Introduction
Sepsis remains a challenge in critical patients due
to the potentially life-threatening levels of whole-body
inflammation. As a result, sepsis is a leading contributor to rates
of mortality in intensive care units worldwide (1). Etomidate is a short-acting
intravenous anesthetic, which is well known for its mild repression
of hemodynamics in rapid sequence intubation; however, this
treatment is associated with a period of adrenal steroidogenesis
suppression following injection (2). As adrenal insufficiency or low serum
cortisol can lead to poor outcomes in patients with sepsis, the use
of etomidate in patients with sepsis is controversial. Although
numerous studies have been performed, the effect of etomidate on
mortality rates in sepsis, and the underlying mechanisms
responsible for its effects, remain to be fully elucidated
(3–5). Previous investigation in animals
suggested that etomidate increases mortality rates in septic rats,
although this was not associated with adrenal insufficiency
(6). The present study aimed to
investigate the glucocorticoid-associated anti-inflammatory effect
of etomidate, the apoptosis of lymphocytes and the survival rates
of septic rats following treatment.
Materials and methods
Animals and experimental protocol
Adult female Sprague-Dawley rats (210–240 g) were
purchased from Dalian Medical University (Dalian, China). The rats
(n=25/group) were acclimated under laboratory conditions (12 h
light/dark cycle; 20–24°C) for 1 week prior to experimentation.
Water and food were provided ad libitum throughout the
experiment. Animal experiments were performed in accordance with
the National Institutes of Health Guidelines for the Care and Use
of Laboratory Animals (National Institutes of Health, Bethesda, MD,
USA), and protocols were approved by the Animal Care and Use
Committee of Dalian Medical University (permit no. 20140708-5). All
surgical procedures were performed using aseptic techniques. To
enable continuous injection, right jugular vein catheterization was
performed on the rats under 2–4% isoflurane anesthesia prior to the
start of the experiment protocols. The catheterization procedures
were performed according to instructions previously reported in the
literature (7). Following a
recovery period of 3 days, all rats, with the exception of those in
the sham group, underwent surgery for cecal ligation and puncture
(CLP) under isoflurane anesthesia. The CLP was performed according
to previously described techniques (8). Briefly, the rats were anesthetized by
inhalation of 1–2% isoflurane through a nose cone. Subsequently, a
2-cm midline abdominal incision was made, and one-third of the
distal cecum was ligated and penetrated twice, crosswise, with a
21-gauge needle, which led to the induction of sepsis. Following
this, the cecum was returned and the abdomen was closed. The rats
were injected with 1 ml normal saline with 0.25 µg/ml sufentanil
(Yichang Renfu Pharmaceutical Co., Ltd., Hubei, China)
subcutaneously for postoperative analgesia. The rats were randomly
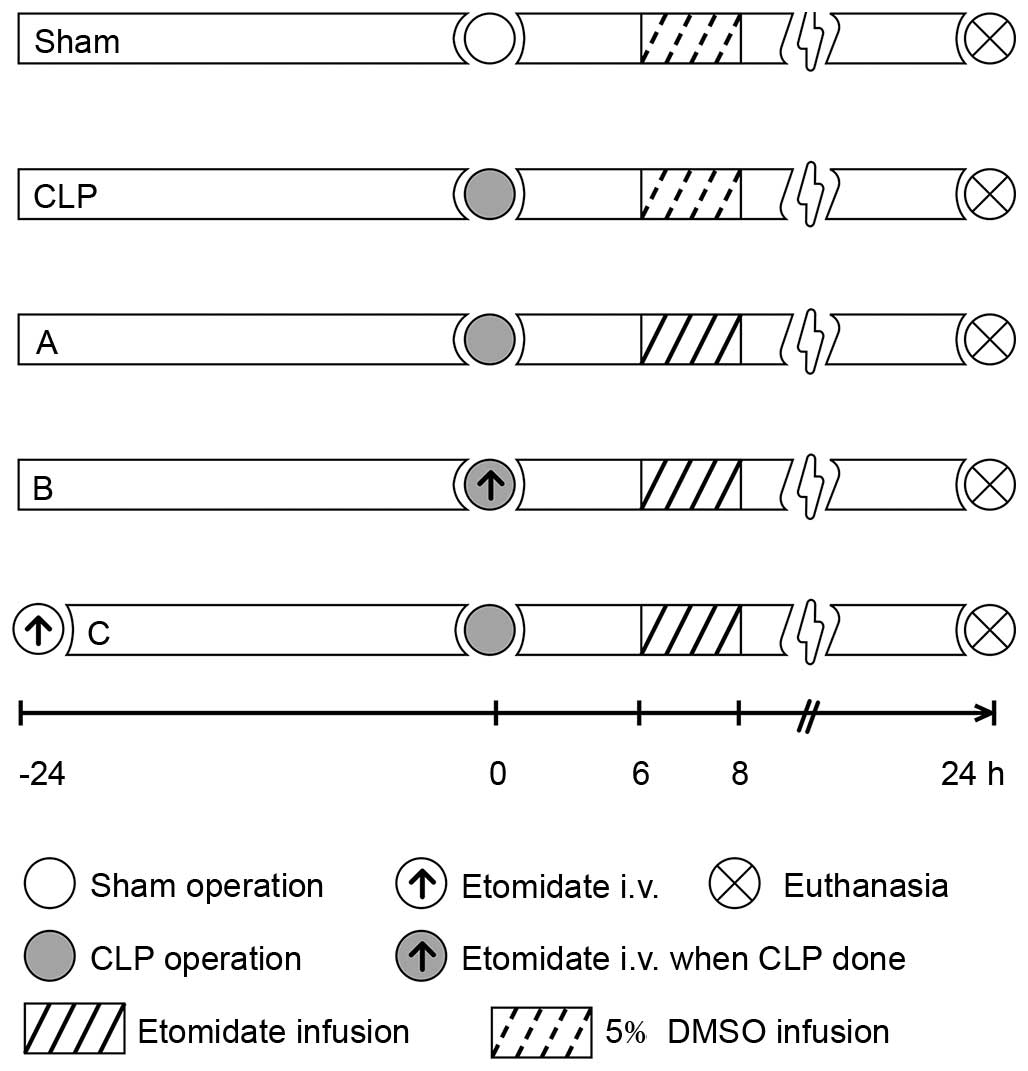
assigned into one of five groups (Fig.
1). All groups were infused with 2 ml of either etomidate
(Enhua Pharmaceutical, Jiangsu, China) or 5% dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) solution
at 1 ml/h for 2 h from 6 h post-surgery. The sham group, in which a
2-cm vertical incision was made on the midline of the abdomen prior
to suturing, was infused with 5% DMSO 6 h post-surgery using a
micro injection pump (BD Biosciences, San Jose, CA, USA) through
the right jugular vein catheter. The CLP group was administered
with a 5% DMSO infusion 6 h post-CLP surgery. Treatment group A was
administered with a 2-h infusion of 2 mg/kg/h etomidate dissolved
in 5% DMSO solution 6 h post-CLP surgery; group B was administered
with a 1-m bolus injection of etomidate (0.6 mg/kg) at the time of
CLP, followed by a 2-h infusion of 2 mg/kg/h etomidate (as in group
A); and group C was administered with 1 ml of etomidate (0.6 mg/kg)
24 h prior to CLP, followed by a 2-h etomidate infusion. Due to the
circadian rhythm associated with corticosterone (CORT), the surgery
was performed between 8:00 and 9:00 a.m. The rats were sacrificed
by inhaled overdose of isoflurane (6–8%, 5 min) 24 h following
surgery, subsequent to which bilateral adrenal glands and an
arterial blood sample were collected. As previous studies have
suggested that female mice are more resistant to CLP, compared with
male mice (9), female rats were
selected in the present study to avoid gender-associated genetic
differences in response to sepsis. Arterial blood samples were
collected 24 h following CLP surgery; the bilateral adrenal glands
and spleens were collected immediately following the sacrifice of
the rats (n=5 per group). Additional rats (n=20 per group), with
the exception of the sham group, were observed for 10 days to
monitor survival rates following surgery. The conditions of the
animals were assessed at 6:00 a.m and 6:00 p.m. each day.
Enzyme-linked immunosorbent assay
The serum levels of tumor necrosis factor-α (TNF-α)
and CORT were measured using a rat ELISA kit (Cusabio, Wuhan,
China) according to the manufacturer's protocol. The minimal
detection levels of TNF-α and CORT were 6.5 and 0.2 pg/ml,
respectively.
RNA isolation and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis. Total RNA from the
adrenal gland was isolated using the RNA prep pure tissue kit
(Tiangen Biotech Co., Ltd., Beijing, China). Total RNA (~90 µg) was
reverse-transcribed using the PrimeScript RT Mix kit (Takara
Biotechnology Co., Ltd., Dalian, China). The following primers were
used: Glucocorticoid receptor (GR), forward
5′-CCGCAGTAGCAGGGTTATTTTC-3′ and reverse
5′-GAAGGGTGGGGAGGATTAGTGT-3′; glucocorticoid-induced leucine zipper
(GILZ), forward 5′-TGGAATGCCAATATGCTCCAG−3′ and reverse
5′-AGGAACAGTCGTTGTCAGGTGAA-3′; and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), forward 5′-GGCACAGTCAAGGCTGAGAATG-3′ and
reverse 5′-ATGGTGGTGAAGACGCCAGTA−3′. A total of 100 ng
complementary DNA was added to a 20-µl reaction for qPCR analysis
with SYBR premix Ex Taq II (Takara Biotechnology Co., Ltd.) and
then amplified using the LightCycler 480 automatic PCR and analysis
system (Roche Applied Science, Indianapolis, IN, USA). GAPDH was
used as an internal control gene. The comparative quantification
(CQ) method was used to calculate the relative expression of the
target gene (10). The qPCR
analysis was performed under the following conditions: Denaturation
for 30 sec at 95°C, followed by 40 cycles of denaturation for 5 sec
at 95°C, annealing for 30 sec at 60°C and cooling for 30 sec at
50°C.
Protein extraction and western blot
analysis
The adrenal glands were homogenized in lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China), and tissue
proteins were extracted using a protein extraction kit (Beyotime
Institute of Biotechnology). The Bradford method was used to
determine the protein concentrations. Equal quantities of the
protein samples (~60 µg) from each group were resolved on 10%
polyacrylamide gels containing 0.1% sodium dodecyl sulfate, and
then transferred onto Immobilon-P PVDF membranes (Merck Millipore).
After blocking with 5% milk in TBS for 1 h at room temperature, the
membranes were probed with rabbit anti-mouse primary antibodies
against inhibitor of nuclear factor (NF)-κB (IκB-α; 1:500; cat. no.
10268-1-AP; Proteintech Group, Inc., Chicago, IL, USA) and
tubulin-α (1:500; cat. no. 11224-1-AP; Proteintech Group, Inc.) at
4°C overnight. Subsequently, the membranes were washed extensively
with TBS-0.1% Tween-20 (TBST) and probed with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody
(1:5,000; cat. no. ab6721; Abcam, Cambridge, UK) conjugated to
horseradish peroxidase (HRP) for 1 h at room temperature. Following
washing with TBST, the immunoreactive bands were visualized using
Luminata Classico Western HRP substrate (Merck Millipore).
Tubulin-α was used as a loading control to normalize IκB-α. The
band intensities were quantified using Quantity One software
(version 4.6.2 for Windows; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Examination of the apoptotic rate of
lymphocytes in the spleen
Lymphocytes were obtained from fresh rat spleens.
Briefly, fresh spleens were isolated and cut into small sections.
Subsequently, several of these sections were mechanically disrupted
on a nylon mesh in a 35-mm dish containing 4–5 ml lymphocyte
separation liquid (Dakewe Biotech Co., Ltd., Shenzhen, China). The
suspension was transferred to a 15-ml centrifuge tube containing
200–500 µl RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and then centrifuged at ~108 × g for 30
min. Finally, the lymphocytes were aspirated and washed twice with
phosphate-buffered saline (pH 7.4; Merck Millipore), following
which the cell density was calculated.
Following adjustment of the cell density to
2×106/ml per sample, a cell apoptosis assay kit
(Biouniquer, Nanjing, China), including fluorescein
isothiocyanate-labeled annexin V and propidium iodide, was used to
determine the apoptotic rate with a flow cytometer (FACS Calibur;
BD Biosciences).
Statistical analysis
The Kolmogorov-Smirnov test was performed to examine
the normality of the data. The quantitative data, which passed
normality assessment, are presented as the mean ± standard error of
the mean. One-way analysis of variance followed by Tukey's test was
performed for comparisons among the groups. Survival curves were
plotted using the Kaplan-Meier method and compared using the
log-rank (Mantel-Cox) test. GraphPad Prism (version 6.0 for Mac;
GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the
data and generate histograms. P<0.05 was considered to indicate
a statistically significant difference.
Results
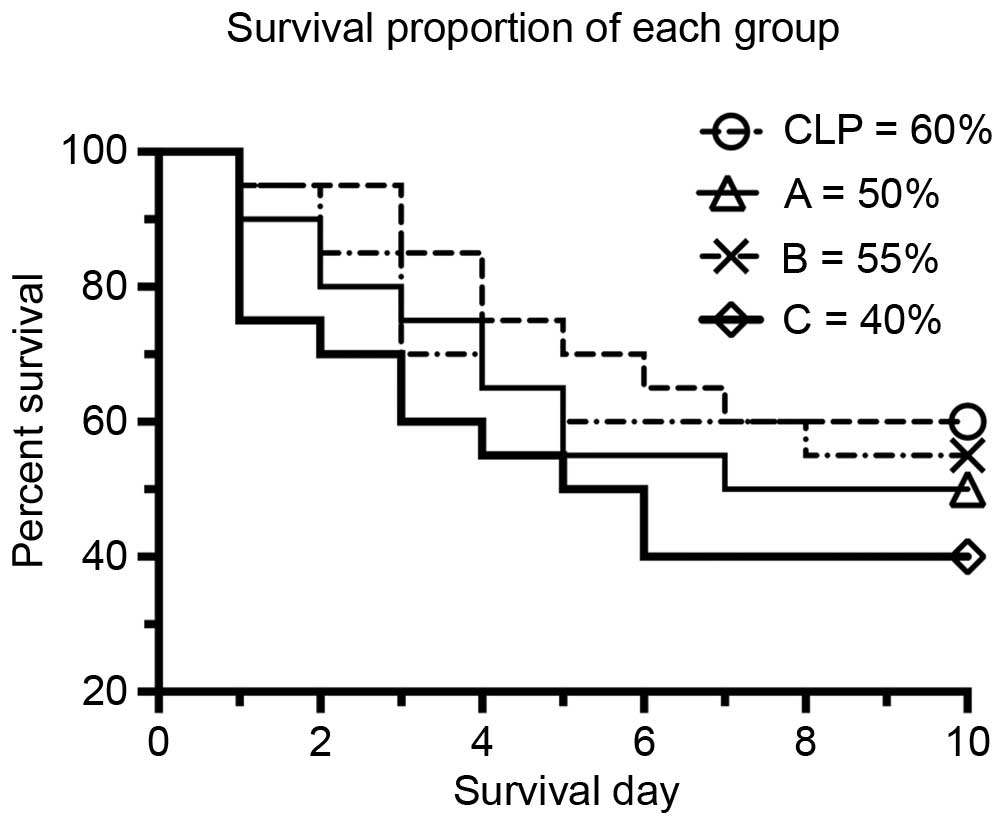
The different etomidate treatment protocols led to
different survival rates (Fig. 2),
which suggested that etomidate had effects on targets other than
the adrenal glands during sepsis. The survival rate in group C was
40%, which was lower, compared with that in the CLP group (60%).
However, no significant difference was found between the treatment
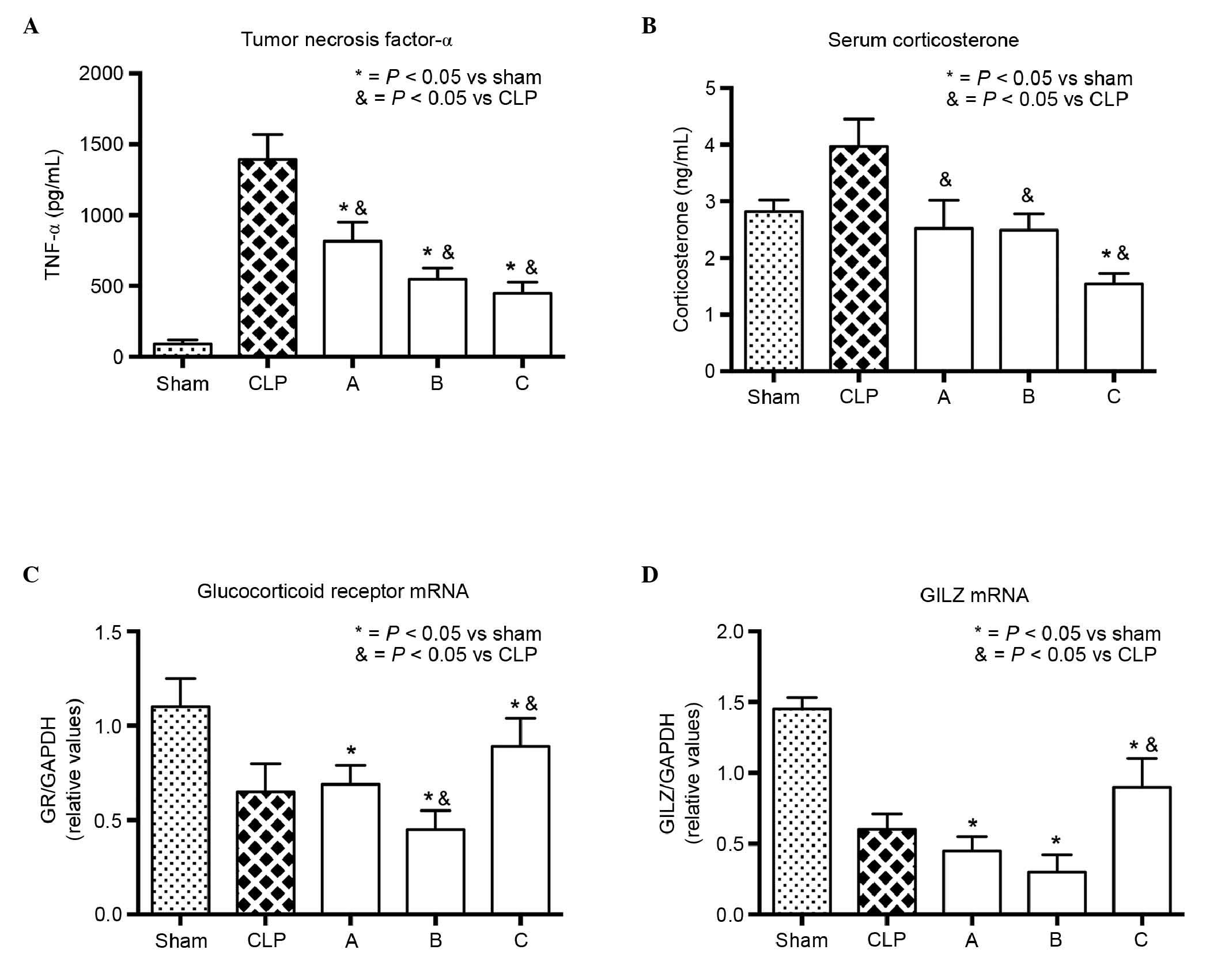
groups. High levels of inflammatory cytokines released during the
early phase of sepsis led to a series of systematic inflammatory
responses throughout the body. TNF-α is one such inflammatory
cytokine, and the level of serum TNF-α can be used to assess the
extent of inflammation. Etomidate treatment inhibited the serum
level of TNF-α (Fig. 3A), which
suggested that inflammation was inhibited in the treated septic
rats. Although it is well known that etomidate inhibits adrenal
steroidogenesis by inhibiting the activity of 11β-hydroxylase,
which coverts 11-deoxycorticosterone to CORT, the serum levels of
CORT in the control and treated groups were not markedly decreased.
However, the level of CORT in group C was significantly lower,
compared with the levels in the other groups (Fig. 3B). This result suggested that the
inhibition of etomidate-induced steroidogenesis may not have
affected the serum levels of CORT over the short period of time, or
that this inhibition may have a delayed effect on the levels of
CORT. The majority of CORT binds to GRs, and the observed decrease
in the mRNA expression level of GR suggested that etomidate may
have inhibited the expression of GR, either directly or indirectly.
However, pretreatment with etomidate (group C) appeared to
alleviate the decline in the mRNA expression of GR (Fig. 3C). The mRNA expression of GILZ, an
important mediator of glucocorticoid action, was in accordance with
that of GR (Fig. 3D), which
further suggested that etomidate may have inhibited the GR
signaling pathway. Furthermore, when NF-κB was activated, IκB-α was
ubiquitinated in the cytoplasm and its expression level decreased.
The expression level of IκB-α in groups A, B and C were
significantly higher, compared with that observed in the CLP group
(Fig. 4). Taken together, the
results presented above suggested that etomidate may inhibit NF-κB
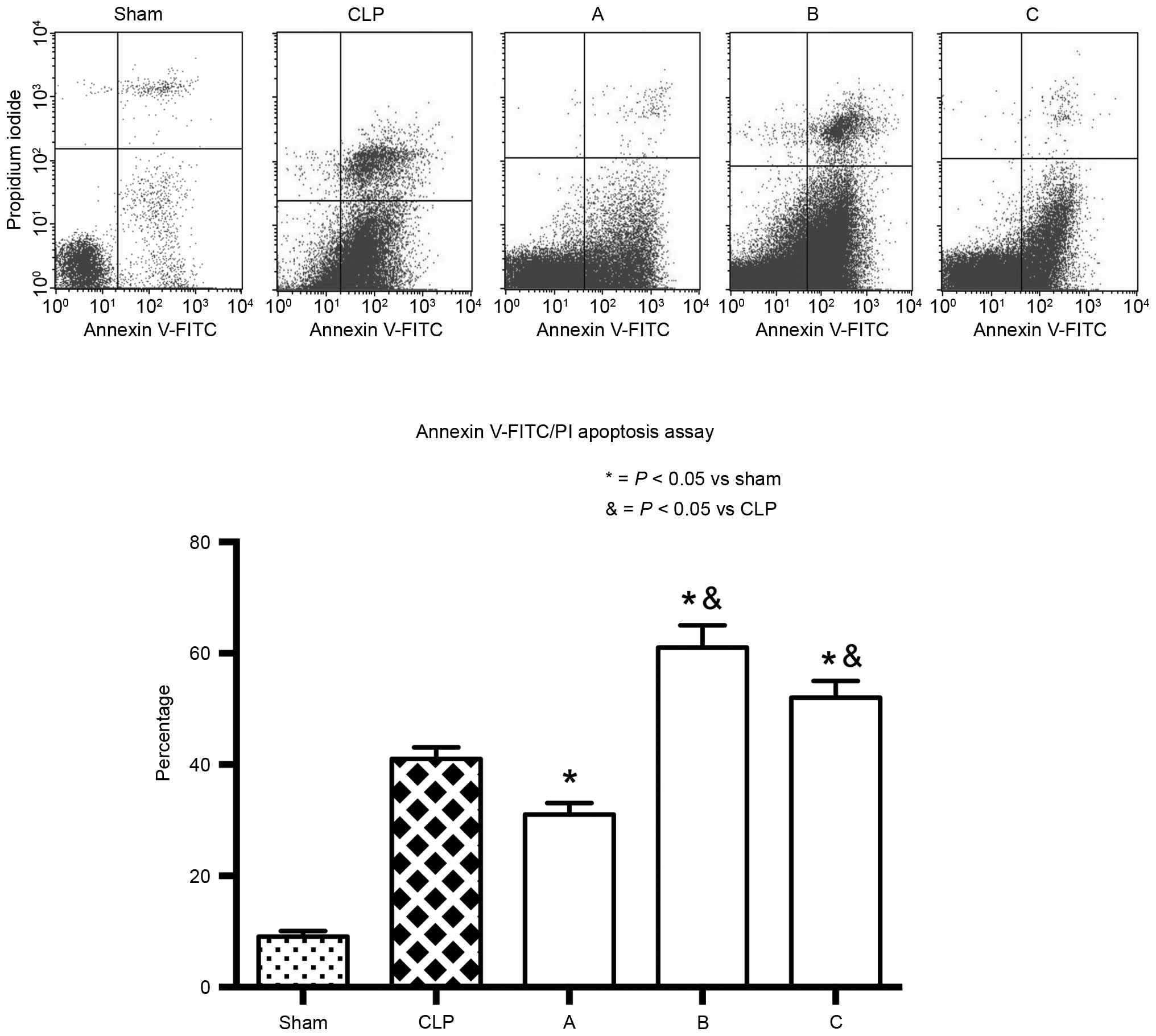
activation through the GR signaling pathway. However, the increased
apoptotic rate of the lymphocytes obtained from groups B and C
suggested that etomidate treatment stimulated lymphocyte apoptosis,
which may harm the immune system and suppress the immune response
(Fig. 5).
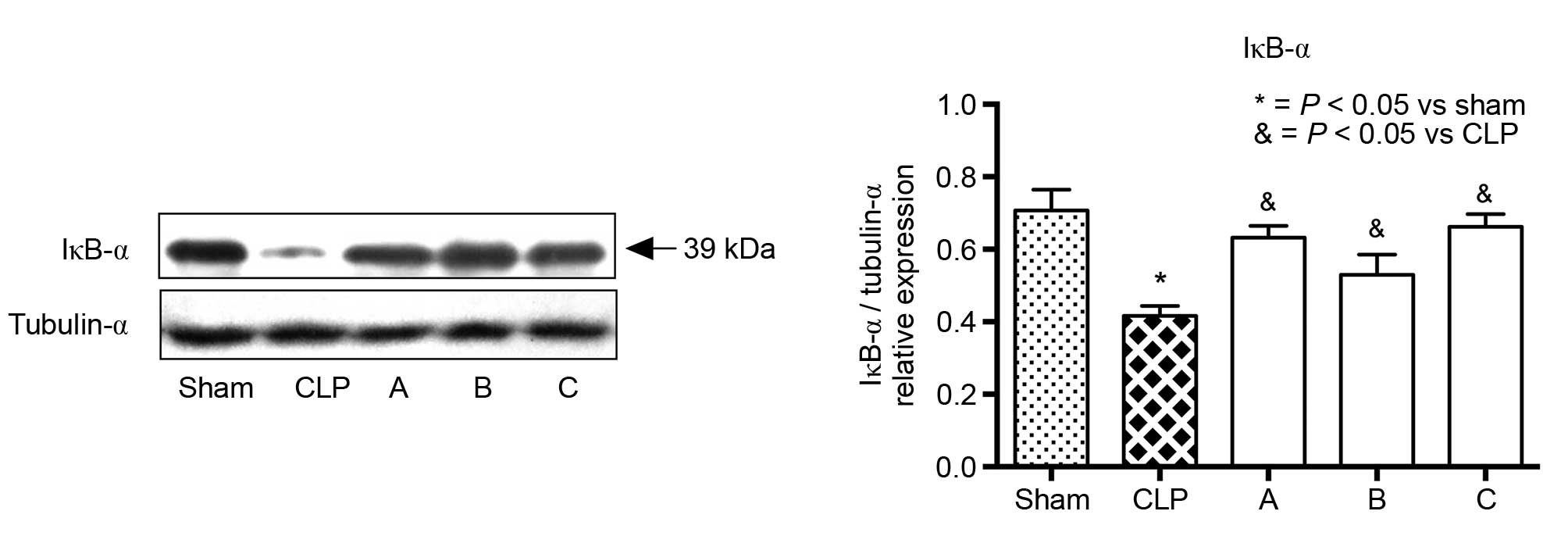 | Figure 4.Expression of IκB-α in the adrenal
glands of each group. Representative blots for the IκB-α proteins
are shown above the histogram. As the activation of NF-κB was
initiated by the signal-induced ubiquitination of IκB-α proteins,
the decreased abundance of IκB-α proteins in the CLP group
suggested NF-κB had been activated. The expression levels of IκB-α
in treatment groups A, B, and C were significantly higher, compared
with that in the CLP group, which suggested that etomidate
treatment inhibited NF-κB activity. Values are presented as the
mean + standard error of the mean (n=5).*P<0.05, compared with
the sham group; &P<0.05, compared with the CLP
group. CLP, cecal ligation and puncture; NF-κB, nuclear factor-κB;
IκBα, inhibitor of NF-κB-α. |
Discussion
Advantages and disadvantages of animal
models
Right jugular vein catheterization and placement of
the catheter at the back of the rat's neck enable intravenous
injections following surgery and infusion for a relatively long
duration. The injection of a 0.6 mg/kg bolus of etomidate and a 2-h
infusion of 2 mg/kg/h etomidate can cause significant sedation
without respiratory depression in rats. The most commonly used
animal models of sepsis are the endotoxemia model, the
bacterial-inoculum model and the CLP model (11). The administration of
lipopolysaccharide (LPS) is one of the most common strategies to
induce sepsis in the endotoxemia model. Although this model is
controlled and standardized, the rapid, transient and
waterfall-like cytokine responses following LPS injection differ
from those observed in human sepsis. Thus, LPS injection may not be
suitable for the investigation of anesthetics during sepsis over a
longer time period. The bacterial inoculum model requires bacterial
growth and quantification prior to administration. By contrast, the
CLP model is a polymicrobial sepsis model, which is readily
reproducible and induces a prolonged, but not excessively high,
elevation of cytokine levels, as observed in humans (11). However, the limitation of the CLP
model is the variability in severity due to differences between
surgical procedures. The present study standardized the key
parameters of CLP surgery, including the length of ligation, the
gauge of the puncture needle and identical post-operative recovery
conditions, to increase the sample size but minimize
variability.
Anti-inflammatory effect of etomidate
and survival rates of treated rats
In the present study, the decreased levels of TNF-α
in groups A, B and C demonstrated the anti-inflammatory effect of
etomidate. Previously, anesthetics and sedatives other than
etomidate have been shown to have anti-inflammatory effects and to
inhibit the activity of NF-κB, including propofol (12), dexmedetomidine (13) and midazolam (14). However, the survival data in the
present study showed that, when etomidate was administered 24 h
prior to surgery, survival rates decreased to 40%, which was lower,
compared with the rate of 60% observed in the CLP group. Therefore,
it appeared that the anti-inflammatory effects of etomidate may not
have improved the outcome of sepsis. Glucocorticoids are a natural
anti-inflammatory agent in the body, and serum levels of CORT
increase to resist inflammation in the presence of a functioning
hypothalamic-pituitary-adrenal axis (15). In the present study, the levels of
CORT were lowest in group C, which made it difficult to conclude
that etomidate induced adrenal insufficiency in sepsis. Considering
that the normal range of CORT levels in humans fluctuates between
55 and 635 nmol/l from day to night, a gold standard is required
for the diagnosis of relative adrenal insufficiency (16). It may be that the decrease in
etomidate-induced CORT occurs 24 h following injection, as it takes
time for etomidate to inhibit the activity of 11β-hydroxylase.
Previous studies (17,18) have revealed that adrenal
suppression persists for >24 h following etomidate infusion.
However, considering the mRNA expression levels of GR and GILZ
observed in the present study, it is possible that etomidate may
have also inhibited the expression of GR in the adrenal gland.
Virtually all the effects of CORT are mediated by the activation of
the GR. According to the commonly accepted theory, GR is a
transcription factor, which exists in its inactive form in the
cytoplasm. Once activated by binding to CORT, the CORT-GR complex
homodimerizes and then migrates to the nucleus, where it binds to
specific DNA to modulate cell function via protein synthesis
(19,20). GILZ, which is upregulated by GR, is
a mediator of the anti-inflammatory effects of CORT. GILZ is also a
promising candidate as a therapeutic anti-inflammatory drug as it
has no detrimental effect of glucocorticoids (21). In the present study, the mRNA
expression levels of GR and GILZ were similar among the groups. In
addition, the levels of IκB in the adrenal gland were not decreased
as expected, which suggested that etomidate also inhibited the
translocation of NF-κB. A previous study (6) observed that etomidate reduces the
level of inflammatory cytokines by inhibiting the activation of
NF-κB. Other studies have reported that the crosstalk between
glucocorticoids and NF-κB results in the inhibition of NF-κB
activity (22), and that this
inhibition can be ascribed to the increased synthesis of IκB
(23). Thus, according to the
expression levels of CORT, GR, GILZ and IκB in the present study,
it was hypothesized that etomidate inhibited the GR signaling
pathway and then inhibited the NF-κB pathway during sepsis.
Effect of etomidate on
lymphocytes
The traditional therapeutic strategies for sepsis
have focused on the uncontrolled inflammatory response, which has
led to attempts to inhibit mediators of inflammation, including LPS
and TNF-α. However, these strategies (24) have largely failed. As a result, a
novel paradigm has emerged, which is focused on the biphasic immune
response of sepsis, with an initial hyperinflammatory phase
followed by an immunoparalysis phase. Of note, the immunoparalysis
phase often determines patient survival rate, as this is a
vulnerable period when patients are at risk of secondary infection
from invading pathogens. The mechanism for immune paralysis appears
to involve the apoptosis of immune cells, particularly lymphocytes.
Weber et al (25)
demonstrated that circulating lymphocytes in patients with severe
sepsis showed accelerated apoptosis. In addition, Muenzer et
al (26) found that the
improved survival rates of CLP-induced septic mice were associated
with decreased lymphocyte apoptosis. Therefore, modulating
lymphocyte apoptosis has increasingly been considered to be an
important stage in sepsis. Another previous study (6) observed a higher mortality rate in the
etomidate pretreatment group; groups B and C showed a higher
lymphocyte apoptotic rate, compared with the CLP control and sham
groups. In addition, Payen et al (27) found that critically ill patients do
not benefit from hydrocortisone used to treat etomidate-induced
adrenal insufficiency. Thus, it is possible that the detrimental
effects of etomidate in sepsis may be ascribed to its effect on
immune suppression rather than adrenal CORT suppression, which
assists in explaining why glucocorticoid supplementary therapy may
not be effective in patients with sepsis.
Limitations
The present study had several limitations. First, GR
protein and the CORT-GR complex are known to translocate from the
cytoplasm to the nucleus following the activation of GR; thus, if
the quantity of GR protein in the cytoplasm and the nucleus had
been determined in the present study, the results may have been
more accurate and convincing. Second, GR protein and activity are
altered during the different phases of sepsis; thus, the inclusion
of additional time points may assist in explaining how the trend of
CORT and GR is affected by etomidate in the hyper-inflammatory and
immunosuppression phases. Finally, the present study only focused
on the expression of adrenal GR. Kanczkowski et al (28) found that immune cells are important
in adrenocortical and adrenal inflammation in sepsis. Therefore,
investigating the expression of GR-associated genes and proteins in
immune cells may provide additional insights. A previous study
(29) reported that
benzodiazepine, a sedative similar to etomidate, augments
γ-amino-butyric acid, suggesting that it may increase mortality
rates in mice with pneumonia. Therefore, the detrimental effect of
etomidate in sepsis may not be due to its inhibition of CORT alone;
etomidate may also inhibit other receptors in immune cells, the
central nervous system and other systems of the human body.
The results of the present study indicated that
etomidate treatment may alleviate inflammation in the adrenal gland
in sepsis through the inhibition of GR and NF-κB translocation.
However, etomidate stimulated the apoptosis of lymphocytes, which
may lead to poor outcomes in sepsis, although additional evidence
is required to investigate this effect. According to the results of
the present study, anesthesiologists may be able to safely use
etomidate once for induction in critical patients. However,
overdose or the repeated use of etomidate in patients with sepsis
may be harmful due to the delayed or accumulated immunosuppression
induced by etomidate. By investigating etomidate-induced
immunosuppression, a more comprehensive understanding of the
immunomodulation and endocrinomodulation in sepsis can be obtained,
and novel therapeutic strategies to treat sepsis may be identified
in the future.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81171791). The
authors would like to thank Jie Zhu (Laboratory of Clinical
Medicine, The Second Hospital of Dalian Medical University) for his
assistance with flow cytometry and the editors of American Journal
Experts for their language editing.
References
|
1
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock: 2012. Crit Care Med.
41:580–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forman SA: Clinical and molecular
pharmacology of etomidate. Anesthesiology. 114:695–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinrich S, Schmidt J, Ackermann A, Moritz
A, Harig F and Castellanos I: Comparison of clinical outcome
variables in patients with and without etomidate-facilitated
anesthesia induction ahead of major cardiac surgery: A
retrospective analysis. Crit Care. 18:R1502014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sunshine J, Deem S, Weiss N, Yanez ND,
Daniel S, Keech K, Brown M and Treggiari MM: Etomidate, adrenal
function, and mortality in critically ill patients. Respir Care.
58:639–646. 2013.PubMed/NCBI
|
|
5
|
Chan CM, Mitchell AL and Shorr AF:
Etomidate is associated with mortality and adrenal insufficiency in
sepsis: A meta-analysis*. Crit Care Med. 40:2945–2953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Li R, Zhu J, Wang Z, Lv S and
Xiong JY: Etomidate increases mortality in septic rats through
inhibition of nuclear factor kappa-B rather than by causing adrenal
insufficiency. J Surg Res. 193:399–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hughey CC, Hittel DS, Johnsen VL and
Shearer J: Hyperinsulinemic-euglycemic clamp in the conscious rat.
J Vis Exp. 24322011.PubMed/NCBI
|
|
8
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Maio A, Torres MB and Reeves RH:
Genetic determinants influencing the response to injury,
inflammation, and sepsis. Shock. 23:11–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dejager L, Pinheiro I, Dejonckheere E and
Libert C: Cecal ligation and puncture: The gold standard model for
polymicrobial sepsis? Trends Microbiol. 19:198–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng M, Ye JS, Wang Y-L, Chen C and Wang
CY: Posttreatment with propofol attenuates
lipopolysaccharide-induced up-regulation of inflammatory molecules
in primary microglia. Inflamm Res. 63:411–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F
and Zhou C, Huang L, Li X and Zhou C: Dexmedetomidine inhibits
inflammatory reaction in lung tissues of septic rats by suppressing
TLR4/NF-κB pathway. Mediators Inflamm. 2013:5621542013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SN, Son SC, Lee SM, Kim CS, Yoo DG,
Lee SK, Hur GM, Park JB and Jeon BH: Midazolam inhibits
proinflammatory mediators in the lipopolysaccharide-activated
macrophage. Anesthesiology. 105:105–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marketon JI and Sternberg EM: The
glucocorticoid receptor: A revisited target for toxins. Toxins
(Basel). 2:1357–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kulstad EB, Kalimullah EA, Tekwani KL and
Courtney DM: Etomidate as an induction agent in septic patients:
red flags or false alarms? West J Emerg Med. 11:161–172.
2010.PubMed/NCBI
|
|
17
|
Fragen RJ, Shanks CA, Molteni A and Avram
MJ: Effects of etomidate on hormonal responses to surgical stress.
Anesthesiology. 61:652–656. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wanscher M, Tønnesen E, Hüttel M and
Larsen K: Etomidate infusion and adrenocortical function. A study
in elective surgery. Acta Anaesthesiol Scand. 29:483–485. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayroldi E, Cannarile L, Migliorati G,
Nocentini G, Delfino DV and Riccardi C: Mechanisms of the
anti-inflammatory effects of glucocorticoids: Genomic and
nongenomic interference with MAPK signaling pathways. FASEB J.
26:4805–4820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vandevyver S, Dejager L and Libert C: On
the trail of the glucocorticoid receptor: Into the nucleus and
back. Traffic. 13:364–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ayroldi E, Macchiarulo A and Riccardi C:
Targeting glucocorticoid side effects: Selective glucocorticoid
receptor modulator or glucocorticoid-induced leucine zipper? A
perspective. FASEB J. 28:5055–5070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling J and Kumar R: Crosstalk between NFkB
and glucocorticoid signaling: A potential target of breast cancer
therapy. Cancer Lett. 322:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auphan N, DiDonato JA, Rosette C, Helmberg
A and Karin M: Immunosuppression by glucocorticoids: Inhibition of
NF-kappaB activity through induction of I kappa B synthesis.
Science. 270:286–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abraham E, Laterre PF, Garbino J,
Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli
W, Anderson P, et al: Lenercept (p55 tumor necrosis factor receptor
fusion protein) in severe sepsis and early septic shock: A
randomized, double-blind, placebo-controlled, multicenter phase III
trial with 1,342 patients. Crit Care Med. 29:503–510. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber SU, Schewe JC, Lehmann LE, Müller S,
Book M, Klaschik S, Hoeft A and Stüber F: Induction of Bim and Bid
gene expression during accelerated apoptosis in severe sepsis. Crit
Care. 12:R1282008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muenzer JT, Davis CG, Chang K, Schmidt RE,
Dunne WM, Coopersmith CM and Hotchkiss RS: Characterization and
modulation of the immunosuppressive phase of sepsis. Infect Immun.
78:1582–1592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Payen JF, Dupuis C, Trouve-Buisson T,
Vinclair M, Broux C, Bouzat P, Genty C, Monneret D, Faure P, Chabre
O and Bosson JL: Corticosteroid after etomidate in critically ill
patients: A randomized controlled trial. Crit Care Med. 40:29–35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanczkowski W, Alexaki VI, Tran N,
Großklaus S, Zacharowski K, Martinez A, Popovics P, Block NL,
Chavakis T, Schally AV and Bornstein SR: Hypothalamo-pituitary and
immune-dependent adrenal regulation during systemic inflammation.
Proc Natl Acad Sci USA. 110:14801–14806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanders RD, Godlee A, Fujimori T, Goulding
J, Xin G, Salek-Ardakani S, Snelgrove RJ, Ma D, Maze M and Hussell
T: Benzodiazepine augmented γ-amino-butyric acid signaling
increases mortality from pneumonia in mice. Crit Care Med.
41:1627–1636. 2013. View Article : Google Scholar : PubMed/NCBI
|