Introduction
Atherosclerosis (AS) is commonly the pathological
basis for numerous cardiovascular and cerebrovascular diseases.
Worldwide, the mortality rate from cardiovascular diseases has been
predicted to reach 36% by 2020 (1). AS develops progressively, however, an
effective treatment against it is yet to be established. Given the
increasing average human life expectancy and the aging population,
AS poses a prominent threat to human health. Therefore, active
research investigating the pathogenesis of AS and establishing
effective therapeutic targets has become a critical issue.
Notable characteristics of AS include arterial
intimal lipid deposition, proliferation of smooth muscle cells
(SMCs) and connective tissues, infiltration and proliferation of
monocytes/macrophages, and the formation of foam cells (which cause
focal intimal fibrous thickening and plaque formation leading to
hardening of the arteries and artery stenosis) (2). Multiple mechanisms have been proposed
to be involved in AS development, including the thrombogenic, lipid
infiltration, homocysteine, and smooth muscle mutation theories,
and also the monoclonal, response-to-injury, oxidative, arginine,
shear stress and stem cell hypotheses (3). However, none of these comprehensively
explain the pathological development of AS. Numerous studies have
suggested that intravascular cytochrome P450 (CYP) oxidase is
involved in AS pathogenesis (4–12).
It has previously been demonstrated that
overexpression of CYP oxidase has a protective effect on tumor
necrosis factor α-induced endothelial cell apoptosis (12). CYP oxidase promotes proliferation
and migration of bovine aortic endothelial cells (9). In vivo, overexpression of CYP
epoxygenase reduces blood pressure (6,10),
proinflammatory protein expression and low-density lipoprotein
(LDL) cholesterol levels in the blood, however it increases
high-density lipoprotein cholesterol (11). Inhibition of CYP oxidase causes
contrasting effects (4). These
results indicate that the CYP oxidase family of proteins is
important in the regulation of cardiovascular diseases.
Based on this functional role of the CYP family, we
hypothesized that genetic and environmental factors downregulate
the expression of CYP oxidase, and lead to its dysfunction. The
protective effect on the blood vessels is subsequently reduced or
lost, leading to the development of AS. The present study used
cytochrome P450 family 2 subfamily J member 2 (CYP2J2), the most
common subtype of CYP oxidase in the human body, for the
investigation. The effects of CYP2J2 overexpression on the
proliferation and migration of human venous endothelial cells,
arterial SMCs and human peripheral monocyte-derived foam cell
formation were investigated. The current study presents a
preliminary report on the effects of CYP2J2 on the occurrence and
development of AS.
Materials and methods
Cell lines and culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from the China Center for Type Culture Collection (Wuhan,
China). They were cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), penicillin (100 U/ml) and streptomycin (100
µg/ml). Primary cultured human aortic smooth muscle cells (HASMC)
were purchased from ScienCell Research Laboratories (San Diego, CA,
USA). These were maintained in Smooth Muscle Cell Medium (cat. no.
1101; ScienCell Research Laboratories). All cells were maintained
at 37°C in a humidified incubator in an atmosphere containing 5%
CO2, and passaged when 90–95% confluent. Trypsin (0.25%)
was used for digestion and passaging.
Peripheral blood mononuclear cell
(PBMC) isolation and foam cell culture
Fresh blood was anonymously collected from 5
coronary heart disease patients with approval from the Guangzhou
General Hospital of Guangzhou Military Area Command of Chinese PLAq
(Guangzhou, China), and all patients provided written informed
consent for the use of samples for the present study. PBMCs were
isolated by density-gradient centrifugation at 400 × g for 30 min
at 20°C using Ficoll-Paque™ PLUS (GE Healthcare Life Sciences,
Uppsala, Sweden). They were then seeded onto 96-well plates at
2×105 cells per well and cultured at 37°C with 5% CO2.
The cells that adhered to the plate after 3 h were considered to be
monocytes and were induced to differentiate into monocyte-derived
macrophages for 2 days in RPMI-1640 medium containing 50 nM
phorbol-12-myristate-13-acetate. The medium was replenished each
day. PBMC-derived macrophages were transformed into foam cells by
incubation with oxidized LDL (ox-LDL; 80 mg/l) for 48 h, and
subsequently infected with lentivirus (LV). After 72 h, the culture
medium and cells were harvested for total cholesterol measurement
and Oil red O staining, respectively.
LV packaging and cell infection
The complete open reading frame of CYP2J2
(NM_000775.2) was amplified by polymerase chain reaction (PCR).
Primer sequences were as follows: Forward,
5′-CCGCTCGAGGCCACCATGCTCGCGGCGATGGGCTC-3′ and reverse,
5′-CGCGGATCCTTACACCTGAGGAACAGCGCAGAG-3′, containing XhoI and
BamHI restriction sites within the 5′ and 3′ termini,
respectively. The amplicon was inserted into the pLVX-IRES-ZsGreen1
plasmid (Clontech Laboratories, Inc., Mountainview, CA, USA), which
was digested with the same enzymes. pLVXs encoding CYP2J2 or empty
vector were generated by transient transfection of 293T cells with
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) following the manufacturer's protocol.
Vector production, concentration, and titration were performed in
accordance with general procedures as previously described
(13,14). For infection, 2×105 HUVECs and foam
cells were subcultured in 6-well culture plates for 24 h prior to
transduction. The cell culture media were then removed and cells
washed twice with PBS followed by addition of 0.5 ml lentiviral
suspension [1×108 IU/ml, multiplicity of infection (MOI)=100]
containing 8 µg/ml polybrene. The cells were incubated at 37°C
overnight. The vector suspension was aspirated, and fresh growth
medium (2 ml/flask) was added to the transduced cells followed by
incubation at 37°C with 5% CO2. The medium was replaced
after 24 h and transduction efficiencies were evaluated on day 3
post-infection. The percentage of green fluorescent protein
(GFP)-positive cells was determined by calculating the number of
GFP-positive and total cells in randomly selected microscopic
fields under a fluorescent microscope (Leica Microsystems GmbH,
Wetzlar, Germany). Samples with >80% GFP-positive cells were
used for subsequent assays.
Cell proliferation assay
Cell proliferation was measured using the CellTiter
96 AQueous One Solution Cell Proliferation Assay kit (MTS; Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. CYP2J2-overexpressed and empty LV-infected HUVEC cells
(1×104) were seeded onto 96-well plates. Following culturing for 0,
24, 48, and 72 h, 10 µl CellTiter 96 AQueous One Solution reagent
was added to each well and further incubated for 4 h at 37°C.
Absorbance at a wavelength of 490 nm was measured using a Multiskan
MK3 microplate reader (Thermo Fisher Scientific, Inc.). The
proliferation ratio was calculated using the following formula:
Proliferation rate (%) = (mean absorbance of LV-CYP2J2-GFP/mean
absorbance of LV-GFP) ×100.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from the harvested cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). To quantify the mRNA levels of CYP2J2, RT
was performed using GoScript Reverse Transcription System (Promega
Corporation) according to the manufacturer's protocol, followed by
qPCR using SYBR-Green qPCR SuperMix (Invitrogen; Thermo Fisher
Scientific Inc.) on the ABI Prism® 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) The PCR cycling program involved an initial denaturation step
at 95°C for 2 min, followed by 40 cycles of 15 sec at 95°C and 32
sec at 60°C. The present study used 18s rRNA as an endogenous
control. The primer sequences used were as follows: CYP2J2,
forward, 5′-AGCACCCTGGACCTCTTCTT-3′ and reverse,
5′-GGCCAATCACTCTGTCAATCT-3′; 18s rRNA, forward,
5′-CCTGGATACCGCAGCTAGGA-3′ and reverse,
5′-GCGGCGCAATACGAATGCCCC-3′. Gene expression was measured in
triplicates, quantified by the 2-ΔΔCq method (15), and normalized to a control.
Western blotting
Cells were lysed using radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China).
Total protein concentration was determined using a bicinchoninic
protein assay kit (Beyotime Institute of Biotechnology). Total
protein (30 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked with 5% milk in Tris-buffered
saline containing 0.05% Tween-20 (TBST) for 1 h at 37°C, incubated
for 1 h with anti-CYP2J2 mouse monoclonal primary antibody (1:500;
cat. no. CF503636, OriGene Technologies, Inc., Beijing, China),
washed three times with TBST, and further incubated with rabbit
anti-mouse IgG (H+L)-horseradish peroxidase (HRP) conjugated
secondary antibody (1:5,000; cat. no. 6170–05, SouthernBiotech,
Birmingham, AL, USA) for 40 min. Subsequently, the membranes were
washed three times with TBST. Membranes were blocked in TBST for 1
h at 37°C for GAPDH detection and incubated for 1 h with
HRP-conjugated monoclonal mouse anti-GAPDH primary antibody
(1:10,000; cat. no. KC-5G5, KangChen Biotech, Shanghai, China), and
washed three times with TBST. All proteins were visualized using an
enhanced chemiluminescence reagent (EMD Millipore).
Transwell migration
Cell migration and invasion were assessed using a
Transwell assay. HUVECs and HASMCs were harvested, and 5×104 cells
in 200 µl RPMI-1640 medium supplemented with 0.1% FBS were placed
in the upper chamber of an insert (pore size, 8 µm). The lower
chamber was filled with medium containing 10% FBS (600 µl). After
24 h of incubation and removal of cells on the upper chamber of the
filter with a cotton swab, the cells on the underside were fixed
with 4% paraformaldehyde for 20 min at 25°C, stained with 0.1%
crystal violet in 20% ethanol for 10 min, and counted in five
randomly selected fields using a phase contrast microscope.
Migrating cells were monitored by imaging at ×200 magnification
under a light microscope (Olympus Corporation, Tokyo, Japan) in
five independent fields for each well. The assays were performed in
triplicate.
Oil red O staining of lipid
accumulation in cells
Culture media was removed from PBMCs and cells were
washed twice with PBS. The cells were then fixed with 4%
paraformaldehyde for 30 min. The Oil Red O working solution was
prepared fresh from its 0.5% (w/v) stock solution, which was
diluted with water at a ratio of 6:4 (Oil Red O:water). Cells were
then incubated with Oil Red O for 30 min at room temperature,
washed gently with PBS three times to remove excess non-specific
staining and observed under a light microscope (Olympus
Corporation).
Quantitation of cellular
cholesterol
Quantification of cellular total and free
cholesterol was performed with culture media using the Total
Cholesterol Quantitation kit (cat. no. F002-1) from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China) and Free
Cholesterol Quantitation kit (cat. no. E1016) from Applygen
Technologies, Inc. (Beijing, China), respectively. The detection
was carried out according to the manufacturer's protocol. All
measurements were conducted in duplicate, and all samples were
analyzed in the same microplate to minimize run-to-run
variability.
Statistical analyses
All data are presented as mean ± standard deviation
and analyzed using SPSS software version 19.0 (IBM SPSS, Armonk,
NY, USA). One-way analysis of variance followed by a least
significant difference post hoc test was used to determine
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of CYP2J2 in HUVEC,
HASMC and foam cells
In order to understand the function of CYP2J2, LVs
expressing CYP2J2 and GFP (LV-CYP2J2-GFP) or GFP alone (LV-GFP)
were packaged and transduced into HUVECs, HASMCs and foam cells at
MOI of 100. LV-CYP2J2-GFP and LV-GFP successfully infected all
three cell types, as presented in Fig.
1A-C. The cells were harvested 72 h post-infection for RT-qPCR
and western blotting analysis of CYP2J2 expression. CYP2J2 mRNA was
significantly overexpressed in HUVECs (P=0.004), HASMCs
(P<0.001) and foam cells (P<0.001), as detected by RT-qPCR
(Fig. 1D-F). CYP2J2 protein levels
were successfully overexpressed in HUVECs, HASMCs and foam cells,
as detected western blotting (Fig.
1G-I).
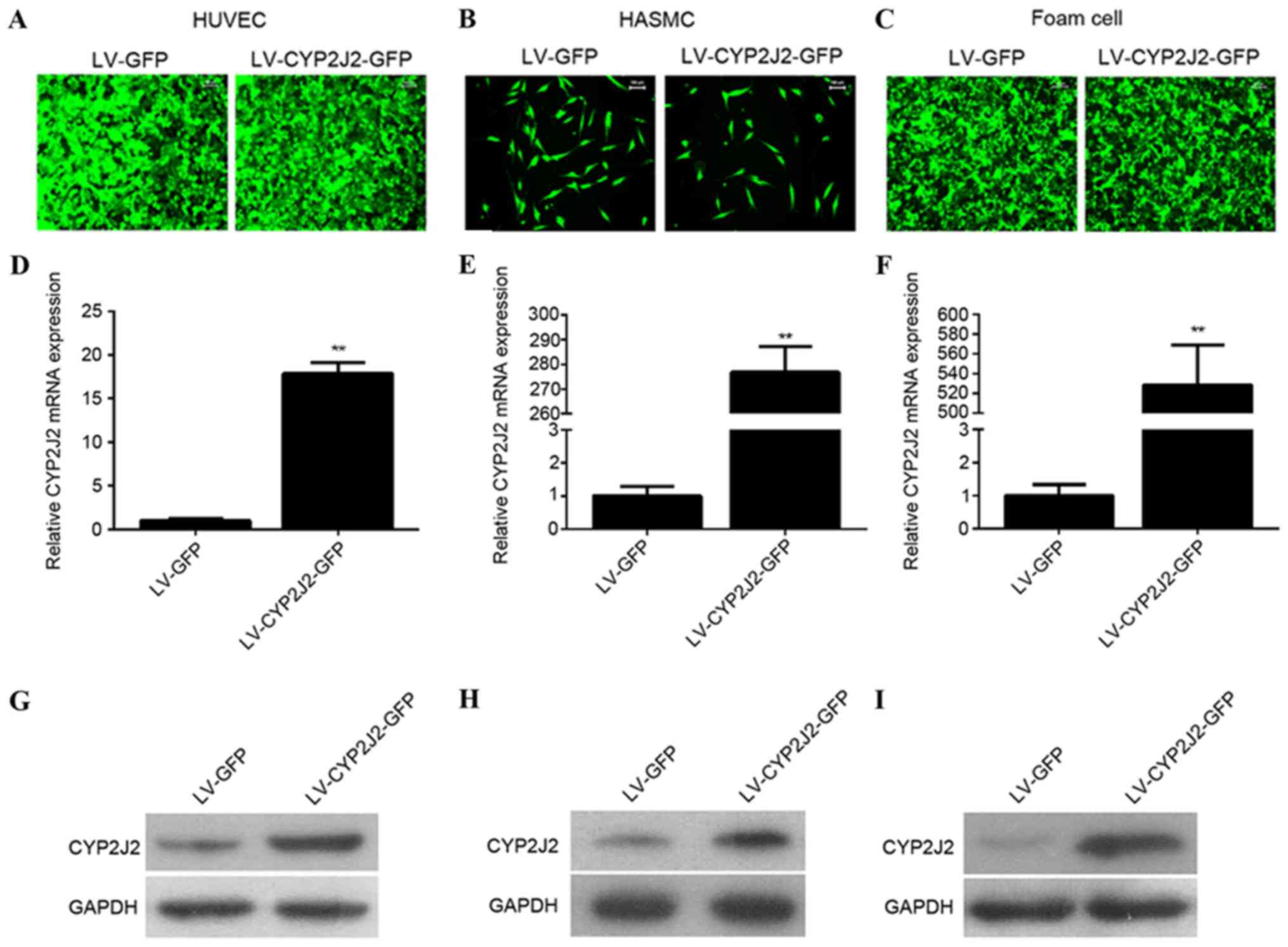 | Figure 1.CYP2J2 is successfully overexpressed
in HUVECs, HASMCs, and foam cells infected with LV-CYP2J2. (A)
HUVEC, (B) HASMC and (C) foam cells were successfully infected with
LV-CYP2J2-GFP or LV-GFP at MOI=100. Reverse
transcription-quantitative polymerase chain reaction revealed that
CYP2J2 was overexpressed in (D) HUVECs, (E) HASMCs and (F)
foam cells. Western blot analysis verified that CYP2J2 was
overexpressed in (G) HUVECs, (H) HAMSCs and (I) foam cells. CYP2J2,
cytochrome P450 family 2 subfamily J polypeptide 2; HUVECs, human
umbilical vein endothelial cells; HASMCs, human arterial smooth
muscle cells; LV, lentivirus; GFP, green fluorescent protein.
**P<0.01 vs. LV-GFP group. |
CYP2J2 overexpression promotes
proliferation of HUVECs and suppresses proliferation of HASMCs
To elucidate the possible function of CYP2J2 in
atherosclerosis, the present study evaluated the effect off CYP2J2
on HUVEC and HASMC proliferation. Following 12 h of culture, CYP2J2
overexpression did not significantly affect HUVEC and HASMC
proliferation compared with the LV-GFP-transduced cells (P=0.079).
However, after 24, 48 and 72 h of culture, CYP2J2 overexpression
increased HUVEC proliferation by 10.0, 19.6 and 21.3%,
respectively, compared with the LV-GFP cells (Fig. 2A). However, HASMC proliferation was
decreased by and 9, 18 and 21% at 24, 48 and 72 h, respectively,
compared with the LV-GFP cells (Fig.
2B).
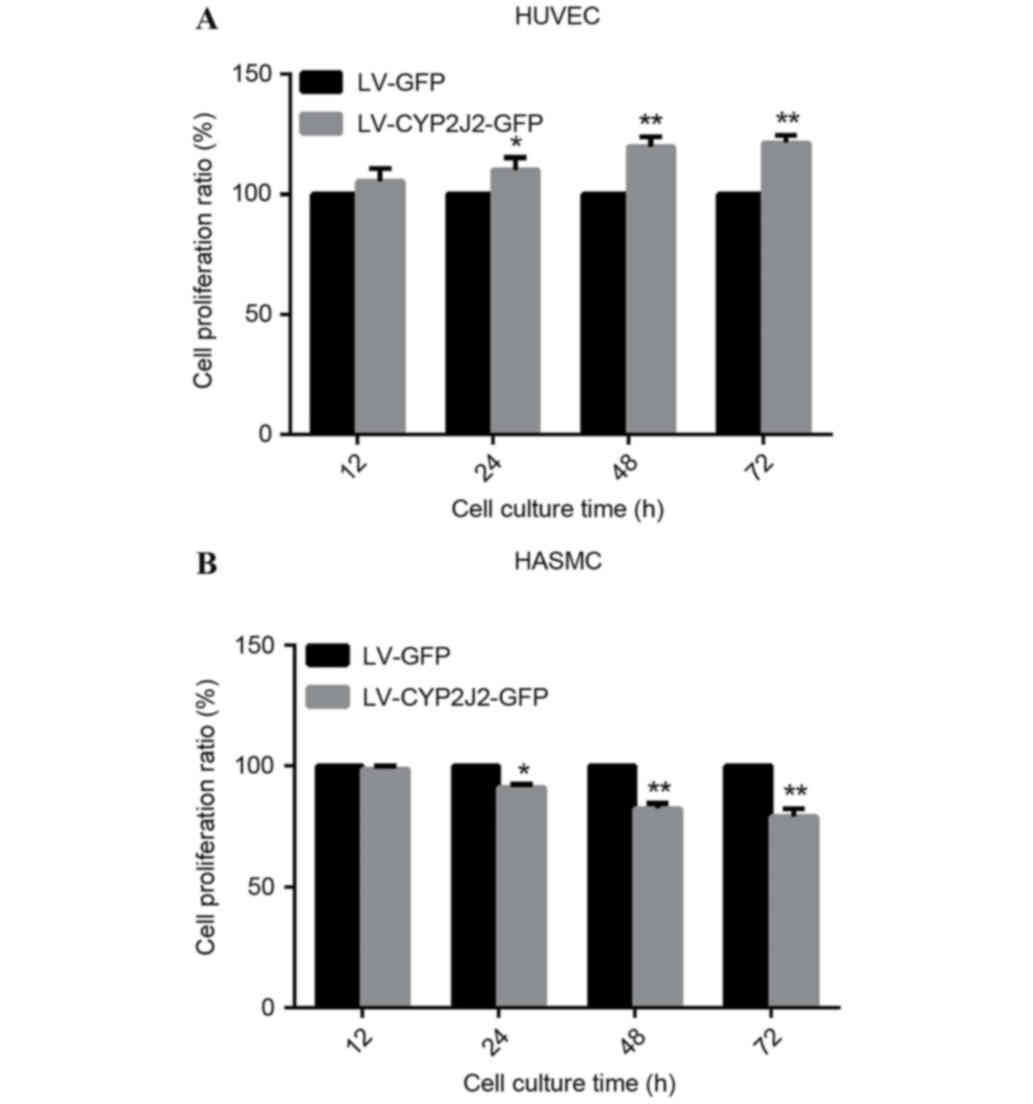 | Figure 2.CYP2J2 overexpression promotes HUVEC
and suppresses HASMC proliferation. Following infection for 72 h
with LV-CYP2J2-GFP or LV-GFP, cells were harvested for cell
proliferation assays. Absorbance at a wavelength of 490 nm was
measured at 12, 24, 48, and 72 h time-points for (A) HUVECs and (B)
HASMCs. Data are expressed as the mean ± standard deviation.
*P<0.05, **P<0.01 vs. LV-GFP. CYP2J2, cytochrome P450 family
2 subfamily J polypeptide 2; HUVECs, human umbilical vein
endothelial cells; HASMCs, human arterial smooth muscle cells; LV,
lentivirus; GFP, green fluorescent protein. |
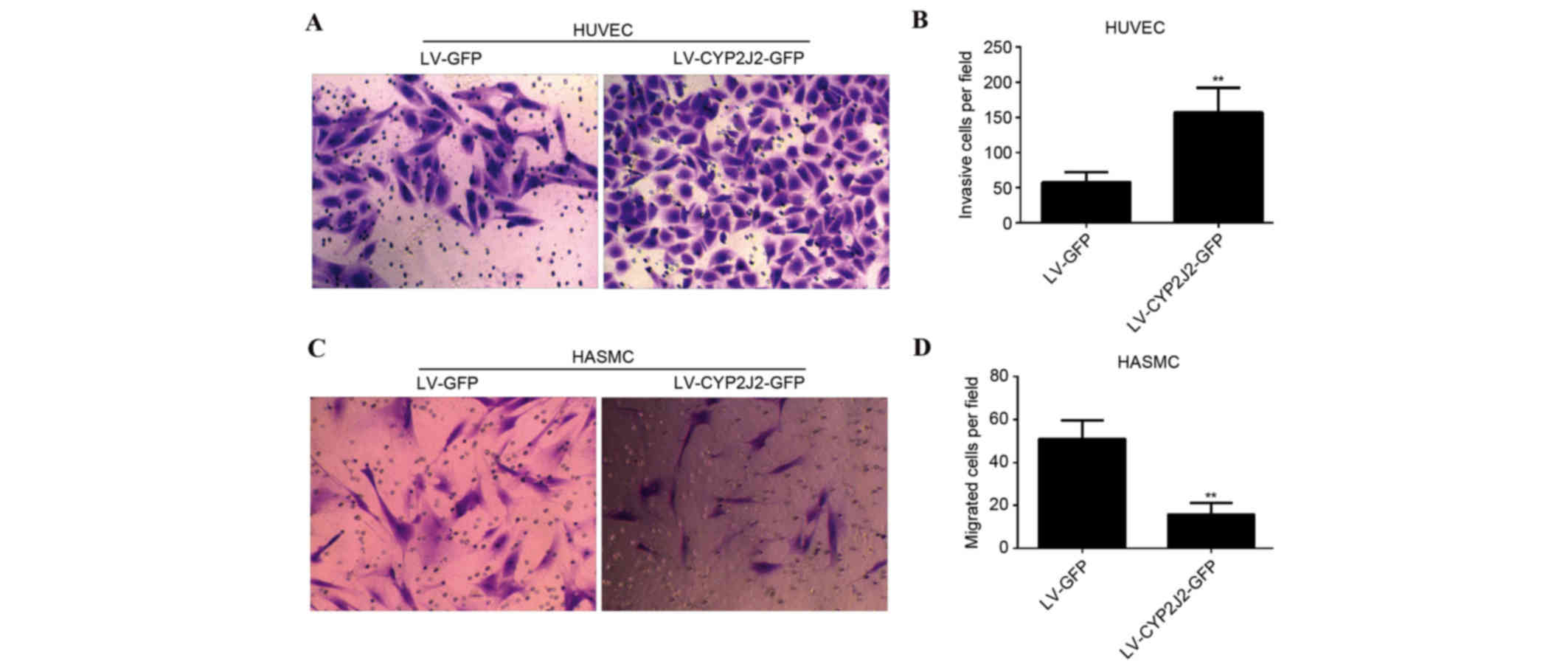
Overexpression of CYP2J2 promotes
migration of HUVECs and inhibits migration of HASMCs
The effect of CYP2J2 overexpression on HUVEC and
HASMC migration was subsequently evaluated. The number of HUVECs
that passed through the membrane into the lower chamber was
significantly greater for LV-CYP2J2-GFP cells compared with LV-GFP
cells (P=0.002). The number of migrating cells upon infection with
LV-GFP and LV-CYP2J2-GFP was 58±14 and 157±35, respectively
(Fig. 3A and B). The number of
LV-CYP2J2-GFP HASMCs that passed through the membrane into the
lower chamber was significantly reduced compared with LV-GFP cells
(P=0.006). The number of migrating cells was 51±9 and 16±5 upon
infection with LV-GFP and LV-CYP2J2-GFP, respectively (Fig. 3C and D).
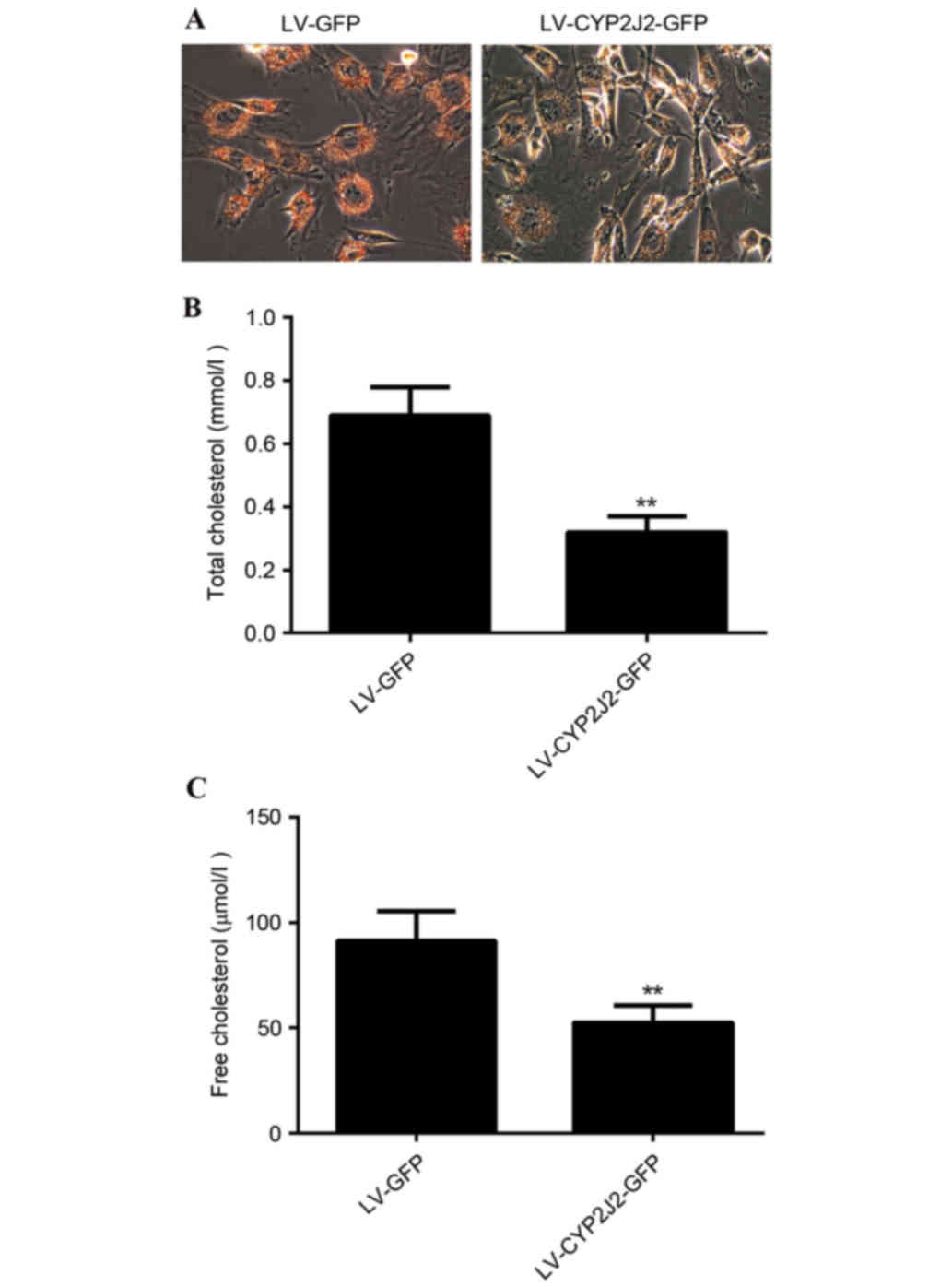
CYP2J2 overexpression suppresses
ox-LDL-induced foam cell formation
It has previously been demonstrated that cholesterol
is involved in the pathogenesis of atherosclerosis, therefore the
present study investigated the effect of CYP2J2 overexpression on
lipid accumulation. PBMC-derived macrophages were treated with
ox-LDL for 48 h, and subsequently infected with LV-GFP or
LV-CYP2J2-GFP. Following 72 h of infection, culture media were
harvested for total cholesterol detection, while the cells were
harvested for Oil Red O staining. Oil Red O staining was used to
determine changes in cellular lipid accumulation following
infection with LV-GFP or LV-CYP2J2-GFP. The results demonstrated
that CYP2J2 overexpression markedly reduced lipid accumulation in
foam cells (Fig. 4A). Furthermore,
quantification of cellular cholesterol content revealed that total
cholesterol (P=0.008) and free cholesterol (P=0.007) concentrations
in LV-CYP2J2-GFP-infected cells were significantly lower compared
with the LV-GFP-infected cells (Fig.
4B and C). CYP2J2 overexpression therefore effectively
suppresses cellular cholesterol accumulation.
Discussion
The CYP oxidase family of proteins and its
metabolites are important in the regulation of the development of
cardiovascular diseases. CYP2J2 is the most common subtype of CYP
oxidase in the human body and its function has been investigated in
various cardiovascular diseases, including ischemic stroke,
myocardial infarction, and coronary heart disease (16–18).
Endothelial-specific CYP2J2 overexpression alleviates high-fat
diet-induced hyperlipidemia in mice (19). In addition, CYP2J2 prevents cardiac
fibrosis by suppressing the transmission of proinflammatory signals
from cardiomyocytes to macrophages (20). CYP2J2 overexpression protects
against susceptibility to arrhythmia in cardiac hypertrophy
(21). Furthermore, polymorphism
in cytochrome P450 epoxygenase CYP2J2 is associated coronary
artery disease risk (22,23). Therefore, based on the importance
of CYP2J2 in cardiovascular diseases, the present study
hypothesized that CYP2J2 may regulate the occurrence and
development of AS. AS is initiated by complex interactions between
circulating factors and various cell types in the vessel walls,
including endothelial cells, lymphocytes, monocytes, and SMCs
(24). Therefore, the function of
CYP2J2 in AS was investigated by observing the effects of its
overexpression on the proliferation and migration of HUVECs, HASMCs
and human peripheral monocyte-derived foam cell formation. The
results provide a mechanistic basis and a novel therapeutic target
for AS.
Endothelial cells are central to the cardiovascular
system by regulating blood circulation and fluidity, vascular tone,
coagulation, inflammatory responses and angiogenesis (9). Proliferation and migration of
endothelial cells is an important process during angiogenesis and
vessel sprouting. The response-to-injury hypothesis, suggests that
endothelial cell injury is a factor in the occurrence and
development of AS (25). It is
therefore important to protect and recover the function of
endothelial cells in AS prevention and therapy. The present study
observed that CYP2J2 overexpression promoted proliferation and
migration of HUVECs. This is supported by a previous study
conducted by Wang et al (9), where the authors demonstrated that
CYP oxidase promotes the proliferation and migration of bovine
aortic endothelial cells (9).
CYP2J2 may therefore exhibit a protective role in the endothelial
cell injury process and promote angiogenesis and vessel sprouting.
In addition, proliferation and migration of vascular smooth muscle
cells (VSMCs), which are the major cell type in blood vessel walls,
have been suggested to foster atherosclerosis development (26,27).
Furthermore, CYP2J2 overexpression suppressed HASMC proliferation
and migration. This observation supports the suggestion that CYP2J2
suppresses VSMC hyperplasia and migration in the process of AS and
protects against it. AS is a chronic disease characterized by the
deposition of excessive cholesterol in the arterial intima
(2). Uncontrolled uptake of
ox-LDL, excessive cholesterol esterification, and/or impaired
cholesterol release leads to accumulation of cholesterol ester
(CE). CE is stored as cytoplasmic lipid droplets and subsequently
triggers the formation of foam cells (28). The results of the present study
revealed that CYP2J2 overexpression suppressed ox-LDL-induced foam
cell formation. Formation of macrophage foam cells in the intima is
a major hallmark of early-stage atherosclerotic lesions. Therefore,
the aforementioned results further support the hypothesis that
CYP2J2 exhibits a protective function against AS.
In conclusion, the present study demonstrated that
overexpression of CYP2J2 promoted an angiogenic phenotype, which
included endothelial cell proliferation and migration. CYP2J2
overexpression suppressed HASMC proliferation and migration, and
ox-LDL-induced foam cell-formation processes that are a requisite
for the occurrence and development of AS. Therefore, CYP2J2 may
exhibit a protective role in the development of AS.
Acknowledgements
The present study was supported by Natural Science
Foundation of Guangdong Province, China (grant no. S2013010011957)
and National Natural Science Foundation of China (grant no.
81402671).
References
|
1
|
Negi S and Anand A: Atherosclerotic
coronary heart disease-epidemiology, classification and management.
Cardiovasc Hematol Disord Drug Targets. 10:257–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z and Xu H: Anti-inflammatory and
immunomodulatory mechanism of tanshinone IIA for atherosclerosis.
Evid Based Complement Alternat Med. 2014:2679762014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Athirakul K, Bradbury JA, Graves JP,
DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X,
et al: Increased blood pressure in mice lacking cytochrome P450
2J5. FASEB J. 22:4096–4108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodiga S, Zhang R, Jacobs DE, Larsen BT,
Tampo A, Manthati VL, Kwok WM, Zeldin DC, Falck JR, Gutterman DD,
et al: Protective actions of epoxyeicosatrienoic acid: Dual
targeting of cardiovascular PI3K and KATP channels. J Mol Cell
Cardiol. 46:978–988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CR, Imig JD, Edin ML, Foley J, DeGraff
LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, et al:
Endothelial expression of human cytochrome P450 epoxygenases lowers
blood pressure and attenuates hypertension-induced renal injury in
mice. FASEB J. 24:3770–3781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Node K, Huo Y, Ruan X, Yang B, Spiecker M,
Ley K, Zeldin DC and Liao JK: Anti-inflammatory properties of
cytochrome P450 epoxygenase-derived eicosanoids. Science.
285:1276–1279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Node K, Ruan XL, Dai J, Yang SX, Graham L,
Zeldin DC and Liao JK: Activation of Galpha s mediates induction of
tissue-type plasminogen activator gene transcription by
epoxyeicosatrienoic acids. J Biol Chem. 276:15983–15989. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Wei X, Xiao X, Hui R, Card JW,
Carey MA, Wang DW and Zeldin DC: Arachidonic acid epoxygenase
metabolites stimulate endothelial cell growth and angiogenesis via
mitogen-activated protein kinase and phosphatidylinositol
3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 314:522–532.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao B, Li X, Yan J, Yu X, Yang G, Xiao X,
Voltz JW, Zeldin DC and Wang DW: Overexpression of cytochrome P450
epoxygenases prevents development of hypertension in spontaneously
hypertensive rats by enhancing atrial natriuretic peptide. J
Pharmacol Exp Ther. 334:784–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Zhang XA and Wang DW: The roles of
CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in
cardiovascular and malignant diseases. Adv Drug Deliv Rev.
63:597–609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang S, Lin L, Chen JX, Lee CR, Seubert
JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, et al: Cytochrome
P-450 epoxygenases protect endothelial cells from apoptosis induced
by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling
pathways. Am J Physiol Heart Circ Physiol. 293:H142–H151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zufferey R, Dull T, Mandel RJ, Bukovsky A,
Quiroz D, Naldini L and Trono D: Self-inactivating lentivirus
vector for safe and efficient in vivo gene delivery. J Virol.
72:9873–9880. 1998.PubMed/NCBI
|
|
14
|
Dull T, Zufferey R, Kelly M, Mandel RJ,
Nguyen M, Trono D and Naldini L: A third-generation lentivirus
vector with a conditional packaging system. J Virol. 72:8463–8471.
1998.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Zhao JH, Ma PJ, Su LL, Tao SB and Ji
SB: Association of CYP2J2 gene polymorphisms with ischemic stroke.
Int J Clin Exp Med. 8:8163–8167. 2015.PubMed/NCBI
|
|
17
|
Kumar AS Arun, Kumar SS, Umamaheswaran G,
Kesavan R, Balachandar J and Adithan C: Association of CYP2C8,
CYP2C9 and CYP2J2 gene polymorphisms with myocardial infarction in
South Indian population. Pharmacol Rep. 67:97–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CR, North KE, Bray MS, Couper DJ,
Heiss G and Zeldin DC: CYP2J2 and CYP2C8 polymorphisms and coronary
heart disease risk: The atherosclerosis risk in communities (ARIC)
study. Pharmacogenet Genomics. 17:349–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Chen G, Li N, Dai M, Chen C, Wang
P, Tang H, Hoopes SL, Zeldin DC, Wang DW and Xu X: CYP2J2
overexpression ameliorates hyperlipidemia via increased fatty acid
oxidation mediated by the AMPK pathway. Obesity (Silver Spring).
23:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Ni L, Duan Q, Wang X, Chen C, Chen
S, Chaugai S, Zeldin DC, Tang JR and Wang DW: CYP epoxygenase 2J2
prevents cardiac fibrosis by suppression of transmission of
pro-inflammation from cardiomyocytes to macrophages. Prostaglandins
Other Lipid Mediat. 116–117, 64–75. 2015.
|
|
21
|
Westphal C, Spallek B, Konkel A, Marko L,
Qadri F, DeGraff LM, Schubert C, Bradbury JA, Regitz-Zagrosek V,
Falck JR, et al: CYP2J2 overexpression protects against arrhythmia
susceptibility in cardiac hypertrophy. PLoS One. 8:e734902013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spiecker M, Darius H, Hankeln T, Soufi M,
Sattler AM, Schaefer JR, Node K, Börgel J, Mügge A, Lindpaintner K,
et al: Risk of coronary artery disease associated with polymorphism
of the cytochrome P450 epoxygenase CYP2J2. Circulation.
110:2132–2136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Q, Fu Z, Ma Y, Yang H, Huang D, Xie X,
Liu F, Zheng Y and Cha E: A novel polymorphism of the CYP2J2 gene
is associated with coronary artery disease in Uygur population in
China. Clin Biochem. 46:1047–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doran AC, Meller N and McNamara CA: Role
of smooth muscle cells in the initiation and early progression of
atherosclerosis. Arterioscler Thromb Vasc Biol. 28:812–819. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Q, Qiu TQ and Yang H: Ligustilide
inhibits vascular smooth muscle cells proliferation. Eur J
Pharmacol. 542:136–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eun SY, Ko YS, Park SW, Chang KC and Kim
HJ: IL-1β enhances vascular smooth muscle cell proliferation and
migration via P2Y2 receptor-mediated RAGE expression and HMGB1
release. Vascul Pharmacol. 72:108–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252. 2013.
View Article : Google Scholar : PubMed/NCBI
|