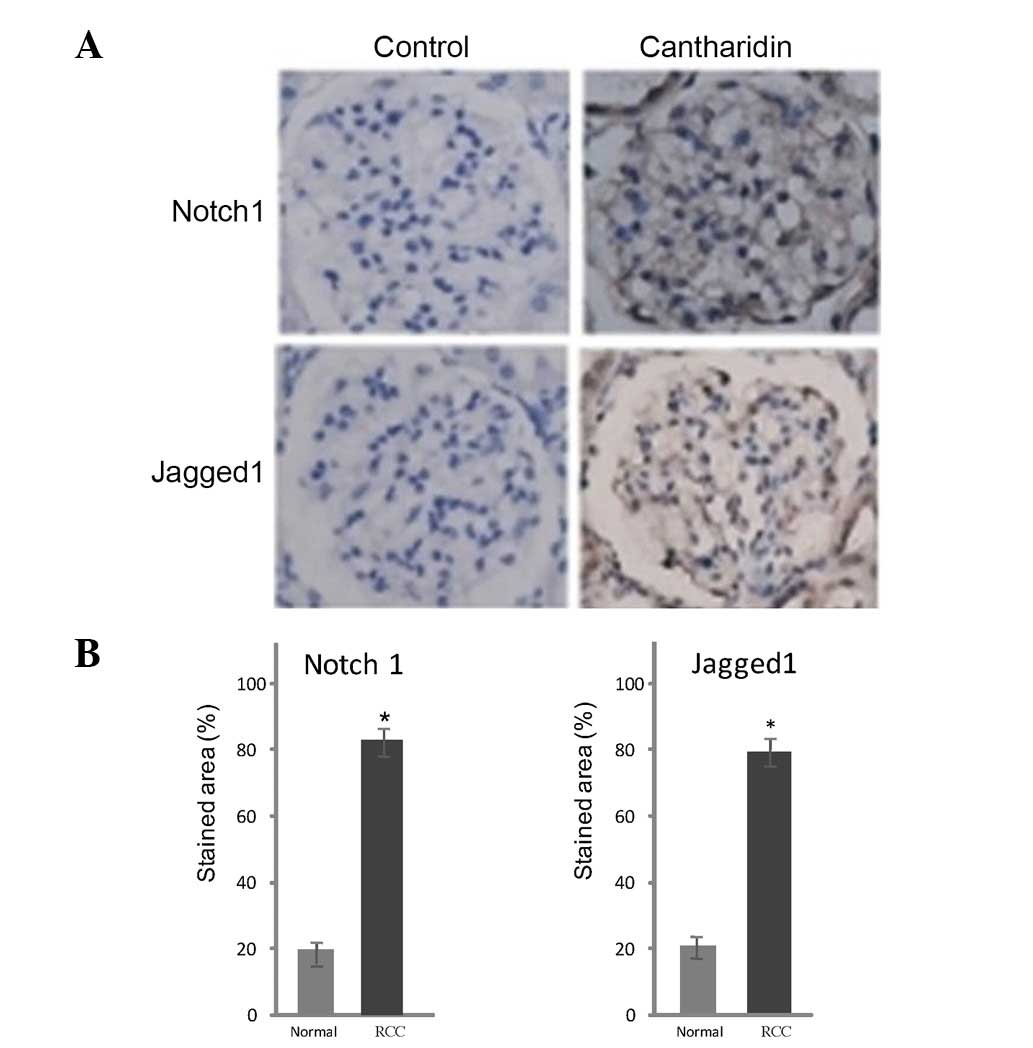
Immunohistochemical analysis
Notch1 and Jagged1 expression was detected in tissue
samples from patients with RCC. Markedly lower levels of these
proteins were detected in control tissues compared with in RCC
tissues (Fig. 5). Treatment of RCC
tissues with cantharidin resulted in a marked reduction in Notch1
and Jagged1 expression after 48 h. Notch1- and Jagged1-positive
staining was 86 and 79% in the RCC tissues (control) and 18 and 21%
respectively in the cantharidin-treated tissues (Fig. 5).
Discussion
Cantharidin is a terpenoid compound, which has been
reported to exhibit promising biological activity (8). Cantharidin induces apoptosis in
various types of cancer cells, including, hepatoma (10), colon (11), bladder (12), breast (13), oral buccal, and leukemia cells
(14). In addition, treatment of
bladder carcinoma cells with cantharidin induces cell cycle arrest
(12). Therefore, the present
study aimed to investigate the effects of cantharidin on
suppression of cell viability, cell cycle arrest and induction of
apoptosis in RCC cell lines. In addition, the effects of
cantharidin on Notch1 and Jagged1 expression were detected in RCC
tissues. The results of the present study revealed that cantharidin
resulted in a marked reduction in cell viability, and induced cell
cycle arrest at G2/M phase and apoptosis.
Apoptosis is a type of programmed cell death, which
is responsible for the elimination of unwanted and damaged cells
from the body. In the case of carcinoma, the process of apoptosis
is disrupted resulting in uncontrolled cell growth. The results of
the present study indicated that treatment of RCC cells with
cantharidin markedly induced apoptosis compared with the control
cells. The Notch pathway exhibits a dual role in the progression of
carcinoma, either by promoting or inhibiting cell proliferation
(15,16). Examination of RCC tissues in the
present study revealed markedly increased Notch1 and Jagged1
expression; however, treatment of the RCC tissues with cantharidin
led to a reduction in Notch1 and Jagged1 expression.
Notch1 expression promotes the progression of tumor
angiogenesis and inhibits the expression of cyclin-dependent
kinase, which is involved in cell cycle regulation (17–20).
The results of the present study demonstrated that treatment with
cantharidin induced cell cycle arrest at G2/M phase;
therefore, it may be suggested that cantharidin-induced cell cycle
arrest, apoptosis induction and cell proliferation inhibition in
ACHN and Caki-1 RCC cells is associated with inhibition of Notch
signaling proteins. In conclusion, cantharidin exhibited an
inhibitory effect on RCC, and may be considered of vital importance
for its treatment. Nevertheless further investigations are required
to identify the precise underlying mechanism. In conclusion,
cantharidin treatment exhibits an inhibitory effect on renal cell
carcinoma. Therefore, it may be of therapeutic importance for the
treatment of renal cell carcinoma.
References
|
1
|
Schrader AJ, Sevinc S, Olbert PJ, Hegele
A, Varga Z and Hofmann R: Gender-specific characteristics and
survival of renal cell carcinoma. Urologe A. 47(1182): 1184–1186.
2008.(In German).
|
|
2
|
Tamaskar I, Choueiri TK, Sercia L, Rini B,
Bukowski R and Zhou M: Differential expression of caveolin-1 in
renal neoplasms. Cancer. 110:776–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ljungberg B, Hanbury DC, Kuczyk MA,
Merseburger AS, Mulders PF, Patard JJ and Sinescu IC: European
Association of Urology Guideline Group for renal cell carcinoma:
Renal cell carcinoma guideline. Eur Urol. 51:1502–1510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: TARGET Study Group: Sorafenib in advanced clear-cell
renal-cell carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu K, Xu L, Zhang L, Lin Z and Hou J: High
Jagged1 expression predicts poor outcome in clear cell renal cell
carcinoma. Jpn J Clin Oncol. 41:411–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Curry SC, Carlton MW and Raschke RA:
Prevention of fetal and maternal cyanide toxicity from
nitroprusside with coinfusion of sodium thiosulfate in gravid ewes.
Anesth Analg. 84:1121–1126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Honkanen RE: Cantharidin, another natural
toxin that inhibits the activity of serine/threonine protein
phosphatases types 1 and 2A. FEBS Lett. 330:283–286. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clarke PR, Hoffmann I, Draetta G and
Karsenti E: Dephosphorylation of cdc25-C by a type-2A protein
phosphatase: Specific regulation during the cell cycle in Xenopus
egg extracts. Mol Biol Cell. 4:397–411. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YN, Chen JC, Yin SC, Wang GS, Tsauer
W, Hsu SF and Hsu SL: Effector mechanisms of norcantharidin-induced
mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer.
100:158–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK,
Kuo YS, Chiang CP and Kuo MY: Norcantharidin-induced apoptosis in
oral cancer cells is associated with an increase of proapoptotic to
antiapoptotic protein ratio. Cancer Lett. 217:43–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Williams LA, Moller W, Merisor E, Kraus W
and Rosner H: In vitro anti-proliferation/cytotoxic activity of
cantharidin (Spanish Fly) and related derivatives. West Indian Med
J. 52:10–13. 2003.PubMed/NCBI
|
|
14
|
Yi SN, Wass J, Vincent P and Iland H:
Inhibitory effect of norcantharidin on K562 human myeloid leukemia
cells in vitro. Leuk Res. 15:883–886. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng AP and Aster JC: Multiple niches for
Notch in cancer: Context is everything. Curr Opin Genet Dev.
14:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi R, An H, Yu Y, Zhang M, Liu S, Xu H,
Guo Z, Cheng T and Cao X: Notch1 signaling inhibits growth of human
hepatocellular carcinoma through induction of cell cycle arrest and
apoptosis. Cancer Res. 63:8323–8329. 2003.PubMed/NCBI
|
|
18
|
Ridgway J, Zhang G, Wu Y, Stawicki S,
Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I,
et al: Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng Q, Li S, Chepeha DB, Giordano TJ, Li
J, Zhang H, Polverini PJ, Nor J, Kitajewski J and Wang CY:
Crosstalk between tumor and endothelial cells promotes tumor
angiogenesis by MAPK activation of Notch signaling. Cancer Cell.
8:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sjölund J, Johansson M, Manna S, Norin C,
Pietras A, Beckman S, Nilsson E, Ljungberg B and Axelson H:
Suppression of renal cell carcinoma growth by inhibition of Notch
signaling in vitro and in vivo. J Clin Invest. 118:217–228. 2008.
View Article : Google Scholar : PubMed/NCBI
|