Introduction
Acute kidney injury (AKI), previously defined as
acute renal failure, is a common and devastating complication among
hospitalized patients with acute illnesses, particularly among
those with a life-threatening illness (1). AKI is characterized by aberrant
alterations in kidney function within hours to days following onset
(2). According to a systematic
study reviewing AKI incidence between the years of 2004 and 2012,
the worldwide incidence rates in adults and children were 21.6 and
33.7%, respectively, and the corresponding mortality rates were
23.9 and 13.8%, respectively (3).
Although clinicians have made significant progress in AKI treatment
(4), the prognosis of AKI remains
poor. This may be due, in part, to the lack of effective strategies
that enable early diagnosis of AKI, which may lead to neglecting
appropriate opportunities for intervention during the early stages
of the disease (5). Therefore, the
development of efficient biomarkers for the early diagnosis of AKI
has become an important area of research worldwide during the last
decade (6).
Kidney injury molecule-1 (KIM-1) is a type I
transmembrane glycoprotein primarily expressed on the surface of T
cells, and possesses two extracellular domains (7). KIM-1 expression is low in normal
kidneys, but is significantly increased in proximal tubule cells
following kidney injury. Upon injury, the extracellular domains of
KIM-1 separate from the cell surface and enter the urine through a
metalloproteinase-dependent process (7). It has been demonstrated that urinary
KIM-1 concentration is markedly increased within h following kidney
injury (8). Therefore, detecting
the level of urinary KIM-1 may potentially be an effective method
for the early diagnosis of AKI. Urinary KIM-1 levels have, thus
far, been permitted by the US Food and Drug Administration to be
used as an early diagnostic biomarker of drug-induced AKI in
preclinical studies (5).
However, whether urinary KIM-1 may be used as an
early diagnostic biomarker of obstructive AKI requires further
validation. Obstructive AKI is the nephropathy-induced form of AKI
caused by congenital or acquired urinary tract obstruction
(9). In the present study, the
potential of urinary KIM-1 as an early diagnostic biomarker of
obstructive AKI was investigated by establishing a unilateral
ureteral obstruction (UUO) rat model. In addition, a colloidal
gold-based immunochromatographic strip for the detection of KIM-1
was developed, with the aim of providing a novel technique for the
rapid diagnosis of AKI.
Materials and methods
Reagents
Chloral hydrate was bought from Qilu Pharmaceutical
Co. Ltd. (Jinan, China). A hematoxylin and eosin (HE) staining kit
was obtained from Beijing Disinbio Science Technology Co. Ltd.
(Beijing, China). A KIM-1 enzyme-linked immunosorbent assay (ELISA)
kit was purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). Immunohistochemistry (IHC) kits for KIM-1, α-SMA and vimentin
were obtained from BD Biosciences (Franklin Lakes, NJ, USA). Human
KIM-1 monoclonal antibody (McAb; cat. no., BAF1750), goat
anti-mouse IgG (cat. no., BAF007) and the goat anti-human
polyclonal antibody (PcAb) KIM-1 (cat. no., AF1750) were obtained
from R&D Systems, Inc. A fiberglass membrane SB06, polyester
fiber membrane VL78, absorbent paper CH27 and a PVC plastic back
plate were purchased from Shanghai Goldbio Technology Co., Ltd.
(Shanghai, China), and the nitrocellulose membrane was obtained
from Merck Millipore (Darmstadt, Germany).
Animals and design
Wistar rats (20 male and 20 female; age, 6–8 weeks;
weight, 180–220 g) were provided by the Experimental Animal Center
of Jilin University (Changchun, China). All rats were housed in a
pathogen-free facility with free access to water and standard
laboratory chow. The temperature was maintained at 20–25°C and a
12/12 h light/dark cycle was simulated. Prior to establishment of
the UUO model, the rats were randomly assigned into four equal
groups, consisting of a Sham group and three UUO groups. The Sham
group was used as the control group, whereby ligation and severing
of the ureter was not performed during the modeling processes. The
three UUO groups were UUO1, UUO3 and UUO7, and consisted of rats
that were sacrificed at 1, 3 and 7 days post-UUO, respectively. The
mental state and diet of rats in each group was recorded every day,
and body weight was measured before and after UUO modeling.
Following UUO modeling, the body weights were determined before the
rats were sacrificed. The present study was approved by the Animal
Ethical Committee of Jilin University (Changchun, China). Following
blood collection, rats were sacrificed by cervical dislocation.
Rats in UUO1, UUO3 and UUO7 groups were sacrificed at 1, 3 and 7
days post-UUO, respectively. Those in the Sham group were
sacrificed at 7 days after the modeling operation.
UUO modeling
Rats were first acclimated to laboratory conditions
for 7 days and fasted for 12 h, before they were anesthetized by
intraperitoneal injection of 10% chloral hydrate at a dose of 2
mg/kg and fixed in a supine position. On the left-hand side of the
middle abdomen, a 2-cm lengthwise incision was made. The left
ureter was isolated and ligated in the renal pelvis and in the
upper third of the ureter before it was severed between the two
ligatures. The incised abdominal skin was subsequently sutured
layer-by-layer. This procedure was conducted under sterile
conditions. For rats in the sham group, the same procedure was
performed without ligating or severing the ureter.
Collection of urine and blood, and
evaluation of kidney function
One day prior to sacrifice, rats were placed in
metabolic cages and their urine was collected over a period of 24
h. During this process, rats were fasted but had free access to
water. The total volume of urine from each rat over 24 h was
measured, and 3 ml of the urine was centrifuged at 400 × g for 10
min at room temperature. The supernatant was collected and
transferred to Eppendorf tubes, before storing at −20°C for
downstream analysis. A total of 5 ml blood was harvested from
vessels near the eyeball by removing the eyeball, before
centrifuging at 600 g for 10 min at room temperature. Serum was
collected and stored at −20°C until further analysis. Kidney
function was evaluated by examining the level of urea nitrogen and
creatinine in rat urine and blood, which was performed using a 7150
Biochemical Analyzer (Hitachi, Tokyo, Japan) according to the
manufacturer's instructions.
Kidney tissue specimen collection
Both kidneys from each rat were obtained immediately
following sacrifice and were analyzed for morphological alterations
in appearance and internal structures. Following removal of the
capsule, kidneys were rinsed with saline and weighed using scales.
They were then divided into several pieces, quickly frozen with
liquid nitrogen and stored at −80°C. Prior to analysis, kidney
tissues were paraffin-embedded and divided into sections (3 µm in
thickness) according to the description by Wu et al
(10).
Histological examination of kidney
damage
Following deparaffinization and rehydration, tissue
sections were stained with HE for histopathological observation
using a Hematoxylin-Eosin Staining kit. Histological alterations in
the renal tubule interstitium were assessed according to the
quantitative measurements of tubular damage, characterized by
tubular epithelial swelling, degeneration, necrosis, tubular
ectasia, inflammatory cell infiltration and tubular interstitial
fibrosis (11). Ten kidney
sections from each rat were randomly selected and evaluated at ×200
magnification. The degree of kidney damage was scored according to
the following criteria described by Leelahavanichkul et al
(11): 0, normal; 1, area of
damage =<25% of tubules; 2, area of damage =25–50% of tubules;
3, area of damage =50–75% of tubules; and 4, area of damage
=75–100% of tubules.
ELISA
Urinary KIM-1 levels were detected using the KIM-1
ELISA kit according to the manufacturer's instructions (R&D
Systems, Inc.). Urine samples with coating buffer were added to
96-well plates (100 µl/well) and incubated overnight at 4°C. The
remaining protein-binding sites were then blocked by incubating
samples for 1.5 h with blocking buffer containing 2% fetal bovine
serum at 37°C. Primary mouse anti-rat KIM-1 monoclonal antibodies
(dilution, 1:1,000) and goat anti-mouse IgG secondary antibodies
(dilution, 1:2,000) were added in order to bind specifically with
the target antigen. Following treatment with 3,3-diaminobenzidene
(DAB) solution from the ELISA kit and stop buffer, the plates were
read at 490 nm using a microplate reader (DG5031, Shanghai Jinggong
Industrial Co., Ltd, Shanghai, China).
IHC analysis
Protein expression of KIM-1, α-SMA and vimentin in
kidney tissues was detected using an IHC kit according to the
manufacturer's instructions (BD Biosciences). Following
deparaffinization and rehydration, tissue sections were incubated
with 1% H2O2 for 5–10 min to inactivate
endogenous peroxidases, and the antigen was retrieved by
microwaving for 5 min in citrate buffer. The sections were
subsequently blocked with goat serum for 20 min at room temperature
and incubated with primary antibodies (mouse anti-rat KIM-1 McAb,
dilution, 1:200, BAF1750, R&D Systems, Inc.; mouse anti-rat
α-SMA McAb, dilution, 1:200, BSM-33187M, BIOSS, Beijing, China;
mouse anti-rat McAb, dilution, 1:200, V5255, Sigma-Aldrich;
Merck-Millipore, Darmstadt, Germany) overnight at 4°C. Sections
were then incubated with the ready-to-use secondary antibody
conjugated to horseradish peroxidase (SP-0022, BIOSS) at room
temperature for 40 min. After rinsing with phosphate-buffered
saline, the sections were incubated in streptaridin-peroxidase
mixture (BIOSS) at room temperature for 20 min, followed by DAB
solution for 10 min at room temperature. The sections were then
counterstained with hematoxylin and mounted with neutral balsam.
Brown staining observed under the microscope indicated KIM-1, α-SMA
or vimentin protein expression. Semi-quantitative analysis was
performed according to staining intensity and area, and was scored
as 0 (no staining or extremely weak), 1 (mild, staining area
<25%), 2 (moderate, staining area involving 26–50%), 3 (strong,
staining area involving 51–75%) or 4 (strong, staining area
>76%).
Preparation of colloidal gold-McAb
KIM-1 conjugates
Colloidal gold with a diameter of 40 nm was
purchased from Shanghai Kinbio Tech Co., Ltd (Shanghai, China). The
optimal pH for binding colloidal gold with protein was equal to the
isoelectric point (pI) of the protein plus 0.5 (12). The pI of human KIM-1 McAb was 7.4,
so the optimal pH for binding colloidal gold with human KIM-1 McAb
was 7.9. The minimal McAb KIM-1/colloidal gold ratio at which
colloidal gold is stabilized with McAb KIM-1 was determined by
mixing 100 µl colloidal gold (pH 7.9) with different volumes (0–50
µl; 5 µl increments) of McAb KIM-1 (100 µg/ml). At the minimal
ratio or higher, the mixtures will maintain their red color due to
the stabilized colloidal gold by using enough McAb KIM-1, or the
color will change from red to blue accompanied with the deposition
of redundant colloidal gold. Therefore, the minimal McAb
KIM-1/colloidal gold ratio can be determined by observing the color
change of these mixtures. The actual volume of McAb KIM-1 used was
the minimum calculated volume, based on the minimal ratio plus 10%
of this volume. Colloidal gold and KIM-1 McAb were mixed according
to the actual usage ratio to obtain the colloidal gold-McAb KIM-1
conjugate solution. This was then purified through a two-step
centrifugation. The conjugate solution was centrifuged at 600 × g
for 10 min at 4°C, and the supernatant was collected and
centrifuged again at 8,000 × g for 60 min at 4°C. The precipitate
was harvested and resuspended with 0.01 M phosphate-buffered saline
supplemented with 0.02% NaN3 and 1% bovine serum
albumin, whereby the purified colloidal gold-McAb KIM-1 conjugate
solution was obtained.
Development of a colloidal gold-based
immunochromatographic strip
The immunochromatographic strip consisted of five
sections, which were sourced from Shanghai Goldbio Technology Co.,
Ltd., unless stated otherwise. The section consisted of the
following components: A sample pad fiberglass membrane (SB06), a
conjugate pad polyester fiber membrane (VL78), an analyzing
nitrocellulose membrane (Merck Millipore), an absorbent pad (CH27)
and a plastic back plate. The conjugate pad was pretreated with the
purified colloidal gold-McAb KIM-1 conjugates, and then dried in an
incubator at 37°C for 30 min. A control (C) line and test (T) line
were marked on the analyzing membrane with goat anti-mouse IgG (2
mg/ml; BAF007, R&D Systems, Inc.) and goat anti-human PcAb
KIM-1 (200 µg/ml, dilution, 1:200, AF1750, R&D Systems, Inc.),
respectively. The sample pad, pretreated conjugate pad, analyzing
membrane and absorbent pad were pasted on a plastic back plate. The
detection limit of the colloidal gold-based immunochromatographic
strip was determined by detecting normal urine specimens
supplemented with KIM-1 at the final concentrations of 0, 50 and
100 ng/ml, and 1 and 10 µg/ml, respectively.
Statistical analysis
Statistical analyses of rat weight, kidney weight,
kidney function and ELISA assays among the experimental groups were
performed using one-way analysis of variance. Comparisons between
two groups were further analyzed with the Student-Newman-Keuls
post-hoc test. All the statistical analyses were performed with the
SPSS software program (version, 19.0; IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Obstructive AKI was successfully
induced by the UUO operation
Rats in all experimental groups survived the UUO
operation, however UUO rats exhibited signs of depression, which
was accompanied by a distinct reduction in food intake. The average
body weights of rats in the UUO3 and UUO7 groups were significantly
decreased following UUO modeling (P=0.036 and P=0.048,
respectively), and the decrease was time-dependent (Fig. 1A). Conversely, the weight of the
left kidney in UUO groups was consistently higher than that of the
right kidney (Fig. 1B), which
reached statistical significance in the UUO7 group (P=0.032). By
contrast, the body and kidney weight of the Sham group demonstrated
no significant alterations before and after UUO modeling
(P>0.05).
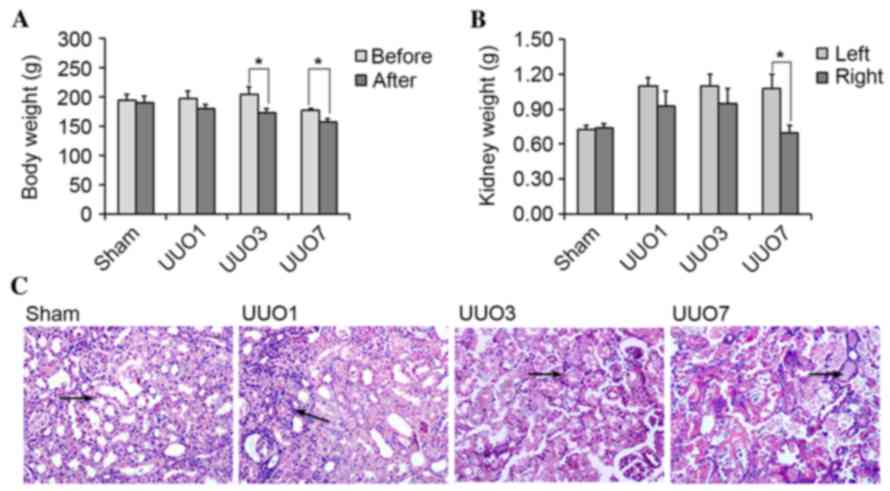 | Figure 1.Alterations in body weight, kidney
weight and micro-morphology following UUO operation. (A) The body
weight of rats at 1 (UUO1), 3 (UUO3) and 7 (UUO7) days following
UUO decreased following UUO operation (*P<0.05 vs. before UUO).
(B) The weight of the left kidney was greater than that of the
right kidney in the UUO groups following the UUO operation
(*P<0.05 vs. the left kidney). (C) Kidney injury at the
micro-morphological level increased in a time-dependent manner in
the UUO group as shown by the arrows. In the Sham group, no clear
damage was observed; In UUO1 group, kidney tubules were enlarged
marginally and the renal interstitium was infiltrated by few
inflammatory cells. In the UUO3 group, alterations such as renal
interstitial edema, inflammatory cell infiltration and vacuolar
degeneration of tubular epithelial cells were clear. In the UUO7
group, the damage was progressed further, which was characterized
by tubular epithelial necrosis, renal interstitial broadening, and
tubular interstitial fibrosis. UUO, unilateral ureteral
obstruction. |
With regards to kidney morphology, the left kidney
in rats from all UUO groups appeared larger and was darker in
color, when compared with corresponding right kidney, and the left
ureter above the ligatures exhibited obvious enlargements.
Following a transverse cut in the kidney, ischemic injury was
observed in the left kidney, particularly in the renal cortex.
Renal pelvis and calyces were enlarged and were filled with turbid
brown urine. Renal papillae were smooth and disappeared. The renal
parenchyma was thinner with an indistinct boundary between the
renal cortex and the medulla. The above alterations in the
appearance and internal structures of left kidneys in UUO groups
were time-dependent. By contrast, rat kidneys in the Sham group
exhibited no substantial alterations (data not shown).
Histopathological observations suggested that
kidneys in the Sham group demonstrated no obvious damage and were
scored as 0 (data not shown). In UUO groups, kidneys exhibited mild
damage one day following the UUO operation (scored as 1; data not
shown). Three days later, the damage became evident, as indicated
by the presence of enlarged tubules, vacuolar degeneration of
tubular epithelial cells, renal interstitial edema and inflammatory
cell infiltration (scored as 2; data not shown). Following seven
days, these pathological alterations progressed with tubular
epithelial necrosis, renal interstitial broadening, and tubular
interstitial fibrosis (scored as 3; data not shown). Representative
tissue sections with HE staining are shown in Fig. 1C.
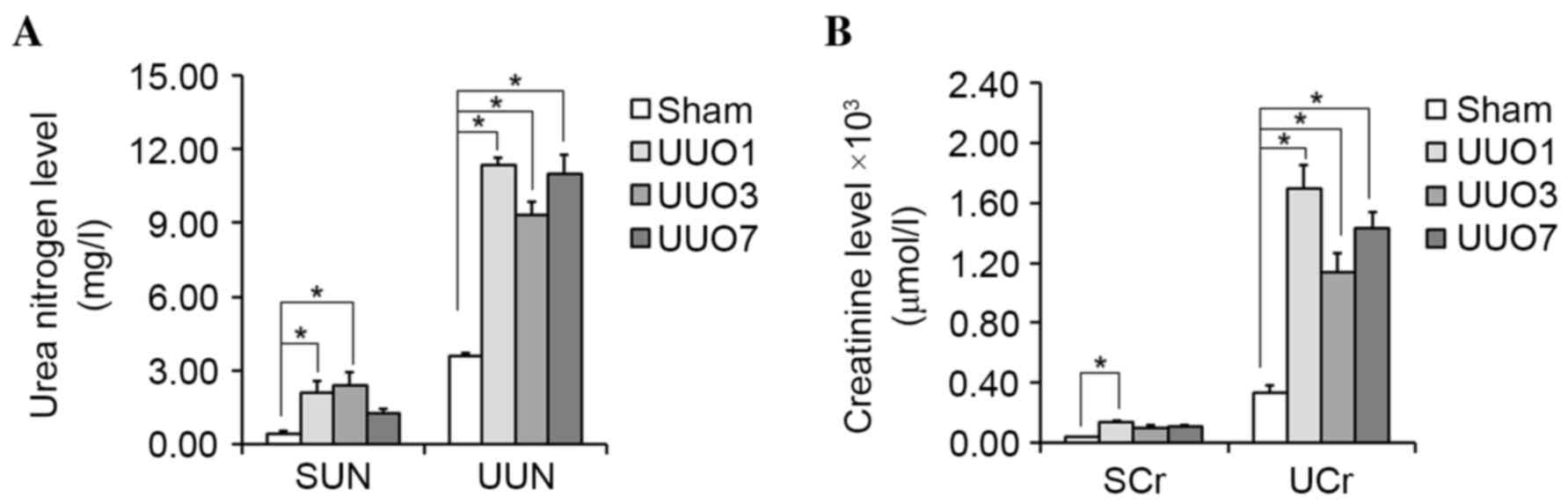
Sham and UUO groups demonstrated significant
differences in kidney function. In the majority of cases, the serum
and urinary urea nitrogen levels were significantly higher in UUO
groups when compared with the Sham group (P=0.027 and P=0.023 for
SUN of UUO1 and UUO3 group vs. Sham group, respectively; P=0.018,
P=0.020 and P=0.017 for UUN of UUO1, UUO3 and UUO7 group vs. Sham
group, respectively; Fig. 2A).
Similar results were observed in the serum and urinary creatinine
levels (P=0.022 for SCr of UUO1 group vs. Sham group; P=0.016,
P=0.025 and P=0.019 for UCr of UUO1, UUO3 and UUO7 group vs. Sham
group, respectively; Fig. 2B).
These results suggest that kidney dysfunction had been successfully
induced in the UUO groups. The significant difference in kidney
morphology and kidney function, between Sham and UUO groups
suggested that obstructive AKI was successfully induced by the UUO
operation.
KIM-1 is a valuable biomarker for the
early diagnosis of obstructive AKI
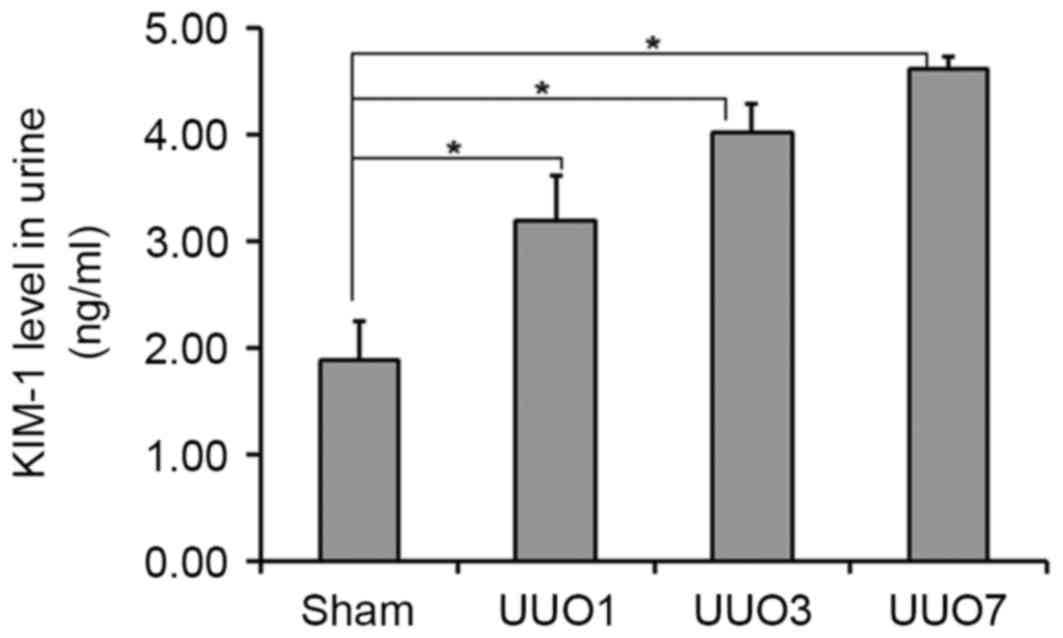
Urinary KIM-1 levels were detected using an ELISA
assay. The urinary KIM-1 concentrations of rats in the UUO1, UUO3
and UUO7 groups were 3.20±0.42, 4.02±0.26 and 4.62±0.10 ng/ml,
respectively; all of which were significantly higher than that of
Sham group (1.89±0.36 ng/ml; P=0.031, P=0.026 and P=0.015,
respectively; Fig. 3). In
addition, urinary KIM-1 levels in the UUO groups displayed a
time-dependent increase, correlating with the increasing kidney
damage. The same result was subsequently demonstrated with IHC
analysis, in which KIM-1 protein expression increased with the
increasing area of kidney damage in UUO1, UUO3 and UUO7 groups with
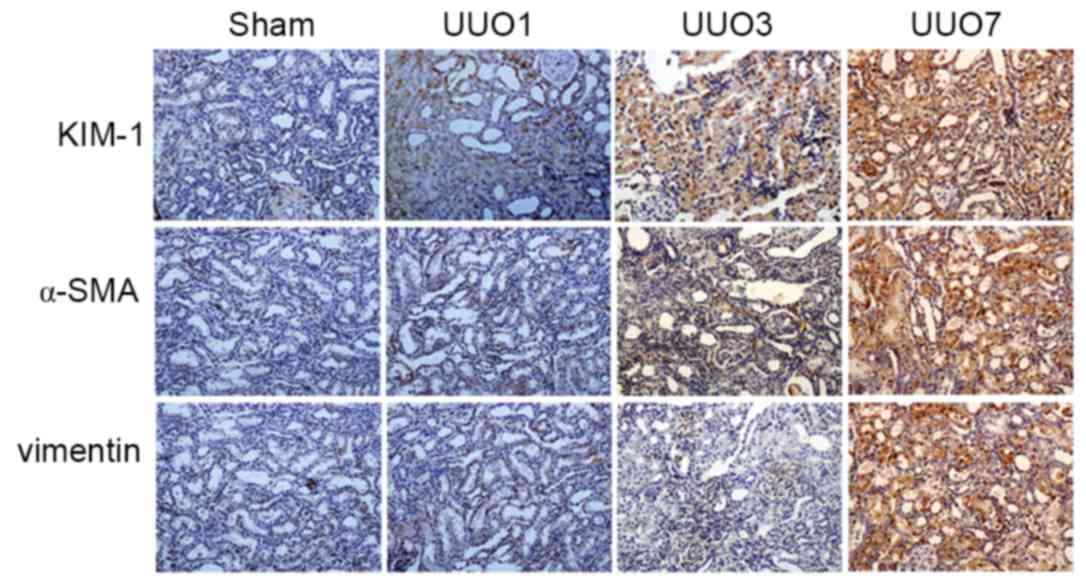
IHC scores of 1, 2 and 3, respectively (Table I). Conversely, the Sham group
exhibited no obvious KIM-1 expression and was scored as 0 (Fig. 4 and Table I). KIM-1 expression was highly
correlated with the development of obstructive AKI, and may
therefore be a potential biomarker of obstructive AKI.
 | Table I.Evaluation of KIM-1, α-SMA and
vimentin expression in Sham, UUO1, UUO3 and UUO7 groups by scoring
of immunohistochemical assays. |
Table I.
Evaluation of KIM-1, α-SMA and
vimentin expression in Sham, UUO1, UUO3 and UUO7 groups by scoring
of immunohistochemical assays.
|
|
| Score |
|---|
|
|
|
|
|---|
| Group | KIM-1 | α-SMA | Vimentin |
|---|
| Sham | 0 | 0 | 0 |
| UUO1 | 1 | 0 | 0 |
| UUO3 | 2 | 1 | 1 |
| UUO7 | 3 | 2 | 2 |
To evaluate whether KIM-1 may be a more sensitive
marker for early obstructive AKI compared with other biomarkers,
the expression of α-SMA and vimentin, which have previously been
identified as the effective AKI markers (13) were examined. The IHC scores are
shown in Table I. Similar to α-SMA
and vimentin, KIM-1 was not detected in the Sham group, but was
induced in the UUO groups in a time-dependent manner. Notably,
KIM-1 expression was detected in the UUO1 group where obstructive
AKI was induced within 24 h of the UUO operation (Table I). By contrast, α-SMA and vimentin
were not detected in this group, which indicates that KIM-1
demonstrated a higher sensitivity for the detection of obstructive
AKI when compared with the other established biomarkers.
Representative results are displayed in Fig. 4. Therefore, KIM-1 was considered to
be a valuable biomarker for the early diagnosis of obstructive
AKI.
Development of a colloidal gold
immunochromatographic strip for the rapid detection of urinary
KIM-1
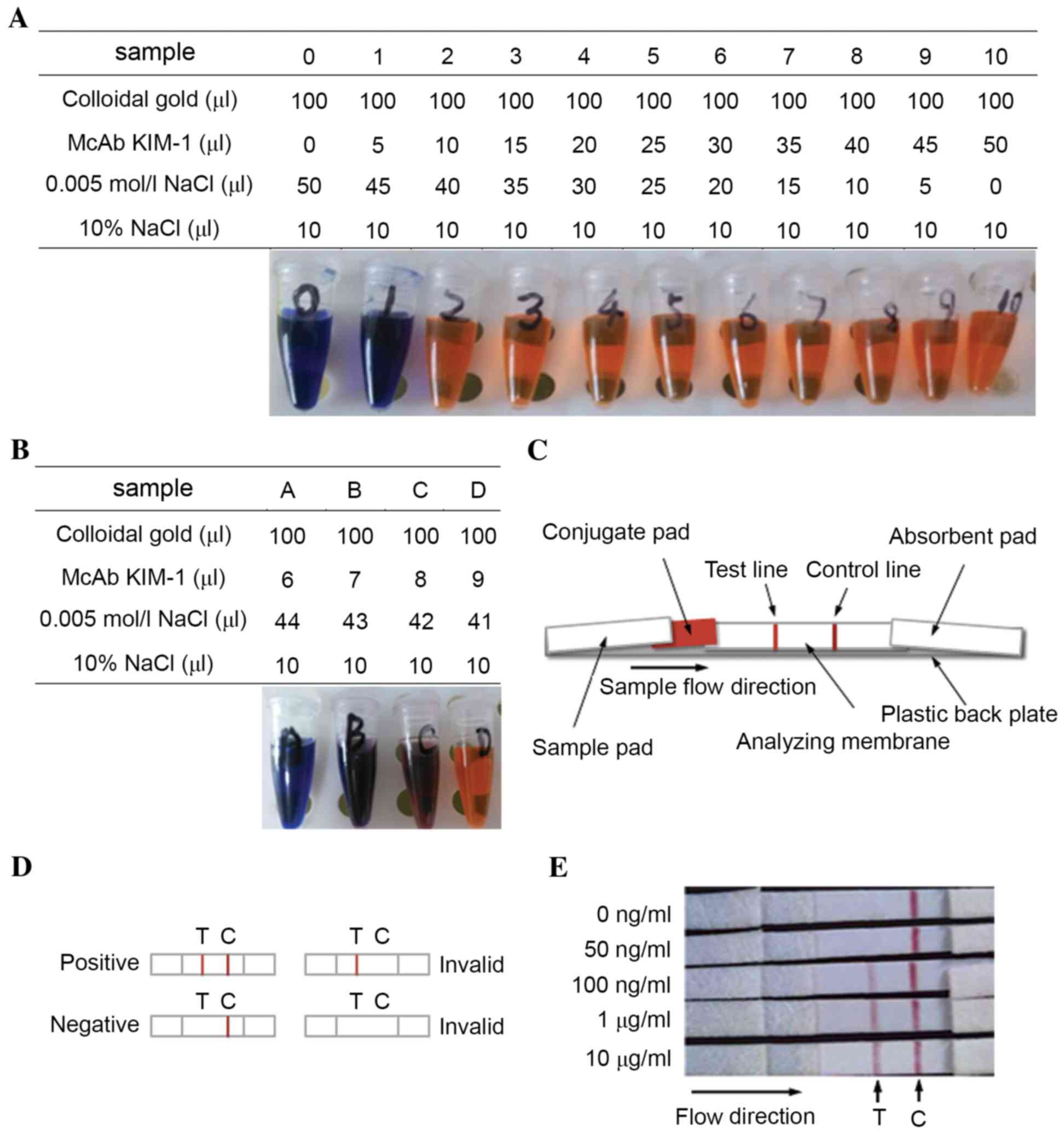
Colloidal gold (pH 7.9) and a McAb of KIM-1 were
combined according to the volumes listed in Fig. 5A. Mixtures 2–10 maintained a red
color, which meant that these tubes contained enough McAb KIM-1 to
stabilize colloidal gold. Therefore, the minimal volume of McAb
KIM-1 (100 µg/ml) required to stabilize 100 µl colloidal gold was
determined to be between 5 and 10 µl. In order to identify the
optimum volume of KIM-1 required to stabilize 100 µl colloidal
gold, 6–9 µl of KIM-1 were tested (Fig. 5B). Following 2 h, mixture D
maintained its red color, thus the minimal volume of McAb KIM-1
(100 µg/ml) required to stabilize 100 µl colloidal gold was
determined to be 9 µl, and the actual volume was calculated as 9 µl
× (1+10%)=9.9 µl. Based on this volume, a colloidal gold-McAb KIM-1
conjugate solution was obtained and purified for utilization in the
preparation of animmunochromatographic strip.
Each component of the immunochromatographic strip
was prepared according to the aforementioned methods. The strip was
assembled as shown in Fig. 5C.
Prior to detection, urine samples were centrifuged at 600 g for 20
min at room temperature, and the supernatant was collected for the
following test. A detection test was performed by adding the sample
to the sample pad of the strip and the results were determined
within 5–10 min. Schematic representations of positive, negative
and invalid results are shown in Fig.
5D.
To evaluate the sensitivity of the strip, normal
human urine was collected and divided into five individual samples,
which were spiked with KIM-1 to final concentrations of 0, 50 and
100 ng/ml, and 1 and 10 µg/ml. The samples were then analyzed using
the strip in triplicate, and the representative results are shown
in Fig. 5E. Samples with KIM-1
concentrations of ≥100 ng/ml were positive, while samples that
contained <100 ng/ml KIM-1 were negative. Therefore, the
detection limit of the strip was determined to be 100 ng/ml.
Discussion
Early diagnosis of obstructive AKI remains
challenging due to the lack of sensitive and specific biomarkers
(14). A recent cohort study
indicated that urinary KIM-1 levels were significantly higher in
children with severe hydronephrosis when compared with those with
mild and non-obstructive forms (9). The authors suggested that increased
urinary KIM-1 levels were associated with increased obstruction. An
additional study involving 90 patients with obstructive nephropathy
demonstrated that urinary KIM-1 content was markedly higher in AKI
patients when compared with non-AKI patients (15). In addition, this study indicated
that urinary KIM-1 was a long-term predicator of renal outcome in
obstructive nephropathy patients with AKI (15). Based on these results, it was
hypothesized that urinary KIM-1 may be a useful biomarker for the
early diagnosis of obstructive AKI, which is consistent with the
results of the present study.
In the current study, UUO rat models were
established with sham-operated rats as the control. There was a
lower starting body weight in the UUO7 group, which may due to the
small sample size, however, rats used in the present study are
adult, thus it was assumed that body weight had no decisive effect
on parameters included in the current study. Rats in the UUO groups
exhibited significant macro- and micro-morphological alterations
accompanied by significant kidney dysfunction within hours to days,
when compared with the Sham group. This indicated that obstructive
AKI was successfully induced in rat models following UUO. The
results of the ELISA assays suggested that urinary KIM-1 levels
were significantly higher in the UUO groups, when compared with the
Sham group, and were increased in a time-dependent manner. In
addition, IHC assays further confirmed a time-dependent increase in
KIM-1 expression in rats from the UUO groups, which was not
detected in the kidneys of rats in the Sham group. The results
demonstrated a positive correlation between increasing urinary
KIM-1 levels and increased obstructive AKI. In addition, KIM-1 was
demonstrated to be a more sensitive biomarker of obstructive AKI
than α-SMA and vimentin, as it was detected during mild obstructive
AKI, while α-SMA and vimentin was not. The present study provided
evidence to suggest that urinary KIM-1 may be a valuable biomarker
for the early diagnosis of obstructive AKI.
Although the function of KIM-1 in AKI progression
has not yet been completely elucidated (16), assessing KIM-1 levels may provide
additional information, when combined with other diagnostic
parameters, as early diagnostic biomarkers in preclinical and
clinical studies (5). The present
study provided evidence for the application of urinary KIM-1 as an
early diagnostic biomarker of obstructive AKI, which may be useful
for the identification of a therapeutic window during the early
stages of the disease. While its applications have not yet reached
clinical use, it is necessary to develop an efficient detection
method for urinary KIM-1.
Rapidity is a core principle for effective disease
diagnosis. The existing detection methods for KIM-1 include IHC,
ELISA and western blotting; however these methods do not meet
clinical diagnostic requirements due to their time-consuming and
complicated detection methods. In the present study, a rapid
detection method for KIM-1 was developed, based on the colloidal
gold immunochromatographic assay, which detected urinary KIM-1
within 5–10 min. This method has been widely adopted in a number of
fields, including medicine, food and pharmaceuticals (17–19).
With the advantage of convenience and rapidity, colloidal
gold-based immunochromatographic strips are a promising strategy in
clinical practice for the rapid diagnosis of AKI. However, in the
present study, the detection limit of the strip (100 ng/ml) was
unsatisfactory, thus the sensitivity of the strip requires further
optimization. The present study is a preliminary attempt, and
future studies will be conducted to improve the sensitivity and
specificity of the strip.
In conclusion, the present study confirmed that
urinary KIM-1 may be a useful biomarker for the early diagnosis of
obstructive nephropathy-induced AKI, which may provide a useful
strategy for identifying opportunities for early intervention in
the treatment of this disease. In addition, the development of a
rapid detection system for urinary KIM-1 was attempted, which
provided an insight into the rapid diagnosis of AKI. However,
future studies will aim to improve the detection limit via
systematic analysis, in order to develop a readily available
immunochromatographic strip for the rapid and sensitive detection
of urinary KIM-1.
Acknowledgements
The present study was supported by the Science and
Technology Development Project of Jilin Province (grant nos.
20140307006YY and 20150101228JC) and the National Nature Science
Foundation of China (grant no. 81302818).
References
|
1
|
Belayev LY and Palevsky PM: The link
between acute kidney injury and chronic kidney disease. Curr Opin
Nephrol Hypertens. 23:149–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rewa O and Bagshaw SM: Acute kidney
injury-epidemiology, outcomes and economics. Nat Rev Nephrol.
10:193–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Susantitaphong P, Cruz DN, Cerda J,
Abulfaraj M, Algahtani F, Koulouridis I and Jaber BL: Acute Kidney
Injury Advisory Group of the American Society of Nephrology: World
incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol.
8:1482–1493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moon KH, Ko IK, Yoo JJ and Atala A: Kidney
diseases and tissue engineering. Methods. 99:112–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dieterle F, Sistare F, Goodsaid F,
Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ,
Vonderscher J, et al: Renal biomarker qualification submission: A
dialog between the FDA-EMEA and predictive safety testing
consortium. Nat Biotechnol. 28:455–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ronco C: Acute kidney injury: From
clinical to molecular diagnosis. Crit Care. 20:2012016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charlton JR, Portilla D and Okusa MD: A
basic science view of acute kidney injury biomarkers. Nephrol Dial
Transplant. 29:1301–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han WK, Bailly V, Abichandani R, Thadhani
R and Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel
biomarker for human renal proximal tubule injury. Kidney Int.
62:237–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wasilewska A, Taranta-Janusz K, Dębek W,
Zoch-Zwierz W and Kuroczycka-Saniutycz E: KIM-1 and NGAL: New
markers of obstructive nephropathy. Pediatr Nephrol. 26:579–586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L, Zhang Y, Ma X, Zhang N and Qin G:
The effect of resveratrol on Fox01 expression in kidneys of
diabetic nephropathy rats. Mol Biol Rep. 39:9085–9093. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leelahavanichkul A, Yasuda H, Doi K, Hu X,
Zhou H, Yuen PS and Star RA: Methyl-2-acetamidoacrylate, an ethyl
pyruvate analog, decreases sepsis-induced acute kidney injury in
mice. Am J Physiol Renal Physiol. 295:F1825–F1835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paek SH, Lee SH, Cho JH and Kim YS:
Development of rapid one-step immunochromatographic assay. Methods.
22:53–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ucero AC, Benito-Martin A, Izquierdo MC,
Sanchez-Niño MD, Sanz AB, Ramos AM, Berzal S, Ruiz-Ortega M, Egido
J and Ortiz A: Unilateral ureteral obstruction: Beyond obstruction.
Int Urol Nephrol. 46:765–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie Y, Xue W, Shao X, Che X, Xu W, Ni Z
and Mou S: Analysis of a urinary biomarker panel for obstructive
nephropathy and clinical outcomes. PLoS One. 9:e1128652014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue W, Xie Y, Wang Q, Xu W, Mou S and Ni
Z: Diagnostic performance of urinary kidney injury molecule-1 and
neutrophil gelatinase-associated lipocalin for acute kidney injury
in an obstructive nephropathy patient. Nephrology (Carlton).
19:186–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wasung ME, Chawla LS and Madero M:
Biomarkers of renal function, which and when? Clin Chim Acta.
438:350–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyoshi-Akiyama T, Narahara K, Mori S,
Kitajima H, Kase T, Morikawa S and Kirikae T: Development of an
immunochromatographic assay specifically detecting pandemic H1N1
(2009) influenza virus. J Clin Microbiol. 48:703–708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Omidfar K, Kia S, Kashanian S, Paknejad M,
Besharatie A, Kashanian S and Larijani B: Colloidal nanogold-based
immunochromatographic strip test for the detection of digoxin
toxicity. Appl Biochem Biotechnol. 160:843–855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shim WB, Kim JS, Kim MG and Chung DH:
Rapid and sensitive immunochromatographic strip for on-site
detection of sulfamethazine in meats and eggs. J Food Sci.
78:M1575–M1581. 2013. View Article : Google Scholar : PubMed/NCBI
|