Introduction
Natural immunity is the primary line of defense in
the resistance of the body against the invasion of foreign
pathogens. Induction of the immune response is dependent on
recognition and binding of pattern recognition receptors (PRRs),
which are found on the surface of natural immune cells, towards the
pathogen-associated molecular patterns (PAMPs), thus resulting in
activation of signaling pathways and the release of proinflammatory
cytokines (1). Toll-like receptors
(TLRs) are the most important innate PRRs that mediate the
generation of proinflammatory cytokines and initiate the immune
response. At present, 14 types of TLR have been identified in
mammals, which serve important roles in recognizing foreign
antigens, promoting the innate immune response, and promoting
activated signaling transduction in cells (2,3).
Although the number of dendritic cells (DCs) is
<1% of mononuclear cells in peripheral blood, their surface is
rich with antigen-presenting molecules [major histocompatibility
complex class I(MHC I) and MHC II], costimulatory molecules
[cluster of differentiation (CD)80/B7-1, CD86/B7-2, CD40 and CD44]
and adhesion molecules [intercellular adhesion molecule (ICAM)-1,
ICAM-2, ICAM-3, lymphocyte function-associated antigen (LFA)-1,
LFA-3]. Therefore, DCs are considered the strongest professional
antigen-presenting cells (APCs) (4). DCs express various PRRs (5), including TLRs, nucleotide-binding
oligomerization domain-like receptors, retinoic acid-inducible gene
I-like receptors and C-type lectins; these PRRS are able to
initiate the transduction of cell signaling and the expression of
related genes.
It has been reported that in response to
lipopolysaccharide (LPS), TLRs promote interleukin-1 (IL-1)-induced
signal transduction in order to mediate activation of other
antigen-induced immune cells; this process has been confirmed by
TLR2- and TLR4-mediated cellular responses (6). IL-1 receptor-associated kinases
(IRAKs) are factors in the TLR/IL-1 receptor (IL-1R) signaling
pathway, which has an important role in regulating autoimmunity
(7). Through kinase and adapter
capabilities, IRAKs are able to initiate the inflammatory cascade,
and ultimately lead to the expression of immune-associated genes.
LPS is able to promote the differentiation of DCs (8), via induction of the TLRs signaling
pathway, which regulates the differentiation and function of DCs
(9). After binding of LPS to TLR4,
regulation of the transcription of various genes occurs via nuclear
factor-κΒ (NF-κΒ) and mitogen-activated protein kinase (MAPK)
pathways, thus promoting the maturation of DCs. As a key factor in
the TLR/IL-1R signaling pathway, IRAK-1 has an important role in
the differentiation and maturation of DCs.
In the present study, RNA interference (RNAi) was
performed to silence the gene expression of IRAK-1 in DCs. The
present study aimed to determine the underlying mechanisms of
IRAK-1 towards the differentiation and maturation of DCs, as well
as to investigate its effects on DC physiological functions,
including antigen presentation and the promotion of lymphocyte
activation.
Materials and methods
Extraction and culture of DCs
Female C57 BL/6 mice (age, 4 weeks; weight, 13–15 g;
specific-pathogen-free level; Experimental Animal Center of Chinese
Medical University, Shenyang, China) were selected for use in the
present study. The mice were housed with a 12 h light/dark cycle,
at 22±2°C, and free access to food and water. Following
intraperitoneal injection of 4% chloraldurate (0.01 ml/g body
weight), the mice were sacrificed by cervical spine dislocation in
order to obtain the femurs, from which the bone marrow cells were
collected and placed into 10% fetal bovine serum (FBS)-containing
1640 medium (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany).
The cells were cultured at 37°C in an atmosphere containing 5% CO2
for 24 h. Subsequently, the supernatant was discarded, 10 µg/ml
IL-4, 10 µg/ml granulocyte-macrophage colony-stimulating factor
(GSM-CF) and 10% FBS-containing 1640 medium were added and the
cells were cultured for a further 6 days; half of the medium was
changed every 24 h. The supernatant was then discarded and the
cells were resuspended in 10% FBS-containing 1640 medium
supplemented with IL-4 and GSM-CF. Subsequently, the cells were
counted, re-seeded into 6-well plates (1×106/ml) and
cultured at 37°C in an atmosphere containing 5% CO2 for further
experimentation. The study was approved by the ethics committee of
Shengjing Hospital of China Medical University (Shenyang,
China).
Identification and purification of
bone marrow mesenchymal stem cells (BMSCs)-originated DCs
The DCs were incubated with anti-CD11c
phycoerythrin-conjugated antibody (50 µg/ml; cat. no. 12-0114-82;
eBioscience, Inc., San Diego, CA, USA) and isotype matched
antibodies for 30 min at 4°C. After incubation, cells were washed
twice with PBS, and then fixed in 2% paraformaldehyde
(Sigma-Aldrich; Merck Millipore) for 1 h at 4°C. Cellular
fluorescence was measured by FACScan (BD Biosciences, San Jose, CA,
USA), and data were analyzed by FlowJo 7.6.1 software (Tree Star,
Inc., Ashland, OR, USA), by calculating the percentage of positive
cells compared with the isotype controls. A CD11c MicroBeads
sorting kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) was
used to further separate DCs differentiated from BMSCs according to
the manufacturer's protocol, and flow cytometry was also used to
detect the ratio of CD11c+ cells.
RNAi
DCs cultured for 6 days and sorted using magnetic
beads were subsequently divided into three groups: LPS + mock
group, LPS + scrambled small interfering (si)RNA group and LPS +
IRAK-1 siRNA group. The LPS + mock group was cultured with IL-4,
GSM-CF and 10% FBS-containing 1640 medium at 37°C in an atmosphere
containing 5% CO2 for 24 h; the LPS + scrambled siRNA and LPS +
IRAK-1 siRNA groups were transfected with negative control siRNA or
IRAK-1 siRNA (Qiagen, Inc., Valencia, CA, USA), respectively, under
the same culture conditions as the LPS + mock group. The siRNA
groups underwent transfection using HiPerFect transfection kit
(Qiagen, Inc.), and were cultured at 37°C in an atmosphere
containing 5% CO2 for 24 h. All three groups were cultured for 24
h, and were then treated with 10 mg/l LPS (Sigma-Aldrich; Merck
Millipore) for 24 h.
Reverse transcription-quantitative
polymerase reaction (RT-qPCR)
After 24 h LPS stimulation, the total RNA was
extracted from the cells using RNAiso Plus kit (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's instructions. The reverse transcription reaction was
performed using PrimeScript™ RT kits (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. The RT reaction
was performed at 37°C for 15 min, followed by 85°C for 5 sec, and
the reaction system (20 µl for each sample) was as follows: 4 µl
PrimeScript Buffer (5X); 1 µl PrimeScript RT Enzyme Mix I; 1 µl
oligo dt Primer (50 µmol/l); 1 µl random hexamers (100 µmol/l); and
13 µl total RNA. The PCR system (15 µl for each sample) was as
follows: 7.5 µl Premix Ex Taq (2X); 0.25 µl forward primer (10
µmol/l); 0.25 µl reverse primer (10 µmol/l); 3 µl cDNA (5 ng/µl);
and 4 µl dH2O. Primers were designed and synthesized by
Takara Biotechnology Co., Ltd. (Dalian, China) with β-actin used as
the internal reference gene (Table
I). After initial denaturation for 15 min at 95°C, the PCR
conditions were as follows: For IRAK-1, 95°C for 30 sec, 50 cycles
of 95°C for 15 sec, 61°C for 15 sec, and 63°C for 34 sec; for TLR4,
95°C for 30 sec, 40 cycles of 95°C for 10 sec, 58°C for 15 sec, and
72°C for 10 sec; for IRAK-4, 95°C for 15 sec, 40 cycles of 95°C for
15 sec, 65°C for 15 sec, and 72°C for 10 sec; for NF-κB p65, 94°C
for 30 sec, 40 cycles of 95°C for 15 sec, 62°C for 15 sec, and 70°C
for 15 sec. The relative expression level was determined using the
2−ΔΔCq analysis method (10).
 | Table I.Primer sequences for quantitative
polymerase chain reaction. |
Table I.
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| IRAK-1 |
CGGACTTCCACAGTTCGAGGTA |
TGACCAGCAAGGGTCTCCAG |
| TLR4 |
TCACCTCTGCCTTCACTAC |
CGTTGGTGCGGTCTATGAG |
| NF-κB p65 |
GGATGGCTACTATGAGGCT | CTAATGGCTTGCTC,
CAGGTCTC |
| IRAK-4 |
ACATGCCCAACGGGTCCTT |
ACCTGATGCCATTTGCTGTCCC |
| β-actin |
TTCCAGCGTTCCTTCTTGGGTAT |
GTTGGCATAGAGGTGTTTACGG |
Flow cytometry
Following 24 h LPS stimulation, DCs in the three
groups were collected, washed twice with flow cytometry buffer and
adjusted to a cell concentration of 1×106/l. The cells
were then incubated with allophycocyanin-CD86 (cat. no. 11-0862),
phycoerythrin (PE)-MHC II (cat. no. 11-0367) and PE-CD40 (cat. no.
12-0401) antibodies (eBioscience, Inc.), at final concentrations of
5 µg/ml, in the dark at 4°C for 30 min. The surface-specific
markers of DCs, namely CD86, MHC II and CD40, were then detected
using flow cytometry.
ELISA assay
Following 24 h LPS stimulation, ELISA kits
(eBioscience, Inc.) were used to detect IL-10 (cat. no. 88-7314),
IL-12 P70 (cat. no. 88-7121) and tumor necrosis factor-α (TNF-α;
cat. no. 88-7324) in the cell supernatant.
Extraction of T lymphocytes
Female C57 BL/6 mice (age, 4 weeks) were sacrificed
and spleens were collected. The spleen samples were placed in PBS
and cut into small pieces using scissors. A 200-mesh sieve was then
used to gently grind the small spleen sections into a single cell
suspension, which was cleared using erythrocyte lysate for 5 min.
The suspension was then centrifuged at 300 × g for 6 min to
discard the supernatant, the cells were resuspended in PBS, and
were placed in 10% FBS-containing 1640 medium for further use.
Sorting of naive T lymphocytes
The Mouse CD4+/CD62L+/CD44 low
Naive Column sorting kit (R&D Systems, Inc., Minneapolis, MN,
USA) was used to sort the spleen single cell suspensions, according
to the manufacturer's protocol. Naive T cells that exhibited the
expression profile CD4+CD62L+CD44−
were obtained and cultured in 10% FBS-containing 1640 medium.
Mixed cell reaction
Following 24 h LPS stimulation, the cells of the
three groups were co-cultured with
CD4+CD62L+CD44− naive T cells; the
ratios of DCs to naive T cells used were 1:1, 1:10, 1:50 and 1:100.
After 48 h co-culture, the MTS assay kit (Promega Corporation,
Madison, WI, China) was used to detect the proliferation of naive T
cells according to the manufacturer's protocol. The absorbance at
detected at 490 nm.
Statistical analysis
All experiments were repeated three times. The data
are presented as the mean ± standard error of the mean, and were
analyzed using SPSS 16.0 statistical software (SPSS Inc., Chicago,
IL, USA). Intergroup comparisons were made using one-way analysis
of variance and least significant difference tests. Prism version
6.01 (GraphPad Software, Inc., La Jolla, CA, USA) was used to
generate the figures. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of BMSCs-originated
DCs
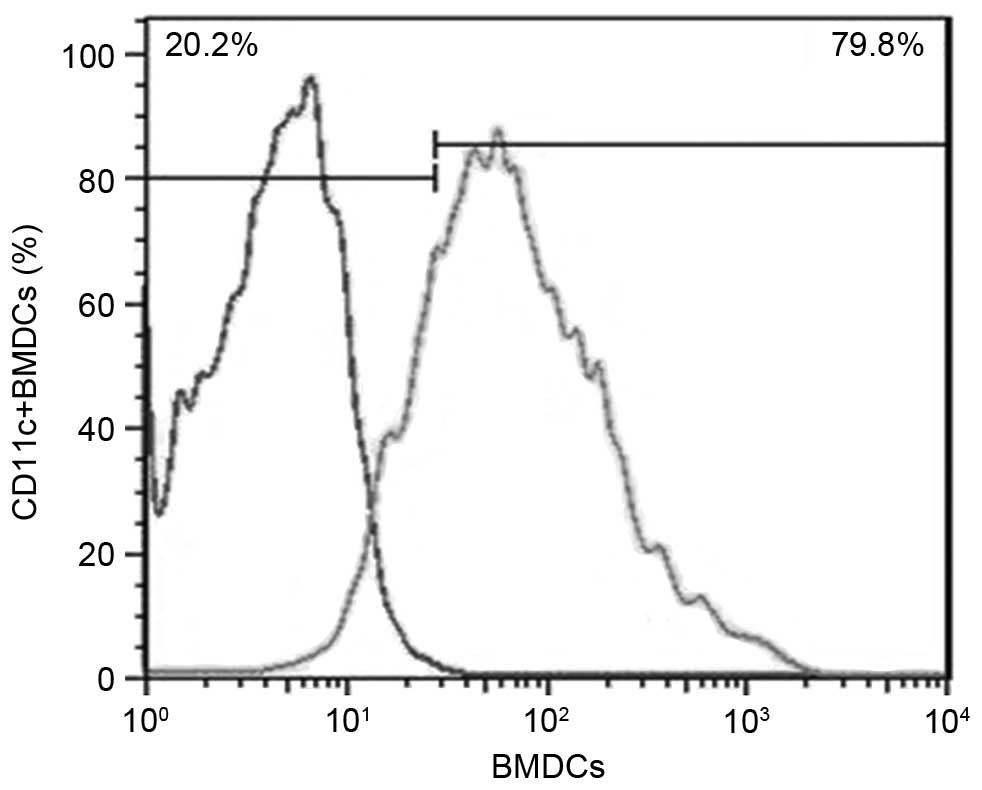
After BMSCs were cultured in medium containing
GSM-CF and IL-4, flow cytometry revealed that the ratio of
CD11c+ cells was 79.8% (Fig. 1). Cells obtained from the
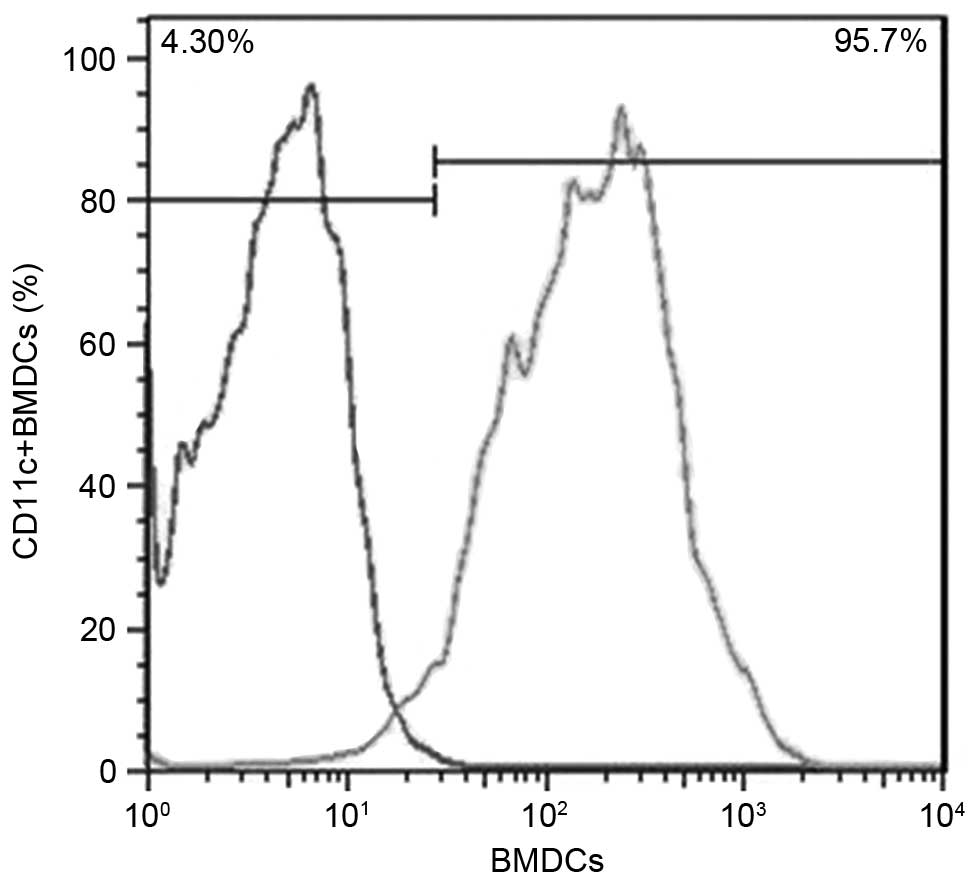
preliminary culture were then further sorted using magnetic beads,
and flow cytometry revealed that the ratio of CD11c+
cells was 95.7% (Fig. 2).
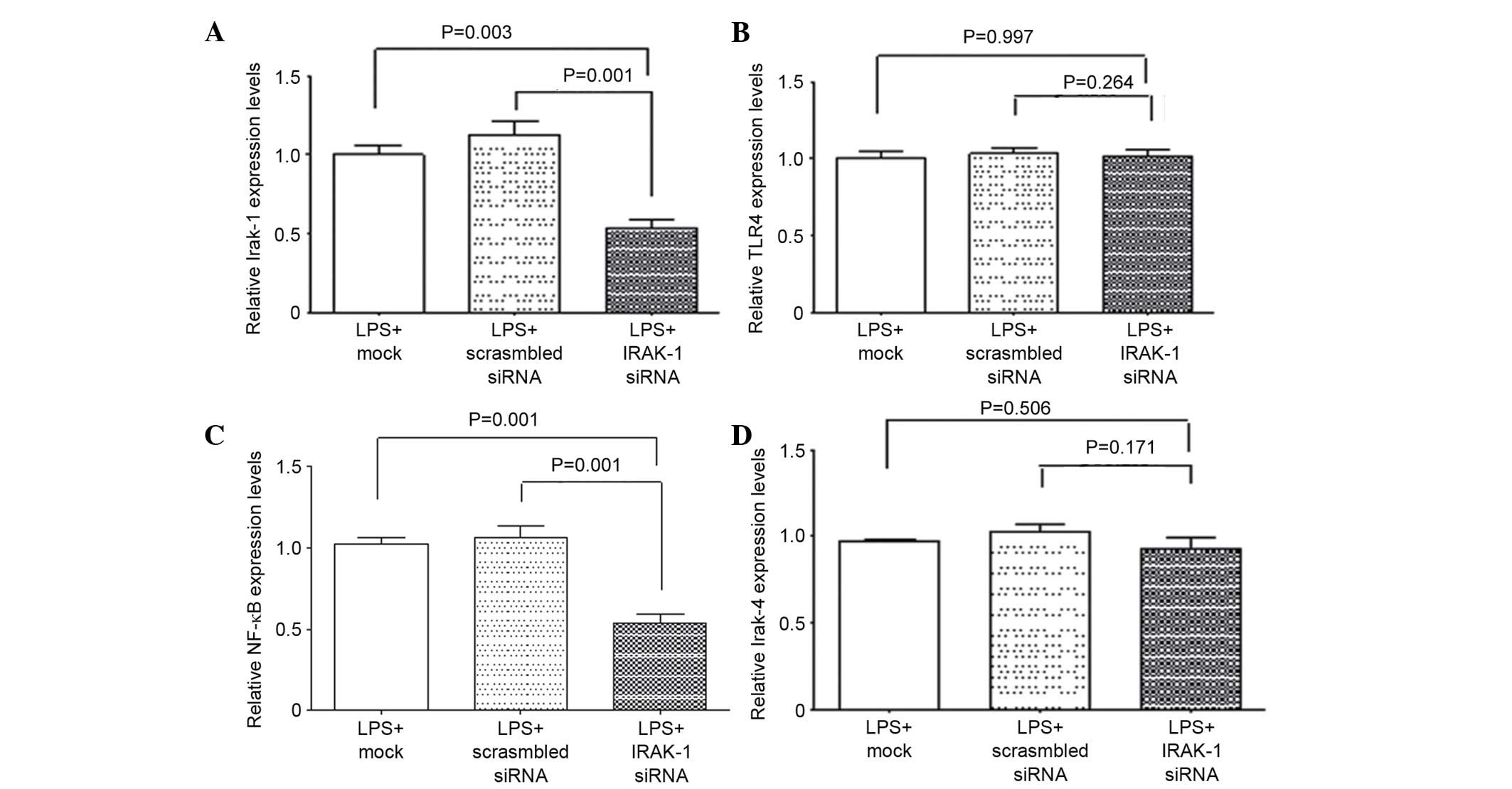
qPCR
qPCR was used to detect mRNA expression levels
(Fig. 3). Following LPS
stimulation, the mRNA expression levels of IRAK-1 in the LPS +
IRAK-1 siRNA group were significantly reduced (P<0.05; Fig. 3A). In addition, the mRNA expression
levels of NF-κB p65 were decreased in the LPS + IRAK-1 siRNA group
(P<0.05; Fig. 3C). There were
no significant differences in the mRNA expression levels of IRAK-1
and NF-κB p65 between the LPS + mock and LPS + scrambled siRNA
groups (Fig. 3A and C), thus
indicating that IRAK-1 RNAi could reduce its expression in DCs,
thus reducing the expression of NF-κB p65. Furthermore, the mRNA
expression levels of TLR4 and IRAK-4 were not significantly
different among the three groups (P>0.05; Fig. 3B and D). These results indicate
that interfering with the gene expression of IRAK-1 has no
significant effects on the mRNA expression levels of the upstream
molecules TLR4 and IRAK-4.
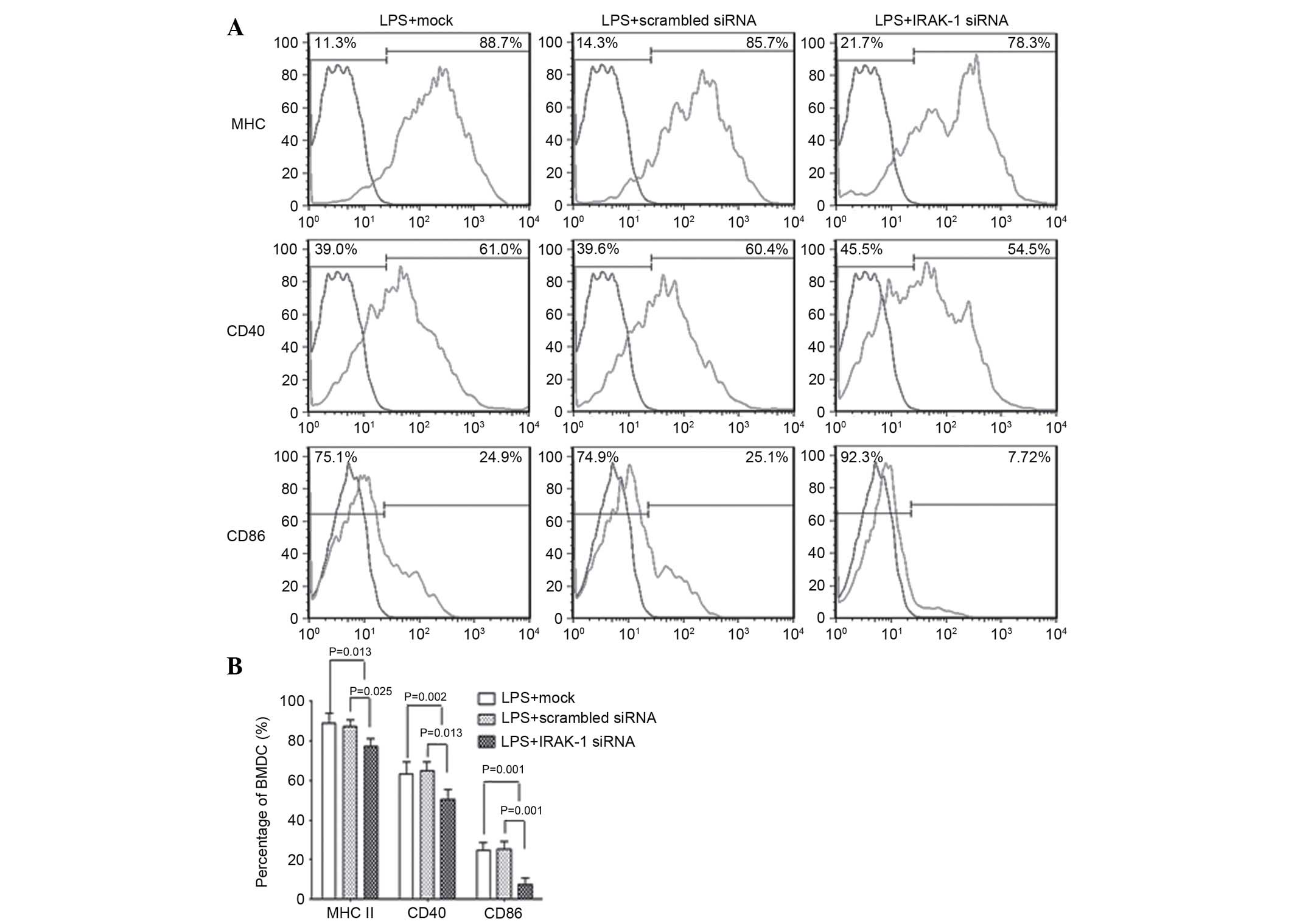
Flow cytometry
Following LPS stimulation, flow cytometry was
performed to detect MHC II, CD40 and CD86 expression among the
three groups. The results demonstrated that the expression levels
of MHC II, CD40 and CD86 in the LPS + mock and LPS + scrambled
siRNA groups were significantly higher compared with in the LPS +
IRAK-1 siRNA group (P<0.05). However, there was no significant
difference between the LPS + mock and LPS + scrambled siRNA groups
(Fig. 4). These results indicate
that following knockdown of IRAK-1 expression with RNAi, LPS
stimulation was able to reduce the expression levels of CD80, MHC
II and CD40 in DCs.
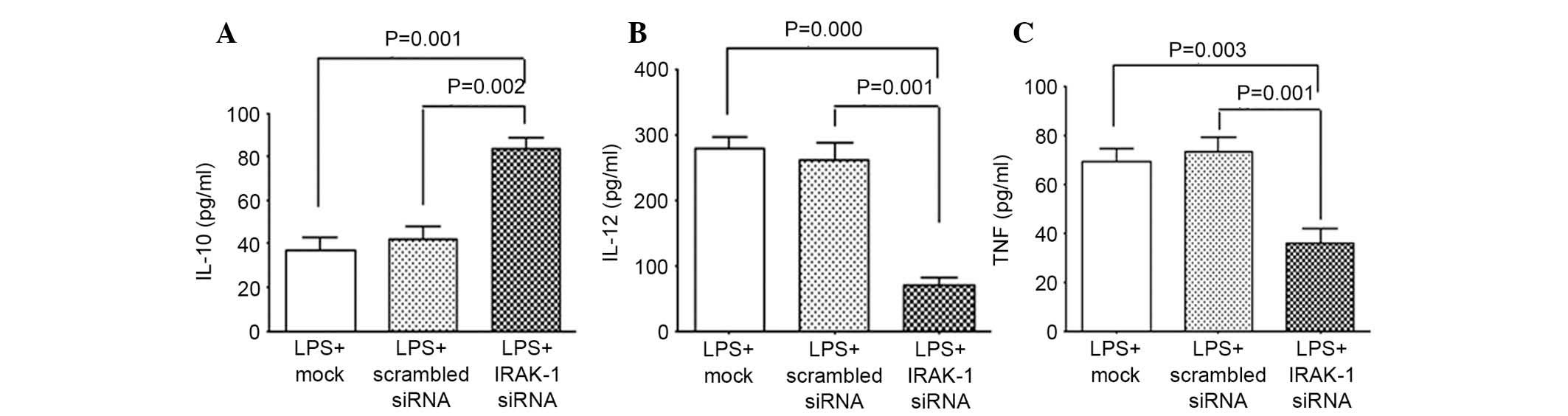
ELISA
ELISA kits were used to determine the release of
cytokines by DCs (Fig. 5). The
results demonstrated that following RNAi-induced knockdown of
IRAK-1 gene expression, LPS stimulation was able to significantly
reduce the expression levels of the inflammatory cytokines IL-12
and TNF-α (Fig. 5B and C), and
promote the expression of the anti-inflammatory cytokine IL-10
(Fig. 5A). There was no difference
in the expression levels of IL-12, TNF-α and IL-10 between the LPS
+ mock and LPS + scrambled siRNA groups; however, when compared
with the LPS + IRAK-1 siRNA group, the expression levels of IL-12
and TNF-α were significantly increased, whereas IL-10 expression
was significantly reduced.
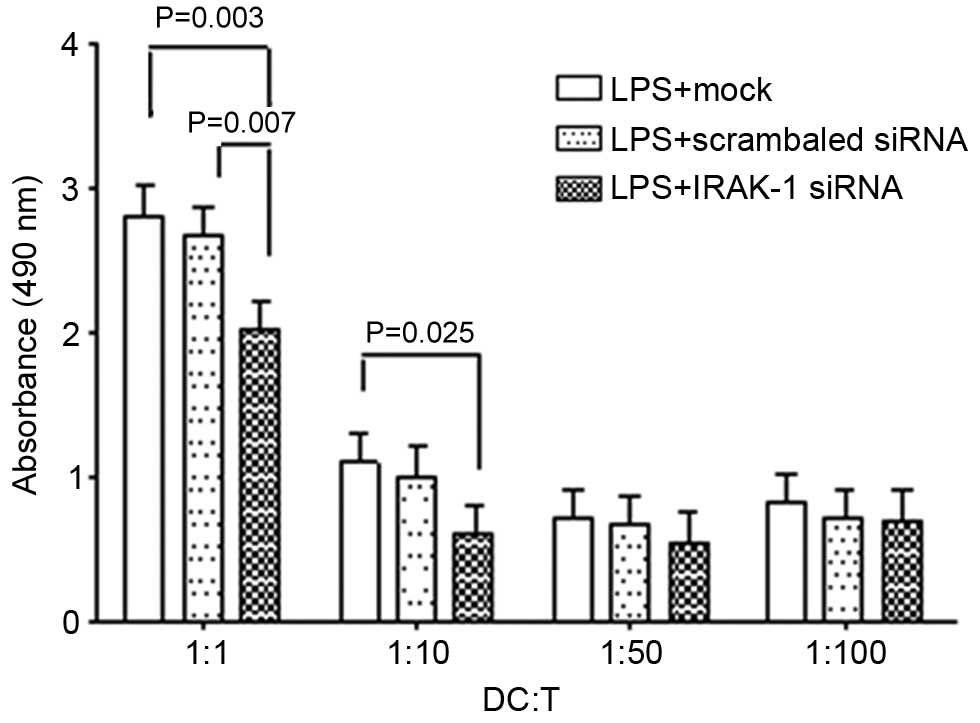
Mixed cell reaction
DCs and spleen-originated naive T cells were
co-cultured, and cell proliferation was subsequently detected using
the MTS assay. The results demonstrated that DCs could
significantly promote the proliferation of naive T cells, which was
also associated with the ratio of cell mixtures; the higher the
proportion of DCs to naive T cells, the more marked the promoting
effects of DCs towards their proliferation (Fig. 6). However, when IRAK-1 gene
expression was suppressed by RNAi, the promoting effects of DCs
towards the proliferation of naive T cells were significantly
reduced.
Discussion
Natural immunity is the primary line of defense in
the resistance of the body against the invasion of foreign
pathogens. PRRs expressed on the surface of natural immune cells
are capable of recognizing and binding PAMPs, thus activating the
cascade of intracellular signals, promoting the release of
inflammatory cytokines and initiating the immune response. TLRs are
the most important innate PRRs, which are able to identify
pathogens when they are still outside the cell, or have just been
phagocytized into inclusion bodies, thus inducing the immune
response. Using such signal transduction molecules as IL-1, human
TLRs mediate LPS-induced activation of immune cells, such as
monocytes. TLRs detect pathogens, and the IL-1R family initiates a
rapid cellular response to the released cytokines. IRAKs are an
important part of the TLR/IL-1R signaling pathway, which serve an
important role in regulating autoimmunity.
Within the IRAKs family, IRAK-1 serves key roles in
the IL-1 signaling pathway, and was the first identified IL-1
receptor kinase. Human and murine IRAK-1 were initially cloned in
1996, and Thomas et al demonstrated that the IRAK-1 gene was
located at Xq29.52-q 29.7 in rats, and at Xq2810 in humans
(11). The molecular weight of
IRAK-1 is ~80 kDa. Following activation it can be expressed as a
~100 kDa modified form, which via various modifications, including
phosphorylation, ubiquitination, acetylation and
polyubiquitination, exerts different functions, including
interferon regulatory factor (IRF)5/IRF7, MAPK, and NF-κB and
signal transducer and activator of transcription signaling pathway
activation (12–14).
It has been reported that in IRAK-1-deficient
macrophages, the expression levels of TNF-α are reduced when TLR2
or TLR4 are activated; this type of TLR-associated damage could
weaken the response against the fatal effects of sepsis caused by
LPS or gram-negative bacilli (15). Deng et al (16) reported that IRAK-1−/−
mice are susceptible to a high dose of S. aureus compared
with wild-type controls. By contrast to the high mortality and
extensive weight loss observed in IL-1R-deficient mice in response
to 1×106 S. aureus, IRAK-1−/− mice are
resistant to this low dose of S. aureus. Furthermore, IRAK-1
has been shown to serve an important role in TLR7- or TLR9-based
interferon (IFN) induction; IRAK-1-deficient plasmacytoid DCs could
not produce IFN-α; however, this deficiency did not affect the
generation of other inflammatory factors, such as IL-6 and TNF-α
(17). Conversely, previous
studies have reported that in IRAK-1-deficient spleen lymphocytes,
when TLR9 was activated, the expression levels of TNF-α and IL-12
were significantly downregulated (16). These differences may be associated
with the different cells selected. Ahmad et al (18) demonstrated that following
inhibition of chondrocyte IRAK-1 by RNAi, the expression levels of
IL-1 stimulation-induced matrix metalloproteinase 13 (MMP-13) were
downregulated, thereby protecting chondrocytes and decreasing the
damage caused by MMP-13. These results suggested that IRAK-1 may
have clear and necessary roles in TLR7- and TLR9-induced IFN-α
downstream induction.
EAE is a T cell-mediated autoimmune disease in mice.
The application of myelin protein and complete Freund's adjuvant is
able to sensitize mice, resulting in the production of clinical
features similar to those observed in human multiple sclerosis. A
previous study reported that TLR4 functional defects could weaken
activation of T helper (Th)17 and Th1 cells in an EAE model,
significantly reducing the symptoms of EAE (19), thus suggesting that TLR4 has a key
mediating role in the occurrence of EAE. Furthermore, Deng et
al (16) demonstrated in
animal studies that IRAK-1-deficient mice were resistant to EAE so
that little or no inflammatory response could be detected in the
central nervous system of the mice, and the functions of Th1 cells
were reduced in such mice. It could be hypothesized that the
possible reason underlying EAE resistance was due to the
suppression of TLR activation during APC-activated IL-1R signaling,
which relies on IRAK-1. Other studies have also confirmed that
IL-1R- and IL-18-deficient mice appear resistant to the EAE model
(20,21). Therefore, it may be speculated that
IRAK-1 is associated with EAE; however, the specific mechanisms
underlying the effects of IRAK-1 on the occurrence and development
of EAE require further investigation.
A previous study reported that the TLR pathway was
able to activate the downstream transcription factor NF-κB, thus
inducing the expression of various proinflammatory genes (22). IRAK-1 is located downstream of TLR2
in this signaling pathway; therefore, it may serve major roles in
signaling transduction from TLR to NF-κB activation. In the
inflammatory responses of various autoimmune diseases, Th17 and Th1
cells serve promoting roles, whereas regulatory T cells (Treg
cells) inhibit this type of regulation; therefore, the responses of
Th17 and Th1 cells may be closely associated with the occurrence
and development of diseases. IRAK-1 has been confirmed to have key
roles in promoting T-cell differentiation towards Th1 and Th17,
which may promote the differentiation of Th17 cells and inhibit the
generation of Treg cells (23).
Therefore, IRAK-1 inhibitors may be considered potential clinical
therapeutic tools for the treatment of autoimmune diseases.
The development process of DCs can be divided into
two stages: Immature DCs and mature DCs, which exert various
functions. Under normal circumstances, the majority of DCs in
vivo, which are predominantly contained inside non-lymphoid
tissues, are in an immature state. Following ingestion of antigens
or stimulation by certain factors (such as LPS, IL-1β and TNF-α),
they may differentiate and become mature. Mature DCs express high
levels of MHC molecules, costimulatory molecules and adhesion
molecules, integrins (beta-1, beta-2), and characteristic markers
(CD1a, CD11c, CD83); secrete cytokines, such as IL-12, IL-2l,
IL-26, IL-28, TNF-α and IFN-α; and stimulate mixed lymphocyte
reaction to enhance its abilities. DCs are associated with various
autoimmune diseases, and have important roles in the occurrence and
development of autoimmune diseases, including multiple sclerosis
and its animal model EAE (24). A
previous study demonstrated that following pretreatment with LPS
and myelin basic protein, the injection of DCs into mice may induce
generation of an EAE model (25).
Furthermore, a previous study demonstrated that LPS could promote
the differentiation of DCs; following the addition of LPS into the
DCs culture system, the most typical form of mature DCs appeared
after 6 days, the expression levels of mature surface markers CD80
and CD86 were increased, the secretion of IL-6 and IL-12 were
significantly increased, and its roles regarding stimulation of the
activation and proliferation of allogeneic T cells were enhanced
(8). Another study reported that
LPS could promote the maturation of DCs predominantly through the
TLRs signaling pathway; after binding with TLR4, LPS may adjust the
transcription of various genes via NF-κΒ and MAPK pathways, thus
promoting DC maturation (9). As a
key factor in the TLR/IL-1R signaling pathway, IRAK-1 may be
involved in the differentiation and maturation of DCs.
Previous studies (26,27)
demonstrated that when BMSCs-originated DCs were treated with LPS,
IRAK-1 expression was significantly upregulated and NF-κB was
upregulated, thus suggesting that LPS-stimulated maturation of DCs
was induced via the TLRs/IL-1Rs pathway. Namely, LPS may bind with
TLRs on the surface of DCs, induce the upregulation of IRAK-1, and
thus promote the expression of NF-κB and initiation of the immune
responses. The present study used RNAi technology to transfect DCs
with IRAK-1 siRNA to inhibit the gene expression of IRAK-1. The
results demonstrated that the differentiation and maturation of DCs
were inhibited; flow cytometry revealed that the expression levels
of DC surface markers: CD80, MHC II and CD40, were significantly
reduced.
IL-10 is a pleiotropic cytokine, the primary
function of which is to limit or terminate inflammatory responses.
IL-10 is able to regulate the growth and differentiation of several
types of cells, including B cells, natural killer cells, cytotoxic
T cells, Th cells, monocytes and DCs (28). IL-12 and TNF-α are well-recognized
inflammatory factors, which promote inflammatory responses and
activate immune cells. Previous studies have suggested that IL-10
has an important role in the occurrence and development of
autoimmune diseases, including multiple sclerosis (29,30).
IL-10 is able to reduce the expression of IL-12 and TNF-α in
various cells, and can downregulate the functions of APCs, thereby
regulating the functions of proinflammatory T cells (31). In particular, IL-10 can limit the
inappropriate amplification of Th17 cells (32). A previous also demonstrated that
IL-10 could inhibit the expression of IL-12 in DCs (33).
The present study demonstrated that following RNAi
to silence the expression of IRAK-1 in DCs, the DCs' abilities to
release IL-12 and TNF-α were decreased, whereas the expression
levels of IL-10 were increased, thus suggesting that the
suppression of IRAK-1 could reduce inflammation. In order to
further assess the functional alterations of DCs, DCs were
co-cultured with naive CD4+ T cells. The results
demonstrated that the promoting effects of DCs towards the
proliferation of CD4+ T cells were significantly
inhibited by IRAK-1 siRNA, this inhibition was associated with the
ratio of DCs to co-cultured lymphocytes; the higher the ratio, the
more obvious the inhibitory effects. These results suggested that
IRAK-1 may serve key roles in the differentiation and maturation of
DCs.
In conclusion, during LPS-mediated differentiation
and maturation of DCs, IRAK-1 may serve key roles. Following the
suppression of IRAK-1, the differentiation and maturation of DCs
was inhibited in several ways; IRAK-1 RNAi reduced the expression
of cell surface factors CD86, CD40 and MHC II; reduced mRNA
expression of NF-κB p65; reduced the expression of proinflammatory
cytokines IL-12 and TNF-α, and enhanced the expression of the
anti-inflammatory cytokine IL-10. In addition, the promoting
abilities of DCs towards the activation and proliferation of T
cells were reduced. Following the completion of further studies
regarding the mechanisms of IRAKs in innate immune processes, novel
drugs and methods may be identified for the clinical treatment of
autoimmune diseases, such as systemic lupus erythematosus and
multiple sclerosis, thus advancing the diagnosis and treatment of
human autoimmune diseases.
Acknowledgements
The present study was supported by the Projects of
Liaoning Provincial Science and Technology Department (grant no.
2012225021) and Program of Basic and Clinical Research Platform of
China Medical University (grant no. CMU201406).
References
|
1
|
Kumar H, Kawai T and Akira S: Pathogen
recognition by the innate immune system. Int Rev Immunol. 30:16–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fischer M and Ehlers M: Toll-like
receptors in autoimmunity. Ann N Y Acad Sci. 1143:21–34. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown J, Wang H, Hajishengallis GN and
Martin M: TLR-signaling networks: An integration of adaptor
molecules, kinases and cross-talk. J Dent Res. 90:417–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Satpathy AT, Wu X, Albring JC and Murphy
KM: Re(de)fining the dendritic cell lineage. Nat Immunol.
13:1145–1154. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pearce EJ and Everts B: Dendritic cell
metabolism. Nat Rev Immunol. 15:18–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiong Y, Qiu F, Piao W, Song C, Wahl LM
and Medvedev AE: Endotoxin tolerance impairs IL-1
receptor-associated kinase (IRAK) 4 and TGF-beta-activated kinase 1
activation, K63-linked polyubiquitination and assembly of IRAK1,
TNF receptor-associated factor 6 and IkappaB kinase gamma and
increases A20 expression. J Biol Chem. 286:7905–7916. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen P: The TLR and IL-1 signalling
network at a glance. J Cell Sci. 127:2383–2390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mihret A, Mamo G, Tafesse M, Hailu A and
Parida S: Dendritic Cells activate and mature after infection with
mycobacterium tuberculosis. BMC Res Notes. 4:2472011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zanoni I, Ostuni R, Capuano G, Collini M,
Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, et
al: CD14 regulates the dendritic cell life cycle after LPS exposure
through NFAT activation. Nature. 460:264–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas JA, Allen JL, Tsen M, Dubnicoff T,
Danao J, Liao XC, Cao Z and Wasserman SA: Impaired cytokine
signaling in mice lacking the IL-1 receptor-associated kinase. J
Immunol. 163:978–984. 1999.PubMed/NCBI
|
|
12
|
Suzuki N, Suzuki S, Duncan GS, Millar DG,
Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, et al: Severe
impairment of interleukin-1 and Toll-like receptor signalling in
mice lacking IRAK-4. Nature. 416:750–756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim TW, Staschke K, Bulek K, Yao J, Peters
K, Oh KH, Vandenburg Y, Xiao H, Qian W, Hamilton T, et al: A
critical role for IRAK4 kinase activity in Toll-like
receptor-mediated innate immunity. J Exp Med. 204:1025–1036. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beinke S, Robinson MJ, Hugunin M and Ley
SC: Lipopolysaccharide activation of the TPL-2/MEK/extracellular
signal-regulated kinase mitogen-activated protein kinase cascade is
regulated by I kappa B kinase-induced proteolysis of NF-kappa B1
p105. Mol Cell Biol. 24:9658–9667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Swantek JL, Tsen MF, Cobb MH and Thomas
JA: IL-1 receptor-associated kinase modulates host responsiveness
to endotoxin. J Immunol. 164:4301–4306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng C, Radu C, Diab A, Tsen MF, Hussain
R, Cowdery JS, Racke MK and Thomas JA: IL-1 receptor-associated
kinase 1 regulates susceptibility to organ-specific autoimmunity. J
Immunol. 170:2833–2842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uematsu S, Sato S, Yamamoto M, Hirotani T,
Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, et al:
Interleukin-1 receptor-associated kinase-1 plays an essential role
for Toll-like receptor (TLR)7- and TLR9-mediated interferon-alpha
induction. J Exp Med. 201:915–923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad R, Sylvester J and Zafarullah M:
MyD88, IRAK1 and TRAF6 knockdown in human chondrocytes inhibits
interleukin-1-induced matrix metalloproteinase-13 gene expression
and promoter activity by impairing MAP kinase activation. Cell
Signal. 19:2549–2557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reynolds JM, Martinez GJ, Chung Y and Dong
C: Toll-like receptor 4 signaling in T cells promotes autoimmune
inflammation. Proc Natl Acad Sci USA. 109:13064–13069. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schiffenbauer J, Streit WJ, Butfiloski E,
LaBow M, Edwards C III and Moldawer LL: The induction of EAE is
only partially dependent on TNF receptor signaling but requires the
IL-1 type I receptor. Clin Immunol. 95:117–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi FD, Takeda K, Akira S, Sarvetnick N
and Ljunggren HG: IL-18 directs autoreactive T cells and promotes
autodestruction in the central nervous system via induction of
IFN-gamma by NK cells. J Immunol. 165:3099–3104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doyle SL and O'Neill LA: Toll-like
receptors: From the discovery of NFKB to new insights into
transcriptional regulations in innate immunity. Biochem Pharmacol.
72:1102–1113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maitra U, Davis S, Reilly CM and Li L:
Differential regulation of Foxp3 and IL-17 expression in CD4 T
helper cells by IRAK-1. J Immunol. 182:5763–5769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galicia G and Gommerman JL: Plasmacytoid
dendritic cells and autoimmune inflammation. Biol Chem.
395:335–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mellanby RJ, Cambrook H, Turner DG,
O'Connor RA, Leech MD, Kurschus FC, MacDonald AS, Arnold B and
Anderton SM: TLR-4 ligation of dendritic cells is sufficient to
drive pathogenic T cell function in experimental autoimmune
encephalomyelitis. J Neuroinflammation. 9:2482012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Yang N, Ni S, Li W, Xu L, Dong P
and Lu M: Pretreatment of lipopolysaccharide (LPS) ameliorates
D-GalN/LPS induced acute liver failure through TLR4 signaling
pathway. Int J Clin Exp Pathol. 7:6626–6634. 2015.
|
|
27
|
Read MA, Cordle SR, Veach RA, Carlisle CD
and Hawiger J: Cell-free pool of CD14 mediates activation of
transcription factor NF-kappa B by lipopolysaccharide in human
endothelial cells. Proc Natl Acad Sci U S A. 90:9887–9891. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian G, Li JL, Wang DG and Zhou D:
Targeting IL-10 in auto-immune diseases. Cell Biochem Biophys.
70:37–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hesse D, Krakauer M, Lund H, Søndergaard
HB, Limborg SJ, Sørensen PS and Sellebjerg F: Disease protection
and interleukin-10 induction by endogenous interferon-beta in
multiple sclerosis? Eur J Neurol. 18:266–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Correa F, Hernangómez-Herrero M, Mestre L,
Loría F, Docagne F and Guaza C: The endocannabinoid anandamide
downregulates IL-23 and IL-12 subunits in a viral model of multiple
sclerosis: Evidence for a cross-talk between IL-12p70/IL-23 axis
and IL-10 in microglial cells. Brain Behav Immun. 25:736–749. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Garra A and Murphy KM: From IL-10 to
IL-12: How pathogens and their products stimulate APCs to induce T
(H)1 development. Nature Immunol. 10:929–932. 2009. View Article : Google Scholar
|
|
32
|
Chaudhry A, Samstein RM, Treuting P, Liang
Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller
W and Rudensky AY: Interleukin-10 signaling in regulatory T cells
is required for suppression of Th17 cell-mediated inflammation.
Immunity. 34:566–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruffell B, Chang-Strachan D, Chan V,
Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS and
Coussens LM: Macrophage IL-10 blocks CD8+ T
cell-dependent responses to chemotherapy by suppressing IL-12
expression in intratumoral dendritic cells. Cancer Cell.
26:623–637. 2014. View Article : Google Scholar : PubMed/NCBI
|