Introduction
Colorectal cancer is one of the most common types of
malignant tumor, with the third highest mortality rate of all
cancers (1). Statistics showed
that in 2008, there were nearly 1 million new cases of colorectal
cancer worldwide and approximately 500,000-related fatalities
(2). In recent years, with social
and economic development, changes of the dietary patterns, aging of
the population and rapid development of endoscopic techniques, the
diagnostic rate of colorectal cancer in China has increased, and
become a serious health problem (3). Compared with 1990s and 1970s, the
morbidity of colorectal cancer in China in urban areas was
increased by 32.0%, while that in rural areas was increased by 8.5%
(4). Furthermore, it has become
the most common type of malignant cancer in China.
Occurrence and progression of tumors is an
evolutionary process with various factors and steps in which gene
expression abnormality is essential (5). With an increasing number of studies
on the pathogenic mechanisms of colorectal cancer, there has been a
focus on molecular markers that are vital in early diagnosis,
clinical stages, individual-based treatment, targeting tumors and
prognosis monitoring. The first identification of a microRNA
(miRNA) molecule was lin-4 in Caenorhabditis elegans
(6). Thus far, nearly 1,500 miRNA
sequences have been determined (6). miRNAs are important in tumor
occurrence and progression (7). At
present, studies of the correlation between certain miRNAs and
cancer have attracted great attention. Numerous studies provided
new ideas for tumor treatment.
JA (jasmonic acid) is a type of plant hormone, whose
methyl derivative is methyl jasmonate. Methyl jasmonate was
initially obtained by separation from jasmine and Tunisian rosemary
oils (8), however, it is now known
to exist universally in plants to stimulate the expression of plant
defence genes and their chemical defence. Studies on methyl
jasmonate were previously restricted to botany and it was
demonstrated to be involved in accelerating organic senescence,
inducing fruit ripening and response of plant diseases and insect
pests (9). However, it has also
been reported that methyl jasmonate could induce cell
differentiation of myelogenous leukemia and inhibit its
proliferation (10). In addition,
methyl jasmonate could induce differentiation and apoptosis of
lymphocytic leukemia cells but did not damage normal lymphocytes
(10,11). The present study demonstrated the
anticancer effect of methyl jasmonate-induced apoptosis of human
colorectal cancer. Furthermore, the anticancer effect of methyl
jasmonate on human colorectal cancer was shown to occur via
downregulation of EZH2 expression by miR-101. Data may suggest that
methyl jasmonate may be developed as a novel drug for the treatment
of colorectal cancer.
Materials and methods
Reagents
RPMI-1640 was purchased from PAN Biotech (Aidenbach,
Germany). Fetal bovine serum (FBS) was purchased from HyClone
(Logan, UT, USA). Cell Counting Kit-8 assay (CCK-8) was obtained
from Hangzhou Evergreen Company (Hangzhou, China). Fluorescein
isothiocyanate-conjugated Annexin V (Annexin V-FITC)/propidium
iodide (PI) was obtained from BD Pharmingen (San Diego, CA, USA).
Bicinchoninic acid (BCA) Protein Assay kit and Lipofectamine 2000
were purchased from Thermo Fisher Scientific Inc. (Waltham, MA,
USA). TRIzol reagent was from Invitrogen Thermo Fisher Scientific
Inc. cDNA Synthesis kit was purchased from Takara Biotechnology
Inc. (Dalian, China).
Cell culture
SW620 human colorectal cancer cells were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and cultured with RPMI-1640 and 10% FBS at 37°C in an
atmosphere of 5% CO2.
In vitro cell viability assay
SW620 cells were plated at a density of
1×104 cells per well in 96-well plates. Briefly, after a
12, 24 or 48 h drug treatment with control, 0.5, 0.75, 1.5 or 2.0
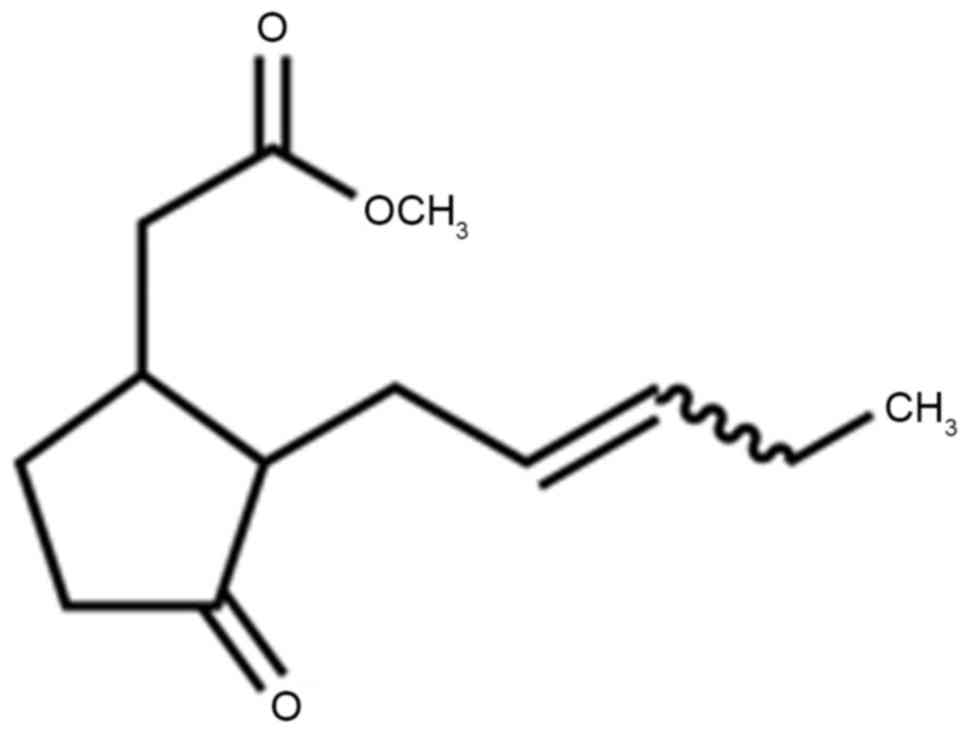
mM methyl jasmonate (structure shown in Fig. 1), SW620 cells were incubated with
10 µl CCK-8 for additional 2 h at 37°C in the dark. Absorbance of
SW620 cells was measured on an Opsys MR spectrophotometer (DYNEX
Technologies, Denkendorf, Germany) at 450 nm.
Apoptotic cells
SW620 cells were plated at a density of
1–2×106 cells per well in 96-well plates. Briefly, after
a 24 h drug treatment with control, 0.5, 0.75 or 1.5 mM methyl
jasmonate, SW620 cells were incubated with Annexin V-FITC and PI
for 30 min in the dark. Apoptotic cells were analyzed by flow
cytometry (FACScan, Becton Dickinson, Mountain View, CA, USA).
Western blot analysis
SW620 cells were plated at a density of
1–2×106 cells per well in 96-well plates. Briefly, after
a 24 h drug treatment with control, 0.5, 0.75 or 1.5 mM methyl
jasmonate, SW620 cells were incubated with 1X cell lysis buffer
(Promega Corporation, Madison, WI, USA). Protein concentration was
measured using a BCA Protein Assay kit. Equal quantities of total
proteins (80 µg) were boiled, separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and blotted onto
polyvinylidene fluoride membranes (Millipore, Darmstadt, Germany).
Following alternative immunoblot analysis with monoclonal mouse
anti-human caspase-3 (1:2,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; cat. no. sc-56050), monoclonal mouse anti-human
EZH2 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA;
cat. no. 3147) and monoclonal mouse anti-human β-actin (1:5,000;
Santa Cruz Biotechnology Inc.; cat. no. sc-130300). Immunoreactive
bands were incubated using the specific goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:5,000;
Beyotime Institute of Biotechnology, Haimen, China; cat. no. A0216)
and the enhanced chemiluminescence system (Amersham Biosciences,
Piscataway, NJ, USA). Band intensities were quantified using the
Image J 2.1.4.7 software (National Institutes of Health, Bethesda,
MD, USA)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
SW620 cells were plated at a density of
1–2×106 cells per well in 96-well plates. Briefly, after
a 24 h drug treatment with control, 0.5, 0.75 or 1.5 mM methyl
jasmonate, SW620 cells were incubated with the TRIzol reagent to
isolate Total RNA. The Transcriptor First Strand cDNA Synthesis kit
(Takara Biotechnology Inc., Dalian, China) was used to transcribe
cDNA from 1–2 µg total RNA. qPCR was performed with 500 ng-1 µg
cDNA using Applied Biosystems 7,700 HT Real-Time PCR System (Thermo
Fisher Scientific, Inc.). PCR reactions were performed under the
following conditions: 40 cycles at 95°C for 15 sec, at 60°C for 40
sec and 72°C for 30 sec analysis was performed at the end. The PCR
primers were as follows: miR-101:
5′-TGCTGTACAGTACTGTGATACGAGTTTTGGCCACTGACTGACTTCAGTTAACAGTACTGTA-3′
and
5′-CCTGTACAGTACTGTTAACTGAAGTCAGTCAGTGGCCAAAACTTCAGTTATCACAGTACTGTAC-3′;
U6: 5′-CTCGCTTCGGCAGCACATA-3′ and 5′-AACGCTTCACGAATTTGCGT-3′. The
relative expression levels were calculated using the
2−∆∆Cq method (12)
Pre-miR-101 construct and stable
transfection
Pre-miR-101-EGFP (5′-TACAGTACTGTGATAACTGAA-3′) and
negative control-EGFP were structured (GenePharma Co., Ltd.,
Shanghai, China) and were transfected into SW620 cells using
Lipofectamine 2000.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Continuous variables were
expressed as the mean ± standard error of the mean. Statistical
comparison between groups was conducted using one way analysis of
variance followed by the Tukey-Kramer method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Methyl jasmonate induces growth
inhibition of human colorectal cancer cells
In order to confirm the anticancer effect of methyl
jasmonate on cell growth of human colorectal cancer cells, the cell
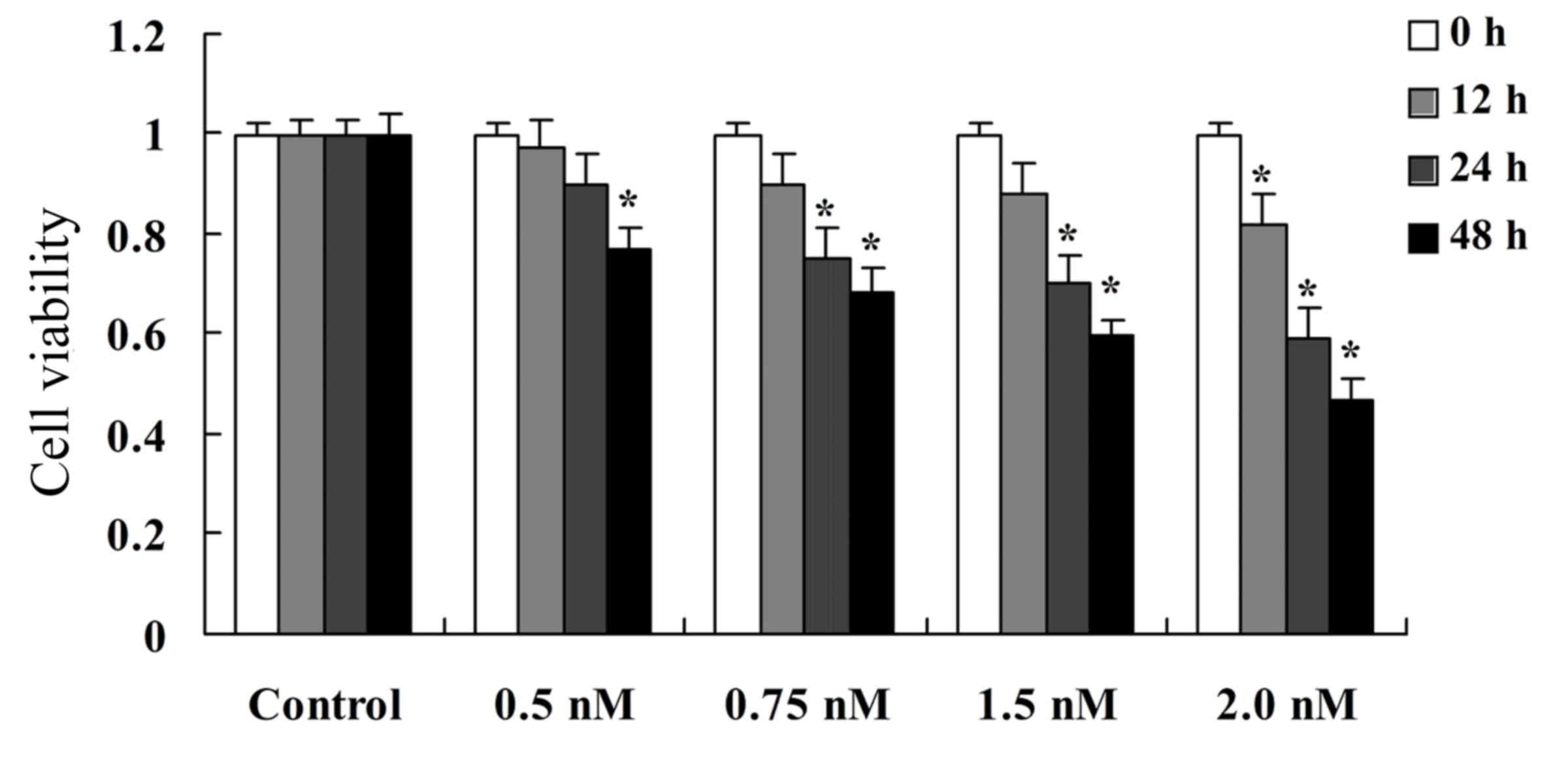
viability of SW620 was determined using CCK-8. The results
indicated that 0.5, 0.75, 1.5 and 2.0 nM methyl jasmonate
significantly inhibited cell viability of SW620 cells in a dose-
and time-dependent manner, when compared with the untreated control
(P=0.0094, P=0.0082, P=0.0042 and P=0.0028, respectively; Fig. 2). This suggests that methyl
jasmonate can potentiate the inhibitory effect on the growth of
colorectal cancer cells.
Methyl jasmonate induces apoptosis of
human colorectal cancer cells
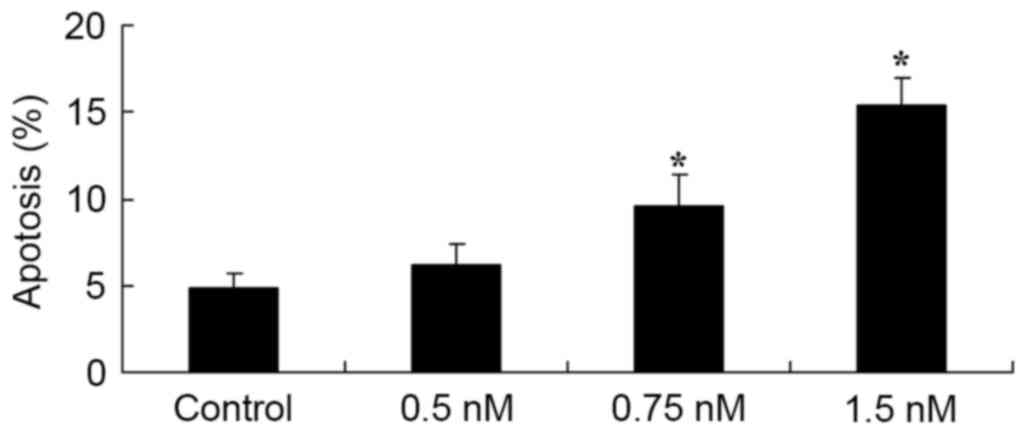
To confirm that cell apoptosis was involved in
methyl jasmonate-induced effect on cell growth of human colorectal
cancer cells, cell apoptosis in SW620 cells was assessed using
(FITC)-conjugated Annexin V (Annexin V-FITC)/propidium iodide (PI)
kit. SW620 cell staining indicated that the administration of 0.75
and 1.5 mM methyl jasmonate increased cell apoptosis of SW620 cells
in a dose-dependent manner, when compared with the untreated
control (P=0.0018 and P=0.0008, Fig.
3).
Methyl jasmonate induces activation of
caspase-3 expression
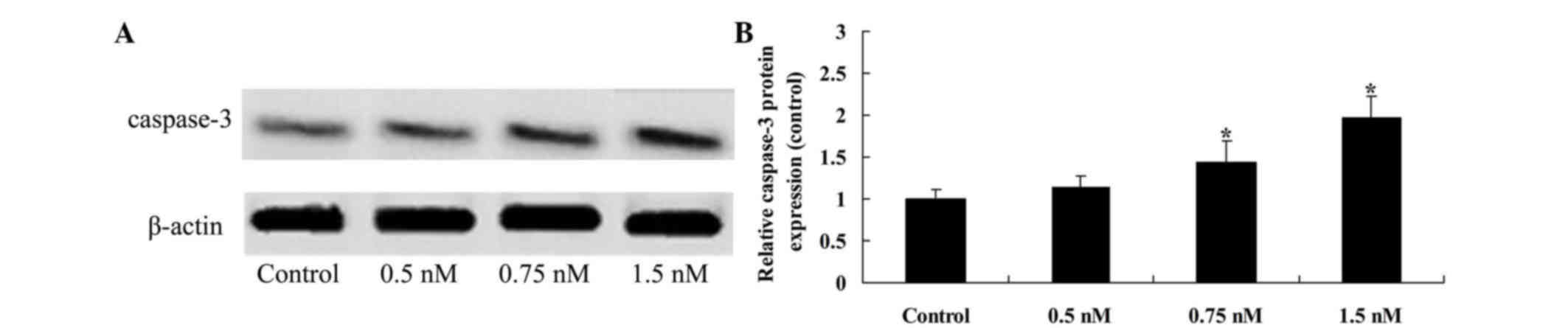
The protein expression of caspase-3 was then
examined by western blot analysis, the critical executioner of
apoptosis. The results indicated that 0.75 and 2.0 mM methyl
jasmonate induced the production of caspase-3 in a dose-dependent
manner in SW620 cells, when compared with the untreated control
(P=0.0034 and P=0.0018, Fig.
4).
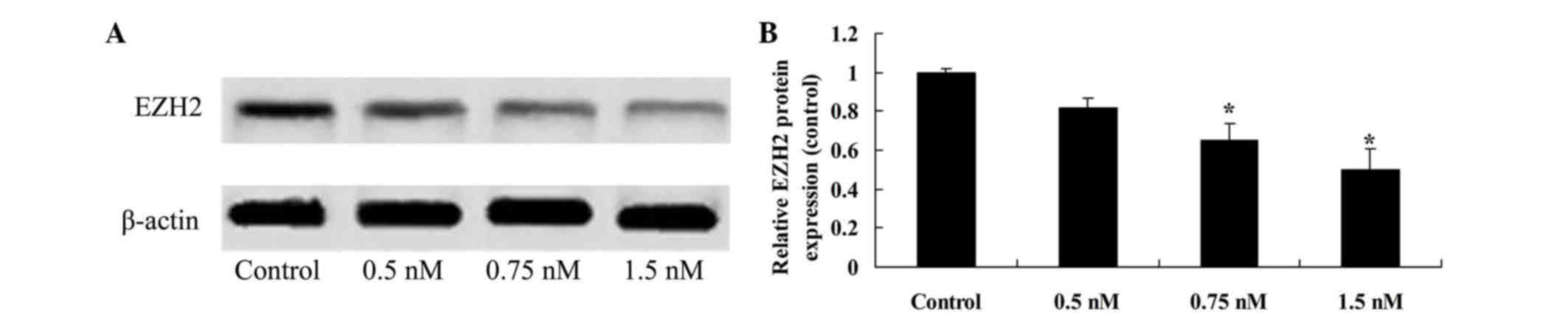
Methyl jasmonate induces inhibition of
EZH2 expression
To confirm the effect of methyl jasmonate on EZH2
protein expression of SW620 cells, EZH2 protein expression was
measured using western blot analysis. It was demonstrated that 0.75
and 2.0 mM methyl jasmonate suppressed the EZH2 protein expression
of SW620 cells, when compared with the untreated control (P=0.0034
and P=0.0018, Fig. 5).
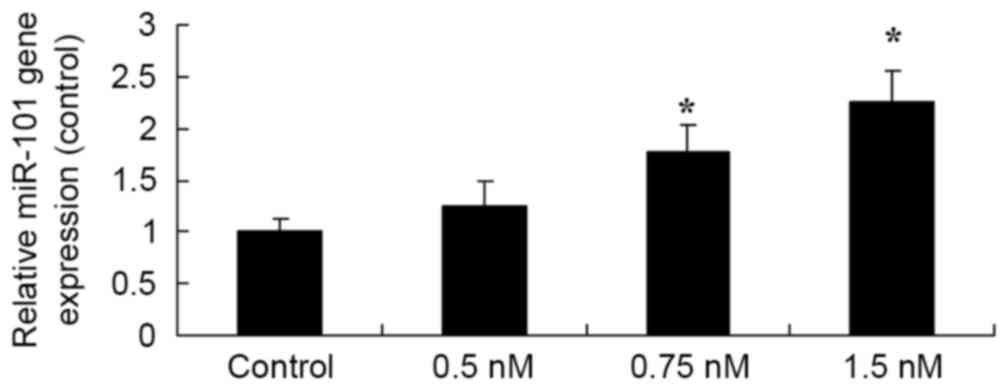
Methyl jasmonate induces upregulation
of miR-101 expression
In order to ascertain the effect of methyl jasmonate
on mediating miR-101 expression, qPCR was conducted to analyze gene
expression Data showed that 0.75 and 2.0 mM methyl jasmonate
upregulated the miR-101 expression in SW620 cells (P=0.0088 and
P=0.0042, Fig. 6).
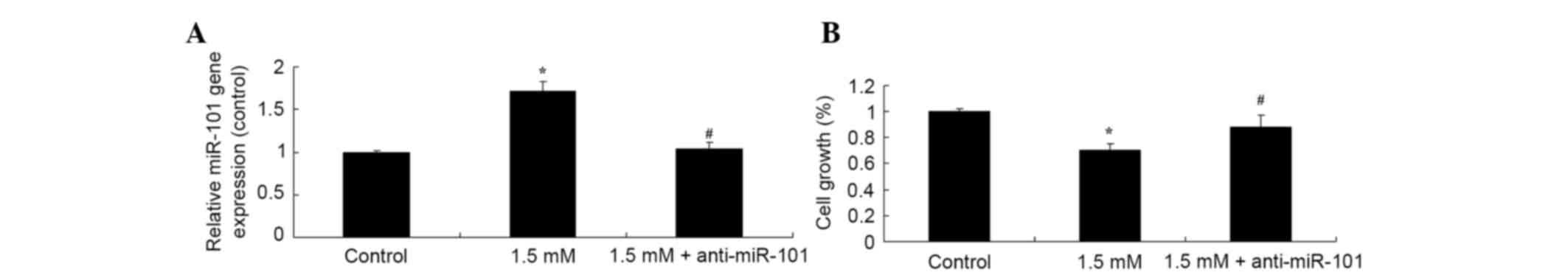
Knockdown of miR-101 suppresses methyl
jasmonate-induced growth inhibition of human colorectal cancer
cells
The mechanisms underlying the effect of methyl
jasmonate on the reduction of cell viability by detecting the
expression of miR-101 were further investigated. The data showed
that knockdown of miR-101 suppresses the miR-101 expression in
SW620 cells and 1.5 mM methyl jasmonate-induced miR-101 expression
(Fig. 7A). In addition, knockdown
of miR-101 reversed the 1.5 mM methyl jasmonate-induced suppression
of SW620 cell growth (P=0.0042, Fig.
7B).
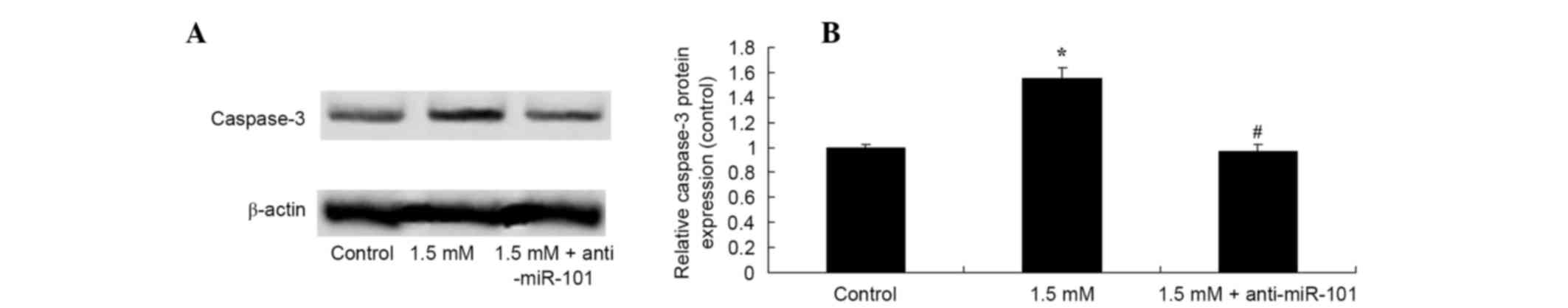
Knockdown of miR-101 suppresses methyl
jasmonate-induced activation of caspase-3 expression
The mechanisms underlying the effects of methyl
jasmonate on caspase-3 expression and the induction of apoptosis of
SW620 cells were investigated. Data showed that knockdown of
miR-101 reversed the 1.5 mM methyl jasmonate-induced increase in
caspase-3 protein expression in SW620 cells (P=0.0036, Fig. 8).
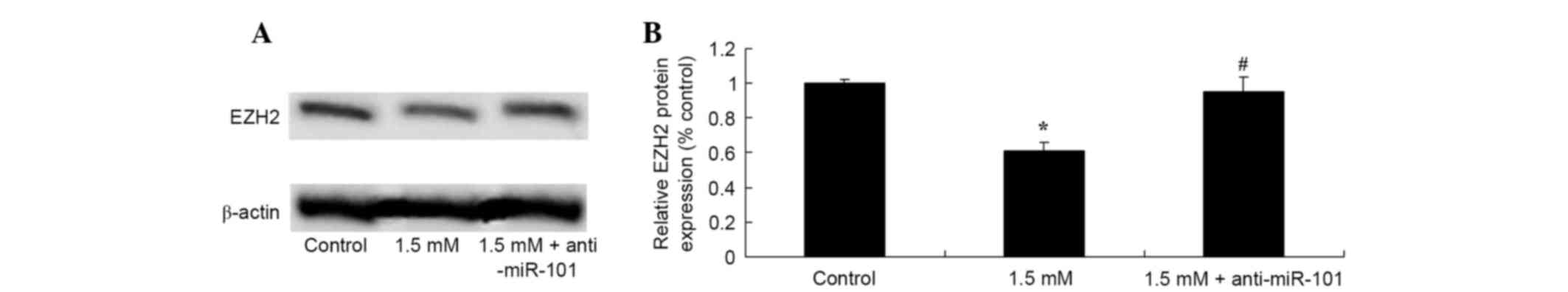
Knockdown of miR-101 suppresses methyl
jasmonate-induced inhibition of EZH2 expression
The mechanisms underlying the effect of methyl
jasmonate on the EZH2 expression of SW620 cells. Results
demonstrated that knockdown of miR-101 reversed the 1.5 mM methyl
jasmonate-induced decrease in EZH2 protein expression in SW620
cells (P=0.0031 and P=0.0004, Fig.
9).
Discussion
Colorectal cancer is one of most common types of
malignant tumor, with the third highest cancer-related mortality
rate (1). However, the
pathogenesis of colorectal cancer needs to be fully determined and
difficulties remain in diagnosis and treatment. Consequently,
morbidity and mortality rates are high. By far, the most effective
treatment method is the combination of radical surgery and
chemotherapy. Five-year survival rates of patients with local
tumors are ~93% (13). However,
nearly 50% patients have metastasis at initial diagnosis (13). In addition, more than one third of
patients with local tumors relapse following surgery (13). In this study, methyl jasmonate
induced growth inhibition, apoptosis and caspase-3 protein
expression in SW620 cells. It was reported that methyl jasmonate
reduces growth of cervical cancer cells (14), human prostate adenocarcinoma cells
(15) and human lung
adenocarcinoma cells (16).
The EZH2 human gene was identified in 1996 (17). EZH2 protein is evidently
upregulated in metastatic prostate cancer and breast cancer
(18). As a transcriptional
repressor protein, EZH2 inhibits transcription of numerous genes
and leads to increased tumor invasion and metastasis (19,20).
Among inhibited genes, the majority inhibit development and
progression of tumors, which increases the expression of
metastasis-promoting genes (21).
Expression of EZH2 protein in colorectal cancer tissues is higher
than that in normal colorectal tissues (22). In addition, it was suggested that
as an oncogene, EZH2 is closely associated with the occurrence and
progression of liver cancer (23).
Methyl jasmonate was also shown to suppress EZH2 protein expression
in SW620 cells. Wang et al (24) demonstrated that methyl jasmonate
sensitizes gembogic acid-induced apoptosis of human bladder cancer
cells via downregulation of EZH2 expression by miR-101.
Increasing evidence suggests that miRNA is crucial
in the carcinogenesis and progression of tumors. A large number of
miRNAs have been demonstrated to be upregulated or downregulated in
various types of tumor (25).
Among occurrence of various tumors, part of miRNA has the function
of tumor-inhibiting factors while part of it has the function of
tumor promoters. miRNA-101 is important in malignant tumors and its
abnormal expression has been confirmed in a number of studies
(26–28). A previous study demonstrated that
miR-101 is associated with the metastasis of cancer and the
inhibition of miR-101 can facilitate metastasis of colorectal
cancer (29). In this study,
miR-101 expression was demonstrated to be upregulated in SW620
cells. Knockdown of miR-101 was shown to suppress the anticancer
effect of methyl jasmonate-induced growth inhibition, caspase-3
protein expression, and inhibition of EZH2 expression in SW620
cells. Wang et al (24)
expounded that methyl jasmonate sensitizes gembogic acid-induced
apoptosis of human bladder cancer cells via downregulation of EZH2
expression through miR-101.
In conclusion, the present study demonstrates that
the anticancer effect of methyl jasmonate suppresses cell growth
and induces apoptosis of SW620 human colorectal cancer cells and
increases caspase-3 expression. These results indicate that its
anticancer effects occur via suppression of the EZH2 pathway and
activation of miR-101 expression in SW620 cells.
References
|
1
|
Negri FV, Musolino A, Naldi N, Bortesi B,
Missale G, Laccabue D, Zerbini A, Camisa R, Chernyschova N, Bisagni
G, et al: Role of immunoglobulin G fragment C receptor
polymorphism-mediated antibody-dependant cellular cytotoxicity in
colorectal cancer treated with cetuximab therapy. Pharmacogenomics
J. 14:14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ,
Park YS, Park JO, Kim SY, Kim TY, Kim JH, et al: Oxaliplatin,
fluorouracil, and leucovorin versus fluorouracil and leucovorin as
adjuvant chemotherapy for locally advanced rectal cancer after
preoperative chemoradiotherapy (ADORE): An open-label, multicentre,
phase 2, randomised controlled trial. Lancet Oncol. 15:1245–1253.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang RX, Wu XJ, Lu SX, Pan ZZ, Wan DS and
Chen G: The effect of COX-2 inhibitor on capecitabine-induced
hand-foot syndrome in patients with stage II/III colorectal cancer:
A phase II randomized prospective study. J Cancer Res Clin Oncol.
137:953–957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weir HK, Thun MJ, Hankey BF, Ries LA, Howe
HL, Wingo PA, Jemal A, Ward E, Anderson RN and Edwards BK: Annual
report to the nation on the status of cancer, 1975–2000, featuring
the uses of surveillance data for cancer prevention and control. J
Natl Cancer Inst. 95:1276–1299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogata-Kawata H, Izumiya M, Kurioka D,
Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H,
et al: Circulating exosomal microRNAs as biomarkers of colon
cancer. PLoS One. 9:e929212014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nouraee N and Calin GA: MicroRNAs as
cancer biomarkers. Microrna. 2:102–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen S and Flescher E: Methyl jasmonate:
A plant stress hormone as an anti-cancer drug. Phytochemistry.
70:1600–1609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheong JJ and Choi YD: Methyl jasmonate as
a vital substance in plants. Trends Genet. 19:409–413. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsumura H, Akimoto M, Kiyota H, Ishii Y,
Ishikura H and Honma Y: Gene expression profiles in differentiating
leukemia cells induced by methyl jasmonate are similar to those of
cytokinins and methyl jasmonate analogs induce the differentiation
of human leukemia cells in primary culture. Leukemia. 23:753–760.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishii Y, Kiyota H, Sakai S and Honma Y:
Induction of differentiation of human myeloid leukemia cells by
jasmonates, plant hormones. Leukemia. 18:1413–1419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015.PubMed/NCBI
|
|
13
|
Mihajlovic M, Vlajkovic S, Jovanovic P and
Stefanovic V: Primary mucosal melanomas: A comprehensive review.
Int J Clin Exp Pathol. 5:739–753. 2012.PubMed/NCBI
|
|
14
|
Milrot E, Jackman A, Kniazhanski T, Gonen
P, Flescher E and Sherman L: Methyl jasmonate reduces the survival
of cervical cancer cells and downregulates HPV E6 and E7, and
survivin. Cancer Lett. 319:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ezekwudo D, Shashidharamurthy R, Devineni
D, Bozeman E, Palaniappan R and Selvaraj P: Inhibition of
expression of anti-apoptotic protein Bcl-2 and induction of cell
death in radioresistant human prostate adenocarcinoma cell line
(PC-3) by methyl jasmonate. Cancer Lett. 270:277–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JH, Lee SY, Oh SY, Han SI, Park HG,
Yoo MA and Kang HS: Methyl jasmonate induces apoptosis through
induction of Bax/Bcl-XS and activation of caspase-3 via ROS
production in A549 cells. Oncol Rep. 12:1233–1238. 2004.PubMed/NCBI
|
|
17
|
Gharpure KM, Chu KS, Bowerman CJ, Miyake
T, Pradeep S, Mangala SL, Han HD, Rupaimoole R, Armaiz-Pena GN,
Rahhal TB, et al: Metronomic docetaxel in PRINT nanoparticles and
EZH2 silencing have synergistic antitumor effect in ovarian cancer.
Mol Cancer Ther. 13:1750–1757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deb G, Thakur VS and Gupta S: Multifaceted
role of EZH2 in breast and prostate tumorigenesis: Epigenetics and
beyond. Epigenetics. 8:464–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsang DP and Cheng AS: Epigenetic
regulation of signaling pathways in cancer: Role of the histone
methyltransferase EZH2. J Gastroenterol Hepatol. 26:19–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferraro A, Boni T and Pintzas A: EZH2
regulates cofilin activity and colon cancer cell migration by
targeting ITGA2 gene. PLoS One. 9:e1152762014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bryant RJ, Cross NA, Eaton CL, Hamdy FC
and Cunliffe VT: EZH2 promotes proliferation and invasiveness of
prostate cancer cells. Prostate. 67:547–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Ma ZB, Li K and Guo GH:
Association between EZH2 polymorphisms and colorectal cancer risk
in Han Chinese population. Med Oncol. 31:8742014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS,
Feng H, Ching AK, Cheung KF, Wong HK, Tong JH, et al: EZH2-mediated
concordant repression of Wnt antagonists promotes
β-catenin-dependent hepatocarcinogenesis. Cancer Res. 71:4028–4039.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Xiang W, Wang M, Huang T, Xiao X,
Wang L, Tao D, Dong L, Zeng F and Jiang G: Methyl jasmonate
sensitizes human bladder cancer cells to gambogic acid-induced
apoptosis through down-regulation of EZH2 expression by miR-101. Br
J Pharmacol. 171:618–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slaby O, Svoboda M, Michalek J and Vyzula
R: MicroRNAs in colorectal cancer: Translation of molecular biology
into clinical application. Mol Cancer. 8:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mirnezami AH, Pickard K, Zhang L, Primrose
JN and Packham G: MicroRNAs: Key players in carcinogenesis and
novel therapeutic targets. Eur J Surg Oncol. 35:339–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng HB, Zheng XG and Liu BP: miRNA-101
inhibits ovarian cancer cells proliferation and invasion by
down-regulating expression of SOCS-2. Int J Clin Exp Med.
8:20263–20270. 2015.PubMed/NCBI
|
|
28
|
Jiang W, Gu W, Qiu R, He S, Shen C, Wu Y,
Zhang J, Zhou J, Guo Y, Wan D, et al: miRNA-101 suppresses
epithelial-to-mesenchymal transition by targeting HMGA2 in
pancreatic cancer cells. Anticancer Agents Med Chem. 16:432–439.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strillacci A, Valerii MC, Sansone P,
Caggiano C, Sgromo A, Vittori L, Fiorentino M, Poggioli G, Rizzello
F, Campieri M and Spisni E: Loss of miR-101 expression promotes
Wnt/β-catenin signalling pathway activation and malignancy in colon
cancer cells. J Pathol. 229:379–389. 2013. View Article : Google Scholar : PubMed/NCBI
|