Introduction
Gastric cancer is a frequently occurring cancer,
which is responsible for ~989,600 novel diagnoses and ~738,000
cases of mortality annually worldwide (1–2). The
goal of this study was to show relationship between Fas (including
its downstream signaling molecules) and gastric cancer. Fas, which
is also known as apoptosis antigen-1 or cluster of differentiation
95, is a member of the death receptor family, a subfamily of the
tumor necrosis factor receptor superfamily (3). Interactions between Fas and its
natural ligand (FasL) or agonistic antibodies induce apoptosis in
responsive cells (3). The
death-inducing signaling complex (DISC), which contains
oligomerized Fas, the adaptor protein Fas-associated death domain
(FADD), two isoforms of procaspase-8, procaspase-10 and cellular
FADD-like interleukin (IL)-1β-converting enzyme-inhibitory protein,
is formed following FasL stimulation (4), resulting in the induction of
programmed cell death or apoptosis (5). Downregulated expression of Fas has
been observed in numerous types of tumor, including head and neck,
esophageal, pancreatic, non-small cell lung and bladder cancer
(4–8).
Gastric cancer with reduced frequency of apoptosis
usually increase during tumor progression (9–12).
The present study investigated Fas expression in specimens from
patients with gastric cancer, in order to determine the involvement
of the Fas signaling pathway in gastric cancer.
Materials and methods
Reagents
The rabbit polyclonal anti-Fas (N-18; cat. no.
sc-714), rabbit polyclonal anti-caspase-3 (H-277; catalog no.
sc-7148), mouse monoclonal anti-caspase-8 (cat. no. sc-81656),
mouse monoclonal anti-poly (adenosine diphosphate (ADP)-ribose)
polymerase 1 (PARP1) (B-10; cat. no. sc-74470) and mouse monoclonal
anti-β-actin (C4; cat. no. sc-47778) antibodies were all purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Polyvinylidene difluoride (PVDF) membranes were purchased from EMD
Millipore (Billerica, MA, USA). The radioimmunoprecipitation buffer
and enhanced bicinchoninic acid assay kit were purchased from
Beyotime Institute of Biotechnology (Haimen, China). The LipoFiter™
Liposomal Transfection reagent was from Hanbio Biotechnology Co.,
Ltd. (Shanghai, China). The Biotin-Streptavidin HRP Detection
System (SP test kit; SP-9000) and diaminobenzidine (DAB)
colorization test kit were purchased from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China). The Annexin
V-phycoerythrin (PE)/7-aminoactinomycin D (AAD) apoptosis detection
kit was obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China). Reverse Transcription-Polymerase Chain Reaction (PCR) kit
was purchased from Sangon Biotech Co., Ltd. (Shanghai, China).
THUNDERBIRD® SYBR® quantification PCR Mix was
purchased from Toyobo Co., Ltd. Primer sequences were synthesized
by Sangon Biotech Co., Ltd.
Human gastric cancer tissue and tissue
microarray
A total of 26 frozen tumor samples and their
corresponding healthy gastric tissues were obtained from 26
patients with gastric cancer diagnosed between 2006 and 2013 at
Jianghan University Medical School (Wuhan, China) including 20
males and 6 females with an average age of 53.5 years.
Formalin-fixed, paraffin-embedded tissue samples were obtained from
113 patients with gastric cancer diagnosed between 2006 and 2013 at
Jianghan University Medical School (Wuhan, China), including 81
males and 32 females with an average age 51.3 years old. All
patients had not treatment before the samples were obtained. The
113 gastric cancer tissues were fixed in 10% neutral buffered
formalin for >24 h within half an hour following surgical
removal and paraffin-embedded with standard procedures,
Furthermore, there were 41 cases of normal gastric tissues that
were paired to 41 cases of gastric cancer tissues from the 113
gastric cancer tissues were also collected. The 41 cases of normal
gastric tissues were collected from the incisal margin where were
away from the tumor at least 5 cm. They were all confirmed by two
pathologists. The normal gastric tissues were also fixed in 10%
neutral buffered formalin and paraffin-embedded, written informed
consent was obtained from all patients. These samples were used
following approval by the Institutional Review Board. The present
study was approved by the ethics committee of the School of
Medicine, Jianghan University (Wuhan, China). A single tissue core,
1.0 mm in diameter and 3–4 mm in depth, was removed from each block
using a manual microarray device (Beecher Instruments Inc., Sun
Prairie, WI, USA) with a total of 113 gastric cancer tissue cores
and 41 paired normal gastric tissues inserted into the recipient
paraffin-block. The tissue microarray was sectioned at 4-µm
thickness. Subsequently, 41 gastric cancer tissues from the 113
gastric cancer tissue and 41 corresponding normal gastric tissues
were selected from the paraffin-embedded samples for
immunohistochemistry (IHC) to analyze Fas expression.
Cell lines
The AGS, BGC823 and SGC7901 gastric cancer cell
lines, and GES-1 healthy gastric cell line were obtained from the
Cell Center of Basic Medicine, Chinese Academy of Medical Sciences
(Beijing, China).
Cell culture
All cells were maintained in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2.
Fas-green fluorescent protein (GFP)
expression plasmid and DNA transfection
The Fas-GFP expression plasmid and the empty
expression control vector (pEGFP-N1) were obtained from Dr. Junfeng
Zhang (Institute of Genetics and Developmental Biology, Chinese
Academy of Sciences, Beijing, China). All transfections were
performed using LipoFiter™ Liposomal Transfection reagent according
to the manufacturer's protocol. The AGS cells were plated
(6×106 cells) in 100 mm dishes in fresh RPMI-1640 medium
containing 10% FBS. The cells were transfected with 4.6 µg Fas-GFP
expression vector or pEGFP-N1 using 4.8 µl LipoFiter™ reagent. The
media was replaced 6 h following transfection and the transfected
cells were incubated at 37°C for 48 h. These transfected cells were
used for subsequent experiments.
IHC
Immunohistochemical staining was performed on 4
µm-thick tumor sections of the tissue microarray using a ‘two-step’
method. The tissue slides were deparaffinized with 100% xylene and
rehydrated gradually in an alcohol series. Antigen retrieval was
performed with immersing the slides in 0.5 mol/l ethylenediamine
tetraacetic acid buffer (pH 8.0), followed by boiling in a water
bath. The endogenous peroxidase activity was attenuated by
incubation in a 3% hydrogen peroxide/methanol buffer for 10 mins at
room temperature. The slides were washed in phosphate-buffered
saline (PBS) and blocked according to the protocol from the
Biotin-Streptavidin HRP Detection System (SP test kit; SP-9000,
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) and subsequently incubated with 1:100 primary antibodies in
PBS overnight at 4°C in a humidified chamber. Following incubation,
the slides were washed with PBS containing 0.05% Tween-20. The
slides were then incubated with the Biotin-Streptavidin HRP
Detection System (SP test kit) (SP-9000, Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) according to the
protocol. The secondary antibody was the Biotin labeled Goat
anti-rabbit/mouse IgG from the SP test kit. The slides were then
washed, incubated with DAB chromogen and washed with tap water,
prior to counterstaining with Mayer's hematoxylin (Sangon Biotech
Co., Ltd).
A total of 100 gastric tumor cells per high
magnification field with five high magnification fields in total
were randomly examined with an Olympus BX51 light microscope
(Olympus Corporation, Tokyo, Japan) and cells that exhibited
Fas-positive staining were scored by two pathologists. The normal
gastric cells and apoptotic cells were examined as negative
controls or positive controls in the same field. The experiments
were performed at least three times to ensure reproducibility of
results. Fas staining was scored as follows: 0, no staining or
staining observed in <10% tumor cells; 1+, faint/barely
perceptible staining detected in ≥10% tumor cells; 2+/3+,
moderate/strong staining, respectively, observed in ≥10% tumor
cells. A score of 0/1+ was considered negative and a score of 2+/3+
was considered positive. The immunostained slides were evaluated
independently by two pathologists in a blinded-manner. In the
majority of cases, the evaluation of the two pathologists was
identical and discrepancies were resolved by re-examination and
consensus.
RNA extraction and reverse
transcription-PCR
Total RNA was extracted from the gastric cancer
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was then reverse transcribed into cDNA using the Reverse
Transcription-PCR kit (M-MuLV First Strand cDNA Synthesis kit; cat.
no. B532435; Sangon Biotech Co. Ltd., Shanghai, China) according to
the manufacturer's protocol. The reaction volume was 20 µl per
sample. Subsequently, a PCR reaction was carried out using the
StepOnePlus Real-Time PCR system (Thermo Fisher Scientific, Inc.)
with 35 cycles with a SYBR-Green-based approach and
SYBR® qPCR mix (LOT:246900, THUNERBIRD; Toyobo Co.,
Ltd., Osaka, Japan) in a final volume of 20 µl including 100 ng
cDNA and 0.4 pmol/µl of each primer. Thermocycling conditions were
as follows: An initial denaturation for 1 min at 95°C and 35 cycles
consisting of denaturation at 95°C for 15 sec, an annealing step at
56°C for 30 sec and an extension step at 72°C for 30 sec. Primers
for Fas, caspase-8, caspase-3, PARP1 and β-actin were designed
using Primer 5.0 (Premier Biosoft International, Palo Alto, CA,
USA) and were used simultaneously in the same reaction. The
following primers were used: Fas, Forward
5′-GGACCCTCCTACCTCTGGTT-3′, reverse 5′-ACCTGGAGGACAGGGCTTAT-3′;
caspase-8, forward 5′-CCAGAGACTCCAGGAAAAGAGA-3′, reverse
5′-GATAGAGCATGACCCTGTAGGC-3′; caspase-3, forward
5′-TGGCATTGAGACAGACA-3′, reverse 5′-GGCACAAAGCGACTG-3′; PARP1,
forward, 5′-TGATGGGTAGTACCTGTACTA-3′, reverse
5′-CAGTTTTATCTACCTGGC-3′; and β-actin, forward
5′-CAACGGCTCCGGCATGTGC-3′ and reverse 5′-CTCTTGCTCTGGGCCTCG-3′. The
relative expression of target genes was calculated by the
2−ΔΔCq method (13).
The data were presented as the relative quantity of target mRNA
normalized to the expression of β-actin mRNA and relative to a
calibrator sample. Each experiment was performed at least three
times.
Western blot analysis
For western blot analysis, gastric cancer and normal
gastric cells were washed with cold PBS and lysed in lysis buffer
containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.25 mM EDTA (pH
8.0), 0.1% SDS, 1% Triton X-100 and 50 mM NaF, supplemented with
MS-SAFE™ Protease and Phosphatase Inhibitor Cocktail and
phosphatase inhibitors, all obtained from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). The protein concentrations were
determined using an Enhanced Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology). The cell lysates were
subsequently mixed with loading buffer (Beyotime Institute of
Biotechnology), separated by 12% SDS-PAGE and transferred onto PVDF
membranes. The PVDF membranes were incubated for 45 min in 2%
(wt/vol) bovine serum albumin (BSA, Boster Biological Technology
Co, Wuhan, China). The membranes were subsequently probed overnight
at 4°C with 1:1,000 (vol/vol) different primary antibodies (the
rabbit polyclonal anti-Fas; N-18; cat. no. sc-714; rabbit
polyclonal anti-caspase-3; H-277; cat. no. sc-7148; mouse
monoclonal anti-caspase-8; cat. no. sc-81656; mouse monoclonal
anti-PARP1; B-10; cat. no. sc-74470; and mouse monoclonal
anti-β-actin; C4; cat. no. sc-47778; antibodies). Then the
membranes were washed with TBS-T (20 mM Tris-HCl; pH 7.6;
containing 150 mM NaCl, 0.1% Tween-20) for 5 mins/3 times at room
temperature. The membranes were subsequently probed with 1:2,000
(vol/vol) appropriate secondary antibodies (goat anti-mouse
IgG-HRP; cat. no. sc-2005; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and goat anti-rabbit IgG-HRP (cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h at 37°C. The membranes were then
washed with TBS-T for 5 mins/3 times at room temperature and
visualized using enhanced chemiluminescence detection reagents (DNR
Bio-Imaging Systems, Ltd., Jerusalem, Israel). The density of the
protein bands was assessed using Totallab analysis software,
version 2.01 (Nonlinear USA, Inc., Durham, NC, USA). The above
antibody were diluted with the 2% (wt/vol) BSA (Boster Biological
Technology Co, Wuhan China).
Apoptotic assay
The cells were stained using an Annexin V-PE/7-AAD
apoptosis detection kit according to the manufacturer's protocol,
in order to detect early apoptotic cells (Annexin
V+/7-AAD−) and necrotic or late apoptotic
cells (Annexin V+/7-AAD+) by flow cytometry.
Briefly, the cells transfected with the Fas-GFP expression vector
or the control vector were collected and re-suspended at a density
of 1×106 cells/ml. The cells were then stained with
Annexin V-PE and 7-AAD in binding buffer, according to the
manufacturer's protocol. Quantification of the apoptotic cells was
performed using a FACScan flow cytometer (Beckman Coulter, Inc.,
Brea, CA, USA) and data were collected and analyzed using CXP
Version 2.2 software (Beckman Coulter, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software version 12.0 (SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard deviation of three replicates and
were analyzed using an unpaired Student's t-test and one-way ANOVA.
The Pearson's correlation coefficient test was used to assess the
strength of the relationship between two continuous variables.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed at least three times to
ensure reproducibility of results.
Results
Fas expression and clinicopathological
features in gastric cancer
Fas expression was examined in 113 samples of
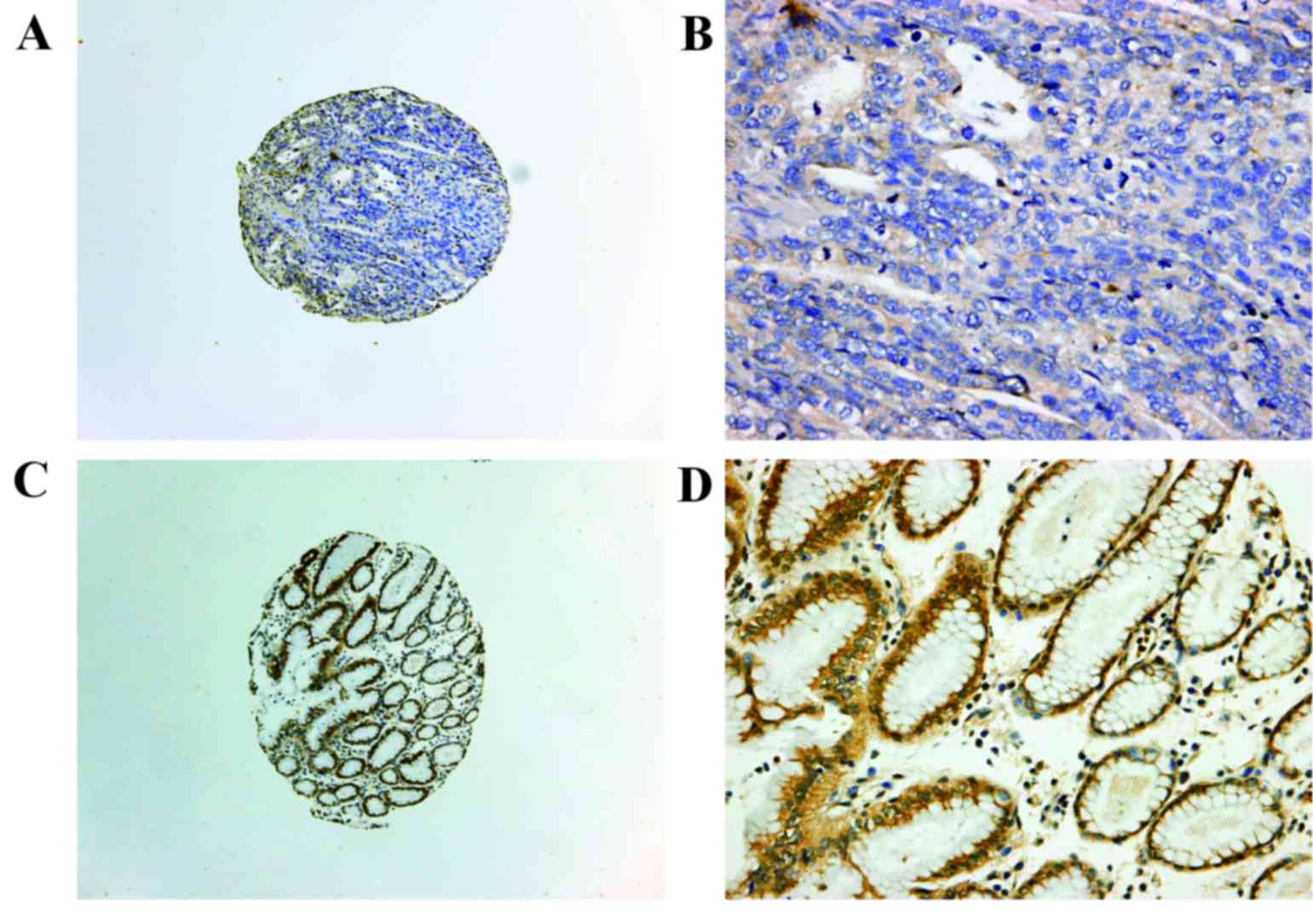
gastric cancer using IHC. Fas expression was primarily detected in
the cytoplasm and on the plasma membrane of gastric cancer cells
(Fig. 1). Positive expression of
Fas was detected in 21 of the 113 samples of gastric cancer.
Downregulated Fas expression was correlated with various factors
including low histological differentiation (P=0.012), male gender
(P=0.00002), and lymph node (P=0.022) and distant metastases
(P=0.026). However, no correlation was observed with age (P=0.617)
and T stage (P=0.173; Table I). A
total of 41 samples of gastric cancer and paired healthy tissues
were examined for Fas expression using IHC. Positive staining of
Fas was detected in 7 of the 41 samples of gastric cancer and in 36
of the 41 corresponding normal tissues (P<0.001; Table II; Fig. 1). These results indicated that Fas
expression was downregulated in gastric cancer.
 | Table I.Fas expression and clinicopathological
features of gastric cancer. |
Table I.
Fas expression and clinicopathological
features of gastric cancer.
|
| Fas expression |
|
|---|
|
|
|
|---|
| Factor | Positive | Negative | P-value |
|---|
| Gender |
| Male | 7 | 74 | P=0.00002 |
|
Female | 14 | 18 |
| Age |
| ≤50
years | 5 | 20 | P=0.617 |
| >50
years | 16 | 72 |
| Histological
differentiation |
| High | 10 | 17 | P=0.012 |
|
Medium | 6 | 28 |
| Low | 5 | 47 |
| T stage |
| T1 | 1 | 18 | P=0.173 |
| T2 | 6 | 21 |
| T3 | 8 | 19 |
| T4 | 6 | 34 |
| N stage |
| N0 | 13 | 32 | P=0.022 |
|
N1-3 | 8 | 60 |
| M stage |
| M0 | 15 | 41 | P=0.026 |
| M1 | 6 | 51 |
 | Table II.Fas expression in T and corresponding
N tissues. |
Table II.
Fas expression in T and corresponding
N tissues.
|
| Fas expression |
|
|---|
|
|
|
|
|---|
| Tissue | Positive | Negative | P-value |
|---|
| T | 7 | 34 | P<0.001 |
| N | 36 | 5 |
|
Fas expression was accompanied by
caspase-8, caspase-3 and PARP1 expression
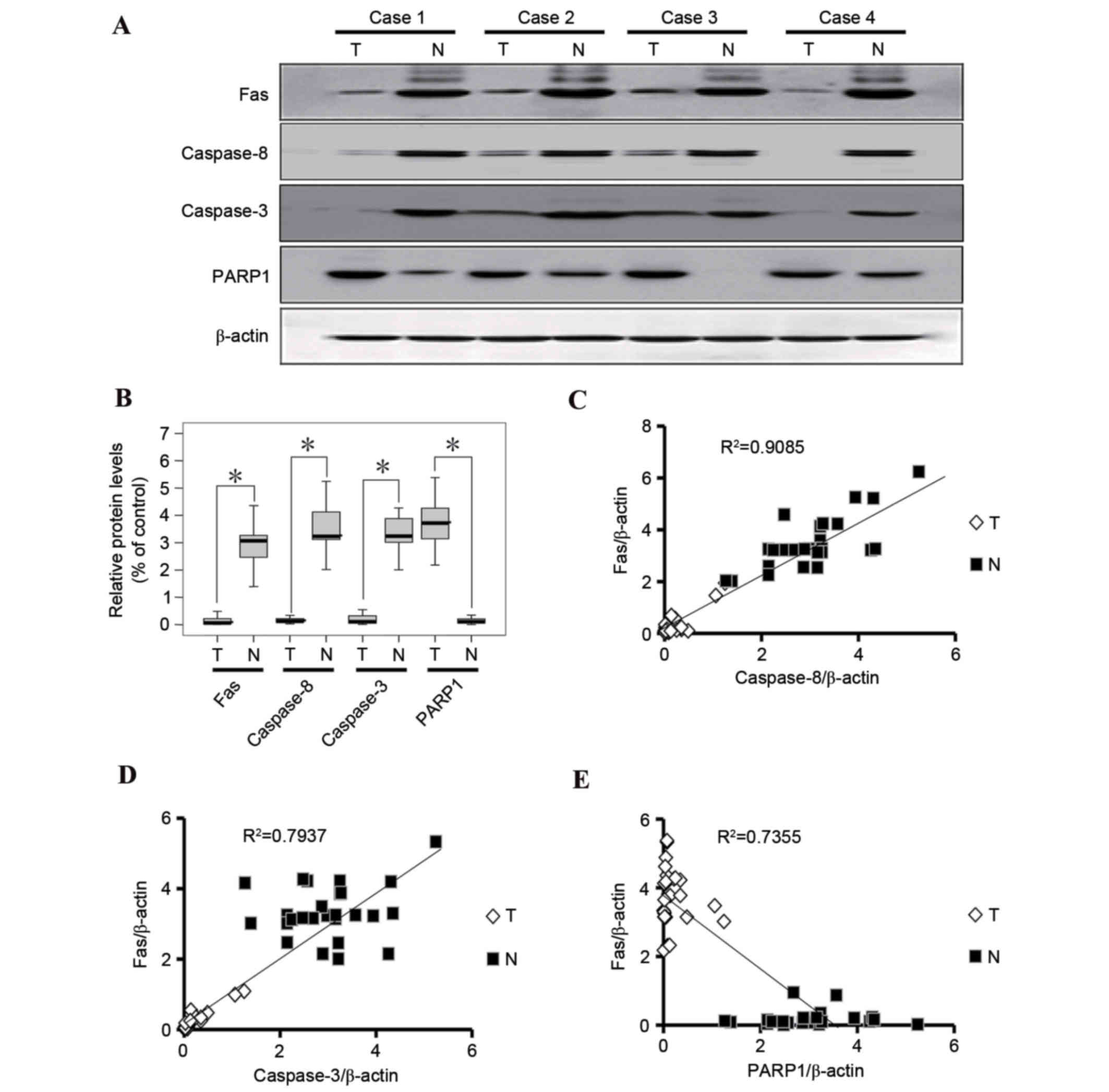
Expression of Fas, caspase-8, caspase-3 and PARP1
was examined in tumor tissues and corresponding healthy tissues of
26 cases of gastric cancer using western blot analysis. Fas,
caspase-8 and caspase-3 expression in gastric cancer tissues was
significantly downregulated (Fig.
2) compared with in paired healthy tissues. However, PARP1
expression was higher in gastric cancer tissues compared with
healthy gastric tissues (Fig. 2).
Fas expression was positively correlated with caspase-8 and
caspase-3 expression, and was inversely correlated with PARP1
expression in tumor and normal gastric specimens (Fig. 2). The expression levels of Fas,
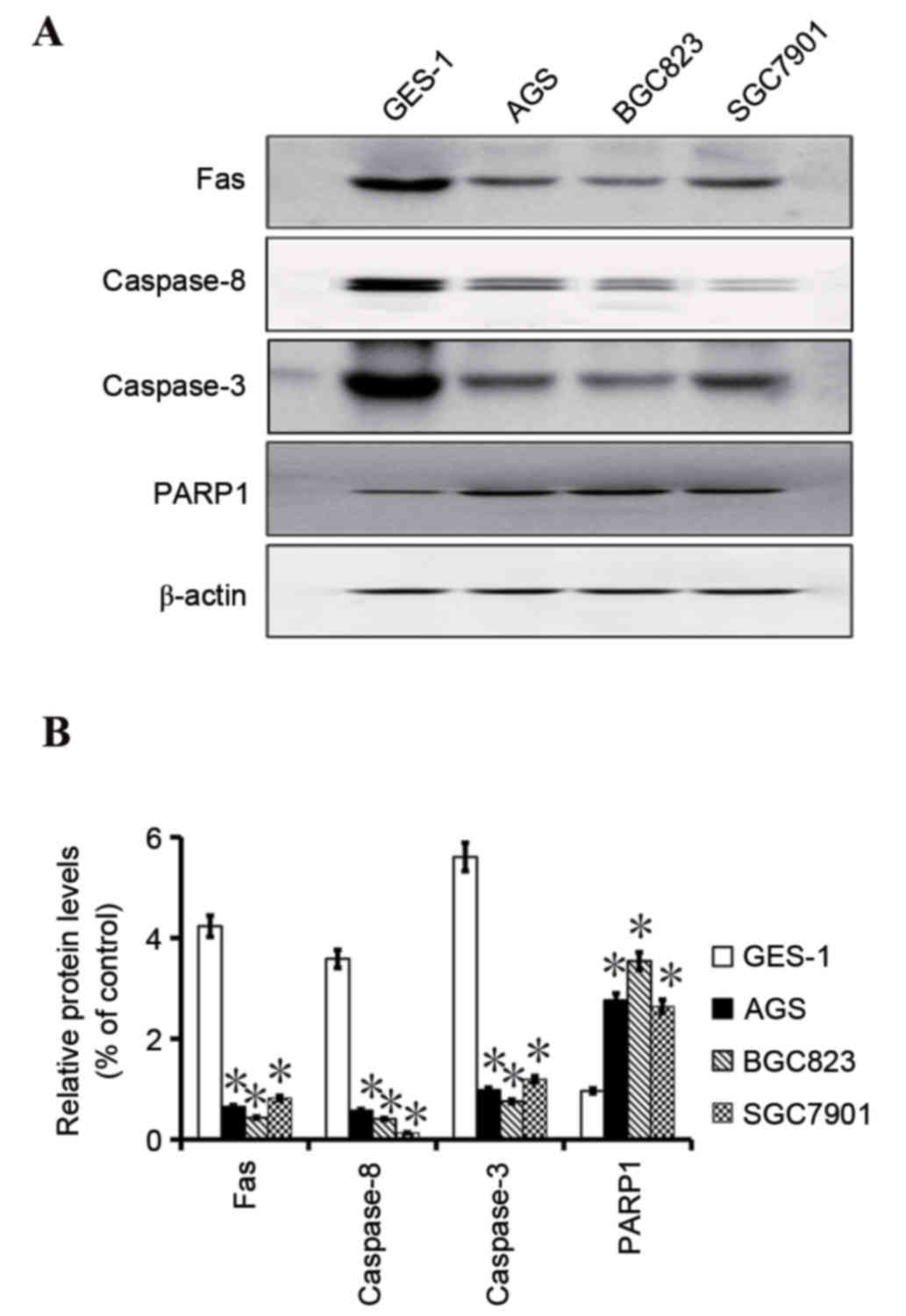
caspase-8, caspase-3 and PARP1 in AGS, BGC823 and SGC7901 gastric
cancer cell lines, and the GES-1 healthy gastric cell line were
also examined. It was observed that Fas, caspase-8 and caspase-3
expression in the gastric cancer cell lines was downregulated
compared with the healthy GES-1 gastric cell line. Conversely,
PARP1 expression was increased in the gastric cancer cell lines
compared with the normal gastric cell line (Fig. 3).
Fas expression and apoptosis in AGS
gastric cancer cells
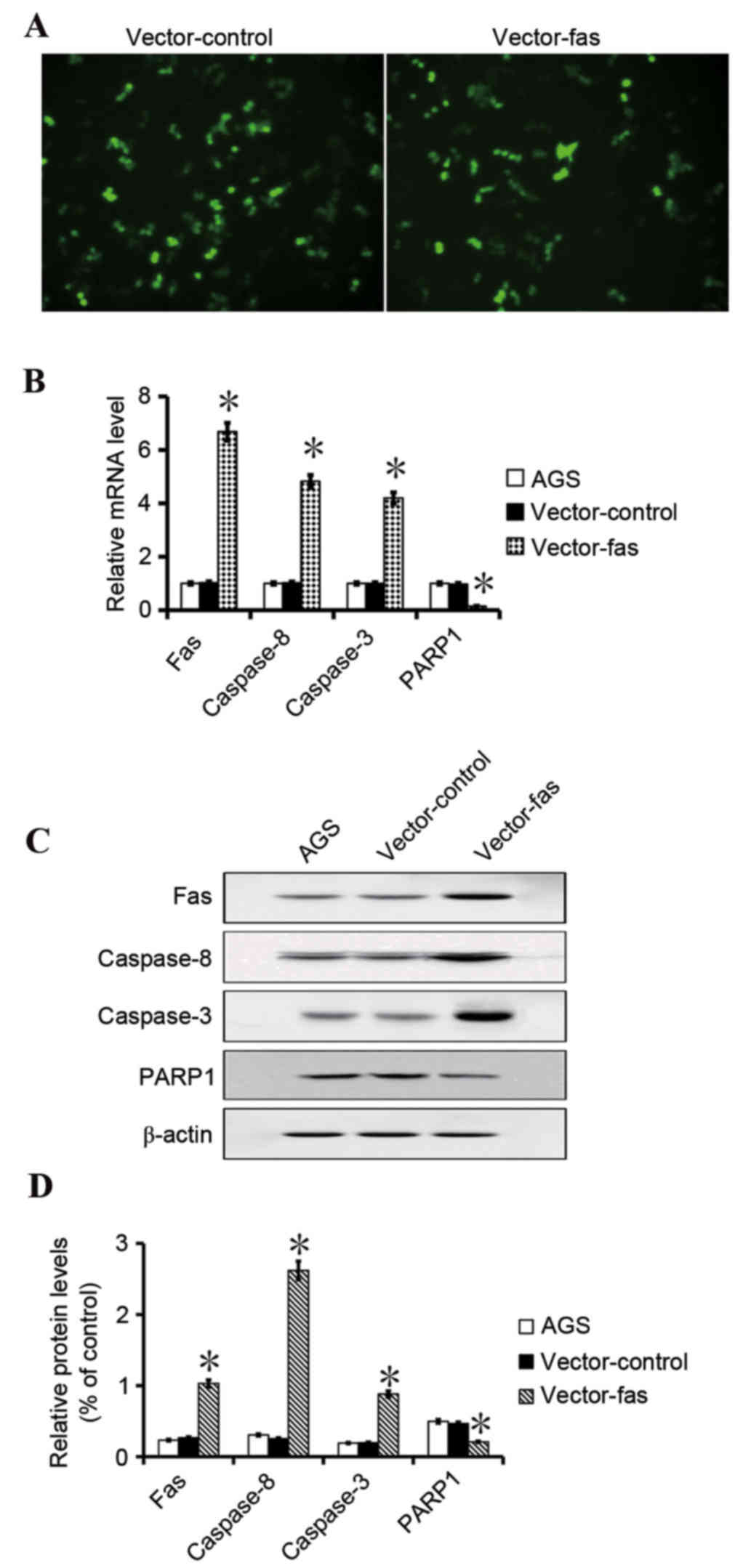
To examine if Fas expression promotes gastric cell
apoptosis, Fas-GFP and the control expression vector were
transfected into gastric cancer AGS cells. The transfection
efficiency of the vectors was similar as revealed by the green
fluorescence in transfected cells (Fig. 4). The PCR and western blotting
assays indicated that Fas, caspase-8 and caspase-3 expression was
increased in the Fas-transfected cells compared with cells
transfected with the control vector (P<0.05; Fig. 4). Western blot analysis and PCR
revealed that the PARP1 expression was lower in the Fas-expressing
cells compared with control cells (P<0.05; Fig. 4). Fas, caspase-8, caspase-3 and
PARP1 expression did not indicate a difference between the parental
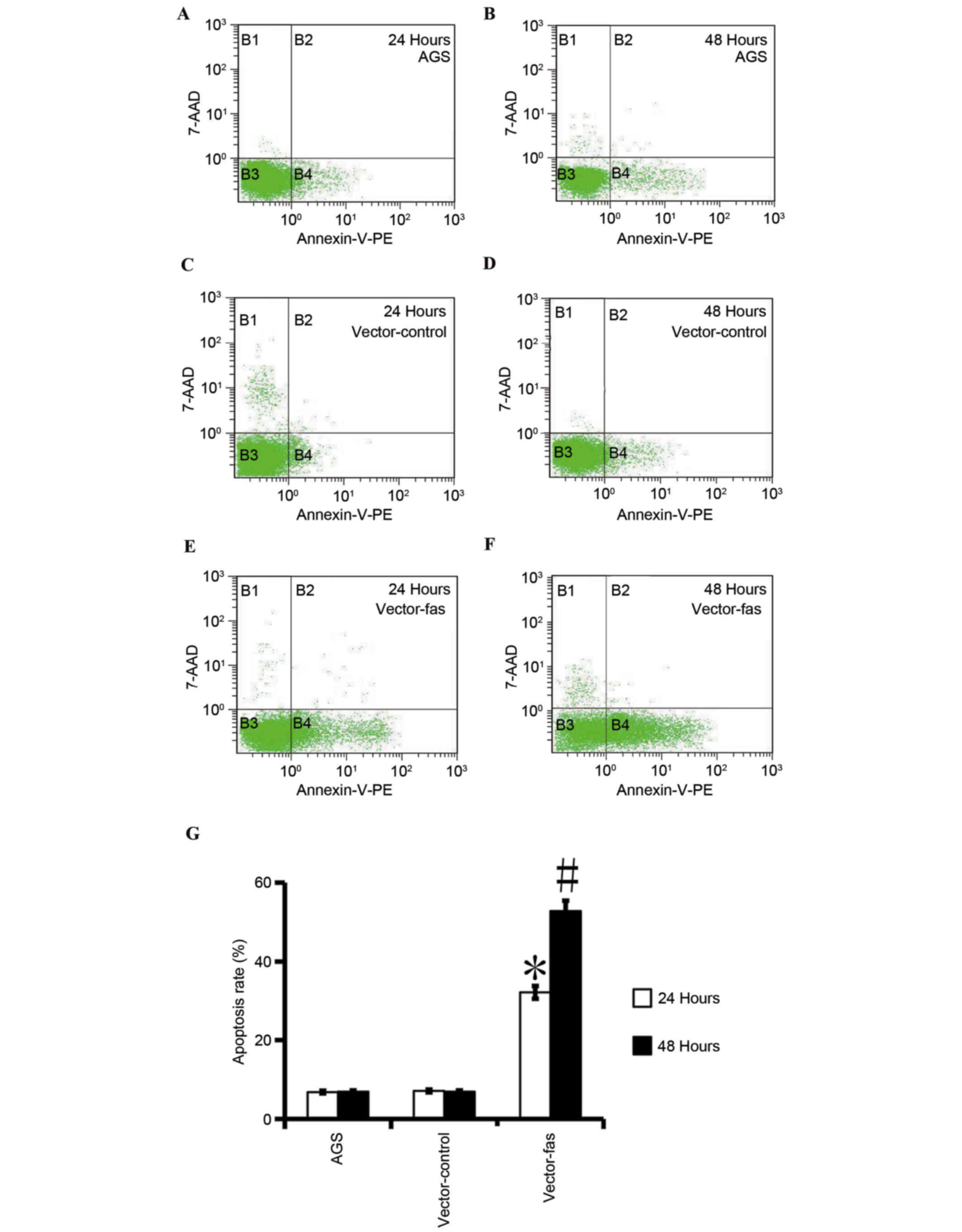
AGS cells and the control cells. The flow cytometry results
revealed that in the Fas-expressing cells, apoptosis was
significantly increased compared with AGS control cells (Fig. 5). These results indicated that
increased Fas expression promotes apoptosis in gastric cancer AGS
cells.
Discussion
Downregulation of Fas expression has been implicated
in tumor progression in various types of cancer, including gastric,
ovarian, lung and renal carcinoma (5,14–18),
and has been hypothesized to result in reduced tumor apoptosis
(19–26). The present study revealed that Fas
expression was correlated with caspase-8, caspase-3 and PARP1
expression in the gastric cancer tissues and cell lines.
Caspase-8 is a primary component of the extrinsic
apoptotic pathway and the first caspase activated in death
receptor-initiated apoptosis (27). Decreased expression of caspase-8
has been previously observed in several types of cancer including
gastric, colorectal and rectal, hepatocellular, and pancreatic
cancers (27). The present study
demonstrated that the caspase-8 expression was lower in gastric
cancer tissues compared with healthy gastric tissues and was
correlated with Fas expression in gastric cancer tissues and cell
lines. Previous studies revealed that functional grade purified Fas
activated caspase 8 in extrinsic apoptosis, and recruited
procaspase-8 to form DISC in extrinsic apoptosis in gastric cancer
cells (28,29). Furthermore, a previous study
reported that Fas facilitates the caspase-8 dimerization and
maturation process (30). The
present study demonstrated that Fas expression was correlated with
caspase-8 expression and this was consistent with previously
reported data (28–30).
Caspase-3 is a member of the cysteine-aspartic acid
protease (caspase)/IL-1β-converting enzyme family (31) and is activated directly by
caspase-8, −9 and −10 via extrinsic and intrinsic pathways to
initiate apoptosis. The present study indicated that expression of
caspase-3 is lower in gastric cancer tissues compared with healthy
gastric tissues. Previous studies revealed that caspase-3
expression was positively associated with Fas expression and FasL
expression in human cells (32,33).
Furthermore, a previous study indicated that patients with low
caspase-3 expression signified an ominous prognosis in gastric
cancer (34). The present study
demonstrated that caspase-3 expression was correlated with Fas
expression, and this was supported by former reports (32,33,35).
PARP1 is a DNA-binding enzyme, which is important in
the base excision repair pathway via detection of DNA strand breaks
and poly ADP-ribosylation of nuclear acceptor proteins responsible
for DNA repair programs and/or apoptotic decision (36). The results of the present study
indicated that PARP1 expression was increased in the gastric cancer
tissues compared with healthy gastric tissues. The results
indicated that greater PARP1 expression may be a trigger or
accompaniment in gastric cancer. In addition, PARP1 expression was
decreased as Fas expression increased in the AGS cells, suggesting
that inhibition of PARP1 expression may be of therapeutic benefit
in patients with gastric cancer.
In summary, the results of the present study
demonstrated that increased expression of Fas and its downstream
signaling molecules caspase-8 or caspase-3 or inhibition of the
expression of PARP1 might improve outcomes in patients with gastric
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470110, 81272754
and 30870981) and the Science Foundation of Health Office of Hubei
Province (grant no. WJ2015Z059).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aguiar PN Jr, Tadokoro H, Forones NM and
de Mello RA: Treating operable patients with gastric cancer:
Macdonald's protocol versus adjuvant chemotherapy. Future Oncol.
11:2247–2249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neumann L, Pforr C, Beaudouin J, Pappa A,
Fricker N, Krammer PH, Lavrik IN and Eils R: Dynamics within the
CD95 death-inducing signaling complex decide life and death of
cells. Mol Syst Biol. 6:3522010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laytragoon-Lewin N, Jönson F, Lundgren J,
Lundgren J, Rutqvist LE, Wikby A, Löfgren S and Lewin F: Perforin,
CD28 and CD95 expression in circulating CD4 and CD8 cells as
predictors of head and neck (H&N) cancer patient survival. Med
Oncol. 31:2902014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He C, Jiang H, Geng S, Sheng H, Shen X,
Zhang X, Zhu S, Chen X, Yang C and Gao H: Expression and prognostic
value of c-Myc and Fas (CD95/APO1) in patients with pancreatic
cancer. Int J Clin Exp Pathol. 7:742–750. 2014.PubMed/NCBI
|
|
6
|
Wang L, Pan XD, Xie Y, Zhang GB, Jiang M,
Zheng L, Wang JH, Shi JF and Zhang XG: Altered CD28 and CD95 mRNA
expression in peripheral blood mononuclear cells from elderly
patients with primary non-small cell lung cancer. Chin Med J
(Engl). 123:51–56. 2010.PubMed/NCBI
|
|
7
|
Gratas C, Tohma Y, Barnas C, Taniere P,
Hainaut P and Ohgaki H: Up-regulation of Fas (APO-1/CD95) ligand
and down-regulation of Fas expression in human esophageal cancer.
Cancer Res. 58:2057–2062. 1998.PubMed/NCBI
|
|
8
|
Watson CJ, O'Kane H, Maxwell P, Sharaf O,
Petak I, Hyland PL, O'Rouke D, McKnight J, Canning P and Williamson
K: Identification of a methylation hotspot in the death receptor
Fas/CD95 in bladder cancer. Int J Oncol. 40:645–654.
2012.PubMed/NCBI
|
|
9
|
Heidari S, Akrami H, Gharaei R, Jalili A,
Mahdiuni H and Golezar E: Anti-tumor activity of ferulago angulata
boiss. Extract in gastric cancer cell line via induction of
apoptosis. Iran J Pharm Res. 13:1335–1345. 2014.PubMed/NCBI
|
|
10
|
Zhao ZG and Shen WL: Heat shock protein 70
antisense oligonucleotide inhibits cell growth and induces
apoptosis in human gastric cancer cell line SGC-7901. World J
Gastroenterol. 11:73–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Guo QL, You QD, Zhao L, Gu HY and
Yuan ST: Anticancer effect and apoptosis induction of gambogic acid
in human gastric cancer line BGC-823. World J Gastroenterol.
11:3655–3659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song MQ, Zhu JS, Chen JL, Wang L, Da W,
Zhu L and Zhang WP: Synergistic effect of oxymatrine and
angiogenesis inhibitor NM-3 on modulating apoptosis in human
gastric cancer cells. World J Gastroenterol. 13:1788–1793. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peter ME, Hadji A, Murmann AE, Brockway S,
Putzbach W, Pattanayak A and Ceppi P: The role of CD95 and CD95
ligand in cancer. Cell Death Differ. 22:885–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Tong S, Guan L, Na F, Zhao W and
Wei L: CD95 rs1800682 polymorphism and cervical cancer risk:
Evidence from a meta-analysis. Tumour Biol. 35:1785–1790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De la Rosa AJ, Gomez MA, Morales S,
Padillo FJ and Muntane J: CD95 signaling in cancer treatment. Curr
Pharm Des. 20:2809–2818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li F, Liu Y, Fu T, Tong W and Zhang A:
Associations of three common polymorphisms in CD95 and CD95L
promoter regions with gastric cancer risk. Tumour Biol.
34:2293–2298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin-Villalba A, Llorens-Bobadilla E and
Wollny D: CD95 in cancer: Tool or target? Trends Mol Med.
19:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu J, Nihal M, Siddiqui J, Vonderheid EC
and Wood GS: Low FAS/CD95 expression by CTCL correlates with
reduced sensitivity to apoptosis that can be restored by FAS
upregulation. J Invest Dermatol. 129:1165–1173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lavrik IN, Golks A, Riess D, Bentele M,
Eils R and Krammer PH: Analysis of CD95 threshold signaling:
Triggering of CD95 (FAS/APO-1) at low concentrations primarily
results in survival signaling. J Biol Chem. 282:13664–13671. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yap R, Veliceasa D, Emmenegger U, Kerbel
RS, McKay LM, Henkin J and Volpert OV: Metronomic low-dose
chemotherapy boosts CD95-dependent antiangiogenic effect of the
thrombospondin peptide ABT-510: A complementation antiangiogenic
strategy. Clin Cancer Res. 11:6678–6685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCabe MJ Jr, Eckles KG, Langdon M,
Clarkson TW, Whitekus MJ and Rosenspire AJ: Attenuation of
CD95-induced apoptosis by inorganic mercury: Caspase-3 is not a
direct target of low levels of Hg2+. Toxicol Lett. 155:161–170.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strand S, Strand D, Seufert R, Mann A,
Lotz J, Blessing M, Lahn M, Wunsch A, Broering DC, Hahn U, et al:
Placenta-derived CD95 ligand causes liver damage in hemolysis,
elevated liver enzymes, and low platelet count syndrome.
Gastroenterology. 126:849–858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takarada S, Imanishi T, Hano T and Nishio
I: Oxidized low-density lipoprotein sensitizes human vascular
smooth muscle cells to FAS (CD95)-mediated apoptosis. Clin Exp
Pharmacol Physiol. 30:289–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amendola A, Poccia F, Martini F, Gioia C,
Galati V, Pierdominici M, Marziali M, Pandolfi F, Colizzi V,
Piacentini M, et al: Decreased CD95 expression on naive T cells
from HIV-infected persons undergoing highly active anti-retroviral
therapy (HAART) and the influence of IL-2 low dose administration.
Irhan Study Group. Clin Exp Immunol. 120:324–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Munker R, Younes A, Cabanillas F and
Andreeff M: Soluble CD95 in the serum of patients with low and
intermediate grade malignant lymphomas: Absence of prognostic
correlations. Leuk Lymphoma. 27:517–521. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elrod HA, Fan S, Muller S, Chen GZ, Pan L,
Tighiouart M, Shin DM, Khuri FR and Sun SY: Analysis of death
receptor 5 and caspase-8 expression in primary and metastatic head
and neck squamous cell carcinoma and their prognostic impact. PLoS
One. 5:e121782010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi LS, Wang H, Wang F, Feng M, Wang M and
Guan WX: Effects of gastrokine-2 expression on gastric cancer cell
apoptosis by activation of extrinsic apoptotic pathways. Mol Med
Rep. 10:2898–2904. 2014.PubMed/NCBI
|
|
29
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014.PubMed/NCBI
|
|
30
|
Stupack DG: Caspase-8 as a therapeutic
target in cancer. Cancer Lett. 332:133–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medema JP, Scaffidi C, Kischkel FC,
Shevchenko A, Mann M, Krammer PH and Peter ME: FLICE is activated
by association with the CD95 death-inducing signaling complex
(DISC). EMBO J. 16:2794–2804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang
YC and Xin Y: Expression of Fas ligand and caspase-3 contributes to
formation of immune escape in gastric cancer. World J
Gastroenterol. 9:1415–1420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nho RS, Peterson M, Hergert P and Henke
CA: FoxO3a (Forkhead Box O3a) deficiency protects Idiopathic
Pulmonary Fibrosis (IPF) fibroblasts from type I polymerized
collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas.
PLoS One. 8:e610172013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Q, Peng J, Liu W, He X, Cui L, Chen X,
Yang M, Liu H, Liu S and Wang H: Elevated cleaved caspase-3 is
associated with shortened overall survival in several cancer types.
Int J Clin Exp Pathol. 7:5057–5070. 2014.PubMed/NCBI
|
|
35
|
Ran X, Diao JX, Sun XG, Wang M, An H,
Huang GQ, Zhao XS, Ma WX, Zhou FH, Yang YG and Miao CM: Huangzhi
oral liquid prevents arrhythmias by upregulating caspase-3 and
apoptosis network proteins in myocardial ischemia-reperfusion
injury in rats. Evid Based Complement Alternat Med.
2015:5189262015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hua RX, Li HP, Liang YB, Zhu JH, Zhang B,
Ye S, Dai QS, Xiong SQ, Gu Y and Sun XZ: Association between the
PARP1 Val762Ala polymorphism and cancer risk: Evidence from 43
studies. PLoS One. 9:e870572014. View Article : Google Scholar : PubMed/NCBI
|