Introduction
Bladder cancer is the fourth most common type of
cancer, and was the cause of >11,510 cases of cancer-associated
mortality among men in the USA in 2014 (1). The majority of bladder cancer cases
are noninvasive, low-grade transitional cell carcinoma (TCC);
however, ≤30% of all diagnosed tumors are classified as invasive
TCC, which is associated with high mortality (2). Notably, among all of the various
types of cancer, bladder cancer exhibits a significantly high
incidence of recurrence post-treatment, and therefore is recognized
as the most expensive cancer to treat. Due to the high prevalence
and frequent tumor recurrence associated with bladder cancer, the
exploration of preventative strategies is required.
Previous epidemiological and scientific studies have
indicated that plant-derived phytochemicals may be beneficial in
the prevention and treatment of cancer (3–5).
Resveratrol is a polyphenolic compound, which is present in grapes
(red wine), knotweed, peanuts, mulberries and other plants.
Numerous studies have suggested that resveratrol may prevent the
progression of various pathologies, including vascular diseases,
ischemic injuries, cancer and neurodegenerative disorders (6–8).
Furthermore, it has previously been confirmed that resveratrol
exerts antiproliferative and/or apoptotic effects on leukemia,
prostate, breast and colon cancers cells in vitro (9–12).
Previous studies regarding the anticancer effects of resveratrol
have focused on its anti-invasive and anti-metastatic activities
(13–15). However, to the best of our
knowledge, there is currently no evidence as to whether resveratrol
may inhibit the migration and invasion of human bladder cancer
cells.
Metastasis is one of the hallmarks of advanced
cancer progression, and cancer cell migration and invasion are
crucial events in bladder cancer metastasis. Matrix
metalloproteinases (MMPs), including MMP-2 and MMP-9, degrade the
basement membrane and extracellular matrix (ECM), and are therefore
considered crucial proteolytic proteinases that facilitate the
invasion of malignant cells (16).
Upregulated MMP expression and activity has been reported to serve
a key role in several types of human cancer with invasive and
metastatic capabilities (17).
The present study aimed to evaluate whether
resveratrol alters metastatic tumor cell progression in
vitro and its underlying mechanism. The results indicated that
resveratrol was able to suppress adhesion, migration and invasion
of T24 cells via the c-Jun N-terminal kinase 1/2 (JNK1/2) and
extracellular signal-regulated protein kinase 1/2 (ERK1/2)
signaling pathways, and the modulation of MMP-2 and MMP-9.
Materials and methods
Reagents
Resveratrol (purity, >99%) was purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). The primary
antibodies mouse anti-MMP-2 (catalog no. sc-13595), mouse
anti-MMP-9 (catalog no. sc-21733), mouse anti-phosphorylated
(p)-ERK1/2 (catalog no. sc-81492), mouse anti-ERK1/2 (catalog no.
sc-135900), mouse anti-p-JNK1/2 (catalog no. sc-293137), mouse
anti-JNK1/2 (catalog no. sc-7345) and mouse anti-β-actin (catalog
no. sc-47778), and a horseradish peroxidase-conjugated goat
anti-mouse secondary antibody (catalog no. sc-2031), were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The
bicinchoninic acid protein assay kit was purchased from Pierce;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Cell culture
The T24 human bladder cancer cell line was obtained
from the Shanghai Institute of Cell Biology, Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in RPMI-1640
medium (HyClone; GE Healthcare, Logan, UT, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS; SAFC Biosciences
Inc., Lenexa, KS, USA), 100 U⁄ml penicillin and 100 mg/l
streptomycin. Cultures were maintained in a humidified atmosphere
containing 5% CO2 at 37°C.
Cell adhesion assay
A 96-well plate was coated with 10 µg/ml fibronectin
(Corning Life Sciences, Corning, CA, USA) and incubated for 60 min,
after which the plate was blocked with 1% bovine serum albumin
(Sigma-Aldrich; Merck Millipore) at 37°C for 30 min. Subsequently,
cells (5×105 cells/ml; 200 µl) were added to each well
and were incubated with resveratrol (0–100 µM) or vehicle (0.1%
dimethyl sulfoxide), for 90 min at 37°C, after which the plate was
gently washed three times with PBS to remove unattached cells. A
total of 20 µl MTT (5 mg/ml) was then added to each well, and the
plate was incubated for 4 h at 37°C. Subsequently, the liquid was
removed and dimethyl sulfoxide was added to dissolve the solid
residue. Following agitation for 10 min, absorbance was measured at
a wavelength of 490 µm using an ELISA plate reader. The adhesion of
cells treated with the vehicle was established as 100%.
Cell migration assay
T24 cells (1×105 cells/well) were allowed
to form a confluent monolayer in 6-well plates, after which cells
were wounded using a 200-µl pipette tip. Following wound
generation, all cells in the plates were treated with resveratrol
at final concentrations of 0, 10, 25 and 50 µM. Images were
captured under a phase contrast microscope at ×100 magnification
(Olympus IX70; Olympus Corporation, Tokyo, Japan) immediately, or
6, 12 or 24 h after wound generation. Image-Pro Plus 5.0 software
(Media Cybernetics, Inc., Rockville, MD, USA) was used to quantify
the cell-free wound area over the period of the experiment. Cell
migration was calculated as the percentage of the remaining
cell-free area compared with the area of the initial wound.
Cell invasion assay
A 24-well Boyden chamber with a polycarbonate
membrane (pore size, 8 µm; Corning Inc., Corning, NY, USA) was used
to analyze cell motility. The membrane was pre-coated with Matrigel
to form a matrix barrier. T24 cells were plated at 5×106
cells/ml in the upper compartment and were cultured in medium
containing resveratrol (0–50 µM) for 24 h. The bottom chambers were
filled with 500 µl medium supplemented with 20% FBS as a
chemoattractant. Cells were incubated at 37°C for 24 h and those
that did not migrate through the pores were removed by scraping the
upper surface of the membrane with a cotton swab. Cells that had
migrated to the lower surface of the membrane were fixed in 100%
methanol for 5 min and were stained with 0.1% crystal violet for 2
min. The cells that invaded through the insert were counted in five
randomly selected microscopic fields (magnification, ×400; Olympus
IX70; Olympus Corporation, Tokyo, Japan) per filter.
Western blot analysis
Cells were harvested 24 h after treatment with
resveratrol (0–50 µM). The harvested cells were washed and lysed
with lysis buffer [10 mmol/l Tris-HCl, 0.25 mol/l sucrose, 5 mmol/l
EDTA, 50 mmol/l NaCl, 30 mmol/l sodium pyrophosphate, 50 mmol/l
NaF, 1 mmol/l Na3VO4, 1 mmol/l PMSF, and 2%
protease inhibitor cocktail (pH 7.5)]. Protein concentration in the
resulting lysate was determined using the bicinchoninic acid
protein assay. Appropriate quantities of protein (20–30 µg) were
separated by electrophoresis in 10–12% Tris-glycine polyacrylamide
gels, and were transferred to nitrocellulose membranes. The
membranes were then blocked with 5% nonfat milk in TBS for 1 h at
37°C, and were incubated overnight at 4°C with the appropriate
primary antibodies at dilutions according to the manufacturer's
protocols (1:200). Subsequently, the membranes were washed with TBS
containing Tween-20 (10 mM Tris-Cl at pH 7.4, 150 mM NaCl, 0.1%
Tween-20) and incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody at a 1:2,000 dilution for
1 h at 25°C. Bound secondary antibody was detected using an
enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All data were analyzed with the SPSS software
version 16.0 (SPSS, Inc., Chicago, IL, USA). All data are presented
as the mean ± standard deviation from at least three different
experiments. Statistical significance was compared between various
treatment groups and controls using one-way analysis of variance
followed by Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
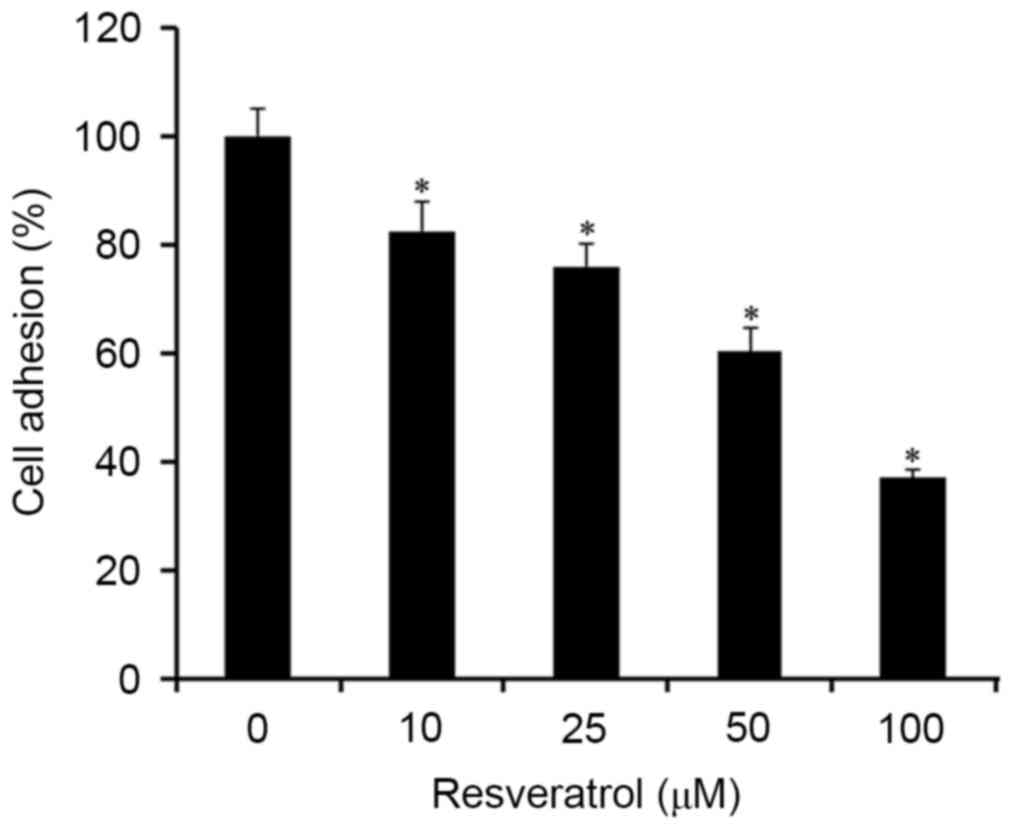
Resveratrol inhibits T24 cell adhesion
to fibronectin
Since the adhesion of tumor cells to the ECM is
considered an important step in the invasive process of metastatic
tumors, the effects of resveratrol on cell adhesion were examined.
The results revealed that incubation of T24 cells with 0–100 µM
resveratrol significantly inhibited cell adhesion to the
fibronectin-coated plates in a concentration-dependent manner
(Fig. 1).
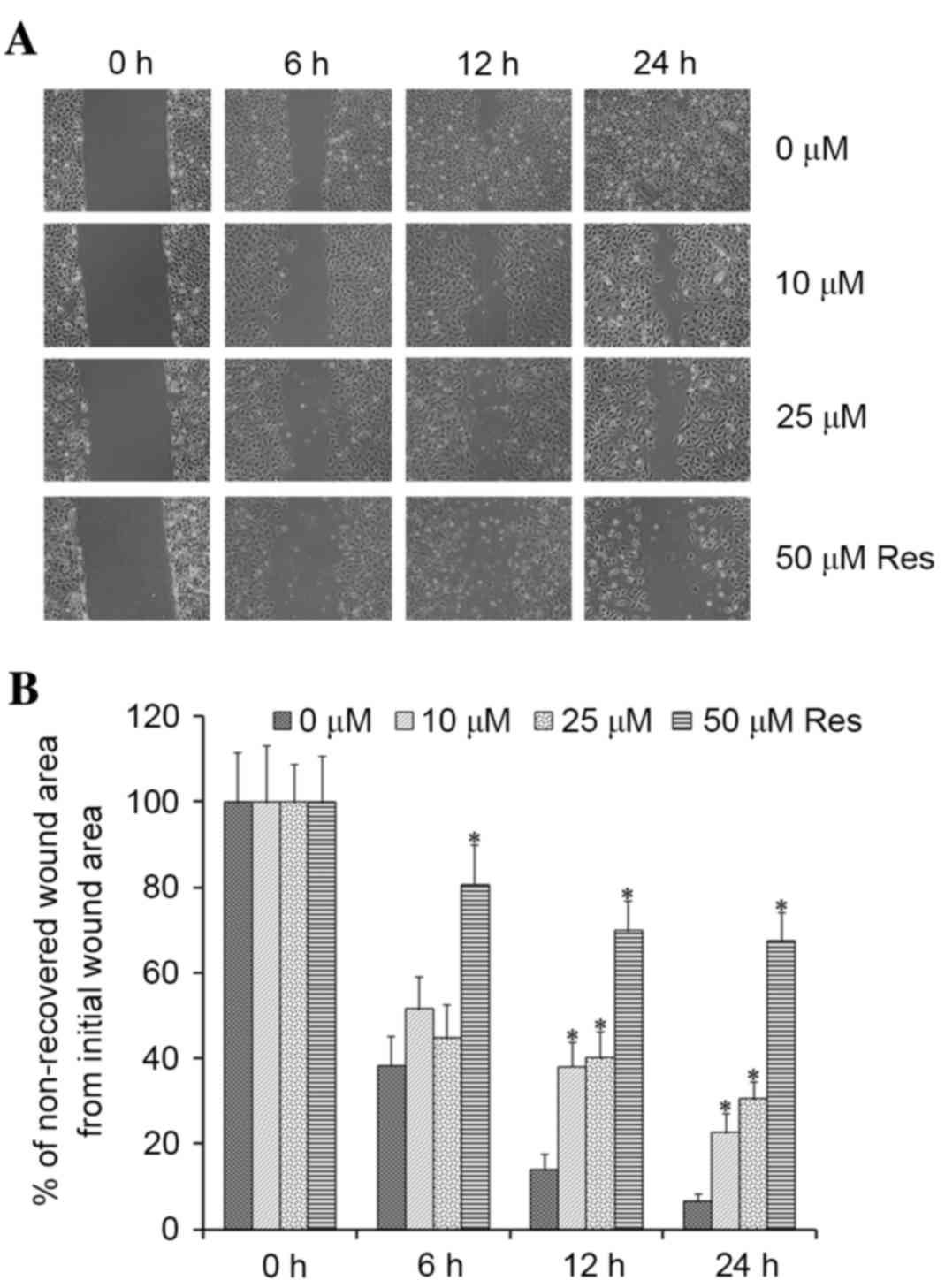
Resveratrol inhibits T24 cell
migration
Cell migration was measured using a wound-healing
assay, which is commonly used to determine the effects of pro- and
anti-migratory agents on cultured cells. T24 cells were treated
with 0–50 µM resveratrol, in order to detect whether the migratory
potential of T24 cells was decreased. As shown in Fig. 2, the wound-healing assay revealed
that cells treated with 10–50 µM resveratrol exhibited reduced
wound closure. These results indicated that treatment with
resveratrol may induce significant suppression of the migratory
capability of T24 cells in a dose-dependent manner.
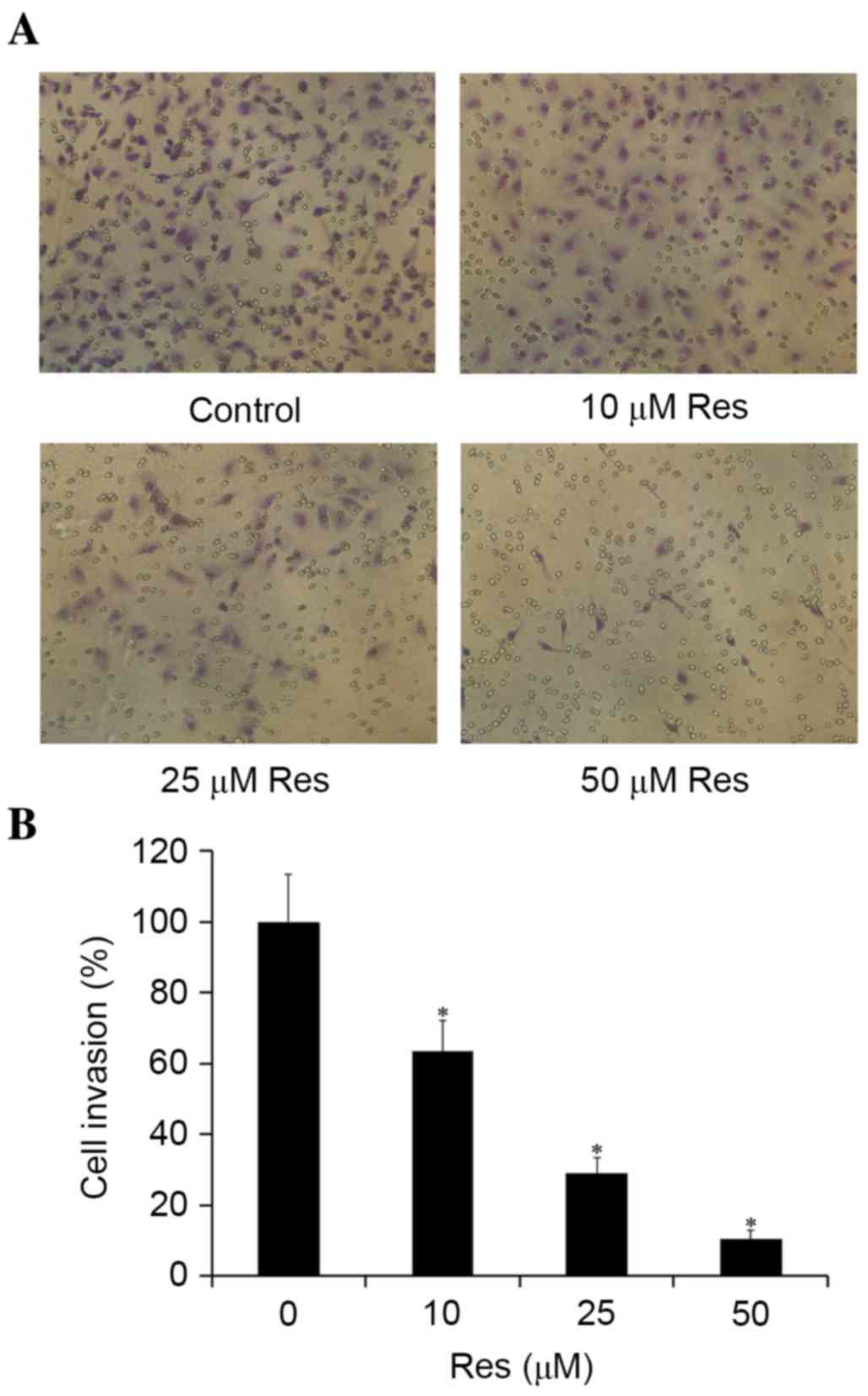
Resveratrol inhibits T24 cell
invasion
The present study further evaluated the
anti-metastatic activity of resveratrol using a Transwell assay.
The ability of T24 cells to permeate through a reconstituted
basement membrane barrier (Matrigel) was determined following
treatment with or without resveratrol. Similar to the migration
assay, resveratrol inhibited the invasion of T24 cells in a
dose-dependent manner. When T24 cells were grown on Matrigel, a
significant reduction in the number of invasive cells was detected
when the cells were treated with 10–50 µM resveratrol for 24 h, as
compared with the control group, with the levels of invasion being
reduced to 63.4 and 10.3% of the control levels at 10 and 50 µM
resveratrol, respectively (Fig.
3).
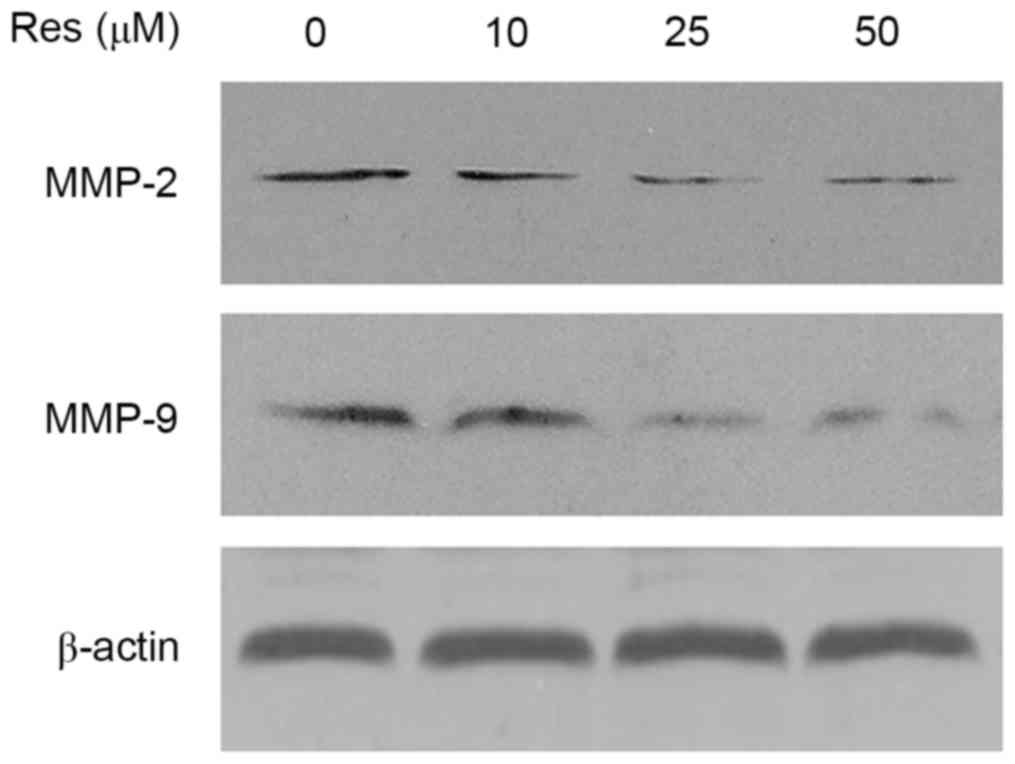
Resveratrol downregulates the protein
expression levels of MMP-2 and MMP-9
It is well known that MMP-2 and MMP-9 serve
important roles in the invasion of cancer cells. The present study
aimed to determine whether resveratrol was able to inhibit the
secretion of MMP-2 and MMP-9 in T24 cells. As presented in Fig. 4, MMP-2 and MMP-9 expression was
suppressed by resveratrol treatment in T24 cells, in a
dose-dependent manner.
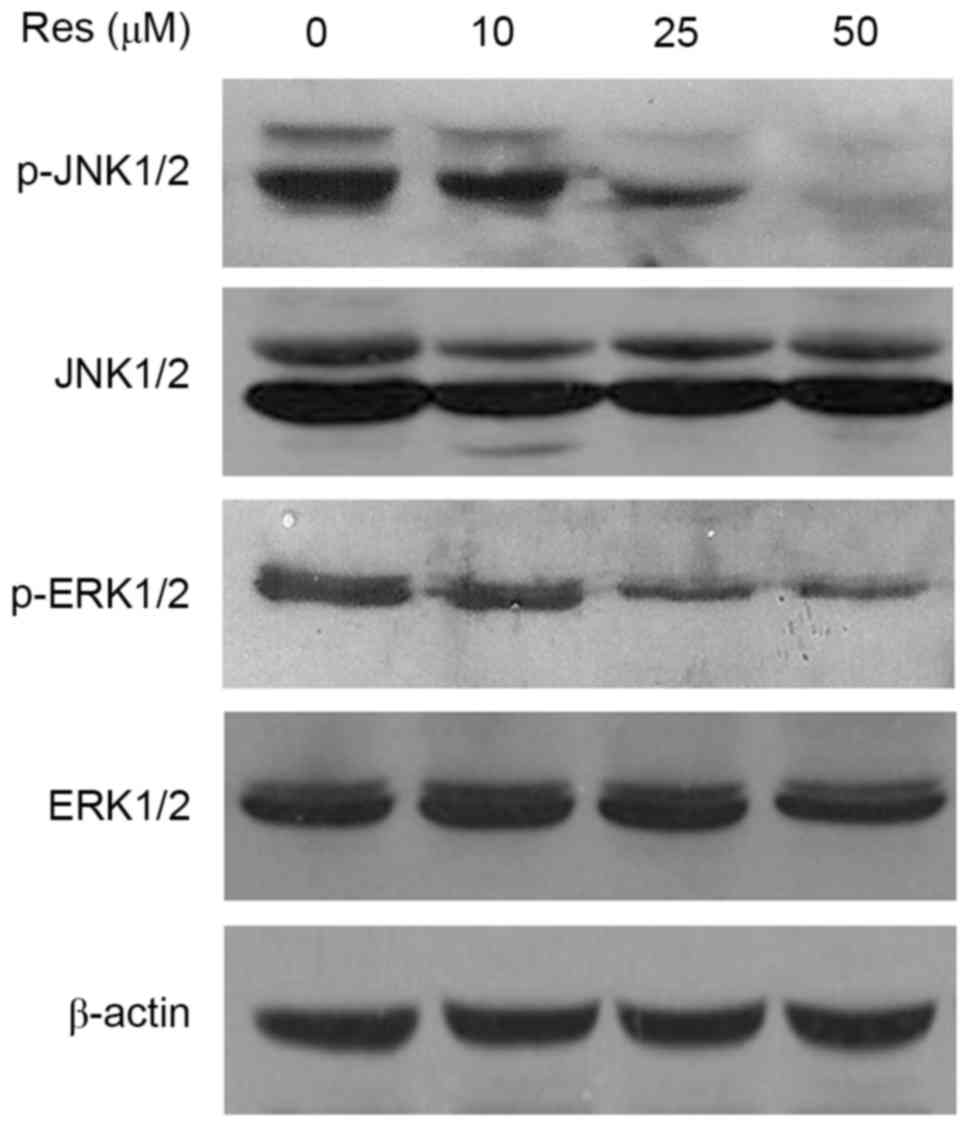
Resveratrol downregulates the
phosphorylation levels of JNK1/2 and ERK1/2
To further investigate the mechanisms by which
resveratrol inhibits cellular adhesion, migration and invasion, the
present study examined alterations in the JNK1/2 and ERK1/2
pathways, which are closely associated with cancer cell metastasis.
The results of western blotting indicated that resveratrol
inhibited the phosphorylation of JNK1/2 and ERK1/2 in T24 bladder
cancer cells in a dose-dependent manner. However, resveratrol had
no marked effect on the total protein levels of JNK1/2 and ERK1/2
(Fig. 5).
Discussion
Metastasis is the most fatal characteristic of
bladder cancer, and is a multistep process that is dependent on
cellular activities, including migration and invasion of cancer
cells (18). In the present study,
adhesion, wound healing and invasion assays demonstrated that
resveratrol was able to effectively inhibit the migration and
invasion of T24 cells in vitro. The present study is the
first, to the best of our knowledge, to present these findings.
MMPs serve an important role in tumor angiogenesis,
metastasis and stimulation of growth factor release from the ECM.
MMP-2 and MMP-9 are major ECM components of the basement membrane,
and have been suggested to be important for the invasive and
metastatic potential of various malignancies, including bladder
cancer. Furthermore, the upregulation of these MMPS has been
associated with the poor prognosis of patients with bladder cancer
(19–21). Therefore, the inhibition of MMP
expression may be considered an early target for the prevention of
cancer metastasis (22,23). The present study demonstrated that
resveratrol decreased the secretion of MMP-2 and MMP-9 protein in a
dose-dependent manner in T24 cells. The results indicated that
resveratrol may act as an important regulator of ECM breakdown
during tumor invasion and metastasis, as a result of its ability to
regulate MMP production.
The mitogen-activated protein kinase (MAPK)
pathways, which are comprised of at least three subfamilies:
ERK1/2, JNK and p38, are frequently activated in the process of
tumor development, and the activation status of MAPK pathways is
essential for successful metastasis (24). Numerous studies have demonstrated
that JNK1/2 and ERK1/2 transcriptionally regulate the expression of
MMP-2 and MMP-9, which results in regulation of cell migration and
invasion (25–27). In human bladder cancer cells,
upregulation of the MAPK pathways may result in migration and
regulation of the expression levels of MMPs (28). Furthermore, it has been reported
that decreased phosphorylation of ERK1/2 and JNK may be involved in
regulation of bladder cancer cell migration (29). Therefore, interrupting MMP
expression and MAPK pathway activation may be a potential approach
used in anti-metastatic therapy. Consistent with this hypothesis,
the present study demonstrated that phosphorylation of JNK1/2 and
ERK1/2 was inhibited, which may be the cause of the downregulation
of MMP-2 and MMP-9.
In conclusion, these findings suggested that
resveratrol exhibits numerous anti-metastatic activities in T24
bladder cancer cells. The possible mechanism underlying
resveratrol-induced inhibition of T24 cell migration and invasion
may be suppression of MAPK activation (JNK and ERK), thus resulting
in the inhibition of MMP-2 and MMP-9. The results of the present
study indicated that resveratrol may be considered a therapeutic
agent in the inhibition of bladder cancer progression, and these
findings provide a novel mechanistic insight into the potential
effects of resveratrol on the suppression of bladder cancer
invasion and metastasis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81201767)
and the Joint Special Funds for the Department of Science and
Technology of Yunnan Province-Kunming Medical University (grant no.
2013FZ277).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging, and grading and
diagnosis. Urology. 66 Suppl 6:S4–S34. 2005. View Article : Google Scholar
|
|
3
|
Khan N, Afaq F, Saleem M, Ahmad N and
Mukhtar H: Targeting multiple signaling pathways by green tea
polyphenol (−)-epigallocatechin-3-gallate. Cancer Res.
66:2500–2505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosono T, Fukao T, Ogihara J, Ito Y, Shiba
H, Seki T and Ariga T: Diallyl trisulfide suppresses the
proliferation and induces apoptosis of human colon cancer cells
through oxidative modification of beta-tubulin. J Biol Chem.
280:41487–41493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Y, Wu J, Li S, Wang X, Liang Z, Xu X,
Xu X, Hu Z, Lin Y, Chen H, et al: Apigenin inhibits migration and
invasion via modulation of epithelial mesenchymal transition in
prostate cancer. Mol Med Rep. 11:1004–1008. 2015.PubMed/NCBI
|
|
6
|
Wang Q, Xu J, Rottinghaus GE, Simonyi A,
Lubahn D, Sun GY and Sun AY: Resveratrol protects against global
cerebral ischemic injury in gerbils. Brain Res. 958:439–447. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Russo A, Palumbo M, Aliano C, Lempereur L,
Scoto G and Renis M: Red wine micronutrients as protective agents
in Alzheimer-like induced insult. Life Sci. 72:2369–2379. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clément MV, Hirpara JL, Chawdhury SH and
Pervaiz S: Chemopreventive agent resveratrol, a natural product
derived from grapes, triggers CD95 signaling-dependent apoptosis in
human tumor cells. Blood. 92:996–1002. 1998.PubMed/NCBI
|
|
10
|
Aziz MH, Nihal M, Fu VX, Jarrard DF and
Ahmad N: Resveratrol-caused apoptosis of human prostate carcinoma
LNCaP cells is mediated via modulation of phosphatidylinositol
3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther.
5:1335–1341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang HY, Shih A, Cao HJ, Davis FB, Davis
PJ and Lin HY: Resveratrol-induced cyclooxygenase-2 facilitates
p53-dependent apoptosis in human breast cancer cells. Mol Cancer
Ther. 5:2034–2042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tessitore L, Davit A, Sarotto I and
Caderni G: Resveratrol depresses the growth of colorectal aberrant
crypt foci by affecting bax and p21(CIP) expression.
Carcinogenesis. 21:1619–1622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Wang H, Liu M, Lin F and Hua J:
Resveratrol abrogates the effects of hypoxia on cell proliferation,
invasion and EMT in osteosarcoma cells through downregulation of
the HIF-1α protein. Mol Med Rep. 11:1975–1981. 2015.PubMed/NCBI
|
|
14
|
Gao Q, Yuan Y, Gan HZ and Peng Q:
Resveratrol inhibits the hedgehog signaling pathway and
epithelial-mesenchymal transition and suppresses gastric cancer
invasion and metastasis. Oncol Lett. 9:2381–2387. 2015.PubMed/NCBI
|
|
15
|
Jiao Y, Li H, Liu Y, Guo A, Xu X, Qu X,
Wang S, Zhao J, Li Y and Cao Y: Resveratrol inhibits the invasion
of glioblastoma-initiating cells via down-regulation of the
PI3K/Akt/NF-κB signaling pathway. Nutrients. 7:4383–4402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
18
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Shi J, Feng J, Klocker H, Lee C
and Zhang J: Type IV collagenase (matrix metalloproteinase-2 and
−9) in prostate cancer. Prostate Cancer Prostatic Dis. 7:327–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kader AK, Liu J, Shao L, Dinney CP, Lin J,
Wang Y, Gu J, Grossman HB and Wu X: Matrix metalloproteinase
polymorphisms are associated with bladder cancer invasiveness. Clin
Cancer Res. 13:2614–2620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin J, Wang Y, Bai Y, Yang K, Mao Q, Lin
Y, Kong D, Zheng X and Xie L: Epigallocatechin-3-gallate inhibits
bladder cancer cell invasion via suppression of NF-κB-mediated
matrix metalloproteinase-9 expression. Mol Med Rep. 6:1040–1044.
2012.PubMed/NCBI
|
|
22
|
Waas ET, Wobbes T, Lomme RM, DeGroot J,
Ruers T and Hendriks T: Matrix metalloproteinase 2 and 9 activity
in patients with colorectal cancer liver metastasis. Br J Surg.
90:1556–1564. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastas
Rev. 22:395–403. 2003. View Article : Google Scholar
|
|
25
|
Crowe DL, Tsang KJ and Shemirani B: Jun
N-terminal kinase 1 mediates transcriptional induction of matrix
metalloproteinase 9 expression. Neoplasia. 3:27–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moon SK, Kim HM, Lee YC and Kim CH:
Disialoganglioside (GD3) synthase gene expression suppresses
vascular smooth muscle cell responses via the inhibition of ERK1/2
phosphorylation, cell cycle progression, and matrix
metalloproteinase-9 expression. J Biol Chem. 279:33063–33070. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang BW, Chang H, Lin S, Kuan P and Shyu
KG: Induction of matrix metalloproteinases-14 and −2 by cyclical
mechanical stretch is mediated by tumor necrosis factor-alpha in
cultured human umbilical vein endothelial cells. Cardiovasc Res.
59:460–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SJ, Cho SC, Lee EJ, Kim S, Lee SB, Lim
JH, Choi YH, Kim WJ and Moon SK: Interleukin-20 promotes migration
of bladder cancer cells through extracellular signal-regulated
kinase (ERK)-mediated MMP-9 protein expression leading to nuclear
factor (NF-κB) activation by inducing the up-regulation of
p21(WAF1) protein expression. J Biol Chem. 288:5539–5552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong Y, Qiu W, Ning X, Yang X, Liu L, Wang
Z, Lin J, Li X and Guo Y: CCDC34 is up-regulated in bladder cancer
and regulates bladder cancer cell proliferation, apoptosis and
migration. Oncotarget. 6:25856–25867. 2015. View Article : Google Scholar : PubMed/NCBI
|