Introduction
Astrocytomas are the most common primary brain tumor
types, which, according to the criteria of the World Health
Organization (1), are classified
to grade I (pilocytic), grade II (diffuse), grade III (anaplastic)
and grade IV (glioblastoma multiforme; GBM) (2,3). GBM
is the most common central nervous system primary malignancy, which
accounts for 60–70% of all gliomas (4). It is known that GBM may develop de
novo or as the consequence of stepwise progression of low-grade
or anaplastic astrocytomas (5,6).
Multiple cancer-associated factors are known to be involved in the
formation and progression of astrocytomas (7–10),
of which activated signal transducer and activator of transcription
3 (STAT3) signaling serves pivotal roles in promoting the growth
and survival of GBMs by triggering multiple oncogenic signaling
cascades (10–12). STAT3 signaling thus emerges as a
key initiator and master regulator of malignant transformation of
glial cells (13), and the central
player in the maintenance and progression of glioblastomas
(14–16). Therefore, it would be of clinical
values to explore the underlying reason(s) leading to STAT3
activation in stepwise carcinogenesis of GBMs.
It has been recognized that STAT3 signaling
transduction can be activated by numerous factors, including
extracellular cytokines, growth factors, hormones and oncoproteins
(17,18). On the other hand, the data obtained
from human and mouse cell lines reveal that the phosphorylation of
STAT3 can be negatively regulated in different manners by a group
of suppressors, including protein inhibitors of activated stats
(PIAS), suppressors of cytokine signaling proteins (SOCS) and SH2
containing tyrosine phosphatase (SHP1 and SHP2) cascades (19–24).
For example, inhibition of PIAS3 results in enhanced proliferation
of glioblastoma cells and PIAS3 overexpression inhibits STAT3
transcriptional activity (25).
However, no comprehensive in vivo data has been available
concerning the expression patterns of those STAT3 negative
regulators and their relevance with STAT3 activation in different
grades of astrocytomas. The present study aims to address the
aforementioned issues.
Materials and methods
Glioblastoma specimens and
tissue-microarray construction
The archived paraffin tissue blocks of 105 cases of
astrocytomas surgical specimens were kindly provided by the doctors
at the Department of Clinical Pathology, Anshan Central Hospital
(Anshan, China). Prior to experiments, hematoxylin and eosin
staining was performed on the sections of those tissue blocks for
morphological re-examination. The representative tumor and, where
possible, tumor surrounding non-cancerous regions in each of the
tissue blocks were determined and marked during the re-examination.
These marked samples were used for glioblastoma tissue microarray
construction, as previously described (26).
Antibodies and their working
concentration
The antibodies used for immunohistochemical staining
are as follows: Rabbit anti-human p-STAT3 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA; cat. no. sc-135649;
1:200), rabbit anti-human PIAS3 polyclonal antibody (Bioworld
Technology, Inc., St. Louis Park, MN, USA; cat. no. BS1467; 1:200),
rabbit anti-human SOCS1 polyclonal antibody (Santa Cruz
Biotechnology, Inc.; cat. no. SC9021; 1:180), rabbit anti-human
SOCS3 polyclonal antibody (Santa Cruz Biotechnology, Inc.; cat. no.
sc-9023; 1:180), rabbit anti-human p-SHP2 polyclonal antibody
(Sangon Biotech Co., Ltd., Shanghai, China; cat. no. D155149;
1:150). A DAB Detection kit (streptavidin-biotin; ZSGB-BIO,
Beijing, China; cat. no. SP-9000) was used for protein
detection.
Immunohistochemical staining
The astrocytomas tissue microarrays in the densities
of 56 tissue spots/cm2 were constructed and subsequently
sectioned in series. The 7-µm thick tissue sections were
respectively used for p-STAT3, SOCS1, SOCS3, PIAS3 and p-SHP2
oriented immunohistochemical staining, as described previously
(27). Briefly, the tissue
sections were washed with PBS and incubated in non-immune animal
serum working solution blocking buffer for 20 min at 37°C. The
primary antibody was applied to the tissue sections overnight at
4°C. Following three washes with PBS, the tissue sections were
incubated with a goat anti-rabbit biotin-labeling generic secondary
antibody (DAB Detection kit) for 20 min at 37°C. Horseradish
peroxidase-labeled streptavidin solution was applied (1:500) to the
slides for 20 min at 37°C. 3,3′-Diaminobenzidine (DAB)-staining was
detected using a DAB Detection kit, according to the manufacturer's
protocol. The array sections without primary antibody incubation
were used as background controls. Based on the labeling density,
two independent researchers, in a blinded manner, evaluated the
staining results and scored them as negative (−), weak (+),
moderate (++) or strong positive (+++) (28).
Statistical analysis
Non-parametric Mann-Whitney tests were applied to
analyze the expression differences between different grade
astrocytoma tissues and non-cancerous brain samples surrounding the
tumor. The data were statistically analyzed by Spearman rank and
bivariate correlation using SPSS 17.0 software. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
According to the classification criteria of World
Health Organization (1), the
astrocytoma specimens are classified as grade I (pilocytic), grade
II (diffuse), grade III (anaplastic) or grade IV (GBM) (2,5). In
the case of GBMs, they may arise primarily (de novo) or are
transformed from the lower-grade astrocytomas (29). The primary and secondary GBMs can
be classified by several factors including the patient age
(5). It has been demonstrated that
primary GBMs usually occurs in patients aged >50 years, while
the secondary glioblastomas are more common among younger patients
(30). Of the 94 surgical
astrocytomas specimens used in the present study, 23 cases were
grouped into grade I (pilocytic), 35 cases to grade II (diffuse),
22 to grade III (anaplastic) and 14 to GBMs. According to the
clinical records, 5/14 GBM patients are >50 years and,
therefore, can be considered as the primary GBMs and the remaining
9 cases as the secondary tumor types. The representative portions
of the tumor, as well as tumor surrounding non-cancerous brain
tissues of the above specimens, were sampled from the tissue blocks
for tissue microarrays construction, as previously described
(31). The prepared tissue
microarrays in the density of 56 tissue spots/microarray were used
for immunohistochemical examination.
A body of evidence demonstrates that STAT3
activation is positively correlated with astrocytomas progression
(25,32,33)
and is critical for the growth and survival of glioblastoma cells
since p-STAT3 proteins trigger or upregulate its downstream gene
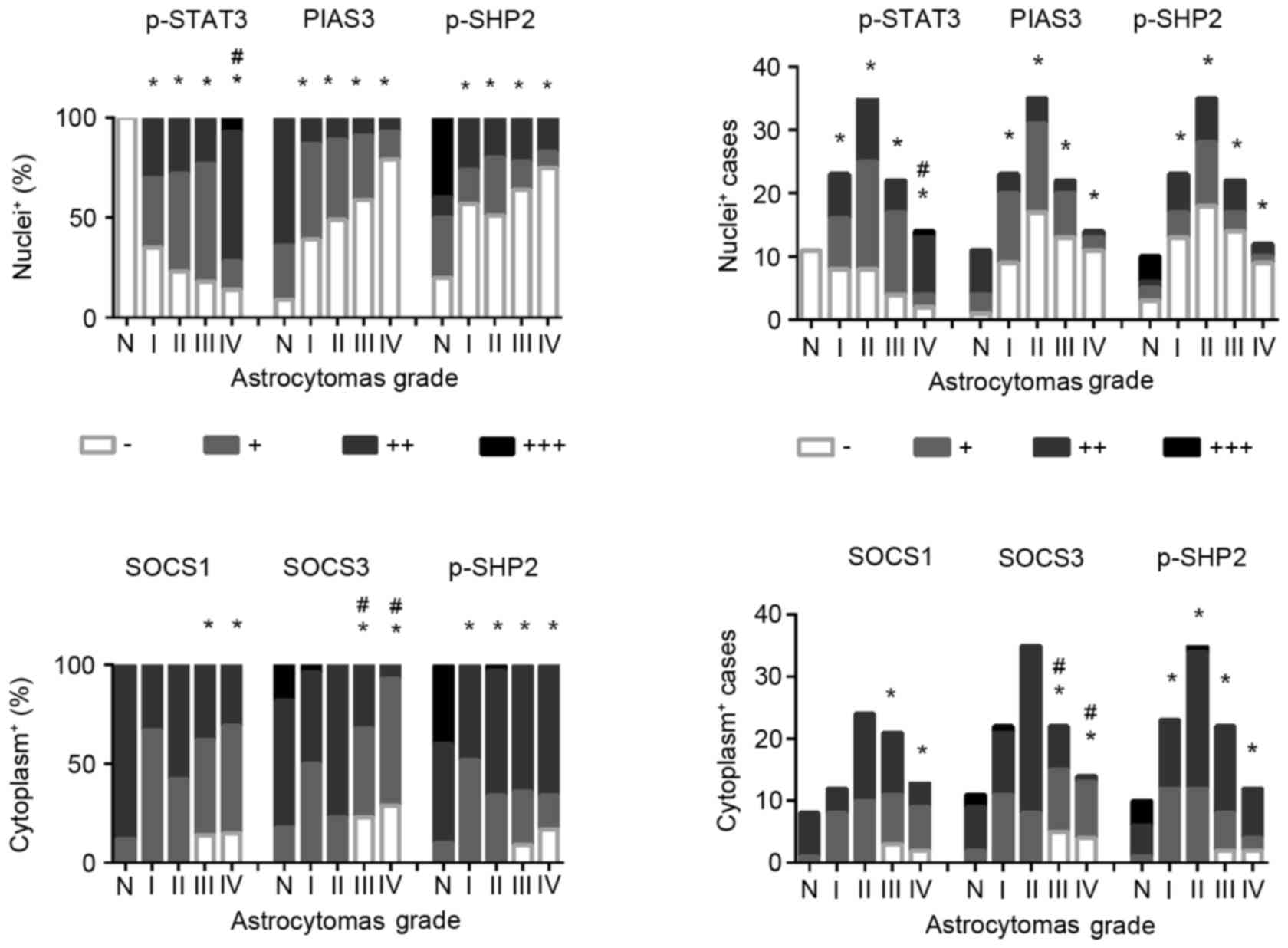
expression following translocation to the nucleus (34). The results of immunohistochemical
staining (Figs. 1 and 2) revealed that p-STAT3 nuclear
translocation was rarely observed in non-cancerous brain tissues
(0/11; 0%), while the frequencies were increased to 65.2% (15/23)
in grade I, 77.1% (27/35) in grade II, 81.8% (18/22) in grade III
and 85.7% (12/14) in grade IV astrocytomas. p-STAT3 nuclear
translocation was observed in all of five GBMs (100%) from patients
>50 years and 7/9 GBMs (77.8%) from patients >50 years.
Statistical analyses revealed the following: i) The frequencies of
p-STAT3 nuclear translocation were significantly increased in the
four subtypes of astrocytomas compared with that of the
non-cancerous brain tissues and ii) that the incidences of p-STAT3
nuclear translocation are closely correlated with tumor grading
[Spearman rank and bivariate correlation (rs)=0.207,
P=0.045]. These results further demonstrated the potential
promoting effects of STAT3 signaling in the stepwise progress of
astrocytomas and de novo formation of GBMs. Further
investigation of the underlying reasons leading to the disordered
STAT3 activation is of potential prognostic and therapeutic value
in the management of astrocytomas.
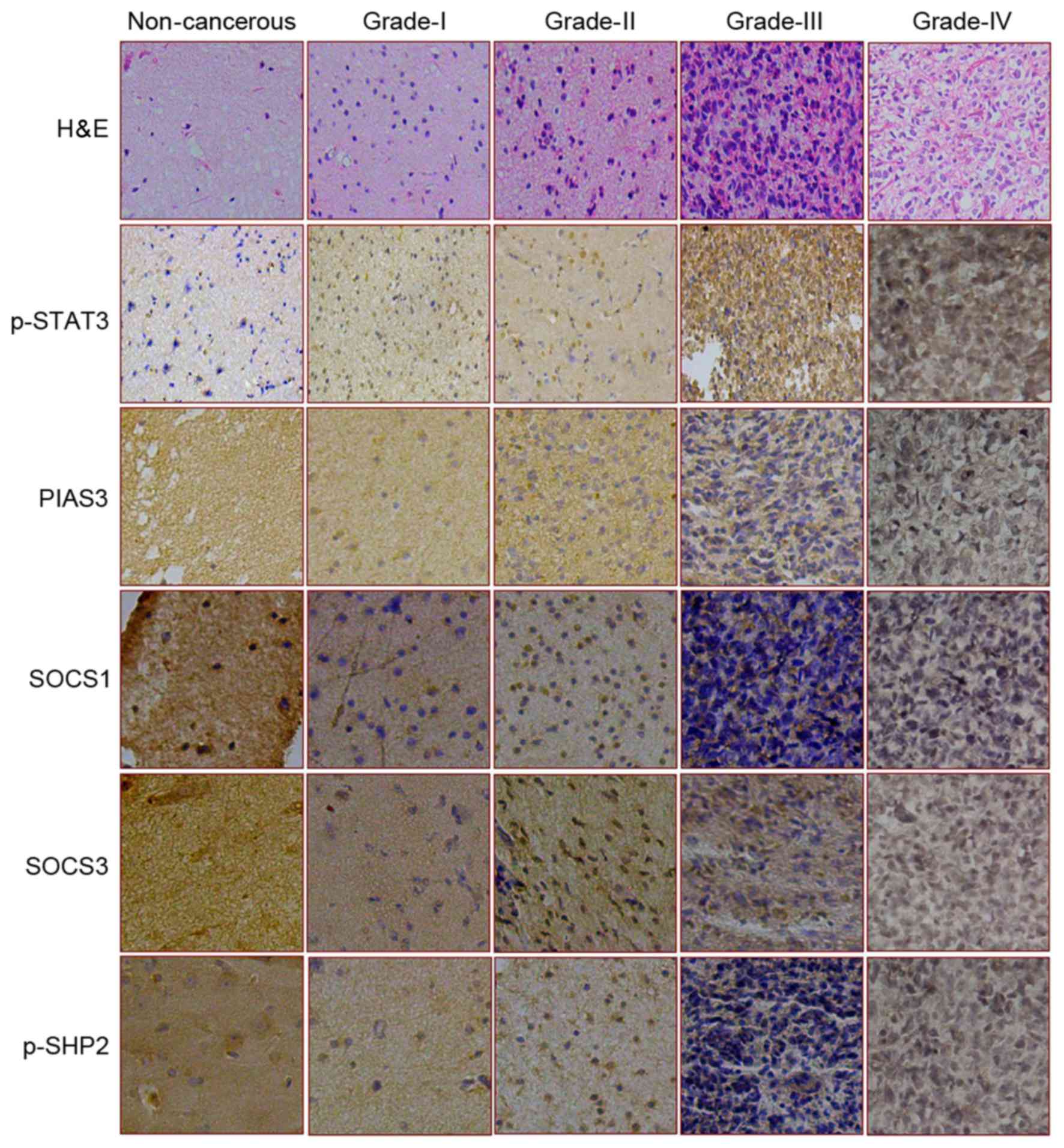 | Figure 1.Expression and intracellular
distribution patterns of p-STAT3 and its four negative regulators
in various brain tissue samples. The expression and distribution of
p-STAT3, PIAS3, SOCS1, SOCS4 and p-SHP2 were assessed by
immunostaining in non-cancerous brain tissue and four grades of
astrocytomas. H&E staining was also used to look at the cells
(magnification, ×100). H&E, hematoxylin and eosin staining; p-,
phosphorylated-; STAT3, signal transducer and activator of
transcription 3; PIAS3, protein inhibitor of activated STAT3; SOCS,
suppressor of cytokine signaling; SHP, SH2 domain-containing
phosphatase. |
It has been recognized that PIAS3 functions as a
negative regulator of STAT-3 signaling by interfering with the
interaction between p-STAT3 and its target genes (19). In agreement with the above notion,
the present in vitro data revealed upregulated expression
and increased nuclear translocation of PIAS3 in
resveratrol-suppressed glioblastoma cells, accompanied by STAT3
inactivation (35). However, no
data concerning the status of PIAS3 in different grades of
astrocytomas has been thus far available, although the activated
STAT3 signaling has been frequently observed in astrocytomas
(15). As shown in Fig. 2, higher levels (++ and +++) of
PIAS3 expression were observed in 63.6% (7/11) of tumor surrounding
brain tissues, which is reduced to 13.1% in grade I (3/23), 11.4%
in grade II (4/5), 9.1% in grade III (2/22) and 7.1% in grade IV
(1/14) astrocytomas. Accordingly, distinct PIAS3 nuclear labeling
is observed in the non-cancerous, but not in the majority (54%) of
tumor tissues (Figs. 1–3). Statistical analyses revealed
significant differences of PIAS3 detection rates between the four
subtypes of astrocytomas and the non-cancerous brain tissues, and
the negative correlation of PIAS3 expression with astrocytomas
formation (P<0.05). Furthermore, the expression of PIAS3 was
negatively-correlated with STAT3 nuclear translocation
(rs=−0.298; P=0.018; Table
I). These results together with our aforementioned in
vitro findings indicated that PIAS3 may serve negative roles in
regulating STAT3 signaling in glioblastoma cells in vitro
and in vivo. Alternatively, PIAS3 downregulation in the four
subtypes of astrocytomas may results in STAT3 activation or
indirectly enhance the biological effects of the upstream STAT3
activators (15). In this context,
the decreased expression of PIAS3 and the lack of PIAS3 nuclear
translocation is an unfavorable prognostic factor of
astrocytomas.
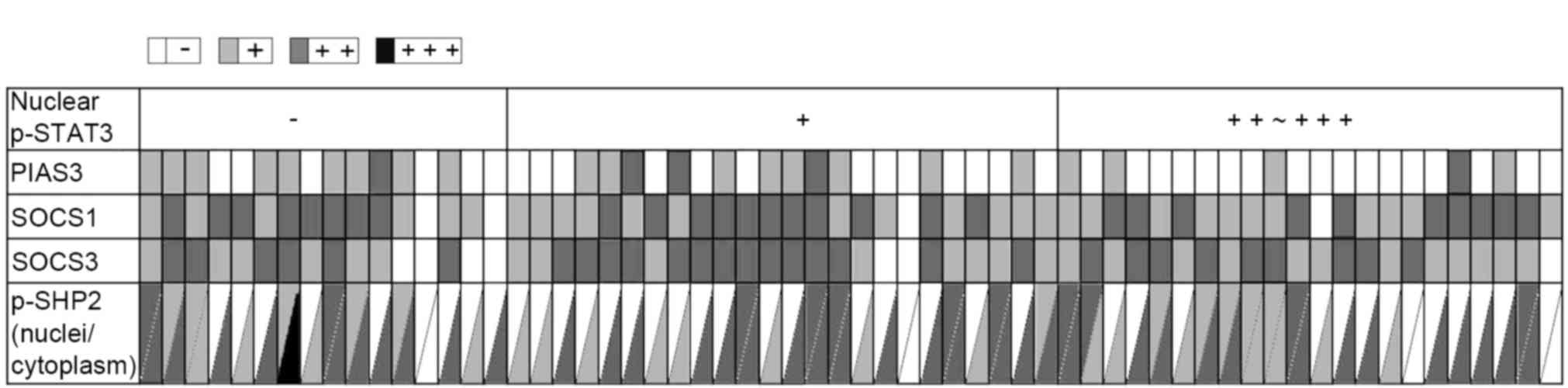 | Figure 3.Relevance of p-STAT3 nuclear
localization with the expression levels of PIAS3, SOCS1, SOCS3 and
p-SHP2 in astrocytoma tissues. The staining results were scored as
negative (−), weak (+), moderate (++) or strong positive (+++), and
are indicated as white, light gray, medium grey and black,
respectively. Each small grid represents an array point and each
vertical represents different antibody expression in the same array
point. p-, phosphorylated-; STAT3, signal transducer and activator
of transcription 3; PIAS3, protein inhibitor of activated STAT3;
SOCS, suppressor of cytokine signaling; SHP, SH2 domain-containing
phosphatase. |
 | Table I.Correlation of p-STAT3 nuclear
translocation with the expression of STAT3 negative regulator in
different grade astrocytoma tissues. |
Table I.
Correlation of p-STAT3 nuclear
translocation with the expression of STAT3 negative regulator in
different grade astrocytoma tissues.
|
| PIAS3a | SOCS1b | SOCS3b | p-SHP2a | p-SHP2b |
|---|
|
|
|
|
|
|
|
|---|
| p-STAT3 | − | + | ++ | r | P-value | − | + | ++ | r | P-value | − | + | ++ | r | P-value | − | + | ++ | r | P-value | − | + | ++ | +++ | r | P-value |
|---|
| − | 6 | 9 | 1 | −0.298 | 0.018 | 2 | 6 | 8 | −0.009 | 0.944 | 4 | 6 | 6 | 0.058 | 0.652 | 9 | 5 | 2 | −0.002 | 0.986 | 1 | 4 | 10 | 1 | −0.124 | 0.337 |
| + | 13 | 8 | 3 |
|
| 1 | 12 | 11 |
|
| 2 | 8 | 14 |
|
| 16 | 3 | 5 |
|
| 1 | 8 | 15 | 0 |
|
| ++ | 16 | 4 | 1 |
|
| 1 | 10 | 10 |
|
| 0 | 12 | 9 |
|
| 12 | 5 | 4 |
|
| 1 | 7 | 13 | 0 |
|
| +++ | 1 | 0 | 0 |
|
| 0 | 1 | 0 |
|
| 1 | 0 | 0 |
|
| 1 | 0 | 0 |
|
| 1 | 0 | 0 | 0 |
|
SOCS1 and SOCS3 are the predominant members of the
SOCS protein family, which work in a classic negative feedback loop
to attenuate STAT3 activity by suppressive binding with
phosphorylated JAK and/or facilitating ubiqitination of JAK in the
cytoplasm (21). SOCS1 and SOCS3
expression in GBMs can be epigenetically regulated in the form of
hypermethylation in CpG island (36,37).
For instance, the methylation of SOCS3 appears to be involved in
the pathogenesis of GBMs and in the resistance of GBMs to
conventional anticancer drugs (38). However, the correlation of SOCS1
and SOCS3 downregulation with STAT3 activation in human
astrocytomas remains to be reported. The present IHC results
revealed that SOCS1 and SOCS3 are expressed in higher levels (++
and +++) in the non-cancerous specimens assessed, while their
levels are decreased (+ or -) in the astrocytomas tissues (Figs. 1–3). Statistical analyses demonstrated that
SOCS3 (rs=−0.400; P=0.000), rather than SOCS1
downregulation (rs=−0.160; P=0.187), is negatively
correlated with the tumor grading (Fig. 2). Nevertheless, neither SOCS1 nor
SOCS3 expression pattern is statistically correlated with p-STAT3
nuclear translocation (rs=−0.009, P=0.944;
rs=−0.058, P=0.652). It has been reported that SOCS3
inactivation by promoter hypermethylation is mutually exclusive to
EGFR activation in glioblastomas and preferentially promotes glioma
cell invasion through the activation of STAT3 and FAK (39). Therefore, it would be possible that
the epigenetically downregulated SOCS3 and SOCS1 may confer on GBM
cells more aggressive biological behaviors, although the relevance
of their downregulation with STAT3 activation cannot be totally
ruled out at present stage.
SHP2 is a non-receptor type protein tyrosine
phosphatase (40) and its
phosphorylated form (p-SHP2) downregulates STAT3 activation by
dephosphorylating active STAT3 complexes both in the cytoplasm and
in the nucleus (41). The statuses
of SHP2 and their relevance with STAT3 signaling in GBMs have been
reported with differeing opinions (42,43).
It was revealed that SHP2-mediated antagonism of STAT3
phosphorylation prevails in the promotion of GBM cell death in
response to EGFR and c-MET co-inhibition (42), while SHP2 can promote glioblastoma
cell growth by suppression of cellular senescence (43). The present immunohistochemical
results using a p-SHP2 specific antibody revealed that cytoplasmic
p-SHP2 staining (++ and +++) was observed in all of the
non-cancerous specimens examined, of which 8 cases (8/10; 80%) were
found with p-SHP2 nuclear translocation (Figs. 1–3). In the case of astrocytomas, the
detection rates of cytoplasmic p-SHP2 are not changed distinctly,
but the frequencies of nuclear p-SHP2 detection are remarkably
decreased in the tumor tissues, in particular in grade III (36.4%,
8/22) and grade IV (25%, 3/12) (Figs.
1 and 3). However, the
statistical analyses revealed no correlation of nuclear
translocation (rs=−0.106, P=0.315) and cytoplasmic
staining (rs=0.065, P=0.536) of p-SHP2 with astrocytomas
grading and p-STAT3 nuclear translocation (nuclei,
rs=−0.002 and P=0.986; cytoplasm, rs=−0.124
and P=0.337; Fig. 2; Table I). Although the present findings
may implicate that the reductive tendencies of p-SHP2 level and
nuclear translocation may be favorable for astrocytomas formation
presumably via preventing cell death (43) and/or reinforcing STAT3 activation
caused by STAT3 activator overexpression and PIAS3 reduction
(25).
In conclusion, SOCS1, SOCS3, PIAS3 and p-SHP2
expression patterns and the frequencies of phosphorylated
STAT3/p-STAT3 nuclear translocation in non-cancerous brain tissues
and the four grades of astrocytomas were profiled by tissue
microarray-based immunohistochemical staining. The results revealed
that p-STAT3 nuclear translocation is progressively common as the
tumor grades increase. By contrast, the expression levels of SOCS1,
SOCS3, PIAS3 and p-SHP2 tended to decrease as the tumor progressed.
Statistical analyses revealed that downregulation of PIAS3 is more
correlated with p-STAT3 nuclear translocation compared with other
STAT3 negative regulators. As a result of the importance of STAT3
activation for the growth and survival of glioblastoma cells, the
decreased expression of PIAS3 can be regarded as an unfavorable
prognostic factor of astrocytomas patients. SOCS1, SOCS3 and p-SHP2
downregulation, and p-SHP2 nuclear translocation in astrocytomas
tissues must have certain biological implications and it would be
of value to further investigate.
Acknowledgements
The authors would like to thank the doctors in the
Department of Clinical Pathology of An-Shan First Hospital for
their co-operation in sample collection and pathological
consultation. This study was supported by grants from the National
Natural Science Foundation of China (nos. 81450016, 81272786 and
30971038), the research fund for PhD supervisors from National
Education Department of China (no. 20122105110005), the Program for
Changjiang Scholar and Innovative Research Team in University
(PCSIRT; IRT13049), the Liaoning Department of Education for key
laboratory (no. L2012317 and L20133453) and the Natural Science
Foundation of Liaoning Province (no. 2013023040 and
2013023050).
References
|
1
|
Kros JM: Grading of gliomas: The road from
eminence to evidence. J Neuropathol Exp Neurol. 70:101–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Holland EC and Cairncross JG:
Glioma classification: A molecular reappraisal. Am J Pathol.
159:779–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suryadevara CM, Verla T, Sanchez-Perez L,
Reap EA, Choi BD, Fecci PE and Sampson JH: Immunotherapy for
malignant glioma. Surg Neurol Int. 6 Suppl 1:S68–S77. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jovcevska I, Kocevar N and Komel R: Glioma
and glioblastoma-how much do we (not) know? Mol Clin Oncol.
1:935–941. 2013.PubMed/NCBI
|
|
6
|
Ohgaki H: Genetic pathways to
glioblastomas. Neuropathology. 25:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahaman SO, Vogelbaum MA and Haque SJ:
Aberrant Stat3 signaling by interleukin-4 in malignant glioma
cells: Involvement of IL-13Ralpha2. Cancer Res. 65:2956–2963. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Li G, Li R, Shen J, He Q, Deng L,
Zhang C and Zhang J: IL-6 promotion of glioblastoma cell invasion
and angiogenesis in U251 and T98G cell lines. J Neurooncol.
100:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jackson C, Ruzevick J, Amin AG and Lim M:
Potential role for STAT3 inhibitors in glioblastoma. Neurosurg Clin
N Am. 23:379–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnston PA and Grandis JR: STAT3
signaling: Anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
See AP, Han JE, Phallen J, Binder Z,
Gallia G, Pan F, Jinasena D, Jackson C, Belcaid Z, Jeong SJ, et al:
The role of STAT3 activation in modulating the immune
microenvironment of GBM. J Neurooncol. 110:359–368. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carro MS, Lim WK, Alvarez MJ, Bollo RJ,
Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, et al:
The transcriptional network for mesenchymal transformation of brain
tumours. Nature. 463:318–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luwor RB, Stylli SS and Kaye AH: The role
of Stat3 in glioblastoma multiforme. J Clin Neurosci. 20:907–911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizoguchi M, Betensky RA, Batchelor TT,
Bernay DC, Louis DN and Nutt CL: Activation of STAT3, MAPK and AKT
in malignant astrocytic gliomas: Correlation with EGFR status,
tumor grade, and survival. J Neuropathol Exp Neurol. 65:1181–1188.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abou-Ghazal M, Yang DS, Qiao W,
Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W,
Sawaya R and Heimberger AB: The incidence, correlation with
tumor-infiltrating inflammation and prognosis of phosphorylated
STAT3 expression in human gliomas. Clin Cancer Res. 14:8228–8235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dinasarapu AR, Gupta S, Ram Maurya M, Fahy
E, Min J, Sud M, Gersten MJ, Glass CK and Subramaniam S: A combined
omics study on activated macrophages-enhanced role of STATs in
apoptosis, immunity and lipid metabolism. Bioinformatics.
29:2735–2743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung CD, Liao J, Liu B, Rao X, Jay P,
Berta P and Shuai K: Specific inhibition of Stat3 signal
transduction by PIAS3. Science. 278:1803–1805. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shuai K: Regulation of cytokine signaling
pathways by PIAS proteins. Cell Res. 16:196–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Miki R, Eeva M, Fike FM, Seligson
D, Yang L, Yoshimura A, Teitell MA, Jamieson CA and Cacalano NA:
Reciprocal regulation of SOCS 1 and SOCS3 enhances resistance to
ionizing radiation in glioblastoma multiforme. Clin Cancer Res.
13:2344–2353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim DJ, Tremblay ML and Digiovanni J:
Protein tyrosine phosphatases, TC-PTP, SHP1 and SHP2, cooperate in
rapid dephosphorylation of Stat3 in keratinocytes following UVB
irradiation. PloS One. 5:e102902010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Funato K, Yamazumi Y, Oda T and Akiyama T:
Tyrosine phosphatase PTPRD suppresses colon cancer cell migration
in coordination with CD44. Exp Ther Med. 2:457–463. 2011.PubMed/NCBI
|
|
24
|
Bixler SL, Sandler NG, Douek DC and
Mattapallil JJ: Suppressed Th17 levels correlate with elevated
PIAS3, SHP2, and SOCS3 expression in CD4 T cells during acute
simian immunodeficiency virus infection. J Virol. 87:7093–7101.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brantley EC, Nabors LB, Gillespie GY, Choi
YH, Palmer CA, Harrison K, Roarty K and Benveniste EN: Loss of
protein inhibitors of activated STAT-3 expression in glioblastoma
multiforme tumors: Implications for STAT-3 activation and gene
expression. Clin Cancer Res. 14:4694–4704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Sun Y, Kong QY, Zhang KL, Wang XW,
Chen XY, Wang Q and Liu J: Combination of nucleic acid and protein
isolation with tissue array construction: Using defined histologic
regions in single frozen tissue blocks for multiple research
purposes. Int J Mol Med. 12:299–304. 2003.PubMed/NCBI
|
|
27
|
Li H, Guo L, Li JW, Liu N, Qi R and Liu J:
Expression of hyaluronan receptors CD44 and RHAMM in stomach
cancers: Relevance with tumor progression. Int J Oncol. 17:927–932.
2000.PubMed/NCBI
|
|
28
|
Ma JX, Li H, Chen XM, Yang XH, Wang Q, Wu
ML, Kong QY, Li ZX and Liu J: Expression patterns and potential
roles of SIRT1 in human medulloblastoma cells in vivo and in vitro.
Neuropathology. 33:7–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bralten LB and French PJ: Genetic
alterations in glioma. Cancers. 3:1129–1140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshida J: Molecular neurosurgery using
gene therapy to treat malignant glioma. Nagoya J Med Sci.
59:97–105. 1996.PubMed/NCBI
|
|
31
|
Xia SL, Wu ML, Li H, Wang JH, Chen NN,
Chen XY, Kong QY, Sun Z and Liu J: CRABP-II- and FABP5-independent
responsiveness of human glioblastoma cells to all-trans retinoic
acid. Oncotarget. 6:5889–5902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schaefer LK, Ren Z, Fuller GN and Schaefer
TS: Constitutive activation of Stat3alpha in brain tumors:
Localization to tumor endothelial cells and activation by the
endothelial tyrosine kinase receptor (VEGFR-2). Oncogene.
21:2058–2065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Shea JJ, Schwartz DM, Villarino AV,
Gadina M, McInnes IB and Laurence A: The JAK-STAT pathway: Impact
on human disease and therapeutic intervention. Annu Rev Med.
66:311–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shu XH, Li H, Sun XX, Wang Q, Sun Z, Wu
ML, Chen XY, Li C, Kong QY and Liu J: Metabolic patterns and
biotransformation activities of resveratrol in human glioblastoma
cells: Relevance with therapeutic efficacies. PloS One.
6:e274842011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tamiya T, Kashiwagi I, Takahashi R,
Yasukawa H and Yoshimura A: Suppressors of cytokine signaling
(SOCS) proteins and JAK/STAT pathways: Regulation of T-cell
inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol.
31:980–985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sutherland KD, Lindeman GJ, Choong DY,
Wittlin S, Brentzell L, Phillips W, Campbell IG and Visvader JE:
Differential hypermethylation of SOCS genes in ovarian and breast
carcinomas. Oncogene. 23:7726–7733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martini M, Pallini R, Luongo G, Cenci T,
Lucantoni C and Larocca LM: Prognostic relevance of SOCS3
hypermethylation in patients with glioblastoma multiforme. Int J
Cancer. 123:2955–2960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lindemann C, Hackmann O, Delic S, Schmidt
N, Reifenberger G and Riemenschneider MJ: SOCS3 promoter
methylation is mutually exclusive to EGFR amplification in gliomas
and promotes glioma cell invasion through STAT3 and FAK activation.
Acta Neuropathol. 122:241–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng GS: Shp2-mediated molecular signaling
in control of embryonic stem cell self-renewal and differentiation.
Cell Res. 17:37–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rakesh K and Agrawal DK: Controlling
cytokine signaling by constitutive inhibitors. Biochem Pharmacol.
70:649–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Furcht CM, Buonato JM, Skuli N, Mathew LK,
Muñoz Rojas AR, Simon MC and Lazzara MJ: Multivariate signaling
regulation by SHP2 differentially controls proliferation and
therapeutic response in glioma cells. J Cell Sci. 127:3555–3567.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sturla LM, Zinn PO, Ng K, Nitta M, Kozono
D, Chen CC and Kasper EM: Src homology domain-containing
phosphatase 2 suppresses cellular senescence in glioblastoma. Br J
Cancer. 105:1235–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|