Introduction
Osteoarthritis (OA) is a multifactorial disease,
which is characterized by degeneration of articular cartilage,
limited intra-articular inflammation with synovitis, and
alterations in periarticular and subchondral bone (1). The majority of individuals >65
years old exhibit radiographic and/or clinical evidence of OA
(2,3). It is widely accepted that the
pathogenesis of OA is complex, and is associated with mechanical
influences, genetic disposition and epigenetic factors.
MicroRNAs (miRNAs/miR) are a group of endogenous,
short, non-coding RNAs, which regulate gene expression by targeting
the 3′-untranslated region (3′UTR) of mRNA. miRNAs have been found
in various organisms, and several of them are evolutionary
conserved. Furthermore, it is estimated that >50% of all human
protein-coding genes are potentially regulated by miRNAs (4). miRNAs have previously been
demonstrated to exist in cartilage and have essential roles during
cartilage development (5).
Appropriate miRNA expression is important for maintaining cartilage
homeostasis, and abnormal miRNA profiles have been shown to be
associated with cartilage diseases (5,6).
A previous study demonstrated that several
aberrantly expressed miRNAs in cartilage are associated with the
pathogenesis of OA (7). In
addition, numerous studies have indicated that miR-140 exhibits a
chondrocyte differentiation-related expression pattern, the
downregulation of which is associated with an enhanced interleukin
(IL)-1β response, which may contribute to the pathogenesis of OA
(6,8,9).
Furthermore, miR-146a is an IL-1β-responsive miRNA, which
contributes to OA pathogenesis by increasing vascular endothelial
growth factor levels (10).
To explore the function of miRNAs during the
pathogenesis of OA and find a target for clinical treatment, the
present study detected the expression levels of nine miRNAs in
cartilage tissue samples from patients with OA, and investigated
the role of candidate miRNAs during the progression of OA.
Materials and methods
Tissue sample collection
Human cartilage was obtained from femoral condyles
and tibial plateaus. OA cartilage samples were obtained from female
patients with OA that underwent total knee arthroplasty (n=33; age,
62±11 years), and normal human cartilage samples were obtained from
individuals within 12 h of death (n=15; age, 60±16 years old). The
normal individuals had no history of joint disease and succumbed to
causes unrelated to arthritic diseases. The cartilage was examined
macroscopically and microscopically to ensure that only normal
tissue was used. All patients with OA were evaluated by a certified
rheumatologist and were diagnosed as having OA according to the
American College of Rheumatology criteria (11). The Ethics Committee Board of the
People's Hospital of Dongying (Dongying, China) approved the use of
human articular tissues. Patients with OA provided written informed
consent, and post-mortem tissues were obtained with the consent of
a family member or authorized individual.
Chondrocyte isolation and culture
Human knee articular chondrocytes were isolated from
9 patients (age, 55–67 years) that underwent joint replacement
surgery for OA. Briefly, cartilage slices (~0.5 cm2)
were initially digested with 0.25% trypsin for 1 h at 37°C, and
were subsequently incubated with 0.04% collagenase type II
overnight in a 37°C water bath and filtered a 20 µm-pore strainer.
Primary cell cultures were maintained at 37°C in a 5%
CO2 atmosphere in Dulbecco's modified Eagle's medium
(DMEM)/F12 (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences), 100 U/ml penicillin and 100 U/ml
streptomycin. Chondrocytes at the third passage were used for
subsequent experiments.
The SW1353 chondrosarcoma cell line was purchased
from the China Infrastructure of Cell Line Resources (Beijing,
China). The cells were cultured in DMEM supplemented with 10% FBS,
100 U/ml penicillin and 100 U/ml streptomycin at 37°C in a
humidified atmosphere.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Single-stranded cDNA was synthesized by using TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and then amplified by using TaqMan Universal PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
together with miRNA-specific TaqMan MGB probes (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR cycling conditions were
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. Primers were supplied by Applied Biosystems (Thermo
Fisher Scientific, Inc.). The expression levels of candidate miRNAs
were measured using stem-loop qPCR with U6 small nuclear RNA as an
internal control. The primers used were purchased from Thermo
Fisher Scientific, Inc. (miR-26a, cat. no. 000405; miR-26b, cat.
no. 000407; miR-27b, cat. no. 002174; miR-125a, cat. no. 002198;
miR-138, cat. no. 02284; miR-140, cat. no. 002234; miR-146a, cat.
no. 000468; miR-181a, cat. no. 000480; miR-199a, cat. no. 002304;
and U6, cat. no. 4451372). Each sample was measured in triplicate
and the experiment was repeated at least three times. The relative
expression levels of the candidate genes were calculated using the
2−ΔΔCq method (12).
For mRNA quantification, first strand cDNA synthesis
was achieved using a Primescript RT reagent kit (Takara Bio, Inc.,
Otsu, Japan). Amplification of cDNA was performed using SYBR Premix
Ex Taq (Takara Bio, Inc.). The primers were synthesized by BGI
(Shenzhen, China) and sequences were as follows: Matrix
metalloproteinase (MMP)-3, forward 5′-CTCGTTGCTGCTCATGAAATTG-3′,
reverse 5′-TCAGGTCTGTGAGTGAGTGATA-3′; MMP-9, forward
5′-GAACTTTGACAGCGACAAGAAG-3′, reverse 5′-CGGCACTGAGGAATGATCTAA-3′;
MMP-13, forward 5′-AGCATCTGGAGTAACCGTATTG-3′, reverse
5′-CCCGCACTTCTGGAAGTATT-3′; cyclooxygenase (COX)2, forward
5′-TCAGTAGGTGCATTGGAATCAA-3′, reverse
5′-GGAGAAACGAAGTGATGAGAAGA-3′; and GAPDH, forward
5′-GACCACTTTGTCAAGCTCATTTC-3′, reverse
5′-CTCTCTTCCTCTTGTGCTCTTG-3′. The PCR cycling condition were 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. The expression levels of GAPDH were used to normalize the
levels of target mRNA, and the relative expression levels of genes
were calculated using the 2−ΔΔCq method (12).
Dual luciferase assay
miRNA mimics, inhibitors and controls were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences
for miRNAs mimics were as follows: miR-26a,
5′-UUCAAGUAAUCCAGGAUAGGCU-3′; miR-26b, 5′-UUCAAGUAAUUCAGGAUAGGU-3′;
and miR-138, 5′-AGCUGGUGUUGUGAAUCAGGCCG-3′. The sequences for
miRNAs inhibitors were as follows: miR-26a,
5′-AGCCUAUCCUGGAUUACUUGAA-3′; miR-26b, 5′-ACCUAUCCUGAAUUACUUGAA-3′;
and miR-138, 5′-CGGCCUGAUUCACAACACCAGCU-3′. The full-length
karyopherin subunit α 3 (KPNA3) 3′UTR was amplified from cDNA of
HEK293T cells and cloned downstream of the firefly luciferase
coding region in the pmirGLO vector (Promega Corporation, Madison,
WI, USA) in order to generate a luciferase reporter vector.
For the luciferase reporter assays, 1×105
SW1353 cells were seeded in 48-well plates. Subsequently, 20 µM
miRNA mimics or inhibitors were transfected into the cells
alongside 100 ng of the reporter vector, using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Scrambled short single-stranded RNA or
double-stranded RNA sequences were used as a control for the miRNA
mimics or inhibitors. Briefly, miRNA mimics or inhibitors were
diluted in DMEM, and Lipofectamine 2000 was also diluted in DMEM.
The mimics, inhibitors and Lipofectamine 2000 dilutions were then
incubated at room temperature for 5 min, before being combined for
20 min at room temperature. The complex (200 µl) was added to each
well of the cell-containing plates, and the plates were incubated
for 4 h. A total of 48 h post-transfection, the cells were
harvested, lysed and assessed using the Dual-Luciferase Assay kit
(Promega Corporation). Each treatment was performed in triplicate
in three independent experiments. The results are expressed as
relative luciferase activity (firefly luciferase
activity/Renilla luciferase activity).
Western blotting
Protein was extracted from the cells using M-PER™
Mammalian Protein Extraction Reagent (Thermo Fisher Scientific,
Inc.); protein samples were quantified using the bicinchoninic acid
method. Protein extracts were subsequently boiled in
SDS/β-mercaptoethanol sample buffer, and 10 µg samples were loaded
into each lane of 10% polyacrylamide gels. The proteins were then
separated by electrophoresis and were blotted onto polyvinylidene
fluoride membranes (Amersham; GE Healthcare Life Sciences, Little
Chalfont, UK) by electrophoretic transfer. The membranes were
blocked in 5% bovine serum albumin in TBS-Tween 20 for 1 h at room
temperature. The membranes were incubated with rabbit anti-KPNA3
polyclonal antibody (1:500; cat. no. ab117578; Abcam, Cambridge,
MA, USA), rabbit anti-NF-κB p65 monoclonal antibody (1:500; cat.
no. ab32536; Abcam), mouse anti-GAPDH monoclonal antibody (1:5,000;
cat. no. sc-32233; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit anti-α-tubulin polyclonal antibody (1:1,000; cat. no.
ab18251; Abcam), rabbit anti-lamin B1 monoclonal antibody (1:1,000;
cat. no. ab13374; Abcam) and mouse anti-β-actin monoclonal antibody
(1:5,000; cat. no. sc-47778; Santa Cruz Biotechnology Inc.) at 4°C
overnight. The specific protein-antibody complexes were
subsequently detected following incubation with horseradish
peroxidase-conjugated goat anti-rabbit (1:5,000) or rabbit
anti-mouse (1:5,000) immunoglobulin G antibodies (cat. nos ab6721
and ab6728, respectively; Abcam) for 2 h at 37°C. The blots were
visualized using an enhanced chemiluminescence kit (Pierce; Thermo
Fisher Scientific, Inc.). The β-actin or GAPDH signals were used as
loading controls for the total cell lysate. The α-tubulin and lamin
B1 signals were used as loading controls for the cytosolic and
nuclear lysates, respectively. The band density was analyzed using
Quantity One software (version 4.6.2; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The experiment was conducted in triplicate and
results were analyzed using Student's t-test.
miRNA transfection and IL-1β
treatment
Chondrocytes were transfected with miRNA mimics,
inhibitors or scrambled control RNA using Lipofectamine 2000. A
total of 48 h post-transfection, the cells were washed three times
with PBS and were incubated for 1 h in serum-starved media (0.5%
FBS). Serum-starved chondrocytes were then stimulated with 10 ng/ml
IL-1β (R&D Systems, Inc., Minneapolis, MN, USA) for 1 h, and
were collected for mRNA and protein expression detection.
NF-κB nuclear translocation
Following various transfections and treatments,
cells were harvested for preparation of the cytoplasmic and nuclear
extracts using NE-PER Nuclear and Cytoplasmic Extraction Reagents
(Thermo Fisher Scientific, Inc.). The NF-κB p65 expression levels
in the extracts were then examined by western blotting.
Statistical analysis
Data were analyzed using SPSS Statistical Package
version 16 (SPSS Inc., Chicago, IL, USA) and are presented as the
mean ± standard deviation. Analyses of two independent groups were
conducted using Welch's unpaired t-test. For multiple comparisons,
one-way analysis of variance was conducted with the Newman-Keuls
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
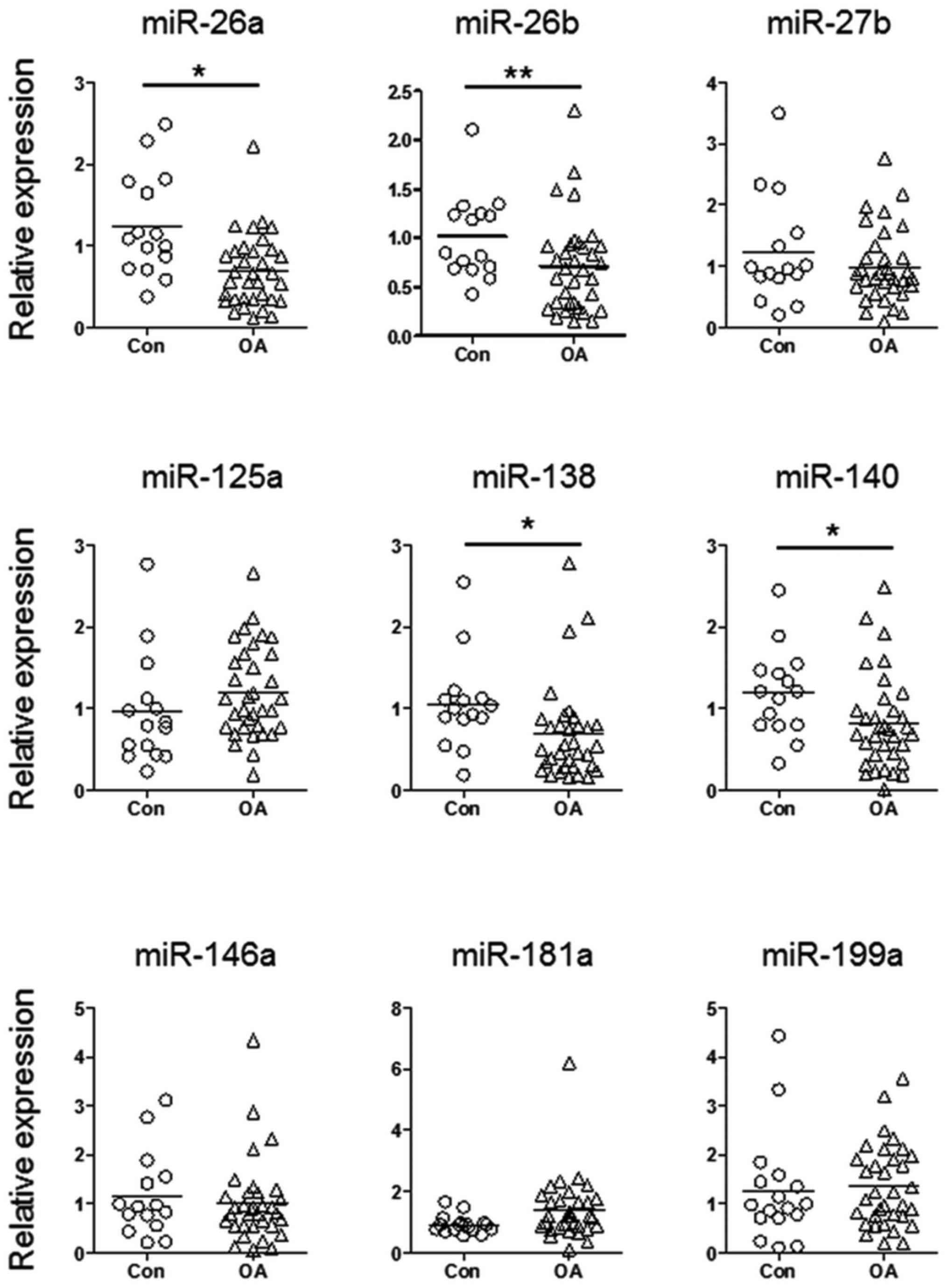
miR-26a, miR-26b, miR-138 and miR-140
are downregulated in patients with OA
To explore the function of aberrantly expressed
miRNAs during the pathogenesis of OA, the present study detected
the expression levels of nine candidate miRNAs in cartilage samples
from patients with OA and normal controls. These candidate miRNAs
have previously been reported to be aberrantly expressed in
cartilage samples from patients with OA, or have been shown to have
a role during chondrogenesis (7,13).
As shown in Fig. 1, miR-26a,
miR-26b, miR-138 and miR-140 exhibited significantly reduced
expression in cartilage samples from patients with OA. Since the
function of miR-140 during the pathogenesis of OA is well
understood (6,14–16),
the present study focused on investigating the biological functions
of miR-26a, miR-26b and miR-138.
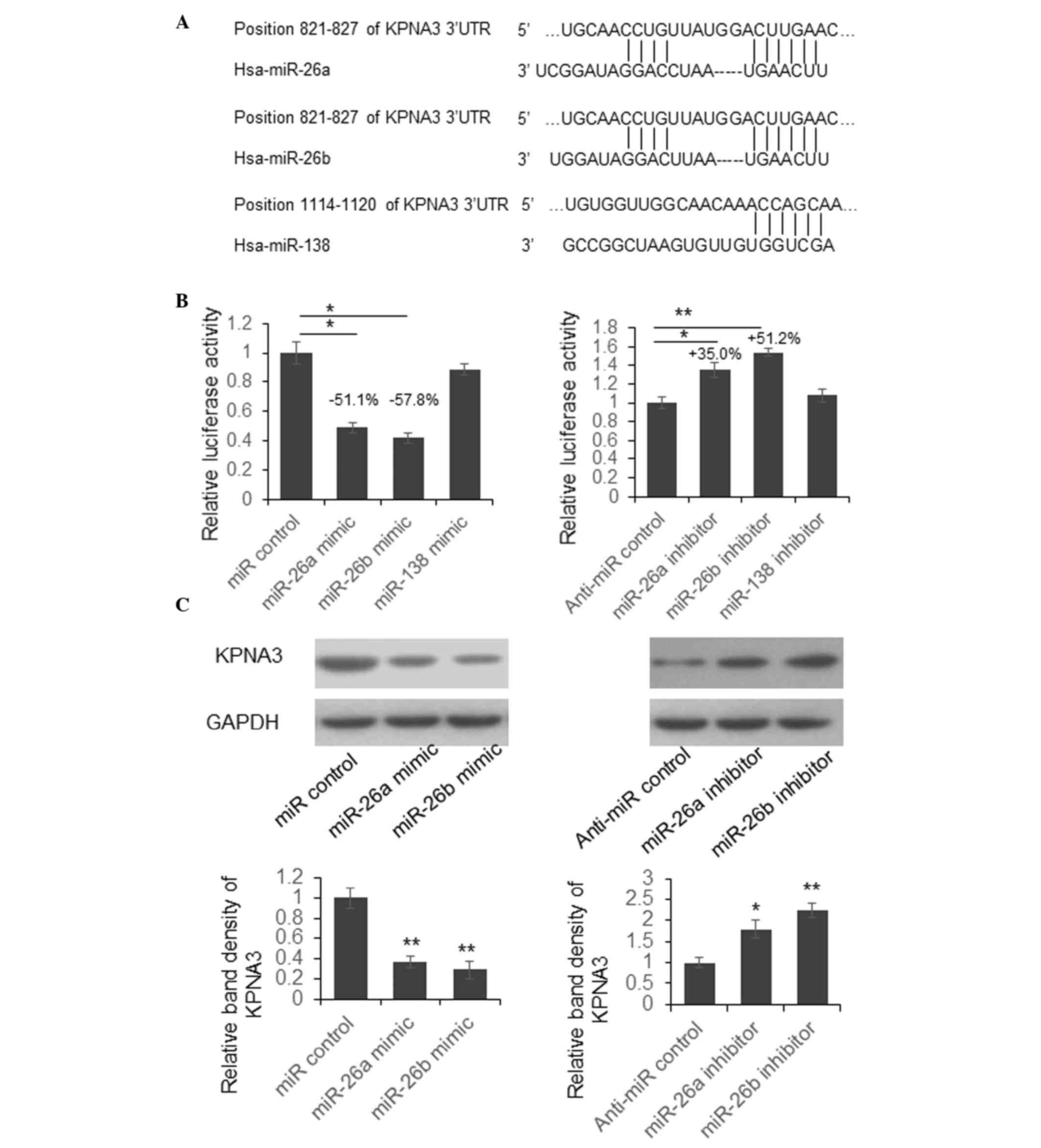
miR-26a and miR-26b suppress KPNA3
expression by targeting its 3′UTR
To investigate the biological function of aberrant
miRNAs during the pathogenesis of OA, the present study predicted
the target genes of miR-26a, miR-26b and miR-138 using the online
bioinformatics tool TargetScan (http://www.targetscan.org/). The predicted
interactions between miRNAs and the 3′UTR of KPNA3 are presented in
Fig. 2A.
To examine whether miR-26a, miR-26b and miR-138 are
able to suppress KPNA3 expression by targeting its 3′UTR, the
present study constructed a luciferase reporter vector by cloning
the full-length KPNA3 3′UTR into the pmirGLO plasmid, downstream of
the firefly luciferase coding region. Dual luciferase assays were
then conducted in SW1353 cells. As presented in Fig. 2B, luciferase activity was
significantly reduced by 51.1 or 57.8% in cells overexpressing
miR-26a or miR-26b. Conversely, luciferase activity was increased
by 35.0 or 51.2% in cells in which miR-26a or miR-26b expression
was suppressed. There was no significant change in luciferase
activity in cells with miR-138 overexpression or downregulation.
These results indicate that miR-26a and miR-26b may suppress
luciferase activity by targeting KPNA3 3′UTR.
To further examine whether endogenous KPNA3
expression was suppressed by miR-26a and miR-26b, SW1353 cells were
transfected with miR-26a and miR-26b mimics or inhibitors. A total
of 48 h post-transfection, the cells were lysed and KPNA3
expression was detected by western blotting. As shown in Fig. 2C, the protein expression levels of
KPNA3 were downregulated following transfection with miR-26a or
miR-26b mimics, whereas KPNA3 expression was upregulated in cells
transfected with miR-26a or miR-26b inhibitors. These results
suggest that endogenous KPNA3 expression may be directly regulated
by miR-26a and miR-26b.
miR-26a and miR-26b modulate NF-κB p65
translocation, and the expression of MMP-3, −9, −13 and COX-2
Previous studies have indicated that KPNA3 is able
to bind to the nuclear localization sequence region of NF-κB p65
and mediate translocation between the cytoplasm and nucleus
(17,18). To determine whether miR-26a and
miR-26b are able to regulate NF-κB signaling by modulating p65
translocation, chondrocytes were transfected with miR-26a and
miR-26b mimics or inhibitors. A total of 48 h post-transfection,
the cells were stimulated with 10 ng/ml IL-1β for 1 h and were
collected for protein expression detection.
As shown in Fig.
3A, the expression levels of miR-26a or miR-26b were
significantly upregulated by miR-26a or miR-26b mimics, and were
reduced by miR-26a or miR-26b inhibitors. Following IL-1β
stimulation, the process of p65 cytoplasmic-nuclear translocation
was arrested following transfection with miR-26a or miR-26b mimics,
and p65 was detained in the cytoplasm (Fig. 3B, left panel). However, the p65
translocation process was promoted following transfection with
miR-26a or miR-26b inhibitors, and increased p65 expression was
detected in the nucleus (Fig. 3B,
right panel).
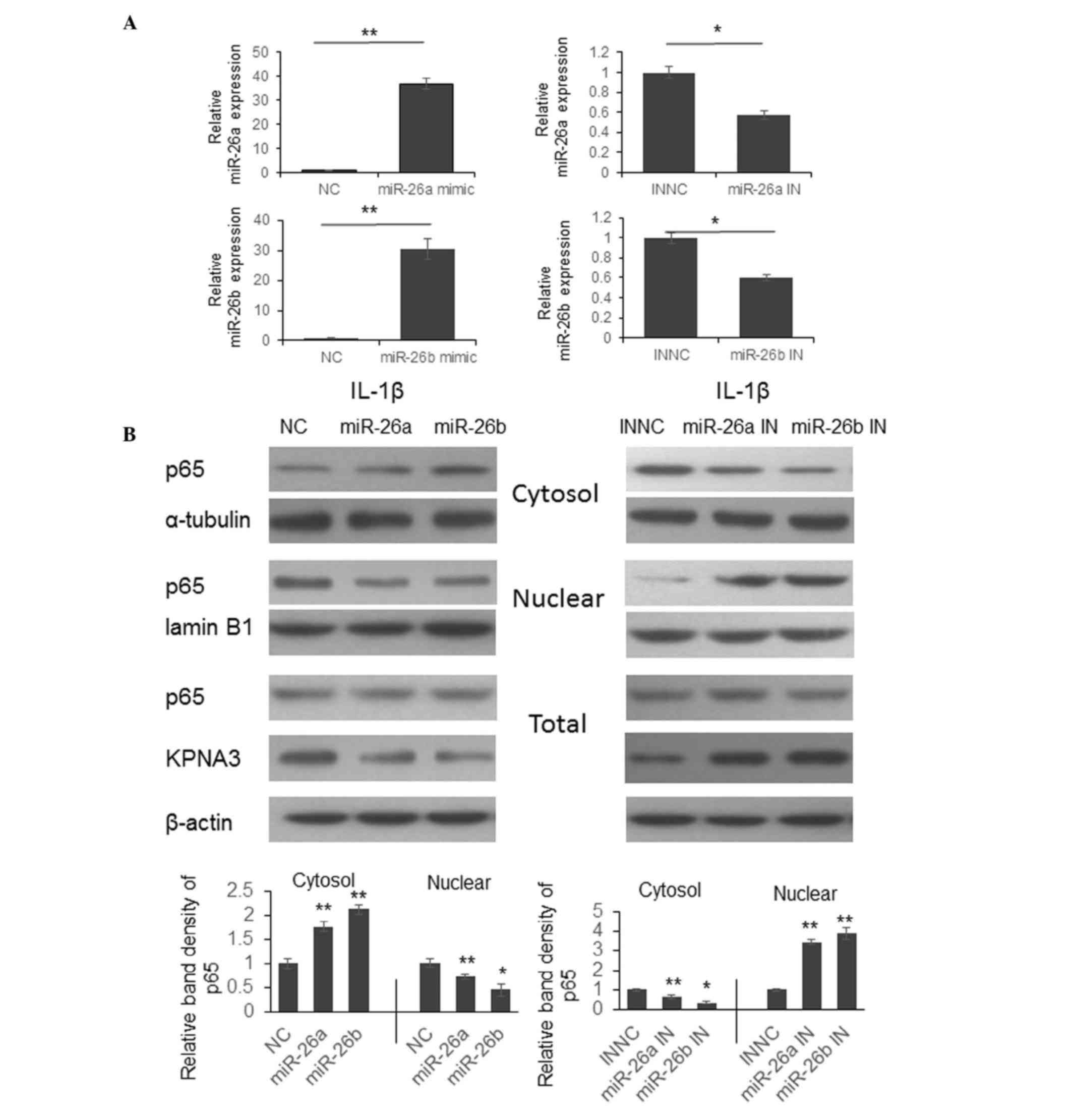 | Figure 3.Downregulation of miR-26a or miR-26b
promotes p65 cytoplasmic-nuclear translocation by upregulating
KPNA3 expression. (A) Chondrocytes were isolated from cartilage
samples from patients with osteoarthritis and were transfected with
miR-26a and miR-26b mimics or inhibitors. A total of 48 h
post-transfection, miR-26a and miR-26b expression levels were
detected. The results were analyzed by Student's t-test.
*P<0.05, **P<0.01. (B) Chondrocytes were transfected with
miRNA mimics or inhibitors for 48 h. The cells were then treated
with IL-1β for 1 h, and the expression levels of p65 were detected
in the cytoplasm, nucleus and total lysate by western
blotting.*P<0.05, **P<0.01. miR/miRNA, microRNA; KPNA3,
KPNA3, karyopherin subunit alpha 3; IL-1β, interleukin-1β; IN,
inhibitor; NC, negative control. |
To explore the function of miR-26a and miR-26b on
modulating NF-κB signaling, the present study examined the mRNA
expression levels of MMP-3, −9, −13 and COX-2. As presented in
Fig. 4, the expression levels of
MMP-3, −9, −13 and COX-2 were significantly downregulated by
miR-26a or miR-26b mimics, and were upregulated by miR-26a or
miR-26b inhibitors. These results indicate that reduced miR-26a and
miR-26b expression may contribute to the pathogenesis and
progression of OA by upregulating NF-κB signaling.
Discussion
miRNAs are a group of endogenous, short, non-coding
RNAs, the function of which was demonstrated to be associated with
the development and homeostasis of articular cartilage. Recently,
increasing reports indicated that an altered miRNA expression
profile is associated with the pathogenesis of OA, however, the
mechanisms were not well understood. The current study examined the
level of nine selected miRNAs in the cartilage samples of 33
patients with OA. In contrast with other reports, upregulation of
miR-146a in cartilage samples from patients with OA was not
detected (19). The expression of
miR-27b and miR-125a was also not altered, in contrast with what
was reported by other researchers, which may be caused by the
differences of samples collection and the genetic background of the
patients in the study (14,20).
However, the current study observed that the levels of miR-26a,
miR-26b, miR-138 and miR-140 were downregulated in the patients
with OA. It was also confirmed that miR-26a and miR-26a function as
an immune response modulators that regulate NF-κB p65 translocation
by targeting KPNA3. To the best of our knowledge, this is the first
report to demonstrate the role of miR-26a and miR-26b as NF-κB
signaling regulators during OA pathogenesis.
The nucleocytoplasmic traffic of large molecules
(>25 nm in diameter) in eukaryotic cells is regulated by
specific nuclear import and export systems. Proteins that contain
classical nuclear localization sequences are imported into the
nucleus by importin α/β heterodimers (21). Although eight importin α members
were found in human cells KPNA3 was confirmed to be the most
important mediator of p50/p65 heterodimer and p50 homodimer
translocation (17). The present
study confirmed that KPNA3 is a direct target of miR-26a and
miR-26b for the first time. Furthermore, upregulation of MMPs and
COX-2 induced by IL-1β in chondrocytes are predominantly modulated
by NF-κB signaling (22,23). The reduction of MMP-3, −9, −13 and
COX-2 mRNA level by miR-26a/b mimics and upregulation by miR-26a/b
inhibitors indicates the functional role of miR-26a/b in regulating
NF-κB signaling. Thus, the current study successfully demonstrated
an association between miR-26a/b reduction and OA pathogenesis,
which may shed light on the mechanism of why altered miRNA
expression is associated with OA.
In conclusion, the present study demonstrated that
miR-26a, miR-26b, miR-138 and miR-140 are downregulated in
cartilage samples from patients with OA. Furthermore, reduced
miR-26a and miR-26b expression induced upregulation of MMPs and
COX-2 by upregulating KPNA3 expression and promoting p65
cytoplasmic-nuclear translocation.
References
|
1
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence RC, Helmick CG, Arnett FC, Deyo
RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder
GG, et al: Estimates of the prevalence of arthritis and selected
musculoskeletal disorders in the United States. Arthritis Rheum.
41:778–799. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shane Anderson A and Loeser RF: Why is
osteoarthritis an age-related disease? Best Pract Res Clin
Rheumatol. 24:15–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirzamohammadi F, Papaioannou G and
Kobayashi T: MicroRNAs in cartilage development, homeostasis and
disease. Curr Osteoporos Rep. 12:410–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyaki S, Nakasa T, Otsuki S, Grogan SP,
Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK and Asahara H:
MicroRNA-140 is expressed in differentiated human articular
chondrocytes and modulates interleukin-1 responses. Arthritis
Rheum. 60:2723–2730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barter MJ and Young DA: Epigenetic
mechanisms and non-coding RNAs in osteoarthritis. Curr Rheumatol
Rep. 15:3532013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyaki S, Sato T, Inoue A, Otsuki S, Ito
Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, et al:
MicroRNA-140 plays dual roles in both cartilage development and
homeostasis. Genes Dev. 24:1173–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura Y, Inloes JB, Katagiri T and
Kobayashi T: Chondrocyte-specific microRNA-140 regulates
endochondral bone development and targets Dnpep to modulate bone
morphogenetic protein signaling. Mol Cell Biol. 31:3019–3028. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Huang J, Dai L, Yu D, Chen Q, Zhang
X and Dai K: miR-146a, an IL-1beta responsive miRNA, induces
vascular endothelial growth factor and chondrocyte apoptosis by
targeting Smad4. Arthritis Res Ther. 14:R752012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. classification of osteoarthritis of
the knee. diagnostic and therapeutic criteria committee of the
american rheumatism association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le LT, Swingler TE and Clark IM: Review:
The role of microRNAs in osteoarthritis and chondrogenesis.
Arthritis Rheum. 65:1963–1974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tardif G, Hum D, Pelletier JP, Duval N and
Martel-Pelletier J: Regulation of the IGFBP-5 and MMP-13 genes by
the microRNAs miR-140 and miR-27a in human osteoarthritic
chondrocytes. BMC Musculoskelet Disord. 10:1482009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Araldi E and Schipani E: MicroRNA-140 and
the silencing of osteoarthritis. Genes Dev. 24:1075–1080. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tardif G, Pelletier JP, Fahmi H, Hum D,
Zhang Y, Kapoor M and Martel-Pelletier J: NFAT3 and TGF-beta/SMAD3
regulate the expression of miR-140 in osteoarthritis. Arthritis Res
Ther. 15:R1972013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fagerlund R, Kinnunen L, Köhler M,
Julkunén I and Melen K: NF-(kappa)B is transported into the nucleus
by importin (alpha)3 and importin (alpha)4. J Biol Chem.
280:15942–15951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agrawal T, Gupta GK and Agrawal DK:
Calcitriol decreases expression of importin alpha3 and attenuates
RelA translocation in human bronchial smooth muscle cells. J Clin
Immunol. 32:1093–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamasaki K, Nakasa T, Miyaki S, Ishikawa
M, Deie M, Adachi N, Yasunaga Y, Asahara H and Ochi M: Expression
of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum.
60:1035–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nugent M: MicroRNAs: exploring new
horizons in osteoarthritis. Osteoarthritis Cartilage. 24:573–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pumroy RA and Cingolani G: Diversification
of importin-alpha isoforms in cellular trafficking and disease
states. Biochem J. 466:13–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh R, Ahmed S, Islam N, Goldberg VM and
Haqqi TM: Epigallocatechin-3-gallate inhibits
interleukin-1beta-induced expression of nitric oxide synthase and
production of nitric oxide in human chondrocytes: suppression of
nuclear factor kappaB activation by degradation of the inhibitor of
nuclear factor kappaB. Arthritis Rheum. 46:2079–2086. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|