Introduction
Cartilage tumors are a common type of bone tumor and
the second most prevalent primary skeletal tumors (1,2). The
main cartilage tumors include osteochondroma, enchondroma,
chondroblastoma, chondromyxoid fibroma and chondrosarcoma.
Chondrosarcoma is a malignant cartilage tumor, highly resistant to
conventional chemotherapy and radiotherapy, and commonly surgical
resection is the only effective treatment (3,4). Due
to the absence of an effective adjuvant therapy, this mesenchymal
malignancy has a poor prognosis. The invasion and metastasis
mechanism of malignant tumors is a complex process, which includes
decomposing the extracellular matrix (ECM), degrading the basal
membrane and invading lymphatic and blood vessels. Chondrosarcoma
invades and metastasizes through this mechanism (5) and inhibiting the ability of
chondrosarcoma cells to decompose ECM can prohibit the relapse and
metastasis of chondrosarcoma.
Matrix metalloproteinases (MMPs) and tissue
inhibitors of matrix metalloproteinases (TIMPs) serve a significant
role in decomposing the ECM. Previous studies have reported that
MMPs decompose all components of the ECM (6,7).
According to their structure and substrate specificity they can be
divided into subgroups of collagenases, gelatinases, stromelysins,
membrane-type MMPs and other MMPs. MMP-1 and MMP-13 are
collagenases, whose biochemical characterization is to decompose
type I, II and III collagen (8).
TIMP-1 is the tissue inhibitor of MMP-1 and MMP-13. It has been
reported that MMPs were highly expressed in some tumor tissues,
such as tumors of the digestive system, the kidneys, ovaries and
lungs (9–11). These tumor cells likely enhance
their invasive capability through MMPs to decompose ECM. It has
been demonstrated that MMP-1 and MMP-13 are highly expressed in
chondrosarcoma (12) and these
high expression levels contribute to the decomposition of type II
collagen.
The mitogen-activated protein kinase (MAPK) pathways
are focal points for diverse extracellular stimuli and regulate the
activities of kinases or transcription factors downstream, thereby
influencing gene expression and cellular responses (13). Three of the most well-known MAPK
pathways have been characterized in detail: Extracellular
signal-regulated kinase 1/2 (ERK1/2), c-jun N-terminal kinases
(JNK) and the p38 pathway (14).
MAPK pathways regulate a number of transcription factors such as
activator protein-1 and nuclear factor-κB, which act independently
or in concert to regulate numerous genes involved in the regulation
of urinary plasminogen activator (u-PA) and MMP expression
(15). Previous studies have
demonstrated that MAPK pathways regulated MMP expression in breast
carcinoma, non-small cell lung carcinoma and liver cancer, however
the association between MAPK pathways and MMPs in chondrosarcoma
remains unclear (16–18). In the present study, the expression
levels of collagenases (MMP-1 and MMP-13) and MAPK pathways in
chondrosarcoma specimens and chondrosarcoma cells were compared on
order to investigate whether MAPK-dependent induction of MMP
expression can enhance the invasive capability of chondrosarcoma by
decomposing cartilage matrix. The present study explored the
relative associations between MAPK pathways and MMPs in
chondrosarcoma, and provides a theoretical basis for curing
chondrosarcoma with MAPK inhibitors.
Materials and methods
Patients and specimen preparation
A total of 79 histologically examined surgical
specimens were obtained from the patients of the Third Hospital of
Hebei Medical University (Shijiazhuang, China) between 2012 and
2014, after approval by the ethics committee of the Third Hospital
of Hebei Medical University. All specimens were divided into three
groups according to degree of malignancy: Normal cartilage tissue
(n=17), enchondroma tissue (n=25) and chondrosarcoma tissue (n=37).
The tissue specimens were obtained from surgical and pathological
records at the hospital. Enchondroma tissue was obtained from 15
male and 10 female patients (age, 34–62 years; mean age, 48 years).
Chondrosarcoma tissue was obtained from 21 males and 16 females
patients (age, 38–72 years; mean age of 56 years). Specimens of
normal cartilage tissue were obtained from knee joints of patients.
Fresh pathological specimens were stored in an ultra-low
temperature freezer before western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). All
specimens were fixed in 4% paraformaldehyde in 0.01 mol/l
phosphate-buffered saline (PBS) before embedding in paraffin
wax.
Cell culture
The human chondrosarcoma cell line (SW1353) was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
and a-modified Eagle's medium supplemented with 10% fetal bovine
serum and maintained at 37°C in a humidified atmosphere of 5%
CO2. The cells were incubated for 48 h and were
pretreated with the ERK1/2 inhibitor, U0126 (10 µmol/l;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), JNK inhibitor,
SP600125 (20 µmol/l; Sigma-Aldrich; Merck Millipore) and p38
inhibitor, SB203580 (10 µmol/l; Sigma-Aldrich; Merck Millipore)
(19). All the compounds were
dissolved in dimethyl sulfoxide (Me2SO).
Immunohistochemistry
Paraffin wax-embedded renal tissue sections (4 µm)
were dewaxed with xylene and rehydrated in graded ethanol
solutions. Endogenous horseradish peroxidase activity was blocked
by pretreatment with 3% H2O2 for 10 min at
room temperature. Antigen recovery was performed using a microwave.
To block nonspecific binding, the sections were incubated at 37°C
for 30 min in PBS containing 10% goat serum. Finally, the sections
were incubated with rabbit polyclonal antibodies against MMP-1
(catalog no. sc-8834; 1:50; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), MMP-13 (catalog no. sc-30073; 1:100; Santa Cruz
Biotechnology, Inc.), TIMP-1 (catalog no. bs-0415R; 1:50; Bioss
Biotechnology, Beijing, China) and type II collagen (catalog no.
sc-28887; 1:50 dilution; Santa Cruz Biotechnology, Inc.), overnight
at 4°C. On the following day, after incubation with the PV-9000
Polymer Detection System (OriGene Technologies, Inc., Beijing,
China), the sections were stained with 3,3-diaminobenzidine and
counterstained with hematoxylin. Negative controls were obtained by
replacing the primary antibody with PBS.
Western blot analysis
Pathological specimens and SW1353 cells were lysed
and protein concentrations were measured by Coomassie brilliant
blue assay. Proteins were loaded and separated on a 10%
SDS-polyacrylamide gel and then transferred to a polyvinylidene
difluoride membrane. The membrane was blocked with 5% dried milk
and incubated overnight at 4°C with rabbit anti-MMP-1 (1:200),
MMP-13 (1:500) TIMP-1 (1:200), type II collagen (1:200)
phosphorylated (p)-ERK1/2 (catalog no. sc-101760; 1:100; Santa Cruz
Biotechnology, Inc.), ERK1/2 (catalog no. 4695; 1:200; Cell
Signaling Technology, Inc., Danvers, MA, USA), p-JNK (catalog no.
sc-135642; 1:100; Santa Cruz Biotechnology, Inc.), JNK (catalog no.
9252; 1:200; Cell Signaling Technology, Inc.), p-p38 (catalog no.
4511; 1:200; Cell Signaling Technology, Inc.), p38 (catalog no.
sc-7149; 1:100; Santa Cruz Biotechnology, Inc.) and β-actin
(catalog no. bs-0061R; 1:1,000; Bioss Biotechnology). After
washing, the membrane was incubated with a horseradish
peroxidase-conjugated chemiluminescent secondary antibody (catalog
no. NA932; 1:1,000; GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA). Proteins were quantified following acquisition and analysis
of the image using the LabWorks software, version 4.5, with the UVP
Imaging Station, (UVP, Inc., Upland, CA, USA). Protein expression
was quantified by comparison with the internal control β-actin.
RT-qPCR
Total RNA was collected using TRIzol Reagent
(Sigma-Aldrich; Merck Millipore) following the manufacturer's
protocol. RNA (1 µg) was reverse transcribed using the oligo (dT)
primer in the presence of avian myeloblastosis virus reverse
transcriptase to produce cDNA. Successful cDNA production was
verified by PCR using the PCR Master Mix (Eppendorf North America,
Westbury, NY, USA). The primers were supplied as a pre-optimized
single tube primer/probe Gene Expression Assay (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The two
oligonucleotide primers were: Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), forward 5′-ACCACAGTCCATGCCATCAC-3′ and
reverse 5′-TCCACCACCCTGTTGCTGTA-3′; MMP-1, forward
5′-AAGCCAGATGCTGAAACCCTG-3′ and reverse
5′-GACCCTTGGAGACTTTGGTGAAT-3′; MMP-13, forward
5′-TTGACCACTCCAAGGACCCAG-3′ and reverse
5′-GAGGATGCAGACGCCAGAAGA-3′; TIMP-1, forward 5′-ACAGGTTTCCGGTTGG-3′
and reverse 5′-CAGGCAGGCAAAGTGAT-3′. Each PCR cycle was conducted
for 33 cycles of 30 sec at 94°C, 30 sec at 55°C and 1 min at 68°C.
The PCR products were then separated electrophoretically in a 2%
agarose DNA gel and stained with ethidium bromide; PCR reactions
were set up with Taqman Universal PCR Master Mix from Applied
Biosystems (Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA
was amplified on the 7900HT Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) at default thermal
cycling conditions: 2 min at 50°C, 10 min at 95°C for enzyme
activation and then 40 cycles of 15 sec at 95°C for denaturation
and 1 min at 60°C for annealing and extension. Results were
analyzed using the relative standard curve method of analysis/ΔCq
method of analysis (20).
Statistical analysis
Data were expressed as the mean ± standard deviation
and were analyzed using one-way analysis of variance with
Bonferroni's post hoc test by SPSS software version 13.0 (SPSS,
Inc., Chicago, IL, USA). In all cases, P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of MMP-1, MMP-13, TIMP-1
and type II collagen in pathological specimens
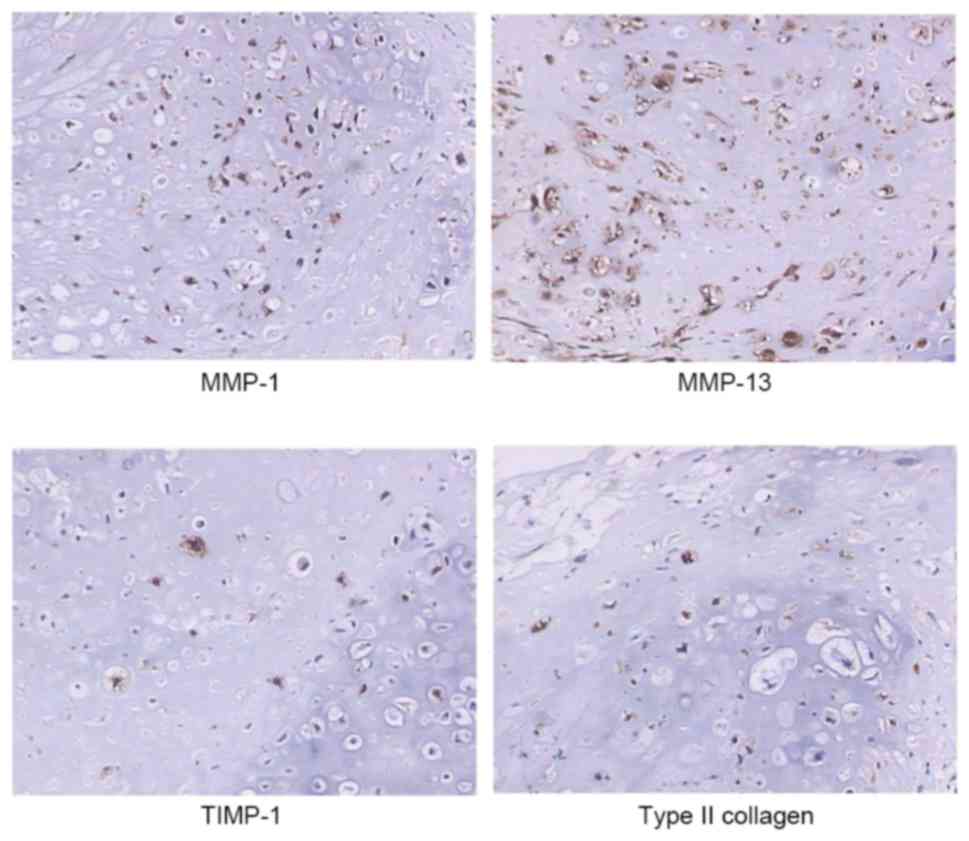
Immunohistochemical results (Fig. 1) demonstrated the protein
expression of MMP-1, MMP-13, TIMP-1 and type II collagen in the
cytoplasm of chondrosarcoma cells. Their protein levels were
evaluated in human normal cartilage, enchondroma, and
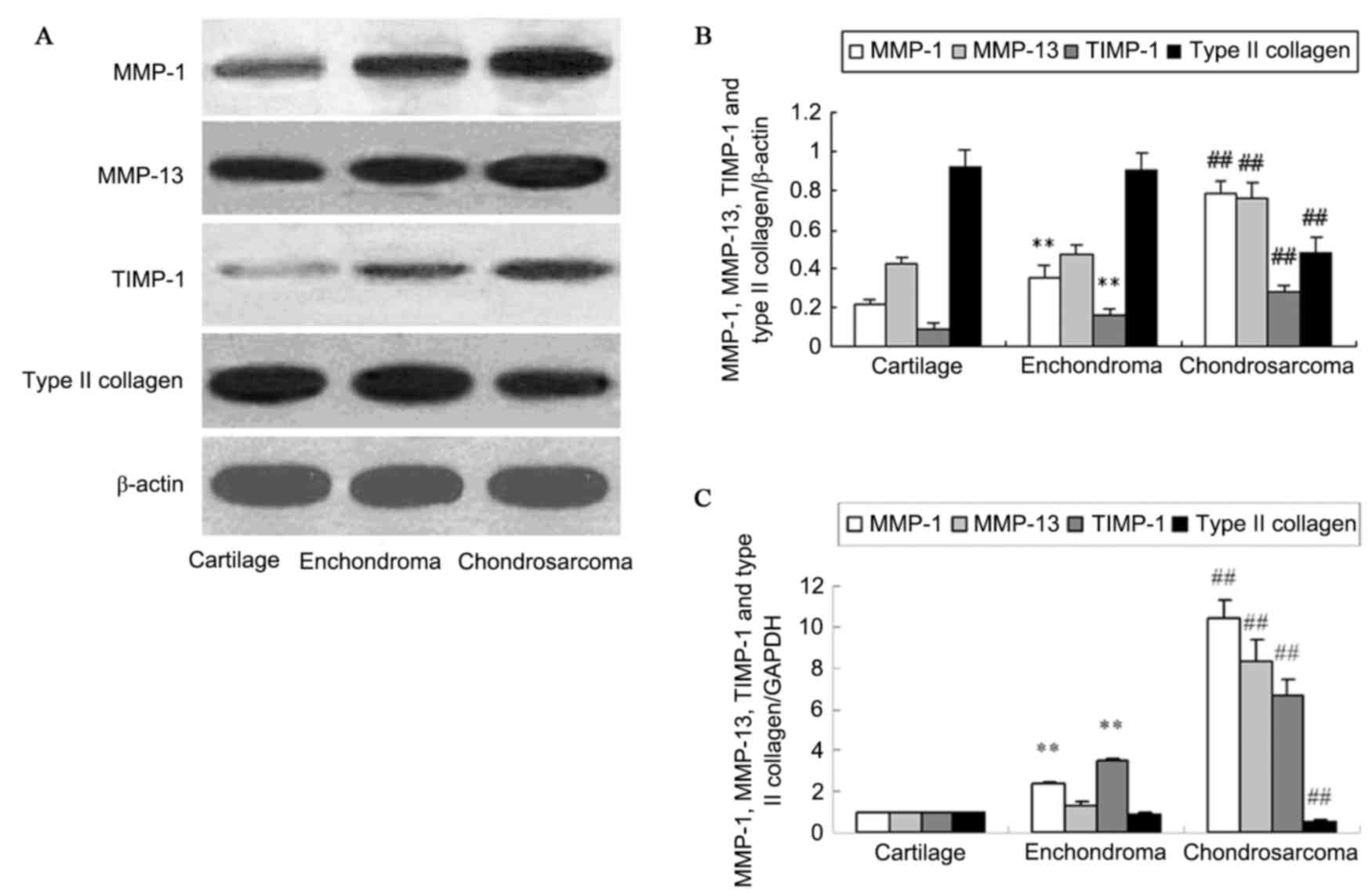
chondrosarcoma tissues by western blotting (Fig. 2A and B). Analysis of protein levels
indicated a significant increase of MMP-1 and TIMP-1 protein in the
enchondroma compared with cartilage tissue (P<0.01). No change
of MMP-13 and type II collagen protein expression was identified in
enchondroma and cartilage tissues (P>0.05). The protein
expression of MMP-1, MMP-13 and TIMP-1 in chondrosarcoma was
significantly higher than that in enchondroma (P<0.01).
Chondrosarcoma tissues exhibited a decreased type II collagen level
compared with that of enchondroma (P<0.01). RT-qPCR demonstrated
similar changes of MMP-1, MMP-13, TIMP-1 and type II collagen mRNA
in normal cartilage, enchondroma and chondrosarcoma tissues
(P<0.01; Fig. 2C).
Expression of MAPK in chondrosarcoma
specimens
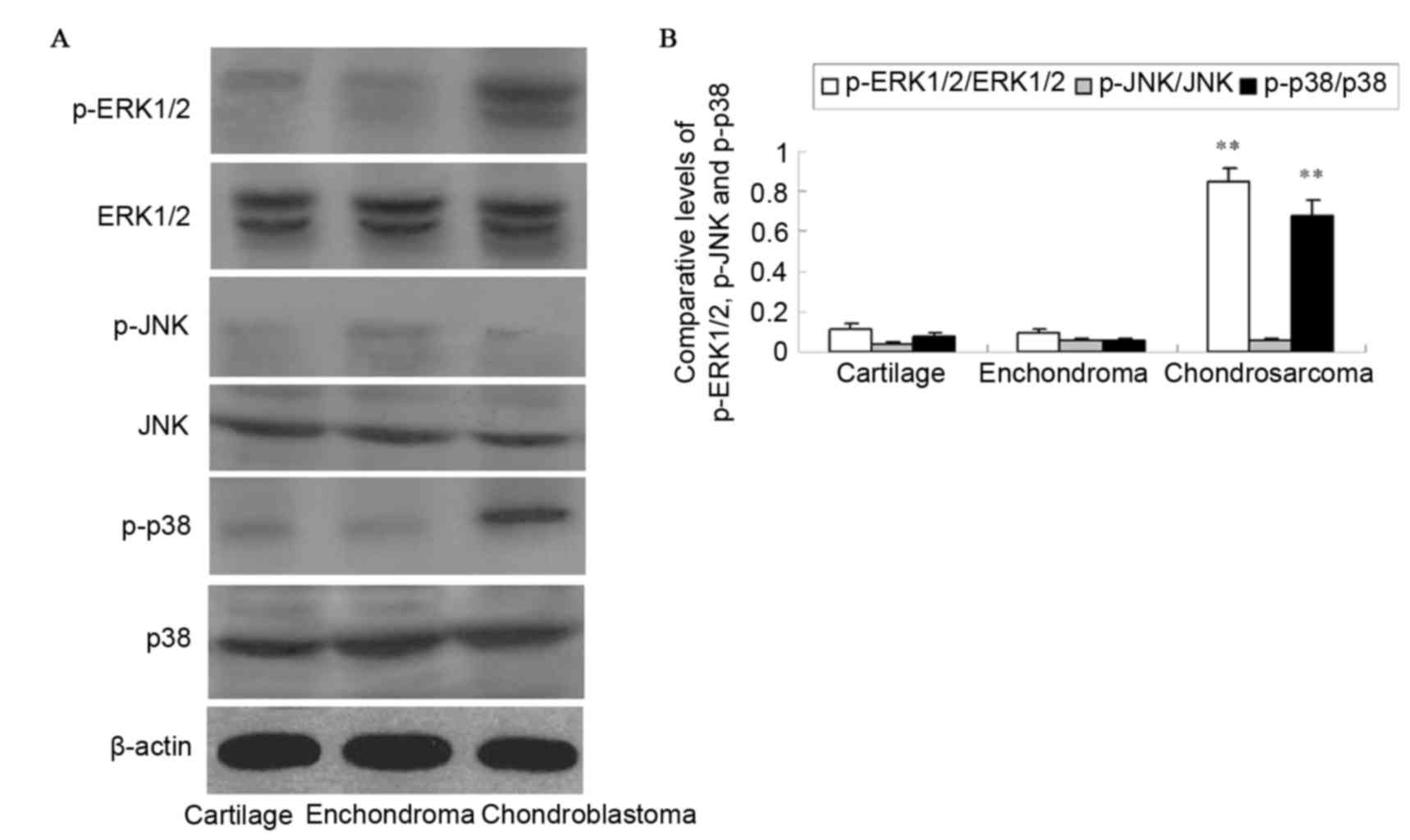
The activation of ERK1/2, JNK and p38 was detected
with antibodies against the phosphorylated forms of the kinases by
western blotting (Fig. 3). The
protein levels of p-ERK1/2 and p-p38 in chondrosarcoma were
significantly increased compared with those in enchondroma and
cartilage tissues (P<0.01). The difference in protein expression
between enchondroma and cartilage tissues was not observed as
significant (P>0.05). No activation of JNK was detected in
pathological specimens (P>0.05).
Activation of ERK1/2 and p38 in human
chondrosarcoma cells
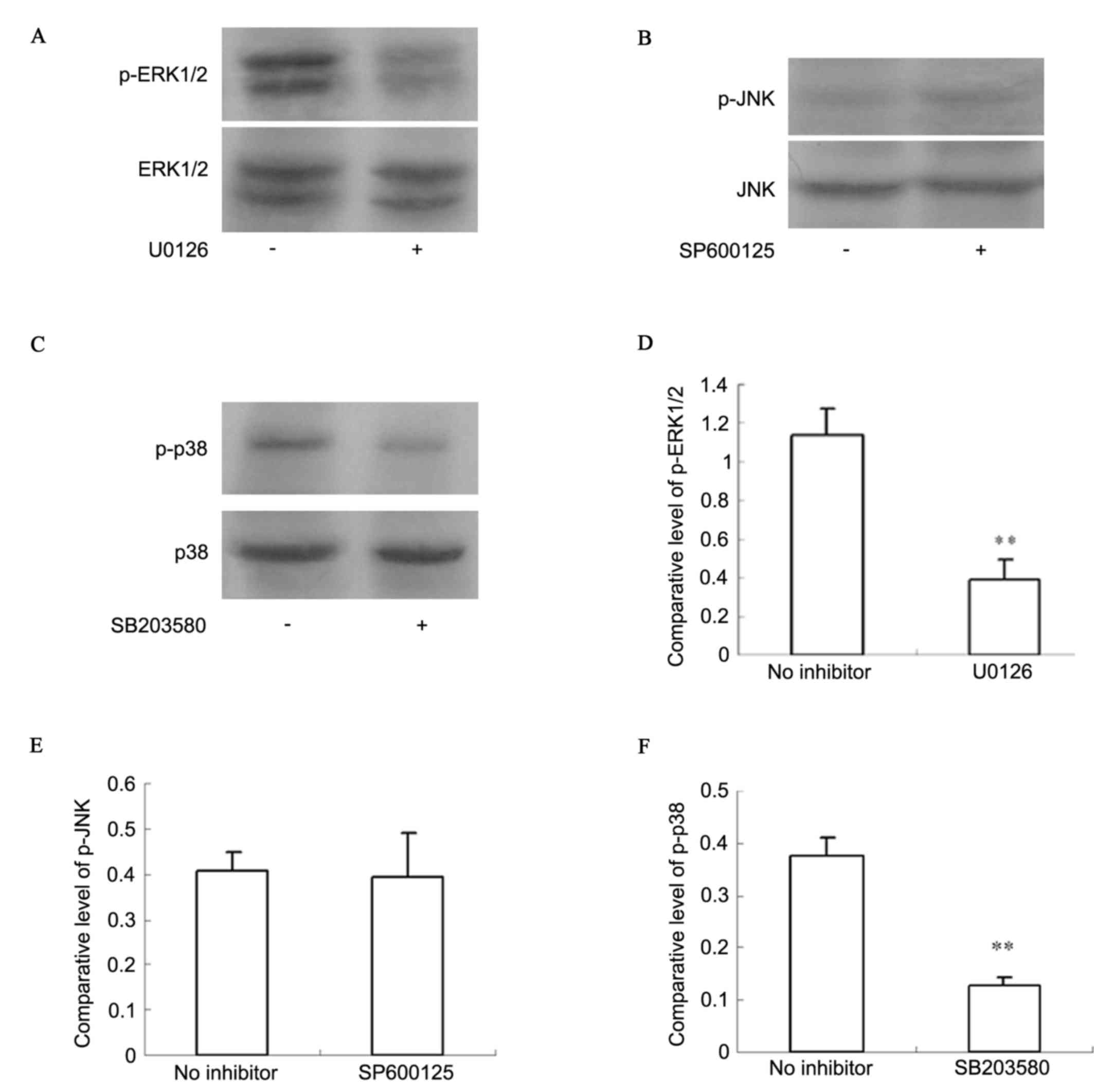
Activation of ERK1/2 and p38 was identified in
chondrosarcoma pathological specimens. Western blot analysis
(Fig. 4) indicated that p-ERK1/2
and p-p38 had higher expression levels in SW1353 cells. With an
ERK1/2 inhibitor (U0126) and a p38 inhibitor (SB203580), activation
of ERK1/2 and p38 was significantly inhibited (P<0.01).
Activation of JNK was not detected in SW1353 cells, whether
pretreated by JNK inhibitor (SP600125) or not (P>0.05).
Expression of MMP-1, MMP-13, TIMP-1
and type II collagen in human chondrosarcoma cells is dependent on
MAPK activity
The present study identified higher expression
levels of MMP-1, MMP-13 and TIMP-1 in clinical chondrosarcoma
specimens together with lower levels of type II collagen, and with
the activation of ERK1/2 and p38. Subsequently, the specific roles
of ERK1/2 and p38 pathways in the regulation of the expression of
MMP-1, MMP-13, TIMP-1 and type II collagen were investigated by
utilizing two selective chemical inhibitors; U0126 to block the
ERK1/2 pathway and SB203580 to inhibit p38 activity. Western blot
analysis (Fig. 5A and B) detected
that ERK1/2 and p38 inhibitors decreased MMP-1, MMP-13 and TIMP-1
protein expression (P<0.01). However, ERK1/2 and p38 inhibitors
increased type II collagen protein level (P<0.01). RT-qPCR
(Fig. 5C) indicated similar
results for MMP-1, MMP-13, TIMP-1 and type II collagen mRNA
expression (P<0.05 or P<0.01).
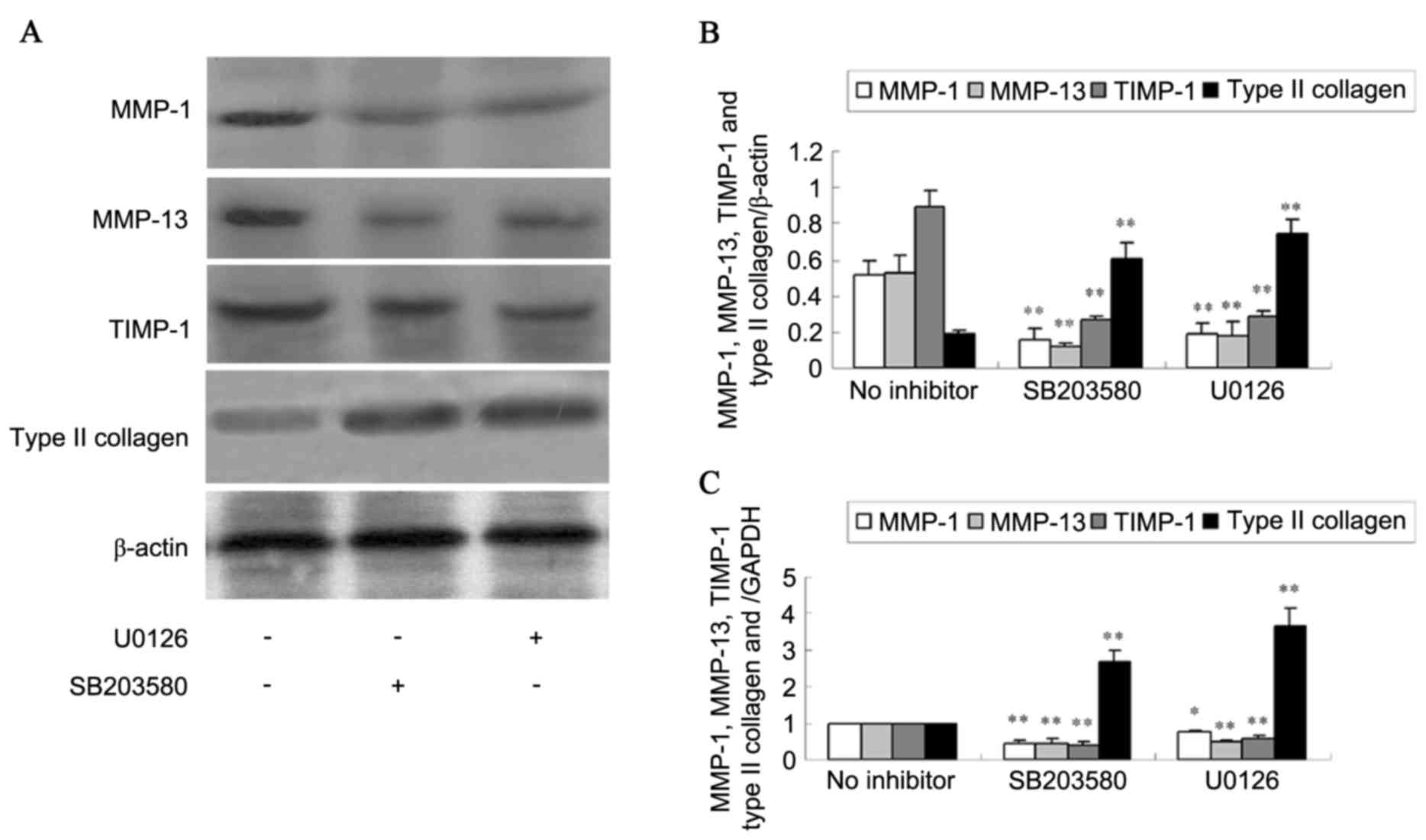 | Figure 5.Expression of MMP-1, MMP-13, TIMP-1
and type II collagen in SW1353 cells treated by MAPK inhibitors.
(A) The protein expression of MMP-1, MMP-13, TIMP-1 and type II
collagen was analyzed using western blotting and (B) the
quantification was presented. (C) The mRNA levels of MMP-1, MMP-13,
TIMP-1 and type II collagen were detected using reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as the mean ± standard deviation. *P<0.05, **P<0.01
vs. no inhibitor. MMP, matrix metalloproteinase; TIMP, tissue
inhibitor of matrix metalloproteinase; MAPK, mitogen-activated
protein kinase. |
Discussion
Unlike osteosarcoma and Ewing's sarcoma, for which
clear increases in long-term survival have been observed as the
result of the use of systemic chemotherapy, chondrosarcoma has a
poor prognosis due to the absence of an effective adjuvant therapy
(21,22). Therefore, it is important to
develop an effective adjuvant therapy to prevent chondrosarcoma
metastasis. Enzymatic degradation of ECM is one of the crucial
steps in tumor invasion and metastasis. MMPs are a family of
structurally associated zinc-dependent neutral endopeptidases that
are collectively capable of degrading essentially all ECM
components, and they are suggested to serve an important role in
ECM degradation in tumor invasion. Each type of MMP degrades a
specific component of the ECM (23,24).
TIMPs are a family of multifunctional agents,
including TIMP-1, TIMP-2, TIMP-3 and TIMP-4, which are a group of
proteinase inhibitors secreted by in vivo cells that can
produce a marked effect on various tumor types by inhibiting MMPs
(25,26). Previous studies have demonstrated
the different expression of MMPs in various types of tumor tissue
(27,28). The results of the present study
demonstrated that enchondroma had a higher expression level of
MMP-1 compared with that of normal cartilage tissue, which suggests
that in benign cartilage tumors, certain MMPs have already been
activated. Furthermore, chondrosarcoma tissue and cells had
significantly higher expression levels of MMP-1 and MMP-13.
Chondrosarcoma cells degrade cartilage matrix that contains type II
collagen through the increasing expression of MMP-1 and MMP-13,
which can enhance the invasion and metastasis of chondrosarcoma
cells.
TIMP-1 is the specific inhibitor of MMP-1 and
MMP-13, and its higher expression level can depress their activity.
It is important to maintain the relative level of MMP-1, MMP-13 and
TIMP-1 to control the proper degradation of ECM. Certain in
vitro experiments have confirmed that TIMP-1 in malignant tumor
cells can depress the expression activity of MMPs and tumor
invasion (29,30). The expression of type II collagen,
the major component in the structure of cartilage tissue, in
chondrosarcoma was decreased, which may be associated with the
degradation of MMPs.
ERK1/2 is commonly implicated in cell proliferation,
differentiation and survival (31), whereas p38 is preferentially
activated by inflammatory cytokines, cellular stresses, withdrawal
of growth factors and proapoptotic stimuli (11). Evidence for the role of MAPKs in
malignant transformation was initially provided by the observation
that constant activation of ERK1/2 by constitutively active Raf or
MAPK/ERK kinase 1/2 results in transformation of the glandular
epithelium (32). Furthermore,
previous studies have demonstrated that the ERK1/2 pathway is
activated in renal and breast carcinoma in vivo (33,34),
providing further evidence that the constant activation of ERK1/2
is involved in the malignant transformation of cells. However, the
consequences of ERK1/2 activation are cell specific, as
demonstrated by the observation that constant activation of the
ERK1/2 cascade by Raf can cause growth arrest in small cell lung
carcinoma cells (35). An
additional study demonstrated that p38 elevated the u-PA expression
level and promoted breast carcinoma invasion (36). Miao et al (37) demonstrated that p38 can encourage
colon carcinoma cell line proliferation, further influencing MMPs
levels and ECM degradation. The results of the present study also
demonstrated activation of ERK1/2 and p38 in chondrosarcoma tissue
and cells. MMP-1, MMP-13 and TIMP-1 overexpression were inhibited
by a selective ERK1/2 inhibitor U0126 and a selective p38 inhibitor
SB203580, indicating that ERK1/2 and p38 activities were essential
for the induction of MMP-1, MMP-13 and TIMP-1 expression.
Furthermore, type II collagen expression was increased by U0126 and
SB203580. However, activation of the JNK pathway was not essential
for enhancement of MMP-1, MMP-13 and TIMP-1 expression in
chondrosarcoma. The results indicated that inhibition of the
activity of distinct MAPKs may serve as a potent method to inhibit
MMPs and increase type II collagen expression in
chondrosarcoma.
The present study demonstrated that expression of
MMP-1 and MMP-13 is dependent on the activity of MAPK pathways in
the succession of chondrosarcoma invasion and metastasis, and has
provided the theoretical basis for chondrosarcoma therapy with MAPK
inhibitors. The identification of targets to inhibit ECM
degradation by MMPs will aid in the treatment of
chondrosarcoma.
Acknowledgements
The authors would like to thank Dr Si-yuan Liu (The
Third Hospital of Hebei Medical University, Shijizhuang, China) for
collecting the pathological specimens.
References
|
1
|
Leddy LR and Holmes RE: Chondrosarcoma of
bone. Cancer Treat Res. 162:117–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
van Rijswijk CS, Krol AD, Taminiau AH and Bovée JV: The clinical
approach towards chondrosarcoma. Oncologist. 13:320–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Gompel JJ and Janus JR: Chordoma and
chondrosarcoma. Otolaryngol Clin North Am. 48:501–514. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan J, Dutton CM and Scully SP: RNAi
mediated MMP-1 silencing inhibits human chondrosarcoma invasion. J
Orthop Res. 32:1467–1474. 2005. View Article : Google Scholar
|
|
5
|
Sakimura R, Tanaka K, Yamamoto S,
Matsunobu T, Li X, Hanada M, Okada T, Nakamura T, Li Y and Iwamoto
Y: The effects of histone deacetylase inhibitors on the induction
of differentiation in chondrosarcoma cells. Clin Cancer Res.
13:275–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taniwaki K, Fukamachi H, Komori K, Ohtake
Y, Nonaka T, Sakamoto T, Shiomi T, Okada Y, Itoh T, Itohara S, et
al: Stroma-derived matrix metalloproteinase (MMP)-2 promotes
membrane type 1-MMP-dependent tumor growth in mice. Cancer Res.
67:4311–4319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JM, Kim A, Oh JH and Chung AS:
Methylseleninic acid inhibits PMA-stimulated pro-MMP-2 activation
mediated by MT1-MMP expression and further tumor invasion through
suppression of NF-kappaB activation. Carcinogenesis. 28:837–847.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiu YC, Yang RS, Hsieh KH, Fong YC, Way
TD, Lee TS, Wu HC, Fu WM and Tang CH: Stromal cell-derived factor-1
induces matrix metalloprotease-13 expression in human chondrocytes.
Mol Pharmacol. 72:695–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon JW, Choi JH, Lee SK, Lee YW, Lee JO,
Kim N, Lee HJ, Seo JS, Kim J, Kim HS, et al: Promoter
hypermethylation of membrane type 3 matrix metalloproteinase is
associated with cell migration in colorectal adenocarcinoma. Cancer
Genet. 208:261–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Yin P, DI D, Luo G, Zheng L, Wei
J, Zhang J, Shi Y, Zhang J and Xu N: IL-6 regulates MMP-10
expression via JAK2/STAT3 signaling pathway in a human lung
adenocarcinoma cell line. Anticancer Res. 29:4497–4501.
2009.PubMed/NCBI
|
|
12
|
Galoian KA, Garamszegi N, Garamszegi SP
and Scully SP: Molecular mechanism of tenascin-C action on matrix
metalloproteinase-1 invasive potential. Exp Biol Med (Maywood).
232:515–522. 2007.PubMed/NCBI
|
|
13
|
Pritchard AL and Hayward NK: Molecular
pathways: Mitogen-activated protein kinase pathway mutations and
drug resistance. Clin Cancer Res. 19:2301–2309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klein AM, Zaganjor E and Cobb MH:
Chromatin-tethered MAPKs. Curr Opin Cell Biol. 25:272–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PN, Hsieh YS, Chiou HL and Chu SC:
Silibinin inhibits cell invasion through inactivation of both
PI3K-Akt and MAPK signaling pathways. Chem Biol Interact.
156:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozen E, Gozukizil A, Erdal E, Uren A,
Bottaro DP and Atabey N: Heparin inhibits Hepatocyte Growth Factor
induced motility and invasion of hepatocellular carcinoma cells
through early growth response protein 1. PLoS One. 7:e427172012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao HJ, Liu T, Mao X, Han SX, Liang RX,
Hui LQ, Cao CY, You Y and Zhang LZ: Fructus phyllanthi tannin
fraction induces apoptosis and inhibits migration and invasion of
human lung squamous carcinoma cells in vitro via MAPK/MMP pathways.
Acta Pharmacol Sin. 36:758–768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen M, Meisser A, Haenggeli L and
Bischof P: Involvement of MAPK pathway in TNF-alpha-induced MMP-9
expression in human trophoblastic cells. Mol Hum Reprod.
12:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative pcr and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwamoto Y: Diagnosis and treatment of
Ewing's sarcoma. Jpn J Clin Oncol. 37:79–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan TW, Yang WH, Lin YT, Hsu SF, Li TM,
Kao ST, Chen WC, Fong YC and Tang CH: Cyr61 increases migration and
MMP-13 expression via alphavbeta3 integrin, FAK, ERK and
AP-1-dependent pathway in human chondrosarcoma cells.
Carcinogenesis. 30:258–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarín C, Gomez M, Calvo E, López JA and
Zaragoza C: Endothelial nitric oxide deficiency reduces
MMP-13-mediated cleavage of ICAM-1 in vascular endothelium: A role
in atherosclerosis. Arterioscler Thromb Vasc Biol. 29:27–32. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daniele A, Zito AF, Giannelli G, Divella
R, Asselti M, Mazzocca A, Paradiso A and Quaranta M: Expression of
metalloproteinases MMP-2 and MMP-9 in sentinel lymph node and serum
of patients with metastatic and non-metastatic breast cancer.
Anticancer Res. 30:3521–3527. 2010.PubMed/NCBI
|
|
25
|
Määtta M, Talvensaari-Mattila A,
Turpeenniemi-Hujanen T and Santala M: Matrix metalloproteinase-2
(MMP-2) and −9 (MMP-9) and their tissue inhibitors (TIMP-1 and
TIMP-2) in differential diagnosis between low malignant potential
(LMP) and malignant ovarian tumours. Anticancer Res. 27:2753–2758.
2007.PubMed/NCBI
|
|
26
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Modulation of uPA, MMPs and their inhibitors by a novel
nutrient mixture in human colorectal, pancreatic and hepatic
carcinoma cell lines. Int J Oncol. 47:370–376. 2015.PubMed/NCBI
|
|
27
|
Kirimlioğlu H, Türkmen I, Başsüllü N,
Dirican A, Karadağ N and Kirimlioğlu V: The expression of matrix
metalloproteinases in intrahepatic cholangiocarcinoma, hilar
(Klatskin tumor), middle and distal extrahepatic
cholangiocarcinoma, gallbladder cancer, and ampullary carcinoma:
Role of matrix metalloproteinases in tumor progression and
prognosis. Turk J Gastroenterol. 20:41–47. 2009.PubMed/NCBI
|
|
28
|
Tang CH, Tan TW, Fu WM and Yang RS:
Involvement of matrix metalloproteinase-9 in stromal cell-derived
factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis.
29:35–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Safranek J, Pesta M, Holubec L, Kulda V,
Dreslerova J, Vrzalova J, Topolcan O, Pesek M, Finek J and Treska
V: Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung
tissue of patients with non-small cell lung cancer (NSCLC) and
benign pulmonary disease. Anticancer Res. 29:2513–2517.
2009.PubMed/NCBI
|
|
30
|
Yamada T, Oshima T, Yoshihara K, Tamura S,
Kanazawa A, Inagaki D, Yamamoto N, Sato T, Fujii S, Numata K, et
al: Overexpression of MMP-13 gene in colorectal cancer with liver
metastasis. Anticancer Res. 30:2693–2699. 2010.PubMed/NCBI
|
|
31
|
Vogel C, Chan A, Gril B, Kim SB,
Kurebayashi J, Liu L, Lu YS and Moon H: Management of
ErbB2-positive breast cancer: Insights from preclinical and
clinical studies with lapatinib. Jpn J Clin Oncol. 40:999–1013.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellington AA, Berhow MA and Singletary KW:
Inhibition of Akt signaling and enhanced ERK1/2 activity are
involved in induction of macroautophagy by triterpenoid B-group
soyasaponins in colon cancer cells. Carcinogenesis. 27:298–306.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wollmann W, Goodman ML, Bhat-Nakshatri P,
Kishimoto H, Goulet RJ Jr, Mehrotra S, Morimiya A, Badve S and
Nakshatri H: The macrophage inhibitory cytokine integrates AKT/PKB
and MAP kinase signaling pathways in breast cancer cells.
Carcinogenesis. 26:900–907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu K, Xu L, Zhang L, Lin Z and Hou J: High
jagged1 expression predicts poor outcome in clear cell renal cell
carcinoma. Jpn J Clin Oncol. 41:411–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Seranno S and Meuwissen R: Progress and
applications of mouse models for human lung cancer. Eur Respir J.
35:426–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Mayer JA, Krisko TI, Speers CW,
Wang T, Hilsenbeck SG and Brown PH: Inhibition of the p38 kinase
suppresses the proliferation of human ER-negative breast cancer
cells. Cancer Res. 69:8853–8861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miao Y, Zhang Y, Wan H, Chen L and Wang F:
GABA-receptor agonist, propofol inhibits invasion of colon
carcinoma cells. Biomed Pharmacother. 64:583–588. 2010. View Article : Google Scholar : PubMed/NCBI
|