Introduction
Hypertension-associated left ventricular hypertrophy
(LVH) is considered to be an independent risk factor of adverse
cardiovascular events, including arrhythmia, myocardial infarction,
congestive heart failure, stroke and kidney failure (1–6). In
spontaneously hypertensive rats (SHRs), prehypertensive treatment
with losartan, a renin-angiotensin system (RAS) inhibitor, may
prevent the development of LVH in the long term. By contrast, the
calcium-channel blocker, amlodipine, another class of
antihypertensive drug, fails to maintain the beneficial effects
following the withdrawal of treatment. Beyond prolongation of the
blood pressure-lowering effect, a decrease in the levels of certain
RAS components in the myocardium, including angiotensin II (Ang II)
and Ang II type 1 receptor (AT1R), may explain the inhibition of
LVH induced by early losartan treatment (7). It is well established that RAS
comprises a number of components, and the various components
collaborate to regulate homeostasis and pathogenesis of the
cardiovascular system (8).
Therefore, it is reasonable to hypothesize that changes occur in
the other components of RAS in the myocardium of losartan-treated
SHRs.
AT1R-associated protein (ATRAP/Agtrap), a
newly identified component of RAS, specifically binds to AT1R,
promotes internalization of the receptor, and downregulates the
AT1R-mediated signaling pathway (9). Overexpression of ATRAP attenuates
cardiomyocyte hypertrophy in vitro (10), reduces oxidative stress and
inflammation in local tissue in vivo (11,12),
and inhibits LVH in chronic Ang II-infused mice (13). Furthermore, an imbalance of AT1R
and ATRAP in the myocardium is associated with the development of
hypertension-associated LVH (14).
However, there are few previously published studies on ATRAP
expression in the myocardium of prehypertensive treated SHRs
(15).
In the present study, prehypertensive SHRs were
treated with losartan or amlodipine. In addition to LVM/BW, cardiac
fibrosis and structure, the expression of ATRAP was observed in the
myocardium of adult rats. It is well known that the expression of
genes may be regulated by genomic variation or epigenetic
modification. The latter includes DNA methylation. Therefore, an
aim of the present study was to measure methylation of the
Agtrap promoter in the myocardium of treated SHRs.
Materials and methods
Animals and pharmacological
treatment
All animal procedures were approved by the
Institutional Animal Care Committee of the First Affiliated
Hospital of Fujian Medical University, China (approval no. 001).
Rats were purchased from Vital River Laboratory Animal Technology
(Beijing, China), and housed (5 rats/cage) at a constant ambient
temperature of 22–24°C and a humidity of 40–60%, and were exposed
to a 12-h light/dark cycle. All the rats were fed standard rat chow
and tap water ad libitum. A total of 72 4-week-old male SHRs
were randomly divided into three groups, and were administered
saline (2 ml/kg per day; the SHR group; n=24), amlodipine (Aml) [10
mg/kg/day; purchased from Pfizer, Inc. (New York, NY, USA); the
SHR-Aml group; n=24], losartan [20 mg/kg per day; purchased from
Merck Sharp & Dohme (Hangzhou, China); the SHR-Los group; n=24]
by gavage for 6 weeks. Wistar Kyoto rats (WKYs), treated with an
equal volume of saline, were used as the control group (WKY; n=24).
At 14, 20 and 32 weeks of age, eight rats in each group were
anesthetized with chloral hydrate (300 mg/100 g, administered
intraperitoneally), and underwent echocardiography prior to
sacrifice.
Measurement of blood pressure and
evaluation of LVH
Systolic blood pressure (SBP) of the conscious rats
was measured using the tail-cuff method (Softron BP-98A; SoftronTM,
Beijing, China) as previously described (16), and was recorded as the mean of
three consecutive readings. Body weight (BW) was measured using a
balance prior to sacrifice. The chests of the rats were opened,
their hearts were removed and blotted dry following sacrifice. The
left ventricular mass (LVM), including the ventricular septum, was
weighed, and LVM/BW was calculated to determine cardiac
hypertrophy.
Determination of cardiac fibrosis
Cardiac fibrosis was measured as previously
described in our laboratory (17).
Briefly, the hearts were immersed into 4% formalin for 24 h,
embedded in paraffin and cut into 4-µm sections for Sirius Red
staining. Sections were observed under a light microscope (CX31;
Olympus Corp., Tokyo, Japan). Images were analyzed using Image Pro
Plus 6.0 software (Media Cybernetics. Inc., Rockville, MD, USA).
The collagen volume fraction (CVF) in the ventricular septum, an
index of cardiac fibrosis, was determined as the percentage of the
Sirius Red-stained area/total ventricular septum area.
Echocardiography for cardiac structure
and function
Rats were anesthetized as described above, and
transthoracic echocardiography was subsequently performed in the
left lateral decubitus position. An M-mode echocardiogram was
obtained from the long-axis view of the left ventricle (LV) using a
vivid 7 echocardiographic system (GE Healthcare, Piscataway, NJ,
USA) with a 10 MHz probe. The left ventricular end-diastolic
dimension (LVEDD), end-diastolic interventricular septum thickness
(IVSTd) and left ventricular ejection fraction (LVEF) were measured
according to the guidelines of the American Society of
Echocardiography (18).
Real-time quantitative reverse
transcription polymerase chain reaction (RT-qPCR) for ATRAP
mRNA
Total RNA from the LV tissue was extracted with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. Total
RNA (2 µg) was reverse-transcribed into complementary DNA (cDNA)
using a RevertAid First Strand cDNA synthesis kit (Thermo Fisher
Scientific, Inc.). RT-qPCR was performed using SYBR Green PCR
Master mix (Takara Bio, Inc., Otsu, Japan) and detected using an
ABI Prism7000 sequence detection system (Applied Biosystems Life
Technologies, Foster City, CA, USA). Thermocycler conditions
consisted of an initial activation step at 95°C (30 min), followed
by a three-step PCR program of 95°C (for 10 sec), 60°C (for 31 sec)
and 72°C (30 sec) for 40 cycles for primer denaturation, annealing
and extension, respectively. The mRNA level was quantified by
calculating the values of the Δ cycle threshold (ΔCq) by
normalizing the average Cq value compared with its
internal control (Gapdh), and then calculating
2-ΔΔCq (19). The
primer sequences were as follows: Agtrap:
5′-ACTCTGTTGATGCCATTG-3′ (forward); 5′-GAAGATGTAATGTAGATAATGTC-3′
(reverse); Gapdh: 5′-CCTGCACCACCAACTGCTTA-3′ (forward),
5′-AGTGATGGCATGGACTGTGG-3′ (reverse).
Western blot analysis for the
expression of ATRAP protein
Western blotting was performed as previously
described in our laboratory, although with minor modifications
(20). Briefly, the total protein
in LV was extracted, and the concentrations were determined using a
bicinchoninic acid protein concentration determination kit
(Beyotime Institute of Biotechnology, Beijing, China). Aliquots of
80 µg protein lysates extracted from the hearts were separated
using 10% SDS-PAGE gels, electro-transferred onto polyvinylidene
fluoride membranes, blocked with 5% non-fat milk in TBS containing
0.05% Tween-20 (TBST), and the protein was probed with anti-ATRAP
antibody (1:200; cat. no. sc134652; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) or anti-β-actin antibody (1:1,000; cat. no.
sc1616; Santa Cruz Biotechnology, Inc) overnight at 4°C. After 3
washes with TBST for 5 min, the membranes were incubated for 1 h at
room temperature with horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. nos. ZB-2301; ZB-2305; OriGene
Technologies, Inc., Beijing, China). Following another 3 washes
with TBST for 10 min, protein bands were visualized using
electrochemiluminescence on high-performance chemiluminescence film
(Lulong Biotech Co., Ltd., Xiamen, China), and were quantified
using Image Pro Plus version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
CpG island analysis of the Agtrap
promoter region
CpG islands in the Agtrap promoter region
were analyzed using the online tool, Methprimer (http://www.urogene.org/methprimer/index.html) with
parameters window100, shift1, observed CpG/expected CpG≥0.60 and
GC%≥50.0%.
Bisulfite-specific polymerase chain
reaction (BSP-PCR) and DNA sequencing for methylation of the Agtrap
promoter
Genomic DNA in LV was extracted using a GenElute
Mammalian Genomic DNA mini-Prep kit (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) following the manufacturer's
protocol, and was subsequently subjected to treatment with the
EpiTect Bisulfite Kit (Qiagen, Hilden, Germany). The
bisulfite-modified DNA was amplified by PCR using BSP-specific
primer pairs [GeneTech (Shanghai) Co., Ltd., Shanghai, China] under
the following conditions: 95°C for 3 min; followed by 40 cycles of
95°C for 15 sec, 54°C for 30 sec, 72°C for 30 sec; and 72°C for 5
min. The PCR products were sequenced using a PyroMark ID sequencer
(Biotage AB, Uppsala, Sweden) according to the manufacturer's
protocol. The percentage of methylated CpG at each site was
quantified using the Pyro Q-CpG software, version 1.0.9 (Biotage
AB). The primers for BSP and DNA sequencing were as follows:
Agtrap: 5′-TGGTAGAGGTTTAGGTAGTAGTAGGAGT-3′ (forward);
5′-biotin-CCAACTCCAAAACAAACTTCCT-3′. (reverse); and
5′-GTTTTGTAGTAAGGGTAAT-3′ (sequencing).
Statistical analysis
Statistical analysis was performed using SPSS
version, 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous data are
presented as the means ± standard deviation. Differences between
groups were compared by one-way analysis of variance, followed by a
least significant difference t-test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
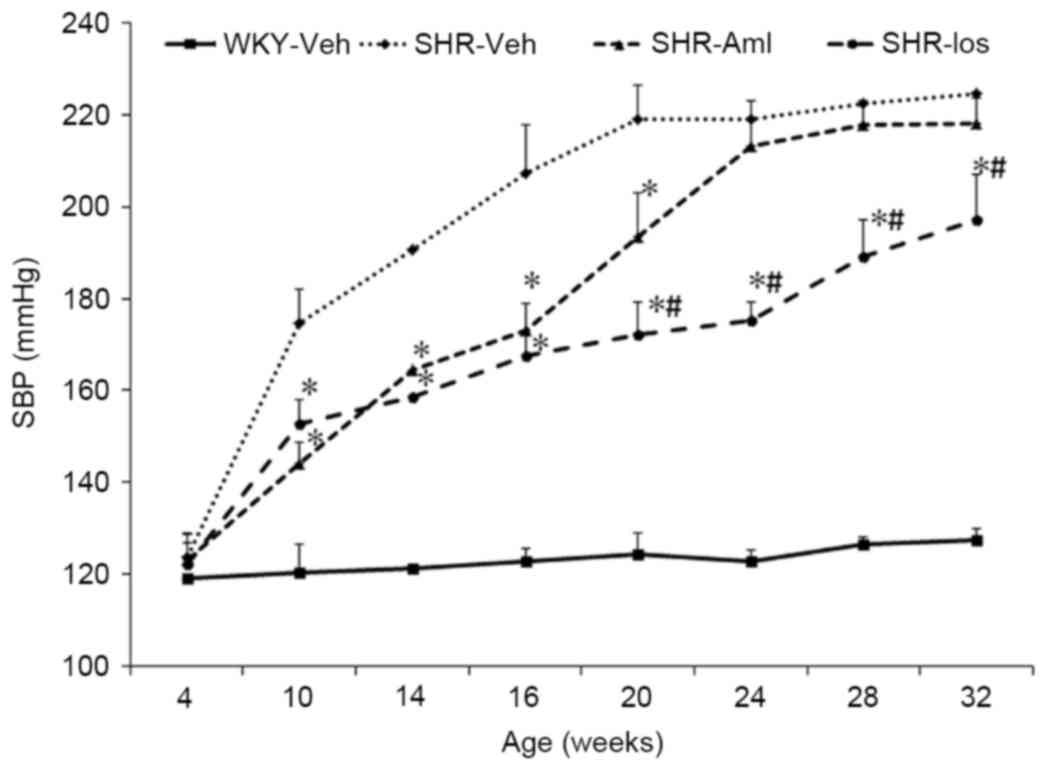
Prehypertensive losartan treatment
reduces SBP in adult SHRs
SBP in the SHR group increased with age, to a
maximum for the rats at 20 weeks of age, and this higher level was
maintained until the rats were 32 weeks old. However, there was no
change of SBP with age in the WKY group. In addition, SBP was lower
in the SHR-Aml and SHR-Los groups compared with SHR before the rats
reached the age of 20 weeks (for the 20-week-old rats in the three
groups: SHR-Aml, 193±16 mmHg and SHR-Los, 172±14 mmHg vs. SHR,
219±14 mmHg; P<0.001). From the age of 24 weeks onwards, SBP in
the SHR-Aml group increased to the level of the SHR group (32 weeks
old: SHR-Aml, 218±12 mmHg vs. SHR, 224±7 mmHg; P=0.281); however,
the rats treated with losartan had a significantly lower SBP until
they reached 32 weeks of age (32 weeks old: SHR-Los, 197±17 mmHg
vs. SHR, 224±7 mmHg; P<0.001) (Fig.
1).
Prehypertensive losartan treatment
inhibits LVH and cardiac fibrosis
LVM/BW (Fig. 2A)
and CVF (Fig. 2B) in the SHR group
were significantly higher compared with the age-matched WKY group
(LVM/BW at 32 weeks of age: SHR 3.38±0.12 mg/g vs. WKY 2.38±0.16
mg/g, P<0.001; CVF, SHR 9.64±0.95% vs. WKY 2.39±0.12%,
P<0.001), and a marked decrease was observed in SHR-Los compared
with the SHR group throughout the study period (LVM/BW at 32 weeks
of age: SHR-Los, 2.86±0.21 mg/g vs. SHR, 3.38±0.12 mg/g,
P<0.001; CVF, SHR-Los 5.76±0.99 % vs. SHR 9.64±0.95%,
P<0.001) (Fig. 2A and B).
LVM/BW in the SHR-Aml group was lower than SHR at 20 weeks of age
(SHR-Aml 2.85±0.20 mg/g vs. SHR 3.06±0.13 mg/g P=0.024); however,
there was no difference between the two groups at 14 and 32 weeks
of age (32 weeks old: SHR-Aml 3.35±0.23 mg/g vs. SHR 3.38±0.12
mg/g: P=0.690) (Fig. 2A). CVF in
the SHR-Aml group was comparable with that of the SHR group at the
same ages throughout the study period (32 weeks old: P=0.590)
(Fig. 2B).
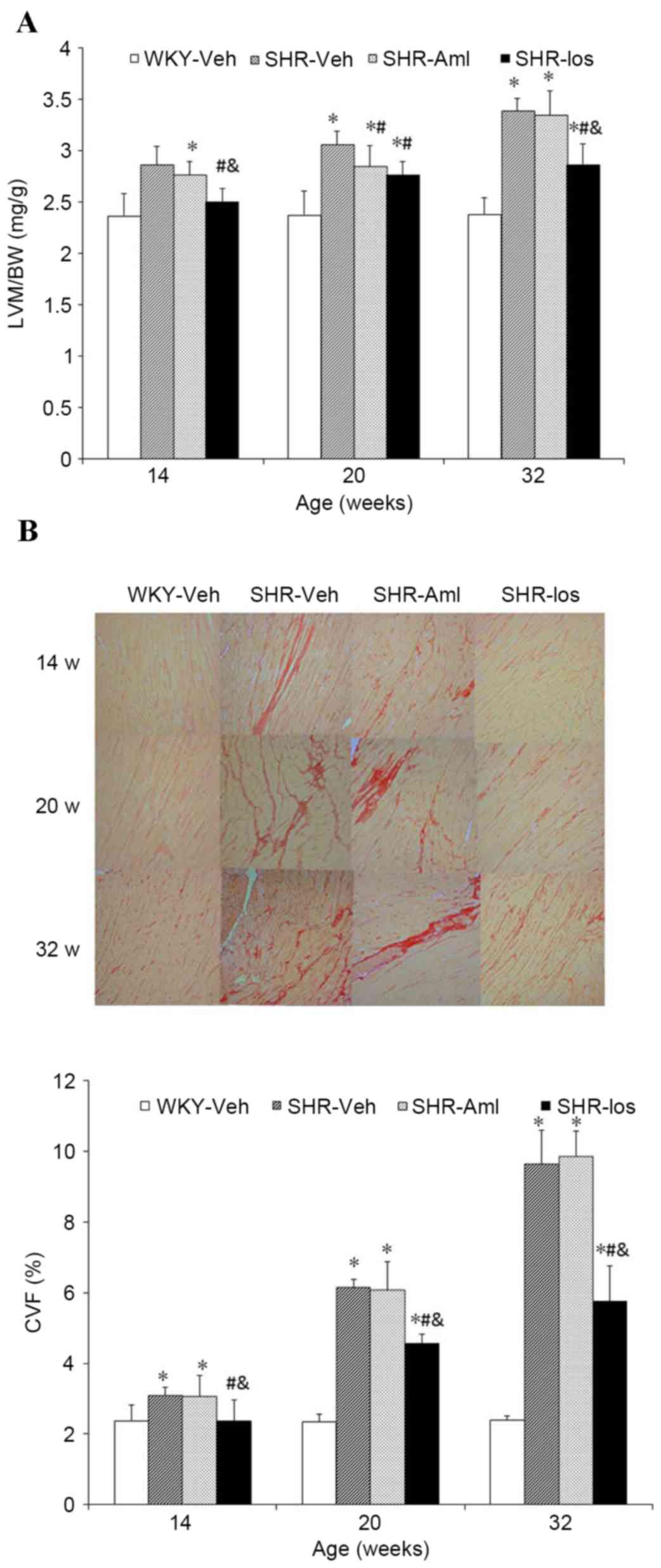 | Figure 2.Long-term effect of prehypertensive
losartan and amlodipine treatment on LVM/BW and CVF in the
myocardium of SHRs. (A) LVM/BW at the different ages. (B)
Representative pictures of myocardial collagen in ventricular
septum at different ages are shown (original magnification, ×10),
and CVF at the different ages. The sections of ventricular septum
were stained with Sirius Red. Myocardial interstitial collagen
appears in red, and cardiac myocytes in yellow. *P<0.05 vs.
age-matched WKY; #P<0.05 vs. age-matched SHR;
&P<0.05 vs. age-matched SHR-Aml. The data are
represented as the means ± standard deviation (n=8). WKY,
vehicle-treated Wistar Kyoto rats; SHRs, spontaneously hypertensive
rats; SHR, vehicle-treated SHRs; SHR-Aml, amlodipine-treated SHRs;
SHR-Los, losartan-treated SHRs; LVM/BW, left ventricle mass/body
weight; CVF, collagen volume fraction. |
The effect of prehypertensive losartan
treatment on cardiac structure and function
The data obtained are shown in Table I. From 10 to 32 weeks old, IVSTd in
the SHR group was greater than in WKY (32 weeks old: SHR 2.56±0.19
mm vs. WKY 1.81±0.20 mm P<0.001), and comparable with SHR-Aml
(32 weeks old: SHR 2.56±0.19 mm vs. SHR-Aml 2.55±0.27 mm, P=0.939);
however, IVSTd was decreased in the SHR-Los group compared with SHR
(32 weeks old: SHR-Los 1.84±0.24 mm vs. SHR 2.56±0.19 mm,
P<0.001). No significant difference was observed in LVEDD and
LVEF among the four groups of age-matched rats throughout the study
period (LVEDD at 32 weeks old: P=0.851; EF: P=0.988).
 | Table I.Echocardiographic evaluation of the
long-term effect of prehypertensive losartan and amlodipine
treatment on left ventricle structure and function in spontaneously
hypertensive rats. |
Table I.
Echocardiographic evaluation of the
long-term effect of prehypertensive losartan and amlodipine
treatment on left ventricle structure and function in spontaneously
hypertensive rats.
| Group | Age (weeks) | IVSTd (mm) | LVEDD (mm) | EF (%) |
|---|
| WKY | 10 | 1.54±0.10 | 7.0±0.6 | 84.2±2.1 |
|
| 20 | 1.76±0.17 | 7.3±0.7 | 84.3±2.6 |
|
| 32 | 1.81±0.20 | 7.3±0.4 | 83.2±1.9 |
| SHR | 10 |
2.32±0.19a | 6.9±0.8 | 81.2±3.1 |
|
| 20 |
2.54±0.17a | 6.9±0.6 | 80.7±2.6 |
|
| 32 |
2.56±0.19a | 7.1±0.9 | 82.7±2.9 |
| SHR-Aml | 10 |
2.30±0.31a | 6.9±0.9 | 82.2±3.1 |
|
| 20 |
2.45±0.38a | 7.1±0.9 | 79.9±4.6 |
|
| 32 |
2.55±0.27a | 7.4±0.5 | 82.7±2.9 |
| SHR-Los | 10 |
1.76±0.27a,b,c | 7.2±0.6 | 83.2±4.1 |
|
| 20 |
1.81±0.22b,c | 7.3±0.9 | 82.9±4.6 |
|
| 32 |
1.84±0.24b,c | 7.3±0.8 | 82.7±5.0 |
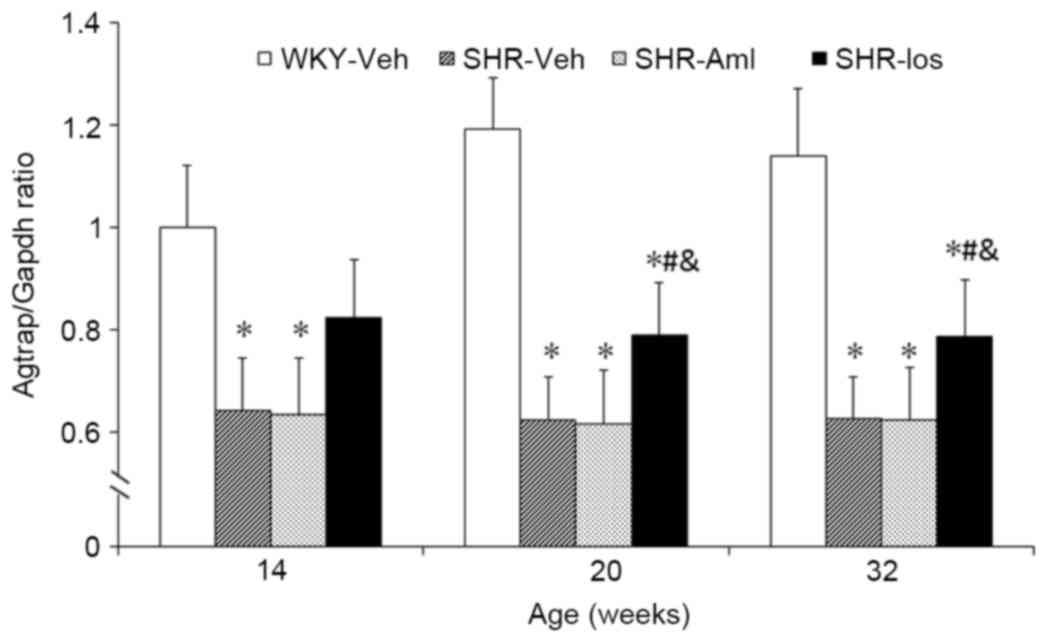
Prehypertensive losartan treatment
upregulates the levels of ATRAP mRNA and protein in the
myocardium
The mRNA level of ATRAP in the SHR group was
decreased compared with age-matched WKY throughout the study period
(32 weeks old: SHR 0.63±0.08 vs. WKY 1.18±0.17, P<0.001). The
expression level of mRNA in the SHR-Los group was elevated compared
with SHR (at 32 weeks of age: SHR-Los, 0.79±0.11 vs. SHR,
0.63±0.08; P=0.014) (Fig. 3). As
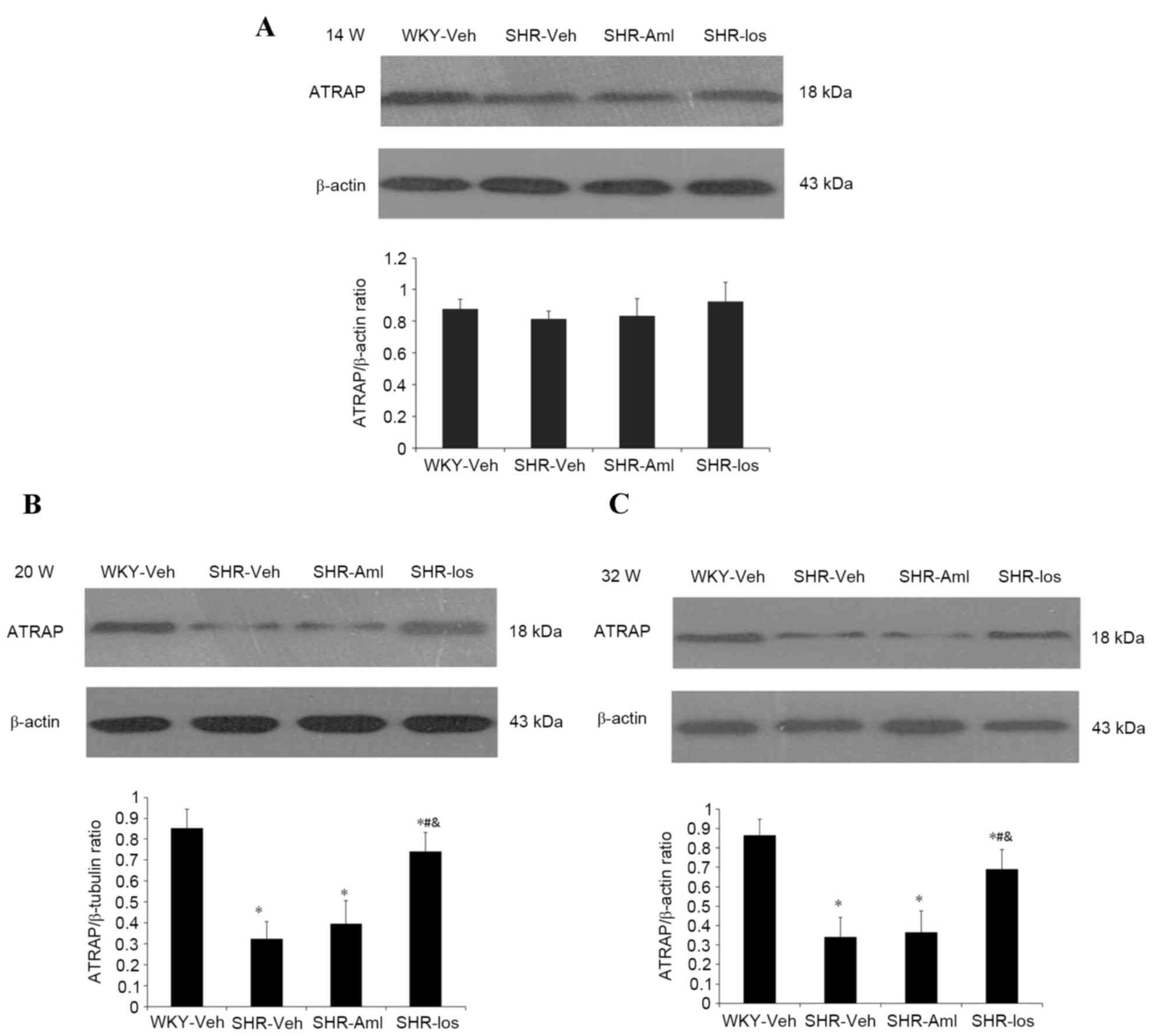
shown in Fig. 4, no significant
differences were observed in the protein level of ATRAP at 14 weeks
among the four groups of rats (P=0.057). However, at the ages of 20
weeks and 32 weeks, the protein level in the SHR group was
decreased compared with age-matched WKY (at 32 weeks of age: SHR
0.34±0.10 vs. WKY 1.04±0.10, P<0.001), and losartan-treated SHRs
demonstrated an increased protein level compared with the SHR group
(at 32 weeks of age: SHR-Los, 0.69±0.10 vs. SHR0.34±0.10;
P<0.001. No significant differences were observed in the
expression levels of ATRAP mRNA and protein between the SHR and
age-matched SHR-Aml groups throughout the study period (mRNA at 32
weeks of age: P=0.883; protein, P=0.638).
Prehypertensive losartan treatment
decreases the methylation of Agtrap promoter in the myocardium
One CpG island was identified in the vicinity of the
transcription start site (TSS) of Agtrap between the
upstream −132 bp and downstream +144 bp (Fig. 5A). As shown in Fig. 5B, changes in the methylation level
among the four groups of the rats were observed at the −120, −117,
−85 and −65 bp sites in the CpG islands, but not at the other CpG
sites (Fig. 5B). The methylation
level at the −120 bp site was higher in the SHR group than in WKY
throughout the study period (at 32 weeks old: SHR 17.6±2.3% vs. WKY
4.8±0.9% P<0.001), and although no significant differences were
observed between the SHR and SHR-Aml groups (at 32 weeks old: SHR
17.6±2.3% vs. SHR-Aml 16.4±2.0%, P=0.187), the level was reduced in
the age-matched SHR-Los group (at 32 weeks old: SHR 17.6±2.3% vs.
SHR-Los 6.4±1.5%, P<0.001) (Fig.
5C). Similar changes were identified at the −117, −85 and −65
bp sites; furthermore, the methylation levels at the three target
sites in the SHR-Los group were comparable with those of the
age-matched WKY group (−117, −85 and −65 bp at 32 weeks old:
P=0.247; Fig. 5D-F).
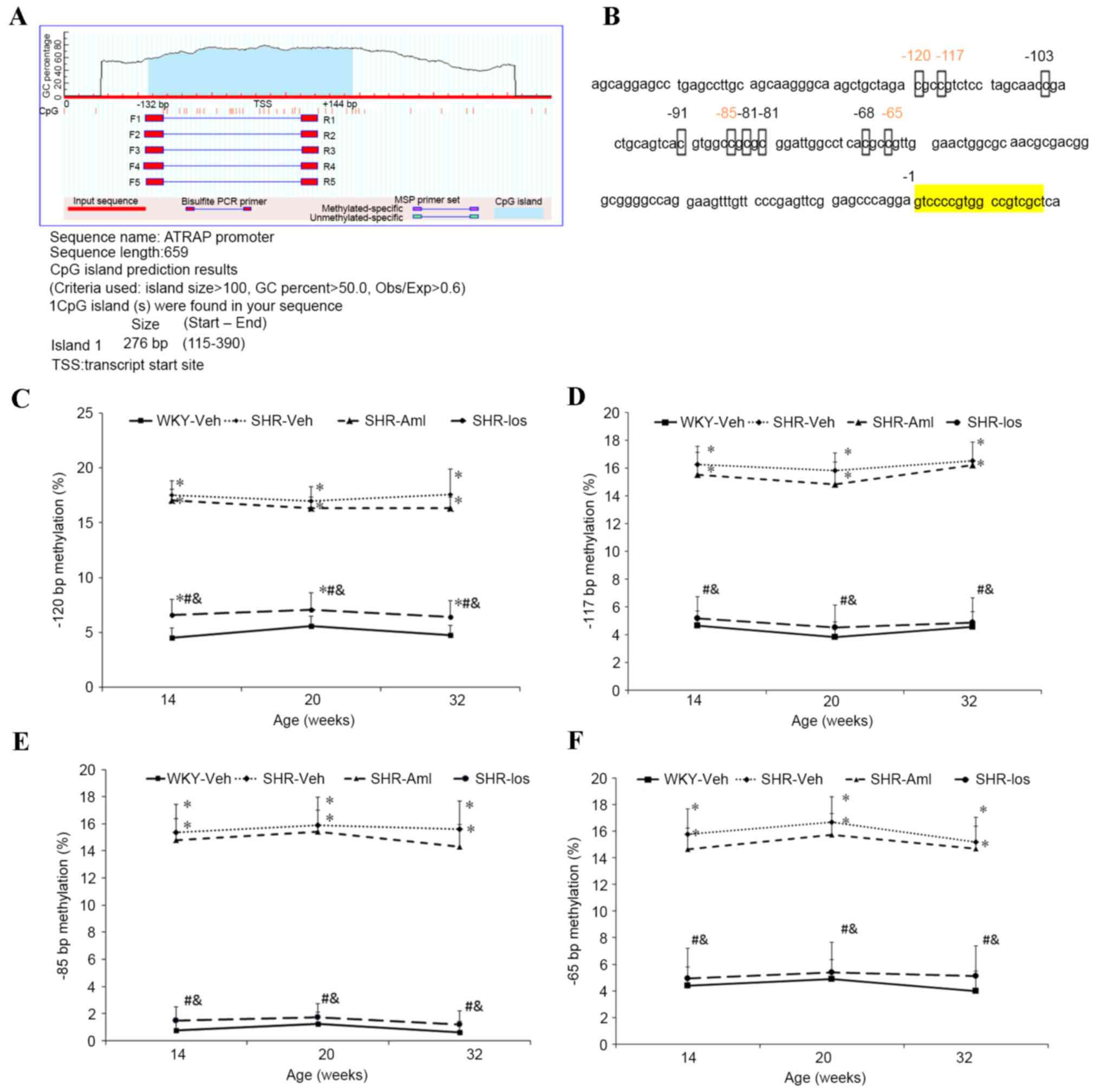 | Figure 5.Long-term effects of prehypertensive
losartan and amlodipine treatment on methylation of the
Agtrap promoter in the myocardium of SHRs. (A) Prediction
result of CpG islands in the Agtrap promoter: The blue zone
corresponds to CpG-pattern-rich regions, whereas the red vertical
lines correspond to positions of the CpG sites. (B) CpG sites in
the vicinity of the Agtrap transcription start site: Red
numbers represent altered CpG sites among the four groups of rats,
whereas the yellow zone represents the transcript start site (C-F)
Quantification of the methylation status of (C) −120 bp, (D) −117
bp, (E) −85 bp and (F) −65 bp CpG sites at the different ages. The
data are shown as the means ± standard deviation (n=8). *P<0.05
vs. age-matched WKY; #P<0.05 vs. age-matched SHR;
&P<0.05 vs. age-matched SHR-Aml. WKY,
vehicle-treated Wistar Kyoto rats; SHRs, spontaneously hypertensive
rats; SHR, vehicle-treated SHRs; SHR-Aml, amlodipine-treated SHRs;
SHR-Los, losartan-treated SHRs; Agtrap, angiotensin II type
1 receptor-associated protein gene. |
Discussion
In the present study, it has been demonstrated that
prehypertensive losartan treatment was more effective than
amlodipine in preventing the progression of LVH and cardiac
fibrosis in adult SHRs. It was also shown that the beneficial
effect of early losartan treatment was associated with the
upregulation of ATRAP expression and hypomethylation of the
Agtrap promoter in the myocardium.
Previous evidence has shown that prehypertensive
patients are more likely to progress to manifest hypertension than
patients with optimal or normal blood pressure. Certain
prehypertensive patients may even be at an increased risk of
mortality from cardiovascular disease (21,22).
However, it has yet to be determined whether early treatment is
able to prevent the pathogenesis of hypertension. The present study
has revealed that prehypertensive losartan treatment was able to
reduce SBP, LVM/BW and CVF in adult SHRs. The TRial Of
Preventing HYpertension (‘TROPHY’) and Prevention of hypertension
with the angiotensin-converting enzyme inhibitor, ramipril
(‘PHARAO’) trials demonstrated that prehypertensive treatment with
RAS inhibitor over a relatively short time period reduced the risk
of incident hypertension (23,24).
In addition, animal studies that featured early L-arginine and
antioxidant supplements or captopril demonstrated a delay in the
increase of blood pressure in adult SHRs (25–27).
Nevertheless, the administration of the antihypertensive drug,
amlodipine, or treatment with a β-blocker led to no effect on
long-term blood pressure in SHRs (28). These findings suggested that,
although prehypertensive treatment is an attractive alternative,
the use of proper medicines remains important.
In the present study, the upregulation of ATRAP
expression was revealed in losartan-treated SHRs, suggesting that
ATRAP may be partly responsible for the beneficial effects elicited
by early losartan treatment on LVH. It may be hypothesized that
ATRAP inhibited LVH through several potential pathways. One
possibility is that ATRAP downregulated p38 mitogen-activated
protein kinase (p38-MAPK) whereas p38-MAPK could mediate negative
inotropic action (29,30). Secondly, ATRAP may inhibit the
signal transducer and activator of transcription 3 (STAT3)
signaling pathway (29). It has
been reported that the STAT3 signaling pathway is associated with
an increase in microtubule stabilization, and a decrease in
contractility in the myocardium (31). Therefore, ATRAP may increase the
contractility of cardiomyocytes. Finally, it is also possible that
ATRAP blocks the calcineurin/nuclear factor of activated T cells
(NFAT) pathway (32) and,
consequently, gene expression associated with cardiomyocyte
hypertrophy is downregulated. Taken together, ATRAP may attenuate
compensatory LVH through different pathways.
LVH occurs due to the adaptation to pressure or
volume overload with an overactivation of protein turnover. Protein
balance is regulated by synthesis and degradation. Li et al
(29) demonstrated that the level
of ATRAP was controlled by proteasome degradation, whereas protein
synthesis may be regulated at the translational and transcriptional
levels, including via the process of methylation modification. It
was predicted that one CpG island would be located in the vicinity
of the TSS of the Agtrap promoter. Our data have further
demonstrated that methylation of the Agtrap promoter was
negatively correlated with the expression of ATRAP mRNA, suggesting
that ATRAP expression may be regulated by methylation modification.
A burgeoning body of evidence has shown that the methylation of
specific genes is involved in the pathogenesis of genetic
hypertension and associated target organ damage (33,34).
For example, Cho et al (35) revealed that methylation of the
Na+-K+ −2Cl− co-transporter 1 was
decreased in the aorta and heart of adult SHRs, and Pei et
al (36) demonstrated that
hypomethylation of ATRAP in the aorta and mesenteric artery of SHRs
was accompanied by increased blood pressure. Furthermore, Watson
et al demonstrated that the DNA methylation inhibitor,
5-azacytidine, was able to prevent the development of LVH in SHRs
(37). Therefore, these studies
suggested that prehypertensive losartan treatment reduces the DNA
methylation level of Agtrap, resulting in the upregulation
of ATRAP expression in the myocardium, which consequently leads to
an inhibition of the development of LVH in adult SHRs.
Methylation is catalyzed by DNA methyltransferases
(38), and it may be triggered by
oxidative stress (39). Further
studies are required to better understand the mechanism of the
hypomethylation of Agtrap.
In conclusion, the results presented in the current
study have demonstrated that hypomethylation of Agtrap in
the myocardium was associated with an increased expression of ATRAP
and an attenuation of LVH in prehypertensive losartan-treated SHRs,
providing a novel insight into the beneficial effects elicited by
an early RAS inhibitor.
Acknowledgements
This study was supported by a grant from a key
program of Fujian Medical University (no. XK201107). The authors
would like to thank Li Liu for her technical assistance in editing
this manuscript.
References
|
1
|
Taylor J: 2013 ESH/ESC guidelines for the
management of arterial hypertension. Eur Heart J. 34:2108–2109.
2013.PubMed/NCBI
|
|
2
|
Chatterjee S, Bavishi C, Sardar P, Agarwal
V, Krishnamoorthy P, Grodzicki T and Messerli FH: Meta-analysis of
left ventricular hypertrophy and sustained arrhythmias. Am J
Cardiol. 114:1049–1052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levy D, Garrison RJ, Savage DD, Kannel WB
and Castelli WP: Prognostic implications of echocardiographically
determined left ventricular mass in the Framingham Heart Study. N
Engl J Med. 322:1561–1566. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okin PM, Devereux RB, Nieminen MS, Jern S,
Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Julius S,
Snapinn S, et al: Electrocardiographic strain pattern and
prediction of cardiovascular morbidity and mortality in
hypertensive patients. Hypertension. 44:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Xue H, Zou Y, Sun K, Fu C, Wang H
and Hui R: Left ventricular hypertrophy, abnormal ventricular
geometry and relative wall thickness are associated with increased
risk of stroke in hypertensive patients among the Han Chinese.
Hypertens Res. 37:870–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charytan D: Is left ventricular
hypertrophy a modifiable risk factor in end-stage renal disease.
Curr Opin Nephrol Hypertens. 23:578–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng F, Lin J, Lin L and Tang H: Transient
prehypertensive treatment in spontaneously hypertensive rats: A
comparison of losartan and amlodipine regarding long-term blood
pressure, cardiac and renal protection. Int J Mol Med.
30:1376–1386. 2012.PubMed/NCBI
|
|
8
|
Horiuchi M, Iwanami J and Mogi M:
Regulation of angiotensin II receptors beyond the classical
pathway. Clin Sci (Lond). 123:193–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez-Ilasaca M, Liu X, Tamura K and Dzau
VJ: The angiotensin II type I receptor-associated protein, ATRAP,
is a transmembrane protein and a modulator of angiotensin II
signaling. Mol Biol Cell. 14:5038–5050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Tamura K, Koide Y, Sakai M,
Tsurumi Y, Noda Y, Umemura M, Ishigami T, Uchino K, Kimura K, et
al: The novel angiotensin II type 1 receptor (AT1R)-associated
protein ATRAP downregulates AT1R and ameliorates cardiomyocyte
hypertrophy. FEBS Lett. 579:1579–1586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakui H, Dejima T, Tamura K, Uneda K,
Azuma K, Maeda A, Ohsawa M, Kanaoka T, Azushima K and Kobayashi R:
Activation of angiotensin II type 1 receptor-associated protein
exerts an inhibitory effect on vascular hypertrophy and oxidative
stress in angiotensin II-mediated hypertension. Cardiovasc Res.
100:511–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maeda A, Tamura K, Wakui H, Dejima T,
Ohsawa M, Azushima K, Kanaoka T, Uneda K, Matsuda M, Yamashita A,
et al: Angiotensin receptor-binding protein ATRAP/Agtrap inhibits
metabolic dysfunction with visceral obesity. J Am Heart Assoc.
2:e0003122013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakui H, Tamura K, Tanaka Y, Matsuda M,
Bai Y, Dejima T, Masuda S, Shigenaga A, Maeda A, Mogi M, et al:
Cardiac-specific activation of angiotensin II type 1
receptor-associated protein completely suppresses cardiac
hypertrophy in chronic angiotensin II-infused mice. Hypertension.
55:1157–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shigenaga A, Tamura K, Wakui H, Masuda S,
Azuma K, Tsurumi-Ikeya Y, Ozawa M, Mogi M, Matsuda M, Uchino K, et
al: Effect of olmesartan on tissue expression balance between
angiotensin II receptor and its inhibitory binding molecule.
Hypertension. 52:672–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baumann M, Sollinger D, Roos M, Lutz J and
Heemann U: Prehypertensive preconditioning improves adult
antihypertensive and cardioprotective treatment. J Pharmacol Exp
Ther. 332:1121–1126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Widdop RE and Li XC: A simple versatile
method for measuring tail cuff systolic blood pressure in conscious
rats. Clin Sci (Lond). 93:191–194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao J, Xie XL, Xie LD, et al: The
influence of naoxintong on myocardial fibrosis in spontaneously
hypertensive rats. Chin J of Integ Med. 6:188–190. 2008.
|
|
18
|
Lang RM, Badano LP, Mor-Avi V, Afilalo J,
Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA,
Kuznetsova T, et al: Recommendations for cardiac chamber
quantification by echocardiography in adults: An update from the
American society of echocardiography and the European association
of cardiovascular imaging. Eur Heart J Cardiovasc Imaging.
16:233–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HF, Xie LD and Xu CS: Role of heat
shock protein 27 phosphorylation in migration of vascular smooth
muscle cells. Mol Cell Biochem. 327:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasan RS, Larson MG, Leip EP, Kannel WB
and Levy D: Assessment of frequency of progression to hypertension
in non-hypertensive participants in the Framingham Heart Study: A
cohort study. Lancet. 358:1682–1686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vasan RS, Larson MG, Leip EP, Evans JC,
O'Donnell CJ, Kannel WB and Levy D: Impact of high-normal blood
pressure on the risk of cardiovascular disease. N Engl J Med.
345:1291–1297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Julius S, Nesbitt SD, Egan BM, Weber MA,
Michelson EL, Kaciroti N, Black HR, Grimm RH Jr, Messerli FH,
Oparil S, et al: Feasibility of treating prehypertension with an
angiotensin-receptor blocker. N Engl J Med. 354:1685–1697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lüders S, Schrader J, Berger J, Unger T,
Zidek W, Böhm M, Middeke M, Motz W, Lübcke C, Gansz A, et al: The
PHARAO study: Prevention of hypertension with the
angiotensin-converting enzyme inhibitor ramipril in patients with
high-normal blood pressure: A prospective, randomized, controlled
prevention trial of the German Hypertension League. J Hypertens.
26:1487–1496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Racasan S, Braam B, van der Giezen DM,
Goldschmeding R, Boer P, Koomans HA and Joles JA: Perinatal
L-arginine and antioxidant supplements reduce adult blood pressure
in spontaneously hypertensive rats. Hypertension. 44:83–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrap SB, Nicolaci JA and Doyle AE:
Persistent effects on blood pressure and renal haemodynamics
following chronic angiotensin converting enzyme inhibition with
perindopril. Clin Exp Pharmacol Physiol. 13:753–765. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen DG, Jin XQ and Wang HJ: Mechanisms
responsible for sustained hypotension after captopril treatment. J
Hypertens. 13:1113–1121. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christensen KL, Jespersen LT and Mulvany
MJ: Development of blood pressure in spontaneously hypertensive
rats after withdrawal of long-term treatment related to vascular
structure. J Hypertens. 7:83–90. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Wang HX, Han QY, Li WJ, Zhang YL, Du
J, Xia YL and Li HH: Activation of the cardiac proteasome promotes
angiotension II-induced hypertrophy by down-regulation of ATRAP. J
Mol Cell Cardiol. 79:303–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vahebi S, Ota A, Li M, Warren CM, De Tombe
PP, Wang Y and Solaro RJ: p38-MAPK induced dephosphorylation of
alpha-tropomyosin is associated with depression of myocardial
sarcomeric tension and ATPase activity. Circ Res. 100:408–415.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ng DC, Ng IH, Yeap YY, Badrian B,
Tsoutsman T, McMullen JR, Semsarian C and Bogoyevitch MA: Opposing
actions of extracellular signal-regulated kinase (ERK) and signal
transducer and activator of transcription 3 (STAT3) in regulating
microtubule stabilization during cardiac hypertrophy. J Biol Chem.
286:1576–1587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Min LJ, Mogi M, Tamura K, Iwanami J,
Sakata A, Fujita T, Tsukuda K, Jing F, Iwai M and Horiuchi M:
Angiotensin II type 1 receptor-associated protein prevents vascular
smooth muscle cell senescence via inactivation of
calcineurin/nuclear factor of activated T cells pathway. J Mol Cell
Cardiol. 47:798–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao D, Dasgupta C, Li Y, Huang X and
Zhang L: Perinatal nicotine exposure increases angiotensin II
receptor-mediated vascular contractility in adult offspring. PLoS
one. 9:e1081612014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Friso S, Pizzolo F, Choi SW, Guarini P,
Castagna A, Ravagnani V, Carletto A, Pattini P, Corrocher R and
Olivieri O: Epigenetic control of 11 beta-hydroxysteroid
dehydrogenase 2 gene promoter is related to human hypertension.
Atherosclerosis. 199:323–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho HM, Lee HA, Kim HY, Han HS and Kim IK:
Expression of Na+-K+ −2Cl- cotransporter 1 is epigenetically
regulated during postnatal development of hypertension. Am J
Hypertens. 24:1286–1293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pei F, Wang X, Yue R, Chen C, Huang J,
Huang J, Li X and Zeng C: Differential expression and DNA
methylation of angiotensin type 1A receptors in vascular tissues
during genetic hypertension development. Mol Cell Biochem. 402:1–8.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watson CJ, Horgan S, Neary R, Glezeva N,
Tea I, Corrigan N, McDonald K, Ledwidge M and Baugh J: Epigenetic
therapy for the treatment of hypertension-induced cardiac
hypertrophy and fibrosis. J Cardiovasc Pharmacol Ther. 21:127–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Subramaniam D, Thombre R, Dhar A and Anant
S: DNA methyltransferases: A novel target for prevention and
therapy. Front Oncol. 4:802014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rexhaj E, Bloch J, Jayet PY, Rimoldi SF,
Dessen P, Mathieu C, Tolsa JF, Nicod P, Scherrer U and Sartori C:
Fetal programming of pulmonary vascular dysfunction in mice: Role
of epigenetic mechanisms. Am J Physiol Heart Circ Physiol.
301:H247–H252. 2011. View Article : Google Scholar : PubMed/NCBI
|