Introduction
Sepsis is a systemic inflammatory response syndrome
with a proven or suspected infectious etiology (1). The early stages of sepsis are
classically characterized by fever and blood hyperdynamics, with
rapidly developing secondary organ injury, and hypothermia and
death as sepsis progresses (1,2). The
mortality rate of sepsis remains high and the therapy remains
intractable.
Dexmedetomidine (DXM) is a selective α2-adrenoceptor
(α2-AR) and imidazoline receptor (IR) agonist (3), which was formally approved for
sedation and adjunct analgesia for patients in the intensive care
unit (ICU) by the US Food and Drug Administration in 1999,
particularly recommended for patients with sepsis (4) Clinical studies of patients with
severe sepsis and other critically ill patients identified that DXM
may marginally improve the survival rate and significantly inhibit
the release of pro-inflammatory factors in patients with sepsis
(5–7). These beneficial effects were
confirmed by an animal study (8),
however the underlying signaling pathways reported from different
animal and cytological models presented a wide range of pathways
including: Toll-like receptor 4 (TLR4)/myeloid differentiation
primary response gene 88 (MyD88)/nuclear factor-κB (NF-κB) or
mitogen-activated protein kinase [c-Jun N-terminal kinase,
extracellular signal-regulated kinases (ERK)1/2] (8,9),
endothelial nitric oxide synthase (eNOS)/nitric oxide (10) and Janus kinase/signal transducers
and activators of transcription (11). How DXM can affect so numerous
signaling pathways or downstream molecules remains unclear.
Caveolae, typically flask-shaped membrane
invaginations expressed in various cell types, are abundant in
lung, muscle and adipose tissues, and have been demonstrated to
participate in the regulation of lipid and glucose metabolism
(12), in addition to the
maturation of immunocytes and inflammation responses (13). Caveolin-1 is a protein that
maintains the morphological and functional integrity of caveolae
(12). Knockout of caveolin-1
decreases the survival rate in mice with cecal ligation and
puncture (CLP)-induced sepsis (14). Jiao et al (15) identified that tyrosine (Tyr) 14
phosphorylation of caveolin-1 induced interaction with TLR4 and
mediated TLR4/MyD88 signaling regulation. Additional studies have
demonstrated that receptors including G-protein coupled receptors
(GPCRs), α-ARs, β-ARs (16), tumor
necrosis factor receptor (17) and
downstream molecules including Gα subunits (Gs, Gi and
Gq) (16), eNOS (18) and ERK1/2 (19), localized to the caveolae. GPCR
activation led to the release and translocation of Gβγ subunits and
subsequent Src-dependent Tyr phosphorylation of caveolin-1
(20). Following these studies, it
was hypothesized that DXM may affect inflammatory pathways through
the influence on caveolin-1.
The present study aimed to evaluate the effect of
DXM on the most frequent clinical manifestations of sepsis and the
expression of caveolin-1 in lung tissues, and to identify the
association between inflammatory pathways and the characteristic
receptors of DXM.
Materials and methods
Animals
A total of 170 male Sprague-Dawley rats aged 8 weeks
(weight, 250–300 g) were obtained from the Animal Center of the
Second XiangYa Hospital of Central South University (Changsha,
China) and housed in the Animal Laboratory Center of the Second
XiangYa Hospital of Central South University, in groups of three
per cage. All rats were weighed and numbered on arrival and
acclimated for 7 days, under 24°C and 48% humidity, a 12-h
light/dark circadian cycle, and were provided with specific
pathogen-free rodent diet and water ad libitum throughout all
experiments. Animals were euthanized with an overdose of
pentobarbital injected after the experiments. All animal procedures
were approved by the Animal Care and Use Committee of XiangYa
Medical College, Central South University (Changsha, China).
Materials
The small-animal-specialized digital thermometer
(AT320; Da-Xiong Ltd., Shenzhen, China) and blood gas analyzer (GEM
Premier 3000; Instrumentation Laboratory Co., Bedford, MA, USA)
were used. The silk suture (Ethilon; Ethicon, Inc., Somerville, NJ,
USA), sodium dodecyl sulfate (SDS) buffer and phosphate-buffered
saline (PBS; Beijing Dingguo Changsheng Biotechnology Co., Ltd.,
Beijing, China) were donated by Dr Lili Jiang (Department of
Anesthesiology, Affiliated Hospital of Qingdao University, Qingdao,
China). DXM and ketamine (HengRui Medicine, Ltd., Nanjing, China)
were purchased from Second XiangYa Hospital of Central South
University. Pentobarbital sodium (Sigma-Aldrich; Merck Millipore,
Darmstadt., Germany), atepamezole (APZ; Axon Medchem, Groningen,
Netherlands), D46G3XP caveolin-1 rabbit monoclonal
antibody [horseradish peroxidase (HRP)-conjugated; 1:10,000;
catalog no. 3267S] and 13E5 β-actin rabbit monoclonal (1:1,000;
catalog no. 4970S) antibodies (Cell Signaling Technology, Inc.,
Danvers, MA, USA) were purchased from respective companies. The
HRP-conjugated secondary antibody (1:1,000; catalog no. CW0103M)
was obtained from Beijing ComWin Biotech Co., Ltd. (Beijing,
China). Enhanced bicinchoninic acid assay (BCA) protein assay kit
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China).
CLP surgery
All rats were anesthetized with pentobarbital sodium
[45 mg/kg, intra-peritoneal (i.p.)] and ketamine (1.5 mg/100 g,
intravenous). Following a previous study (21), a severe polymicrobial septic model
was induced by CLP. Rats were fixed on a surgical board following
the shaving of abdominal hair. Surgical fields were covered with
sterile drapes after disinfection with iodine complex (three
times), then a 1–2 cm lower-midline laparotomy was conducted. The
cecum was exposed and ligated tightly 25% distal to the ileocecal
valve with a 6–0 silk suture, and perforated twice with an 18-gauge
needle on the same side near and distal to the ligation,
respectively. The cecum was squeezed gently to extrude two small
amounts of feces after which the cecum was returned to abdominal
cavity. The abdomen was closed with 3–0 silk sutures in two layers.
Then 5 ml/100 g 37°C normal saline was injected subcutaneously
using a 25G needle prior to the rat being transferred to a recovery
cage. Perioperative body temperature was protected with
temperature-adjusting blankets and monitored at 1 h intervals.
Experimental protocol
Rats were randomly divided into five groups: Control
group (Control); sham surgery (Sham); CLP (CLP); DXM + CLP (DXM);
and DXM + APZ + CLP (APZ). Control rats did not undergo any
procedures. Sham surgery was conducted with laparotomy and cecum
exposure without ligation and puncture. CLP was conducted as
aforementioned. DXM (2 µg/100 g, 10 µg/ml) or an equal volume
normal saline was injected i.p. 10 min prior to surgery. APZ (25
µg/100 g, 1 mg/ml) or an equal volume normal saline was injected
i.p. simultaneously with pentobarbital injection.
Survival rate, anal temperature and
disease severity monitoring, and arterial blood gas (ABG)
analysis
A total of 65 rats were randomly divided into five
groups: Control and Sham (10 per group), CLP, DXM and APZ (15 per
group) as aforementioned. All rats were monitored up to 24 h or
until death, and the time when an animal died was recorded. Left
ventricular blood was collected when rats died or were euthanized
at 24 h for ABG. Anal temperature and disease severity were
determined at pre-set time-points (immediately prior to surgery, 4,
8, 12 and 24 h postoperatively) with a digital thermometer and a
scoring system, murine sepsis score (MSS), developed by Shrum et
al (22). The criteria in MSS
(data not shown) include: Degree of piloerection, spontaneous
activity, response to stimuli, level of consciousness, openness of
eyes, posture and degree of labored breathing. Each criterion was
scored from 0 to 4 and a total MSS score was calculated. Mortality
rises as MSS increases.
Lung tissue collection
At each time-point (immediately prior to surgery, 4,
8, 12 and 24 h postoperatively), thoracotomy was performed on the
rats (three rats per group in control and sham groups, five per
group in the rest of the groups), with pulmonary transfusion of
0.9% normal saline under speed of 120 ml/h for 15 min with drainage
of the blood from left atrium. Then both lungs were removed. The
lower and middle lobes of right lung tissues were snap frozen in
the liquid nitrogen and stored at −80°C for subsequent protein
detection, and the whole left lung was perfused with 1.5 ml 10%
formalin via the bronchus and stored in 10% formalin for
hematoxylin and eosin (H&E) staining and pathological
analyses.
Western blot analysis
Frozen rat lung tissues were homogenized and the
lysates were prepared in ice-cold lysis buffer and centrifuged
(10,000 × g, −4°C, 10 min). The supernatant was collected and
normalized for equal amounts of total protein measured with the BCA
method; 60 µg protein from each sample were separated on a 12%
SDS-polyacrylamide gel (SDS-PAGE) and transferred to PVDF membranes
(Merck Millipore). The membranes were blocked with 5% non-fat milk
and incubated overnight with the primary anti-caveolin-1 antibody
at 4°C, followed by incubation with the HRP-conjugated secondary
antibody for 4 h. Cellular β-actin protein was immunodetected as
the internal standard.
HE staining and histological injury
scoring for lung tissues
Formalin-fixed lungs were embedded in paraffin and
serially sectioned in toto, then stained with H&E. A total of
five images per slide were captured with a microscope (Nikon
Eclipse E200; Nikon Corporation, Tokyo, Japan) at magnifications of
×4 and ×40. Histological changes, including alveolar wall edema,
congestion, hemorrhage and inflammatory cell infiltration under a
magnification of ×40, were scored by a pathologist blinded to the
present study, as previously described (8). Each criterion was scored between 0
(normal) and 5 (severe), and the overall pulmonary inflammation was
categorized according to the sum of the score (0–5, normal to
minimal inflammation; 6–10, mild inflammation; 11–15, moderate
inflammation; 16–20, severe inflammation).
Statistical analysis
Data were presented as the mean ± standard deviation
and analyzed with SPSS software, version 20.0 (SPSS, Inc., Chicago,
IL, USA). Differences between groups were determined by analysis of
variance, followed by a post hoc test (least significant difference
method; LSD) in ABG and histological scoring data. The missing
values of MSS and anal temperature due to mortality were
interpolated with the maximal MSS score and the temperature
measured immediately prior to mortality, respectively. Western
blotting images were read and calculated with Image J2X software
(http://imagej.net/ImageJ2). Then MSS,
anal temperature and caveolin-1 expression data were analyzed with
repeated analysis of variance, followed by post hoc analysis (LSD).
The cumulative survival rates among groups were analyzed with
log-rank χ2 test and the survival curve with the
Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Survival rate
As demonstrated in Fig.
1, the survival rate 24-h post-operatively was 100, 90, 20, 40
and 33.3, respectively, for the Control, Sham, CLP, DXM and APZ
groups. No significant differences were identified between the
Control and Sham groups. CLP surgery markedly decreased survival
rate compared with the Sham group, and survival rate was notably
improved by pre-emptive DXM treatment. The antagonism of APZ did
not significantly decrease the protection of DXM.
 | Figure 1.The effect of DXM and its antagonist
APZ on survival rates of septic rats induced by CLP. Control,
control group (n=10); Sham, sham operation group (n=10); CLP, CLP
operation group (n=15); DXM, DXM pretreatment group (n=15); APZ,
APZ antagonizing group (n=15). The survival rate 24 h after
operation was analyzed. #P<0.05, vs. Sham, DXM and APZ groups,
respectively. DXM, dexmedetomidine; APZ, atepamezole; CLP, cecal
ligation and puncture. |
The severity of disease
The MSS score, which was reported to be highly
predictive of sepsis progression and mortality and was paralleled
with systemic pro-inflammatory factors in CLP-induced mice models
(22), was employed to evaluate
the effect of DXM. Fig. 2A
provides the MSS scoring at pre-set time-points after surgery from
individual rats in different treatment groups without missing data
interpolation. Control rats scored 0 at all time-points.
Sham-operated rats scored similar to CLP-operated rats 4 h
postoperatively and went back to control level 8 h postoperatively,
with the exception of one whose cecum was in the right upper
quadrant and kept high scores after surgery. Scores of CLP-operated
rats increased continuously 8 h postoperatively. DXM pretreatment
resulted in the rise of scoring, however inhibited the rising speed
4 h postoperatively compared with that of CLP; however, APZ partly
antagonized this effect. Fig. 2B
demonstrates the differences of MSS among groups with missing data
interpolation. MSS scores significantly increased after Sham and
CLP surgery and only returned to the control level 8 h after
surgery in the Sham group. DXM markedly increased scores at 4 h and
maintained a similar level until 12 h postoperatively compared with
the CLP group. APZ significantly antagonized the effect of DXM and
reduced the scores until 24 h postoperatively.
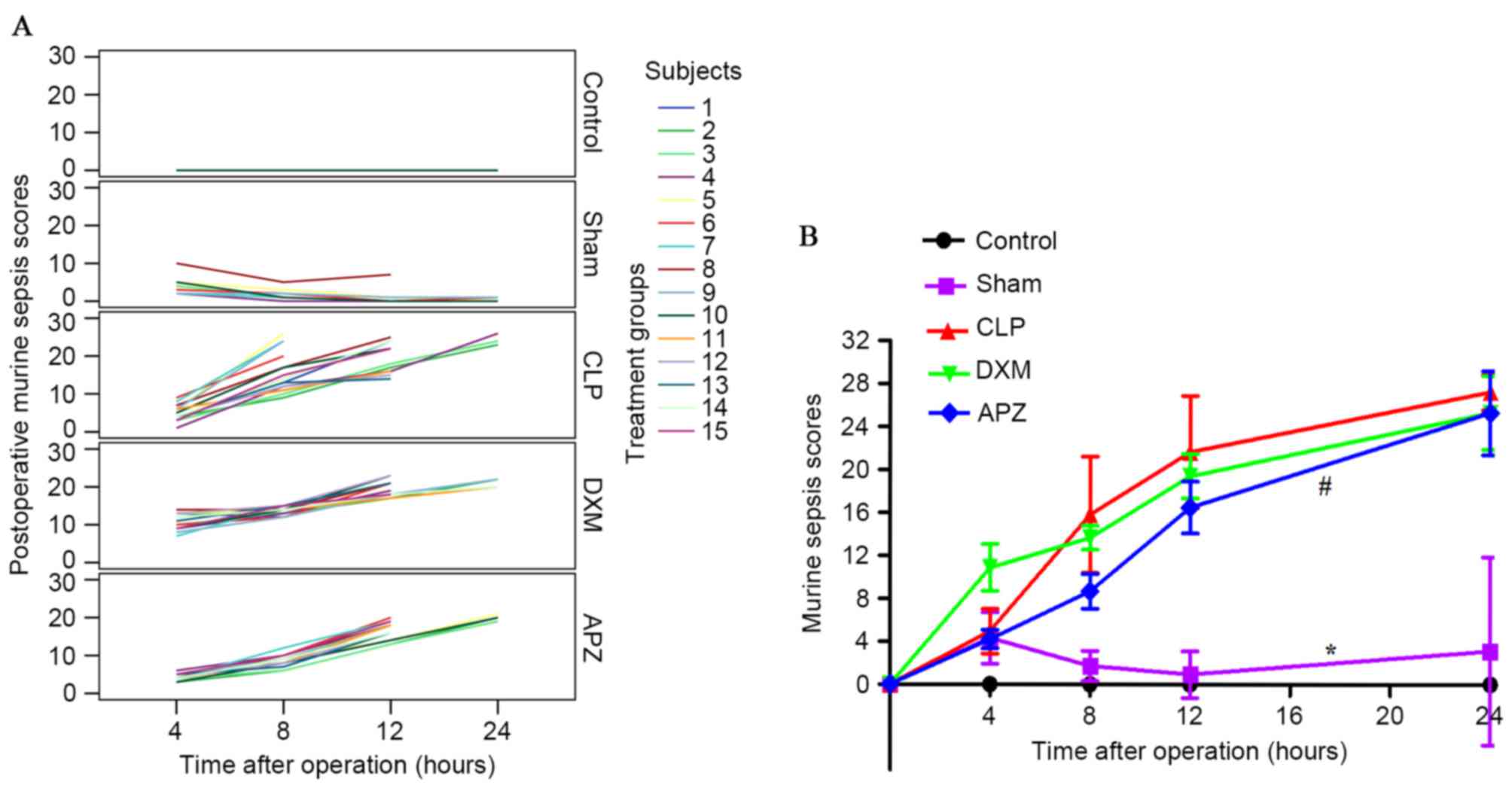 | Figure 2.The effect of DXM and its antagonist
APZ on the MSS of septic rats induced by CLP. Control, control
group (n=10); Sham, sham operation group (n=10); CLP, CLP group
(n=15); DXM, DXM treatment group (n=15); APZ, APZ antagonizing
group (n=15). The subjects represent each numbered rat in each
group. (A) The MSS scoring at pre-set time-points after operation
from individual rats in the different treatment groups without
missing data interpolation. (B) Differences of MSS among groups
with missing data interpolation were analyzed. *P<0.05, vs.
control and CLP groups, respectively; #P<0.05, vs. DXM group.
DXM, dexmedetomidine; APZ, atepamezole; MSS, murine sepsis scores;
CLP, cecal ligation and puncture. |
ABG analysis
As demonstrated in Table I, there was no significant
difference of pH among the groups. Blood lactate and base excess
significantly increased in the CLP group compared with the Sham
group, DXM marginally ameliorated lactate accumulation, and APZ
marginally antagonized these effects, however these were not
significant. Blood glucose (Glu) significantly increased in the
Sham, CLP and DXM groups, however remained higher in the DXM group
compared with the CLP group but (not statistically significant).
Glu was markedly antagonized by APZ.
 | Table I.Arterial blood gas analysis data. |
Table I.
Arterial blood gas analysis data.
| Group | pH | Lac (mmol/ml) | BE (mmol/ml) | Glu (mmol/ml) |
|---|
| Control | 7.42±0.08 | 1.34±0.45 | 0.33±2.77 | 6.40±1.76 |
| Sham | 7.40±0.08 |
1.79±0.33a |
−1.45±3.31a | 9.85±2.31 |
| CLP | 7.32±0.13 | 4.5±1.35 | −4.18±5.04 | 10.85±2.51 |
| DXM | 7.45±0.13 | 3.58±1.01 | −4.10±4.78 | 12.67±5.74 |
| APZ | 7.43±0.14 | 4.02±0.89 | −3.96±3.56 |
7.59±1.86b |
Anal temperature
Fig. 3A
demonstrates the anal temperature (°C) of individual rats at
pre-set time-points after surgery from different treatment groups
without missing data interpolation. Sham-operated rats convergently
maintained a higher temperature without influence on short-term
survival. CLP surgery affected the temperature and survival of
rats. DXM and APZ effectively improved survival rate, however DXM
significantly inhibited the increase of temperature as sepsis
progressed, which was effectively antagonized by APZ. Fig. 3B demonstrates the differences of
anal temperature among groups with missing data interpolation.
Normal anal temperatures fluctuated between 37.0–38.0°C and peaked
at 12 h after surgery (corresponding to 22:00 h). CLP surgery
caused significant fever and hypothermia at different stages of
sepsis compared with the Sham group, and severely disturbed the
circadian rhythm, which was significantly inhibited by DXM
pretreatment. APZ significantly antagonized the effect of DXM.
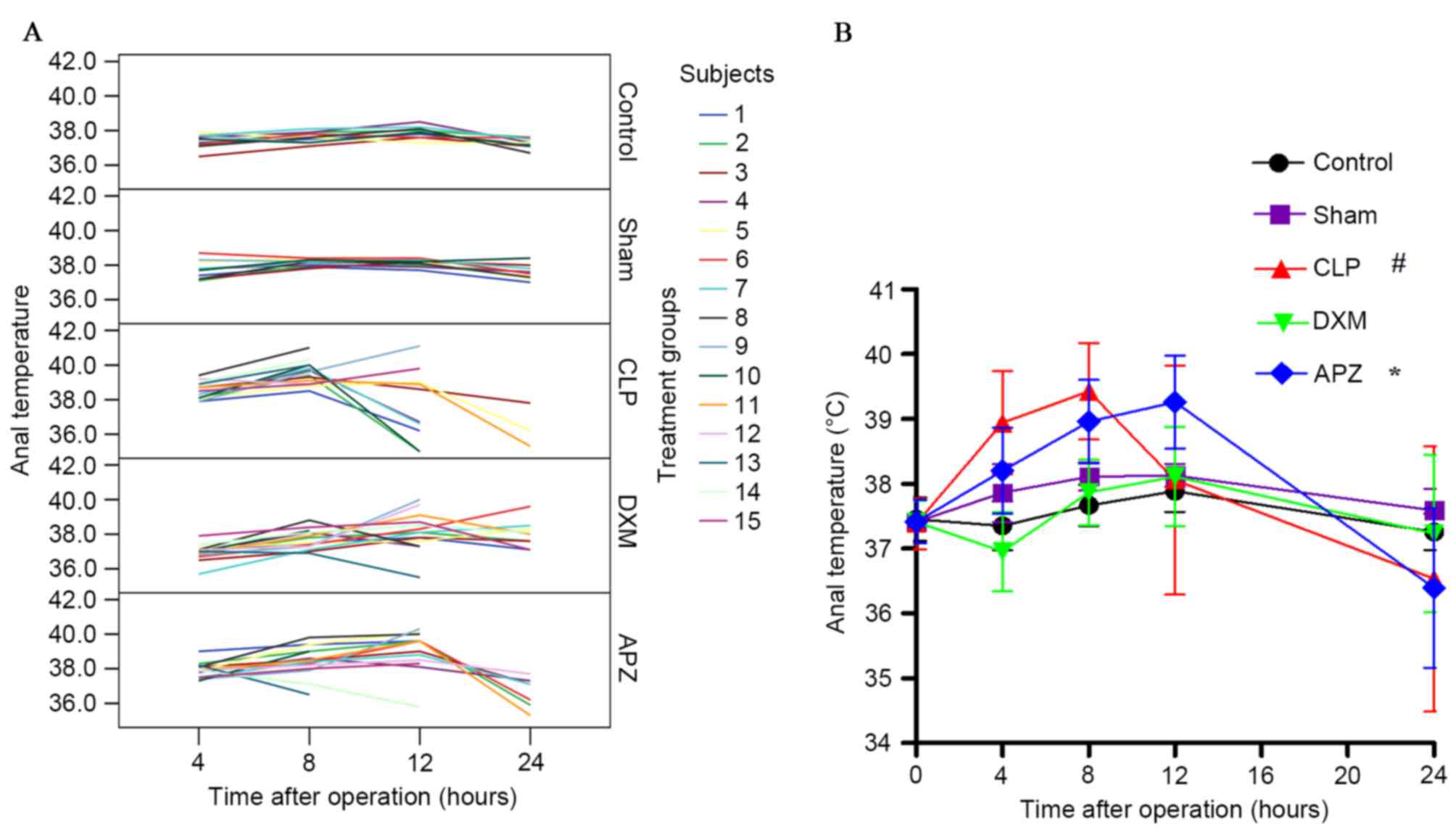 | Figure 3.The effect of DXM and its antagonist
APZ on temperature of rats with CLP-induced sepsis. Control,
control group (n=10); Sham, sham operation group (n=10); CLP, CLP
operation group (n=15); DXM, DXM treatment group (n=15); APZ, APZ
antagonizing group (n=15). The subjects represent each numbered rat
in each group. (A) The anal temperature (°C) of individual rats at
pre-set time-points after operation from different treatment groups
without missing data interpolation. (B) Differences of anal
temperature among groups with missing data interpolation were
analyzed. #P<0.05, vs. Sham and DEX groups; *P<0.05, vs. DXM
group. DXM, dexmedetomidine; APZ, atepamezole; CLP, cecal ligation
and puncture. |
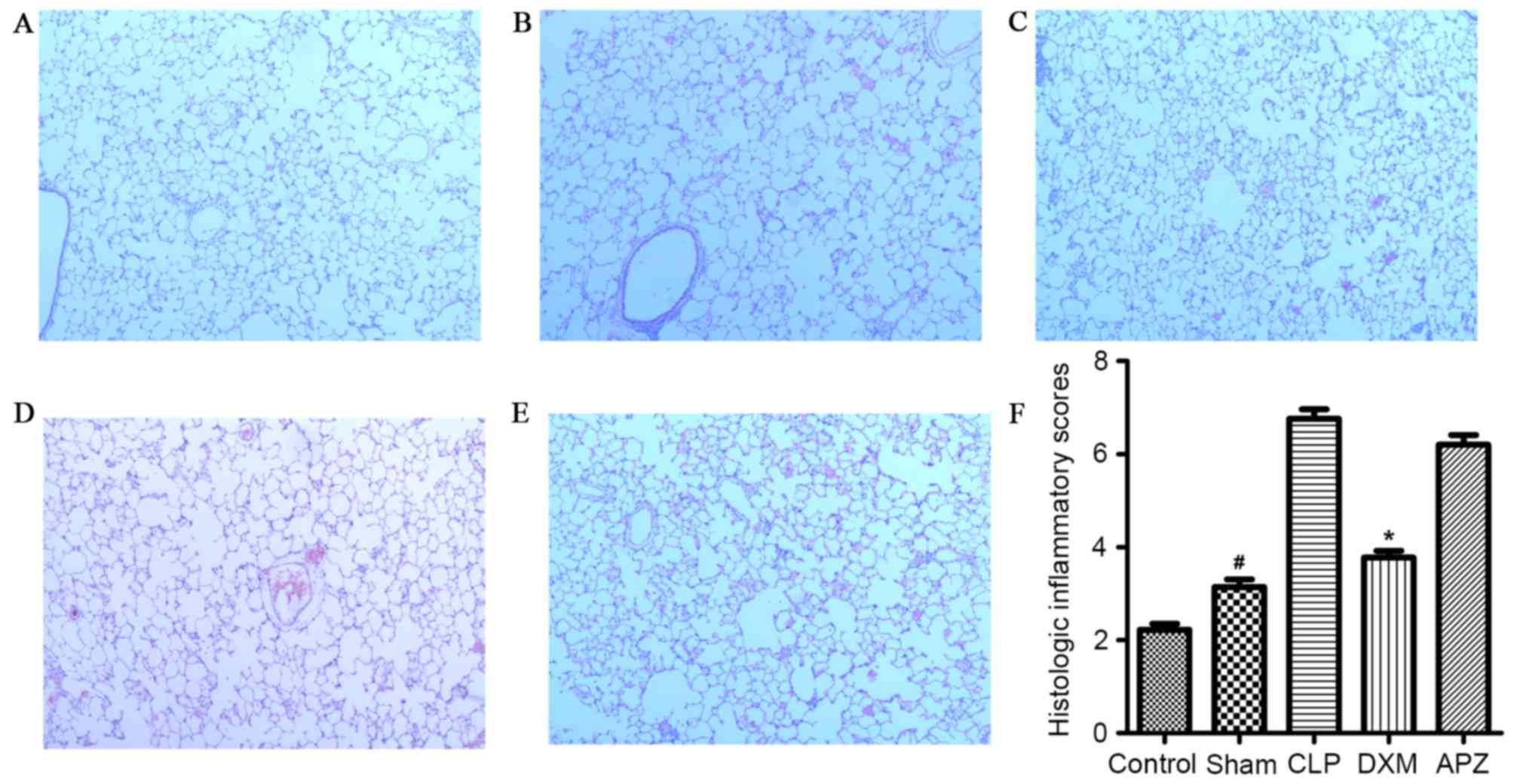
Histological changes of lung
tissues
As demonstrated in Fig.
4, the histological changes due to procedures of tissue
harvesting were minimal (Fig. 4A and
F). Sham surgery caused minimal but significant lung injury
compared with the Control group (Fig.
4B and F). CLP surgery induced mild to moderate injury
(Fig. 4C and F), which was
significantly reduced by DXM pretreatment to a minimal level
(Fig. 4D and F). APZ antagonized
the beneficial effect of DXM (Fig. 4E
and F).
Western blot analysis for caveolin-1
expression in lung
In order to identify whether DXM is able to affect
caveolin-1 expression, the expression of caveolin-1 in lung tissues
was examined. Fig. 5A demonstrates
the caveolin-1 and β-actin bands determined by western blotting in
different groups at pre-set time-points. Fig. 5B demonstrates the diurnal
expression mode of caveolin-1 in the Control group. As indicated in
Fig. 5A and B, rats in the Control
group expressed a stable amount of caveolin-1 with peak expression
at the 8-h time-point (corresponding to ~4:00 p.m.), and the lowest
expression at pre- and 24-h time-point (corresponding to ~8:00
a.m.). Fig. 5C indicates the
average level of caveolin-1 expression during one day in different
treatment groups. Fig. 5D
demonstrates the difference of caveolin-1 expression in different
treatment groups. As indicated in Fig.
5A, C and D, caveolin-1 expression fluctuated in operated rats,
with the exception of the DXM and APZ pretreated groups. DXM
significantly upregulated the expression which was markedly
inhibited by CLP surgery 4 h postoperatively. This effect was
partly antagonized by APZ. DXM and APZ pretreatment effectively
preserved the diurnal rhythm disturbed by CLP surgery.
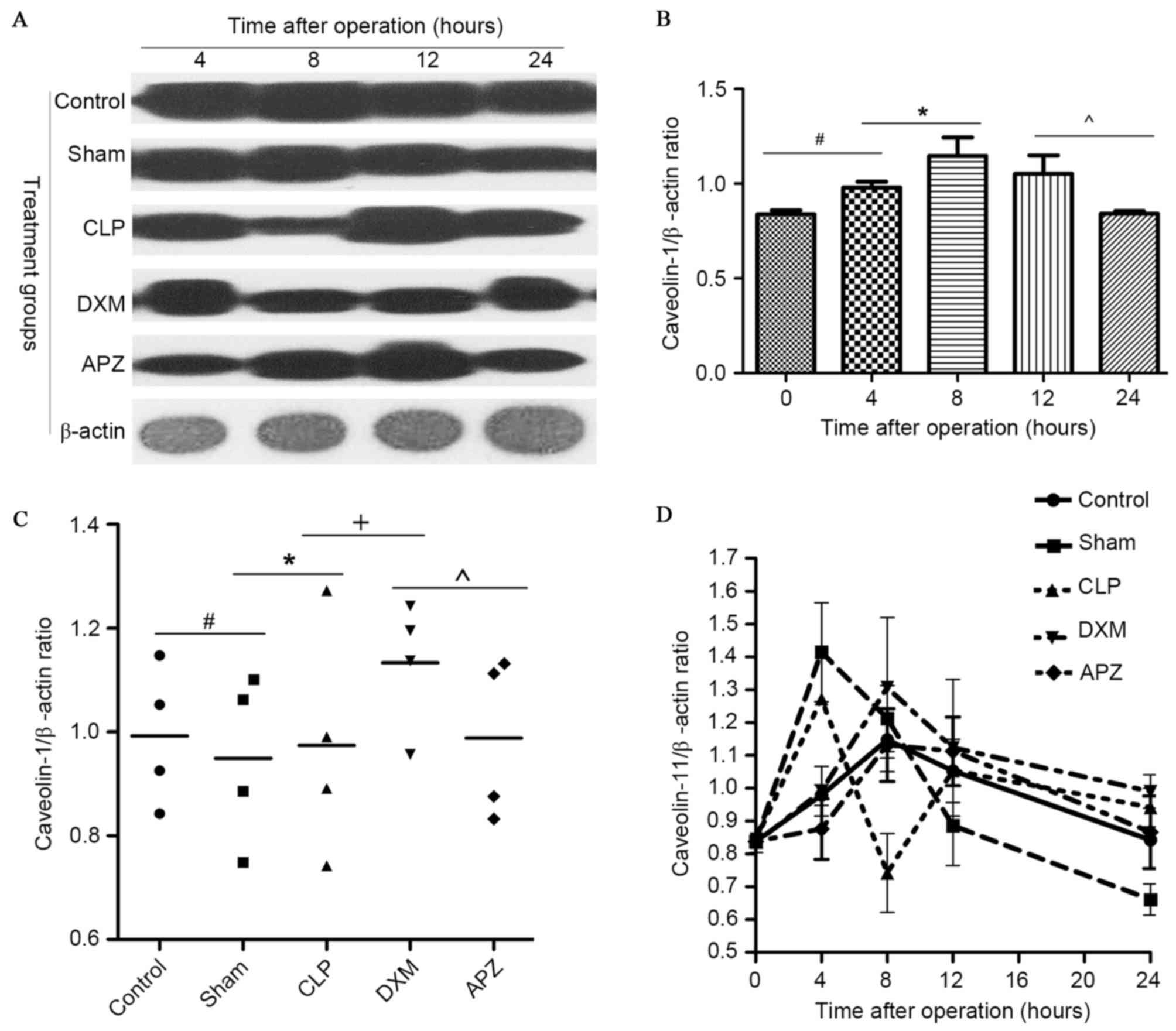 | Figure 5.The effect of DXM and its antagonist
APZ on caveolin-1 expression in lung tissues of rats with sepsis
determined by western blotting. Control, control group (n=10);
Sham, sham operation group (n=10); CLP, CLP operation group (n=15);
DXM, DXM pretreatment group (n=15); APZ, APZ antagonizing group
(n=15). β-actin was used as the internal standard protein. The grey
value of protein band was read by Image J2X and presented as the
mean ± standard deviation. (A) The caveolin-1 and β-actin
expression determined by western blotting in different groups at
pre-set time-points. (B) The diurnal expression mode of caveolin-1
in control group, the tissue was harvested at the time
corresponding to the pre-set time-points in treated groups.
#,*,^P<0.05. (C) The average level of caveolin-1 expression
during a day in different treatment groups, #,*,+,^P<0.05. (D)
The difference of caveolin-1 expression in different treatment
groups. DXM, dexmedetomidine; APZ, atepamezole; CLP, cecal ligation
and puncture. |
Discussion
DXM was recommended as a sedative for septic
patients in the ICU due to its anti-inflammatory effects, however
its underlying mechanisms and long-term effects remain unclear. In
the present study, it was identified that DXM pretreatment at a
single sedative/hypnotic dose (20 µg/kg, i.p.) effectively
decreased 24-h mortality and acute lung injury, and inhibited the
increase of temperature, however it failed to reduce the increase
of blood lactate, glucose and MSS scores. It was demonstrated that
DXM can upregulate the expression and partly preserve the diurnal
rhythm of caveolin-1 in lung tissues. APZ could partly neutralize
the effects of DXM.
DXM pharmaceutically causes a dose-dependent
inhibition of sympathetic tone in the central and peripheral
nervous system and is used for sedation, analgesia, hypotension and
hypothermia, its use resulting in a spontaneous motility decrease
and induction of sleep. All these effects can be inhibited by prior
or simultaneous delivery of APZ (23,24).
The results of the present study agreed with this, even though it
appeared that DXM marginally increased MSS scores while APZ
decreased the scores. Due to the fact that the MSS scoring system
gauged activity, consciousness, response to stimuli and eye opening
as criteria, DXM could have increased these scores due to its
sedative and analgesic activity, and APZ downgraded the scores
through the neutralization of these effects. The present study also
identified that DXM slightly decreased blood lactate and increased
glucose levels, which can be significantly antagonized by APZ. To
the best of our knowledge, stress can induce the rise of blood
glucose, and tissue hypoxia can induce lactate generation. DXM may
have reduced blood glucose due to the inhibition of stress through
sedation and analgesia, however the effect of DXM on blood glucose
and insulin secretion was more complex, since it can directly
inhibit insulin secretion through activation of α2-AR and
imidazoline receptors on pancreatic β cells (25), but also diminished activation of
α1-AR and β-AR through reducing the sympathoadrenal output: The
balance of these factors may have resulted in different glucose
levels (26). These effects on
peripheral sympathetic tone in addition with its central
sympatholysis and vagal effect can significantly reduce systemic
blood pressure. In addition, DXM was reported to enhance
micturition through α2b-AR on the proximal tubule endothelia
(27), which may further decrease
circulatory volume and blood pressure when not under continuous
fluid perfusion. These effects may have contributed to the failure
of DXM to effectively reduce the hypoxia in tissues and the
accumulation of lactate, even following the balance of its
favorable effect in the preservation of capillary perfusion through
attenuation of leukocyte-endothelial interaction and micro-emboli
generation (28). Above all, DXM
may be a promising sedative and antipyretic in patients with
sepsis, and may be promising for clearing blood lactate after
efficient fluid resuscitation. However, DXM may be detrimental to
patients who survive sepsis, with the exception of the unclear
effect on lactate, due to the fact that the effect of central
hypothermia and glucose upregulation could bring patients into a
chronic infectious or susceptible state (29). This indicates that the long-term
effect of DXM on patients with sepsis or critically ill patients
who survive should be further studied to assist clinical
practice.
DXM was reported to inhibit lung injury and improve
survival rate in CLP-induced rats (8), which was in agreement with the
results of the present study. These results indicated that the
anti-inflammatory action of DXM may be mediated by inhibition of
the TLR4/MyD88/NF-κB signaling pathway. The TLR4-dependent
signaling pathway mediates neutrophil sequestration into the lungs
and causes secondary lung injury due to endotoxin or hyperinflation
(30). Additional studies have
implied that the receptors mediate the anti-inflammatory actions of
DXM possibly through α2-AR and IRs, although one study used APZ as
an antagonist in a renal ischemic-reperfusion injury (IRI) rat
model (11) and the other utilized
yohimbine in a lung IRI rats model (9). It is known that DXM binds to α2-AR
and specifically activates the Gαi subunit, which has been
demonstrated to bind with the N-terminus (Residual 82–101) of
caveolin-1 prior to being activated, and to translocate away from
caveolin-1, concomitantly releasing Gβγ subunits after activation
(31). Studies have also
demonstrated that Src Tyrosine kinases, H-ras and eNOS share the
same binding domain of caveolin-1 (32), the Gβγ complex induces activation
of Src after release (33) and the
later phosphorylated caveolin-1 at Tyr14 induces interaction with
TLR4 and mediates MyD88-dependent signaling (15). Deficiency of caveolin-1 would
reduce TLR4 signaling (34).
However, knockout studies have identified that caveolin-1 protects
against sepsis by modulating inflammatory responses, alleviating
bacterial burden and suppressing thymocyte apoptosis in rats with
CLP-induced sepsis (35). In
brief, caveolin-1 may biphasicly regulate the TLR4-dependent
inflammatory responses.
In the present study, the expression of caveolin-1
in the lung was determined and it was identified that DXM can
effectively inhibit the increase of caveolin-1 at 4 h and the
decrease at 8 h postoperatively induced by CLP surgery, and promote
the average expression amount. APZ only partly antagonized the
upregulation effect of DXM. This implied that DXM could exert
influence on the TLR4 pathway-mediated inflammatory responses and
the promote sepsis survival rate through regulation of caveolin-1
expression, however the exact receptors and downstream molecular
events require additional investigation.
In conclusion, preemptive clinical sedative doses of
DXM may upregulate the expression of caveolin-1 downregulated by
sepsis, and may contribute to the inhibition of inflammatory
pathways such as the TLR4-mediated pathways. Furthermore, DXM may
favor the improvement of short-term outcome by the regulation of
other metabolic pathways.
Acknowledgements
The present study was supported by the Provincial
Natural Science Foundation of Hunan, China (grant nos. 2013FJ4082
and 2015SK2085).
References
|
1
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock, 2012. Intensive Care
Medicine. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao H, Siddiqui J and Remick DG:
Mechanisms of mortality in early and late sepsis. Infect Immun.
74:5227–5235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ernsberger P, Giuliano R, Willette RN and
Reis DJ: Role of imidazole receptors in the vasodepressor response
to clonidine analogs in the rostral ventrolateral medulla. J
Pharmacol Exp Ther. 253:408–418. 1990.PubMed/NCBI
|
|
4
|
Barr J, Fraser GL, Puntillo K, Ely EW,
Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et
al: Clinical practice guidelines for the management of pain,
agitation, and delirium in adult patients in the intensive care
unit. Crit. Care Med. 41:263–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandharipande PP, Sanders RD, Girard TD,
McGrane S, Thompson JL, Shintani AK, Herr DL, Maze M and Ely EW:
MENDS investigators: Effect of dexmedetomidine versus lorazepam on
outcome in patients with sepsis: An apriori-designed analysis of
the MENDS randomized controlled trial. Crit Care. 14:R382010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Memiş D, Hekimoğlu S, Vatan I, Yandim T,
Yüksel M and Süt N: Effects of midazolam and dexmedetomidine on
inflammatory responses and gastric intramucosal pH to sepsis, in
critically ill patients. Br J Anaesth. 98:550–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fraser GL, Devlin JW, Worby CP, Alhazzani
W, Barr J, Dasta JF, Kress JP, Davidson JE and Spencer FA:
Benzodiazepine versus nonbenzodiazepine-based sedation for
mechanically ventilated, critically ill adults: A systematic review
and meta-analysis of randomized trials. Crit Care Med. 41 Suppl
1:S30–S38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F
and Zhou C, Huang L, Li X and Zhou C: Dexmedetomidine inhibits
inflammatory reaction in lung tissues of septic rats by suppressing
TLR4/NF-kB pathway. Mediators Inflamm. 2013.5621542013.PubMed/NCBI
|
|
9
|
Jiang L, Li L, Shen J, Qi Z and Guo L:
Effect of dexmedetomidine on lung ischemia-reperfusion injury. Mol
Med Rep. 9:419–426. 2014.PubMed/NCBI
|
|
10
|
Snapir A, Talke P, Posti J, Huiku M,
Kentala E and Scheinin M: Effects of nitric oxide synthase
inhibition on dexmedetomidine-induced vasoconstriction in healthy
human volunteers. Br J Anaesth. 102:38–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L,
Liu C, Wang J, Yang X, Vohra A and Ma D: Dexmedetomidine protects
against renal ischemia and reperfusion injury by inhibiting the
JAK/STAT signaling activation. J Transl Med. 11:1412013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L, Brown D, McKee M, Lebrasseur NK,
Yang D, Albrecht KH, Ravid K and Pilch PF: Deletion of Cavin/PTRF
causes global loss of caveolae, dislipidemia, and glucose
intolerance. Cell Metab. 8:310–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Moore XL, Lee MK, Fernández-Rojo MA,
Parat MO, Parton RG, Meikle PJ, Sviridov D and Chin-Dusting JP:
Caveolin-1 plays a critical role in the differentiation of
monocytes into macrophages. Arterioscler Thromb Vasc Biol.
32:e117–e125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng H, Guo L, Song Z, Gao H, Wang D, Fu
W, Han J, Li Z, Huang B and Li XA: Caveolin-1 protects against
sepsis by modulating inflammatory response, alleviating bacterial
burden, and supressing thymocyte apoptosis. J Biol Chem.
285:25154–25160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G,
Minshall RD, Malik AB and Hu G: Caveolin-1 Tyr14 phosphorylation
induces interaction with TLR4 in endothelial cells and mediates
MyD88-dependent signaling and sepsis-induced lung inflammation. J
Immunol. 191:6191–6199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calizo RC and Scarlata S: A role for
G-proteins in directing G-protein-coupled receptor-caveolae
localization. Biochemistry. 51:9513–9523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D'Alessio A, Kluger MS, Li JH, Al-Lamki R,
Bradley JR and Pober JS: Targeting of tumor necrosis factor
receptor 1 to low density plasma membrane domains in human
endothelial cells. J Biol Chem. 285:23868–23879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Bakhshi FR, Shajahan AN, Sharma T,
Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG,
Skidgel RA, et al: Nitric oxide-dependent Src activation and
resultant caveolin-1 phosphorylation promote eNOS/caveolin-1
binding and eNOS inhibition. Mol Biol Cell. 23:1388–1398. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vetterkind S, Poythress RH, Lin QQ and
Morgan KG: Hierarchical scaffolding of an ERK1/2 activation
pathway. Cell Commun Signal. 11:652013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banquet S, Delannoy E, Agouni A, Dessy C,
Lacomme S, Hubert F, Richard V, Muller B and Leblais V: Role of
Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor
coupling to endothelial NO synthase in mouse pulmonary artery. Cell
Signal. 23:1136–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toscano MG, Ganea D and Gamero AM: cecal
ligation puncture procedure. J Vis Exp. 28602011.PubMed/NCBI
|
|
22
|
Shrum B, Anantha RV, Xu SX, Donnelly M,
Haeryfar SM, McCormick JK and Mele T: A robust scoring system to
evaluate sepsis severity in an animal model. BMC Res Notes.
7:2332014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Virtanen R: Pharmacological profiles of
medetomidine and its antagonist, atipamezole. Acta Vet Scand Suppl.
85:29–37. 1989.PubMed/NCBI
|
|
24
|
Madden CJ, Tupone D, Cano G and Morrison
SF: α2 adrenergic receptor-mediated inhibition of thermogenesis. J
Neurosci. 33:2017–2028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi T, Kawano T, Eguchi S, Chi H,
Iwata H and Yokoyama M: Effects of dexmedetomidine on insulin
secretion from rat pancreatic β cells. J Anesth. 29:396–402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Restitutti F, Raekallio M, Vainionpää M,
Kuusela E and Vainio O: Plasma glucose, insulin, free fatty acids,
lactate and cortisol concentrations in dexmedetomidine-sedated dogs
with or without MK467: A peripheral α-2adrenoceptor antagonist. Vet
J. 193:481–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cussac D, Schaak S, Gales C, Flordellis C,
Denis C and Paris H: alpha(2B)-adrenergic receptors activate MAPK
and modulate proliferation of primary cultured proximal tubule
cells. Am J Physiol Renal Physiol. 282:F943–F952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miranda ML, Balarini MM and Bouskela E:
Dexmedetomidine attenuates the microcirculatory derangements evoked
by experimental sepsis. Anesthesiology. 122:619–630. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drewry AM, Fuller BM, Skrupky LP and
Hotchkiss RS: The presence of hypothermia within 24 hours of sepsis
diagnosis predicts persistent lymphopenia. Crit Care Med.
43:1165–1196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu G, Malik AB and Minshall RD: Toll-like
receptor 4 mediates neutrophil sequestration and lung injury
induced by endotoxin and hyperinflation. Crit care med. 38:194–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Okamoto T, Chun M, Sargiacomo M,
Casanova JE, Hansen SH, Nishimoto I and Lisanti M: Evidence for a
regulated interaction between heterotrimeric G proteins and
caveolin. J Biol Chem. 270:15693–15701. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Couet J and Lisanti MP: Src tyrosine
kinases, Galpha subunits, and H-Ras share a common
membrane-anchored scaffolding protein, caveolin. Caveolin binding
negatively regulates the auto-activation of Src tyrosine kinases. J
Biol Chem. 271:29182–29190. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shajahan AN, Tirupathi C and Minshall RD:
Gbetagamma activation of Src induces caveolae-mediated endocytosis
in endothelial cells. J Biol Chem. 279:48055–48062. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mirza MK, Yuan J, Gao XP, Garrean S,
Brovkovych V, Malik AB, Tiruppathi C and Zhao YY: Caveolin-1
deficiency dampens Toll-like receptor 4 signaling through eNOS
activation. Am J Pathol. 176:2344–2351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng H, Guo L, Song Z, Gao H, Wang D, Fu
W, Han J, Li Z, Huang B and Li XA: caveolin-1 protects against
sepsis by modulating inflammatory response, alleviating bacterial
burden, and supressing thymocyte apoptosis. J Biol Chem.
285:25154–25160. 2010. View Article : Google Scholar : PubMed/NCBI
|