Introduction
Acute kidney injury (AKI) is a common disease,
associated with a high morbidity and mortality; the reported
incidence of AKI varies from 5% of all hospitalized patients to
30–50% of patients in intensive care units (1). At present, no therapeutic agents have
been proven to prevent AKI (2).
Kidney ischemia-reperfusion (IR) is known to be a major cause of
AKI. Ischemic injury is present in ~50% of patients with AKI
(3) and is induced by several
factors, including hypotension, hypoperfusion, hypoxia, oxidative
stress and renal vasoconstriction (4). Methods to ameliorate IR injury (IRI)
may provide an effective therapeutic strategy for the treatment or
prevention of ischemic AKI.
It is well known that inflammation serves an
important role in AKI. IR has previously been shown to initiate
pathogenesis in vascular endothelial cells and tubular epithelial
cells, and may result in a loss of immune system homoeostasis in
the kidney (2). Macrophages belong
to the family of mononuclear phagocytes and perform various
critical roles in homeostasis, surveillance, immune response, and
tissue injury and repair (5,6).
Macrophages have been reported to importantly contribute to
ischemic AKI. In addition, macrophages have been identified as
important regulators of wound healing from primarily initiating an
inflammatory response, to later facilitating tissue repair and
downregulating inflammation (7).
The diverse functions of macrophages are attributed to the
plasticity of the macrophage phenotype, which includes classically
activated macrophages (M1) and alternatively activated macrophages
(M2) (8). M1 macrophages secrete
various proinflammatory mediators and have been reported to serve
critical roles in some inflammatory diseases, including
inflammatory bowel disease, atherosclerosis and insulin resistance
in obesity (9–11). Conversely, M2 macrophages
counterbalance M1-induced inflammation and promote tissue repair
(12,13).
Atorvastatin (ATO) is one of the most effective
pharmaceutical agents for the treatment of cardiovascular disease.
In addition to its lipid lowering properties via the inhibition of
3-hydroxy-3-methylglutaryl coenzyme A reductase, ATO also possesses
several pleiotropic effects, including anti-inflammatory and
antioxidant properties, thus modulating effects on endothelial
function and vascular wall structure (14). It has previously been reported that
treatment with ATO protects the kidney from IRI in rats, and
promotes human monocyte differentiation toward M2 macrophages
(15,16).
The present study aimed to investigate whether ATO
has the capacity to facilitate rat monocyte differentiation toward
M2 macrophages in a rat model of renal IRI. In addition, the
underlying mechanisms were investigated.
Materials and methods
Animals
Male Sprague-Dawley rats (weight, 200–250 g; age, 7
weeks), were purchased from Guangzhou University of Chinese
Medicine Laboratory Animal Center (Guangzhou, China). All animal
procedures conducted in the present study were performed strictly
in accordance with the recommendations of the National Institutes
of Health Guide for the Care and Use of Laboratory Animals (1996)
and the present study was approved by Huadu District People's
Hospital (Guangzhou, China). The rats had ad libitum access
to food and water, were housed under controlled conditions of
23±3°C room temperature and 55±15% relative humidity, and were
maintained under a 12 h light-dark cycle.
The 40 rats were randomly assigned to the following
four groups (n=10): IR + ATO group, rats with IRI were administered
a single intravenous dose of ATO (10 mg/kg) 30 min prior to
reperfusion; IR group, rats with IRI were administered intravenous
saline; sham group, sham-operated rats were administered
intravenous saline; and control group, rats were administered
intravenous saline. Saline was administered at the same volume as
the ATO.
To generate the IRI model, surgery was conducted as
previously described (17).
Briefly, following administration of general anesthesia (3%
napental at 30 mg/kg), the rats underwent bilateral incisions and
the right kidney was removed. The left renal artery was exposed and
was then occluded for 60 min. Subsequently, the incisions were
closed to allow the rats to recover and the organ was allowed to
reperfuse for 24 h. The sham group underwent the same operation;
however, the left renal artery was not clamped.
Serum preparation and tissue
collection
All rats were sacrificed ~24 h after surgery by
injection by 3% sodium pentobarbital (30 mg/kg). Blood samples were
collected via cardiac puncture and were left to clot at room
temperature. Following centrifugation at 3,000 × g for 5 min
at 4°C, the extracted serum samples were pipetted into a clean tube
and were stored at −80°C until further use. After collecting blood
samples from the heart, the kidneys were immediately dissected and
were preserved in 10% neutral buffered formalin for further
immunohistological examinations. During the experiment, urine
samples were collected using metabolic cages.
Measurement of serum creatinine (Scr)
and creatinine clearance rate (Ccr)
The levels of Scr and urinary creatinine were
determined using an automatic biochemistry analyzer (ADVIA 1800;
Siemens AG, Munich, Germany). Based on the levels of urinary
creatinine, Scr, urine volume and body weight, Ccr was calculated
according to the following formula (18): Ccr = urinary creatinine (µmol/l) ×
urinary volume (ml/kg/min) / Scr (µmol/l).
Histological examination
The fixed kidneys were cut into transverse sections
and embedded in paraffin. Sections (~4 µm) were prepared and
processed for staining with hematoxylin and eosin (0.1% hematoxylin
for 6 min at room temperature; 0.5% eosin for 2 min at room
temperature), periodic acid-Schiff (oxidized in 0.5% periodic acid
solution for 5 min at room temperature; Schiff reagent for 15 min
at room temperature) and periodic acid-methenamine silver (oxidised
in 0.5% periodic acid solution for 15 min at room temperature;
methenamine silver working solution for 1 h at 60°C). Renal tubular
injuries, including renal necrosis, brush border detachment,
tubular cast formation and tubular dilation, were analyzed at 20
randomly selected high power fields under light microscopy
(magnification, 400x) in the renal outer medulla. According to the
ratio of tubular injury, the degrees of tubular injury were scaled
from 0–5, as follows: 0, not present; 1, ≤10%; 2, 11–25%; 3,
26–50%; 4, 51–75%; 5, ≥76%.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The 4-µm sections underwent TUNEL staining using the
In Situ Apoptosis Detection kit (Roche Diagnostics,
Indianapolis, IN, USA) according to the manufacturer's protocol, to
determine the rate of apoptosis in the kidney. Briefly, after
dewaxing and dehydrating, the sections were incubated with protease
for 30 min at 37°C and then with TUNEL reaction mixture for ~20 min
at 37°C in the dark. Following treatment with converter-peroxidase
solution at 37°C for 10 min in a moisture chamber, the sections
were incubated with 3,3′-diaminobenzidine solution for 5–6 min at
room temperature. Apoptotic cells were characterized by dark brown
staining in the nuclei. Each section was examined at six randomized
high power fields under a fluorescence microscopy (magnification,
400x).
ELISA
TNF-α and IFN-γ concentrations were determined in
the collected serum samples using Rat TNF-α Quantikine ELISA kit
and Rat IFN-γ Quantikine ELISA kit (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocols.
Three independent tests were conducted.
Immunofluorescence staining
Frozen sections were prepared and
immunohistofluoresence analysis was conducted using specific
primary antibodies against rat cluster of differentiation (CD)68
(1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no.
sc-70760), CD206 (1:100; Santa Cruz Biotechnology, Inc.; cat. no.
sc-34577), inducible nitric oxide synthase (iNOS; 1:100;
eBioscience, Inc., San Diego, CA, USA; cat. no. 17-5920-80) and
PPAR-γ (1:400; Santa Cruz Biotechnology, Inc.; cat. no. sc-6284)
overnight at 4°C. In addition, CD68/iNOS, CD68/CD206 and
CD68/PPAR-γ double staining was conducted by incubating sections at
1:100 dilutions with the antibodies overnight at 4°C.
4′,6-Diamidino-2-phenylindole (DAPI) was used to detect nucleate
cells under a fluorescence microscope.
Statistical analysis
Results are presented as the mean ± standard
deviation. Data from the different groups were compared using
one-way analysis of variance followed by Tukey's post-hoc analysis
with SPSS 22.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
ATO treatment significantly alleviates
renal IRI
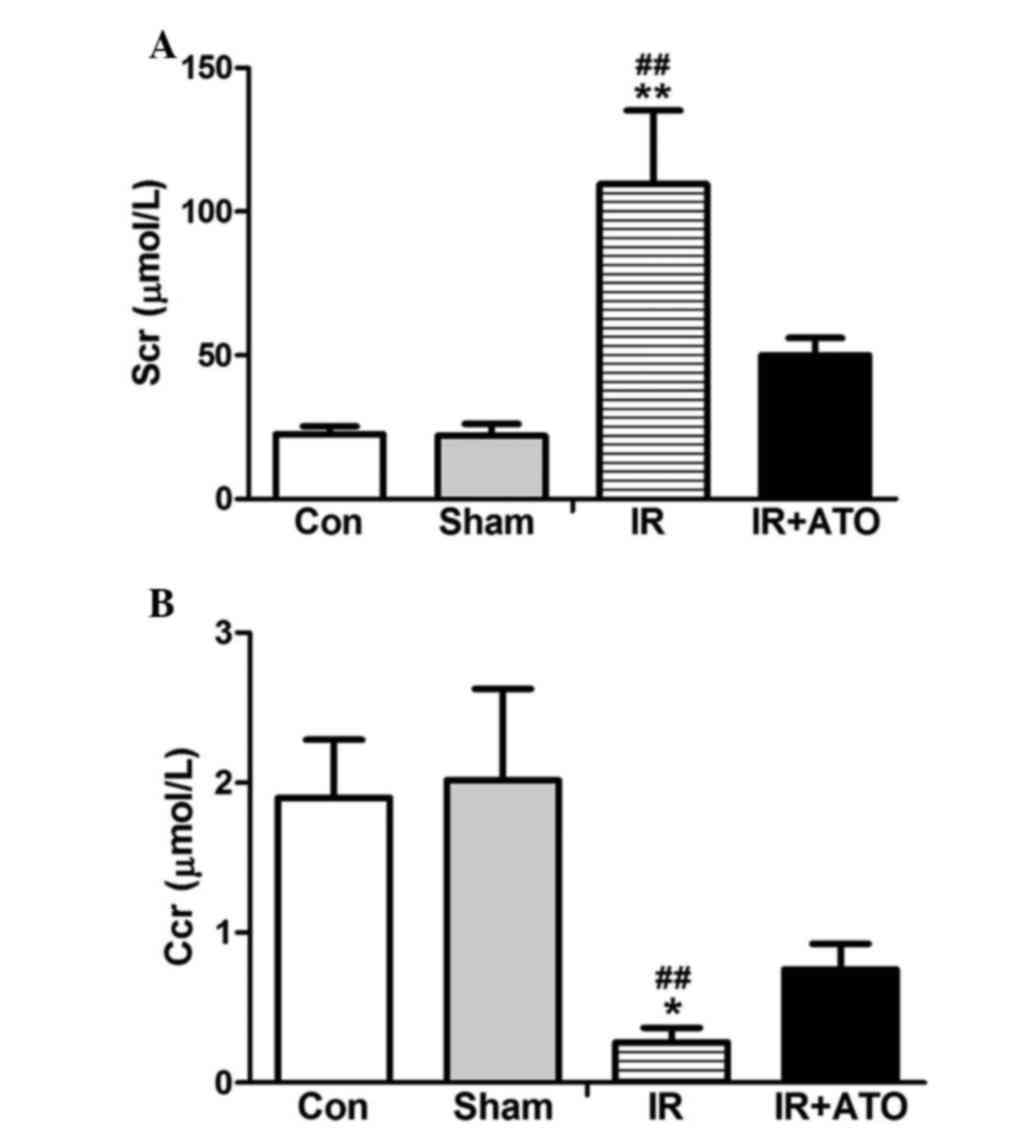
To evaluate the effects of ATO on IRI, Scr levels
were determined in the rats 24 h after IR. As shown in Fig. 1, Scr levels were significantly
increased and the Ccr was significantly decreased in the IR group,
compared with in the control and sham-operated groups. The Scr
levels and the Ccr were comparable between the sham and control
groups. These results indicate that the rat model of renal IRI was
successfully established. In addition, for the IR + ATO group, the
ATO treatment relatively alleviated the Scr increase and the Ccr
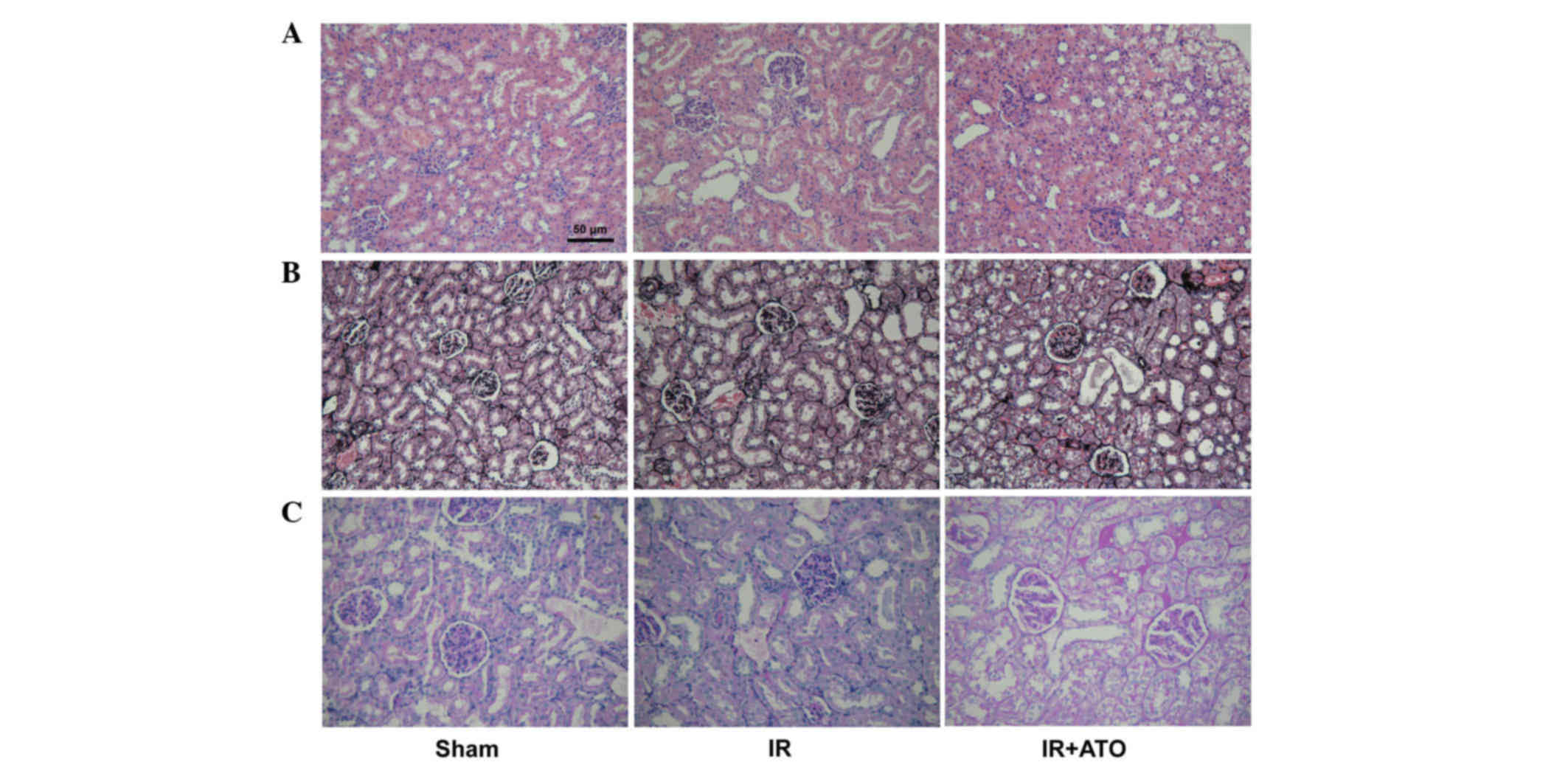
decrease compared with the IR group. Renal pathological alterations
were evaluated in the rats 24 h after IR. Histologically, 24 h
after IR, the control rats developed granular and vacuolar
degeneration in renal tubular epithelial cells, tubular casts,
broader brush detachment and tubular dilation (Fig. 2). Following ATO treatment, the
aforementioned pathological alterations were markedly alleviated.
In addition, no lesions were observed in kidneys of sham-operated
rats. Kidney injury severity was graded among the groups (Table I).
 | Table I.Extent of kidney injury in control,
sham, IR and IR + ATO rats. |
Table I.
Extent of kidney injury in control,
sham, IR and IR + ATO rats.
|
| Number of rats with
tubular injury |
|---|
|
|
|
|---|
| Group | 0 | 1 | 2 | 3 | 4 | 5 |
|---|
| Control | 5 | 5 | 0 | 0 | 0 | 0 |
| Sham | 3 | 7 | 0 | 0 | 0 | 0 |
| IR | 0 | 2 | 4 | 1 | 3 | 0 |
| IR + ATO | 1 | 3 | 3 | 3 | 0 | 0 |
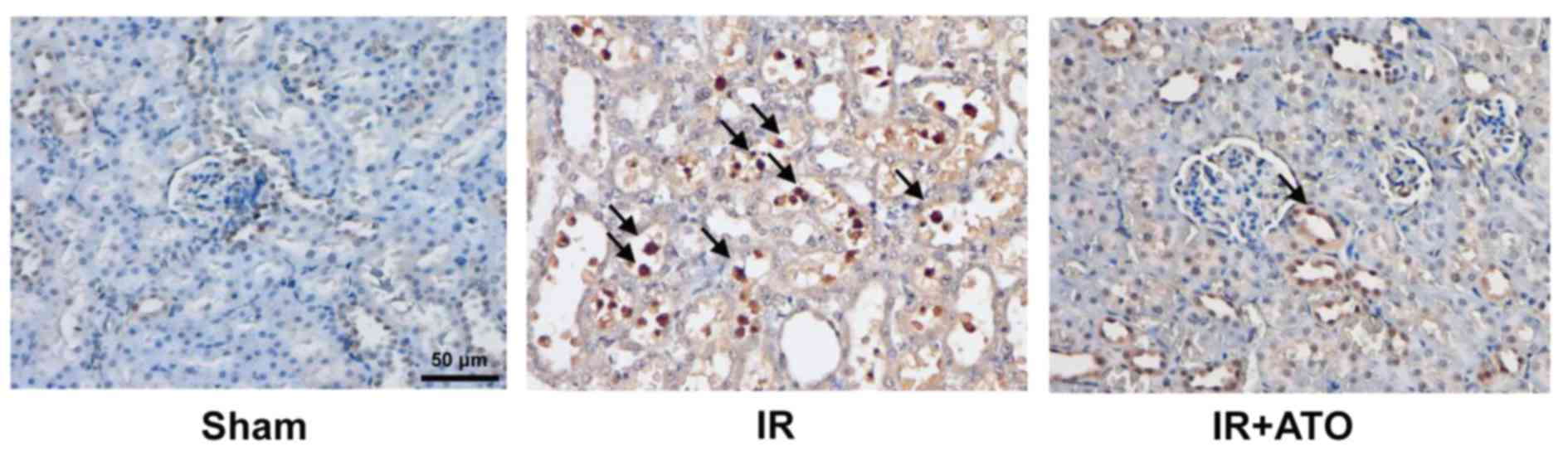
Further analysis by TUNEL assay revealed that IRI
was associated with the increased apoptosis of tubular epithelium
cells (Fig. 3). However, following
treatment with ATO, IR-induced apoptosis was markedly suppressed.
These results indicate ATO treatment may significantly attenuate
renal IRI.
ATO treatment decreases serum TNF-α
and IFN-γ levels
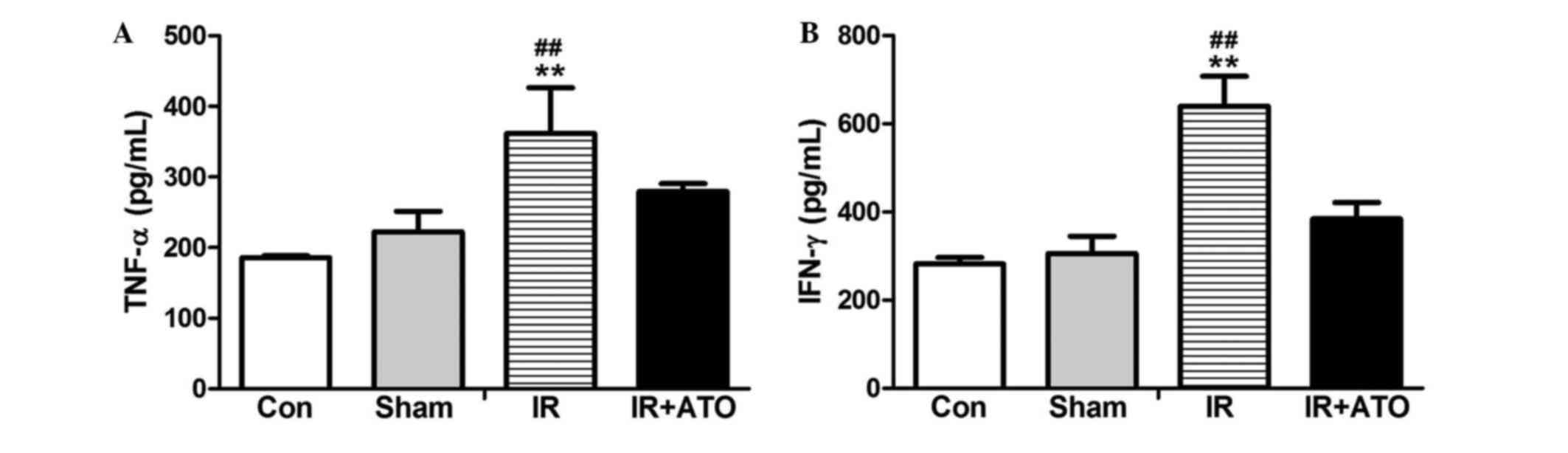
To determine the effects of ATO on proinflammatory
cytokine levels, the serum levels of TNF-α and IFN-γ were detected
24 h after IR. As shown in Fig. 4,
the serum levels of TNF-α and IFN-γ in the sham group were
comparable to the control group. However, the levels were
significantly increased in the IR group compared with the sham
group. Following treatment with ATO, the levels were significantly
decreased compared with the IR group. These results suggest that
ATO treatment may significantly inhibit IRI-induced
inflammation.
ATO treatment accelerates M2
macrophage polarization
To evaluate the effects of ATO on macrophage
activation, M1 and M2 macrophages were detected by double
immunofluorescence staining. Macrophages were detected by red
fluorescence staining with an anti-CD68 antibody (macrophage
marker); M1 macrophages were detected by green fluorescence
staining with an anti-iNOS antibody; and M2 macrophages were
detected by green fluorescence staining with an anti-CD206
antibody. Compared with the sham group, the number of M1
macrophages was markedly increased, whereas the number of M2
macrophages was markedly decreased in IR group. Conversely, M1 and
M2 macrophages in the IR + ATO group were markedly decreased and
increased, respectively, compared with the IR group (Fig. 5). These results indicate that ATO
may promote the M1 to M2 transition in rats with IRI.
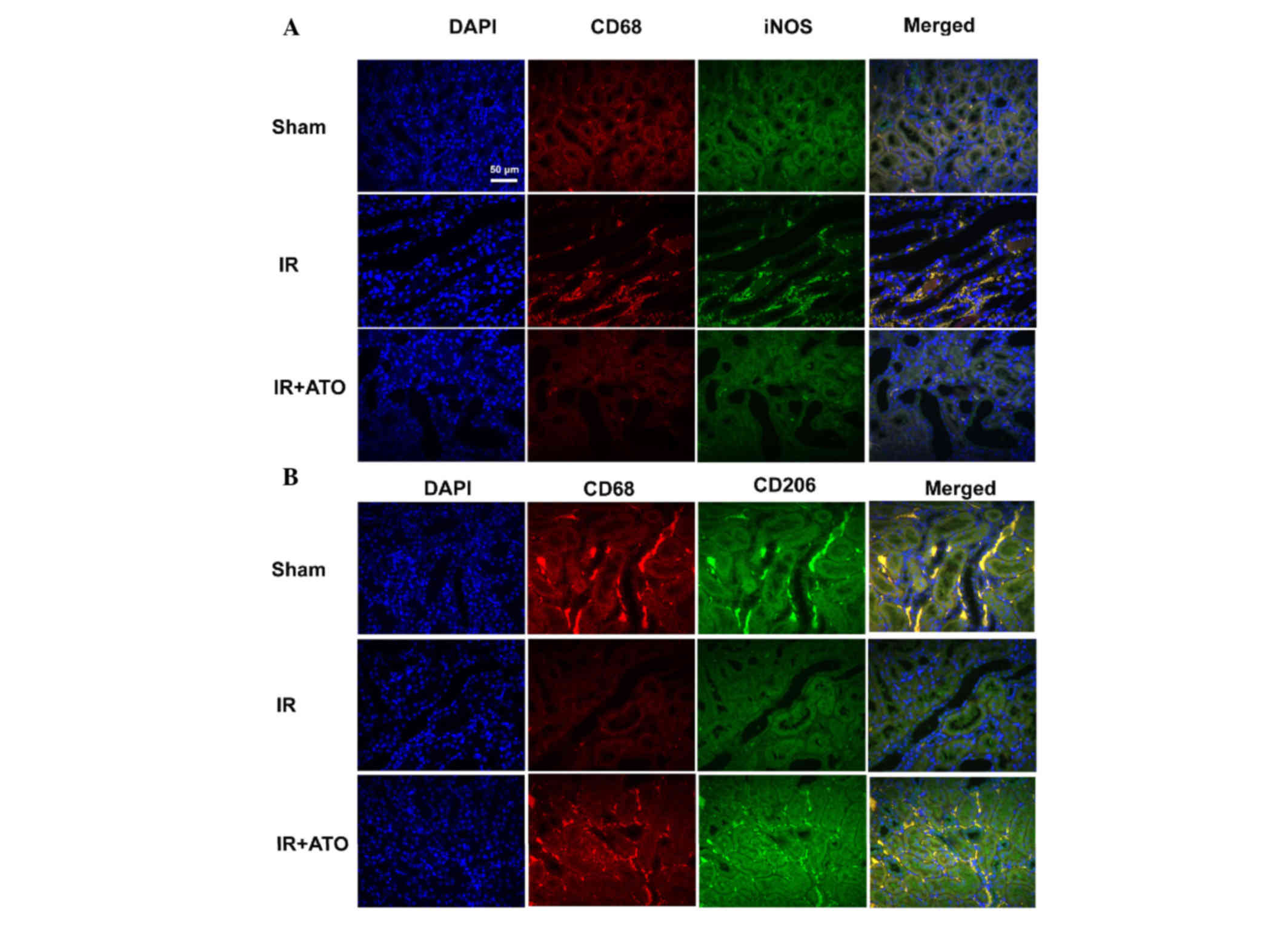 | Figure 5.Representative confocal images of
indirect double immunofluorescence staining in kidney samples from
rats with IR. (A) Double immunofluorescence staining with CD68 and
iNOS in the sham, IR and IR + ATO groups. DAPI (blue), CD68 (red),
iNOS (green), merged (merged DAPI, CD68 and iNOS). (B) Double
immunofluorescence staining with CD68 and CD208 in the sham, IR and
IR + ATO groups. DAPI (blue), CD68 (red), CD208 (green), merged
(merged DAPI, CD68 and CD208). Scale bar, 50 µm. IR,
ischemia-reperfusion; ATO, atorvastatin; CD, cluster of
differentiation; DAPI, 4′,6-diamidino-2-phenylindole. |
ATO treatment increases PPAR-γ
expression in renal macrophages
The effects of ATO on PPAR-γ expression in
macrophages were investigated. Using double immunofluorescence
staining, renal tissues were analyzed for PPAR-γ (green) and CD68
(red). As shown in Fig. 6,
nucleate cells in the renal tissues were labeled with DAPI.
Compared with the sham group, PPAR-γ expression was markedly
decreased in the IR group. Conversely, PPAR-γ expression in
macrophages was increased in the IR + ATO group compared with the
control group, and was markedly increased compared with the IR
group. These results suggest that ATO may induce PPAR-γ expression
in macrophages.
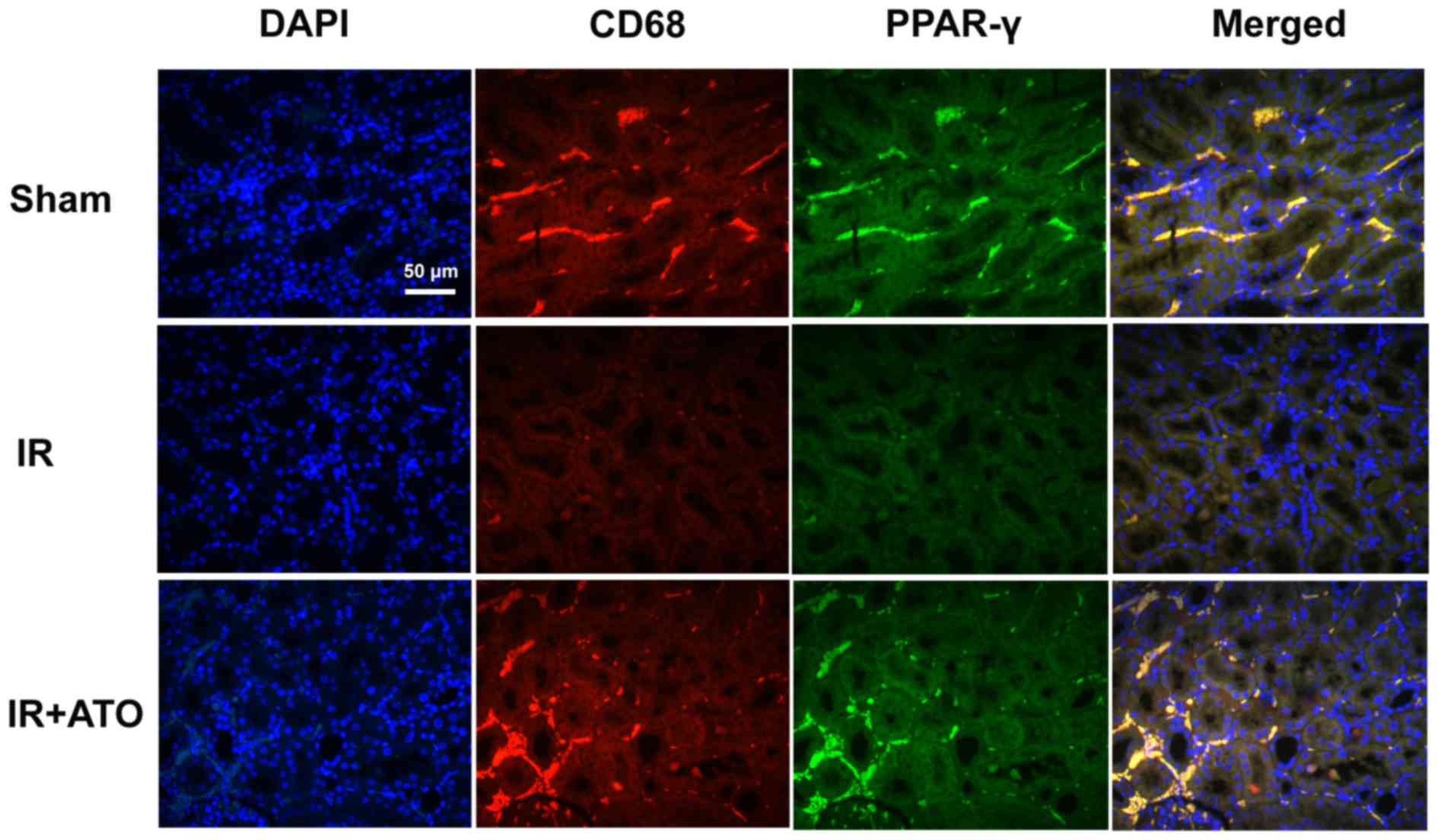 | Figure 6.Representative confocal images of CD68
and PPAR-γ double immunofluorescence staining in kidney samples
from rats with IR. Double immunofluorescence staining with CD68 and
PPAR-γ in the sham, IR and IR + ATO groups. DAPI (blue), CD68
(red), PPAR-γ (green), merged (merged DAPI, CD68 and PPAR-γ). Scale
bar, 50 µm. IR, ischemia-reperfusion; ATO, atorvastatin; CD,
cluster of differentiation; DAPI, 4′,6-diamidino-2-phenylindole;
PPAR-γ, peroxisome proliferator-activated receptor-γ. |
Discussion
There is an urgent requirement for the
identification of therapeutic strategies that protect the kidney
from IRI during surgery or transplantation. The present study
confirmed that treatment with ATO could attenuate renal IRI.
Furthermore, the results of the present study demonstrated that ATO
treatment induced PPAR-γ expression and activation in rat kidney
samples, leading to the differentiation of monocytes into M2
macrophages, which may functionally contribute to the protection of
the kidney from IRI.
The pathophysiology of renal IRI is complex;
however, inflammation is a well known associated factor. As a type
of statin, ATO has been reported to exert protective effects on
injuries associated with inflammation, such as hepatic IRI and
chronic obstructive pulmonary disease (14,19).
The results of the present study revealed that ATO treatment
significantly attenuated renal IRI; this finding is consistent with
the results of a previous report (15). Furthermore, treatment with ATO
significantly decreased IR-induced serum TNF-α and IFN-γ levels. It
has previously been demonstrated that IFN-γ, which is associated
with macrophage activation and neutrophil recruitment, may amplify
the immune response following kidney reperfusion, mediating the
early phase of IRI (2,20). Meldrum et al (21) reported that peak serum TNF-α
expression occurred after 1 h of ischemia and 1 h of reperfusion,
and early kidney TNF-α expression is an important mediator of renal
IRI (22). These findings
suggested that ATO may protect kidneys from IRI by decreasing the
levels of TNF-α and IFN-γ.
Macrophages are an essential component of innate
immunity, which have been reported to be critical in the
pathogenesis of human renal disease in the glomerulus and renal
interstitium (23). A previous
study demonstrated that ablation of renal macrophages can improve
renal injury in certain models, including IRI, glomerulonephritis
and fibrosis (24). It is well
known that the macrophage phenotype is regulated by various
inflammatory cytokines. M1 macrophage polarization can be induced
by T helper (Th) 1 cytokines, including TNF-α and IFN-γ, whereas M2
macrophages can be induced by Th2 cytokines, including interleukin
(IL)-4 and IL-10. During experimental AKI, there is a spontaneous
switch from the M1 to the M2 phenotype (25). Lee et al (26) reported that the proinflammatory M1
phenotype contributes toward IRI early in AKI, whereas the
anti-inflammatory M2 phenotype ameliorates injury and stimulates
repair. The present study demonstrated that ATO significantly
promoted monocyte differentiation toward M2 macrophages and
significantly suppressed M1 polarization. These findings suggested
that ATO may suppress M1 polarization by inhibiting the inductive
effects of TNF-α and IFN-γ on monocytes.
As a member of the nuclear hormone receptor family
of ligand-dependent transcription factors, PPAR-γ has been well
characterized as a potent anti-inflammatory factor that modulates
the immune inflammatory response (16). Initially described as a master
regulator of adipocyte differentiation, PPAR-γ has been
demonstrated to be highly expressed in macrophages and to
functionally contribute to the differentiation of macrophages into
M2 macrophages in vitro and in vivo (27–29).
Consistent with a previous report that ATO promotes PPAR-γ
expression in human monocytes (30), the present results indicated that
in kidney samples collected following IR, ATO significantly
increased PPAR-γ expression levels. All these findings indicated
that the contributory effects of ATO on M2 differentiation may be
mediated by PPAR-γ.
In conclusion, the present study demonstrated that
by promoting M1-M2 differentiation ATO significantly ameliorated
AKI after IR. In addition, the findings of the present study
suggested that this effect is likely mediated by an increase in
PPAR-γ expression and a decrease in TNF-α and IFN-γ levels.
Although the exact underlying mechanism requires further study, the
results of the present study suggested that endogenous and
exogenous factors, which regulate macrophage phenotype, may affect
the occurrence and development of kidney injuries.
Acknowledgements
The present study was supported by the Guangzhou
Medical Key Subject Construction Project (2013–2015; grant no.
XM203190).
References
|
1
|
Devarajan P: Emerging biomarkers of acute
kidney injury. Contrib Nephrol. 156:203–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kinsey GR, Li L and Okusa MD: Inflammation
in acute kidney injury. Nephron Exp Nephrol. 109:e102–e107. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng J, Kohda Y, Chiao H, Wang Y, Hu X,
Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S and Star RA:
Interleukin-10 inhibits ischemic and cisplatin-induced acute renal
injury. Kidney Int. 60:2118–2128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kunzendorf U, Haase M, Rolver L and
Haase-Fielitz A: Novel aspects of pharmacological therapies for
acute renal failure. Drugs. 70:1099–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geissmann F, Manz MG, Jung S, Sieweke MH,
Merad M and Ley K: Development of monocytes, macrophages, and
dendritic cells. Science. 327:656–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ginhoux F and Jung S: Monocytes and
macrophages: Developmental pathways and tissue homeostasis. Nat Rev
Immunol. 14:392–404. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palmer MB, Vichot AA, Cantley LG and
Moeckel GW: Quantification and localization of M2 macrophages in
human kidneys with acute tubular injury. Int J Nephrol Renovasc
Dis. 7:415–419. 2014.PubMed/NCBI
|
|
8
|
Wang Y and Harris DC: Macrophages in renal
disease. J Am Soc Nephrol. 22:21–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunter MM, Wang A, Parhar KS, Johnston MJ,
Van Rooijen N, Beck PL and McKay DM: In vitro-derived alternatively
activated macrophages reduce colonic inflammation in mice.
Gastroenterology. 138:1395–1405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naruszewicz M: Macrophages in the
pathogenesis of atherosclerosis. Advances in science and personal
observations. Kardiol Pol. 32:27–34. 1989.(In Polish). PubMed/NCBI
|
|
11
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mroz RM, Lisowski P, Tycinska A, Bierla J,
Trzeciak PZ, Minarowski L, Milewski R, Lisowska A, Boros P,
Sobkowicz B, et al: Anti-inflammatory effects of atorvastatin
treatment in chronic obstructive pulmonary disease. A controlled
pilot study. J Physiol Pharmacol. 66:111–128. 2015.PubMed/NCBI
|
|
15
|
Wu K, Lei W, Tian J and Li H: Atorvastatin
treatment attenuates renal injury in an experimental model of
ischemia-reperfusion in rats. BMC Nephrol. 15:142014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang O and Zhang J: Atorvastatin promotes
human monocyte differentiation toward alternative M2 macrophages
through p38 mitogen-activated protein kinase-dependent peroxisome
proliferator-activated receptor γ activation. Int Immunopharmacol.
26:58–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paller MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Invest. 74:1156–1164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue W, Lei J, Li X and Zhang R: Trigonella
foenum graecum seed extract protects kidney function and morphology
in diabetic rats via its antioxidant activity. Nutr Res.
31:555–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghobadi H, Lari SM, Pourfarzi F,
Mahmoudpour A and Ghanei M: The effects of atorvastatin on
mustard-gas-exposed patients with chronic obstructive pulmonary
disease: A randomized controlled trial. J Res Med Sci. 19:99–105.
2014.PubMed/NCBI
|
|
20
|
Dong X, Swaminathan S, Bachman LA, Croatt
AJ, Nath KA and Griffin MD: Resident dendritic cells are the
predominant TNF-secreting cell in early renal ischemia-reperfusion
injury. Kidney Int. 71:619–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meldrum KK, Meldrum DR, Meng X, Ao L and
Harken AH: TNF-alpha-dependent bilateral renal injury is induced by
unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ
Physiol. 282:H540–H546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donnahoo KK, Meng X, Ayala A, Cain MP,
Harken AH and Meldrum DR: Early kidney TNF-alpha expression
mediates neutrophil infiltration and injury after renal
ischemia-reperfusion. Am J Physiol. 277:R922–R929. 1999.PubMed/NCBI
|
|
23
|
Kluth DC, Erwig LP and Rees AJ: Multiple
facets of macrophages in renal injury. Kidney Int. 66:542–557.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferenbach DA, Ramdas V, Spencer N, Marson
L, Anegon I, Hughes J and Kluth DC: Macrophages expressing heme
oxygenase-1 improve renal function in ischemia/reperfusion injury.
Mol Ther. 18:1706–1713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westenfelder C: Programmed
anti-inflammatory macrophages protect against AKI and promote
repair through trophic actions. Kidney Int. 81:939–941. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee S, Huen S, Nishio H, Nishio S, Lee HK,
Choi BS, Ruhrberg C and Cantley LG: Distinct macrophage phenotypes
contribute to kidney injury and repair. J Am Soc Nephrol.
22:317–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Penas F, Mirkin GA, Vera M, Cevey Á,
González CD, Gómez MI, Sales ME and Goren NB: Treatment in vitro
with PPARα and PPARα ligands drives M1-to-M2 polarization of
macrophages from T. cruzi-infected mice. Biochim Biophys Acta.
1852:893–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pisanu A, Lecca D, Mulas G, Wardas J,
Simbula G, Spiga S and Carta AR: Dynamic changes in pro- and
anti-inflammatory cytokines in microglia after PPAR-α agonist
neuroprotective treatment in the MPTPp mouse model of progressive
Parkinson's disease. Neurobiol Dis. 71:280–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grip O, Janciauskiene S and Lindgren S:
Atorvastatin activates PPAR-gamma and the inflammatory response in
human monocytes. Inflamm Res. 51:58–62. 2002. View Article : Google Scholar : PubMed/NCBI
|