Introduction
Diabetic retinopathy (DR) is a serious microvascular
complication of diabetes and a major cause of blindness, which
usually affects individuals between 30 and 70 years old (1). In a previous study, neuroinflammation
has been suggested as an early event in the pathogenesis of DR
(2). Diabetes affects the entire
neurovascular unit of the retina, with gradual neurodegeneration,
gliosis, neuroinflammation, vascular abnormalities including plasma
leakage, compromised vascular blood-retinal barrier (BRB), edema,
angiogenesis, and eventual fibrosis, all of which occur at
increasing frequency (3). However,
the treatment options for DR remain limited and are often
associated with adverse effects; therefore, patients with diabetes
have a high risk of eventual blindness. There is an emerging
requirement to develop novel therapeutic approaches for this
devastating disease.
Niacin (vitamin B3 or nicotinic acid) is the most
effective medication for the treatment of atherosclerosis in
current clinical use, which increases high-density lipoprotein
levels, and substantially lowers total cholesterol and triglyceride
levels (4). Niaspan is a prolonged
release formulation of niacin, which is safe to use in patients
with diabetes (5). It has
previously been reported that prolonged niacin treatment may exert
anti-inflammatory effects (6).
Furthermore, niacin has been revealed to inhibit vascular
inflammation by downregulating the nuclear factor-κB (NF-κB)
signaling pathway (7). However,
the anti-inflammatory effects of Niaspan on DR have yet to be
elucidated.
The present study aimed to examine the
anti-inflammatory effects of Niaspan on streptozotocin
(STZ)-induced DR. The results demonstrated that administration of
Niaspan .3 months after the induction of diabetes significantly
improved functional outcome, and inhibited vascular inflammation in
the retina.
Materials and methods
Animals
Adult Male Wistar rats (age, 7 weeks; weight,
225–250 g) were purchased from the Academy of Military Medical
Science (Beijing, China). The rats were housed in specific
pathogen-free conditions (temperature 22±2°C, light/dark cycle
12/12 h) with ad libitum access to food and water. All
procedures involving rats were approved by the Laboratory Animal
Care and Use Committee of Tianjin Medical University (Tinajin,
China), and conformed to the Association for Research in Vision and
Ophthalmology Statement for the Use of Animals in Ophthalmic and
Vision Research (8).
Diabetes induction and treatment
Diabetes was induced via injection of STZ (45 mg/kg;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) into the tail
vein of Wistar rats. Fasting blood glucose levels were determined
using a glucose analyzer 6 days after STZ injection; rats with
fasting blood glucose levels >16.7 mmol/l were identified as
diabetic and were used in the present study (9). Niaspan (China Resources
Pharmaceutical Group Co., Ltd., Beijing, China) was dissolved in
water, and 40 mg/kg/day was administered following STZ injection
(the 7th day following STZ injection). A total of 90 rats were
divided into the following groups: i) Normal control group (control
group; n=30); ii) DR model group without Niaspan treatment (DR
group; n=30); and iii) DR model group treated with Niaspan (Niaspan
group; n=30).
Histological and immunohistochemical
analyses
Rats were anesthetized via injection of chloral
hydrate (concentration:10%; 600 mg/kg) into the tail vein of Wistar
rats in the third month following Niaspan treatment. Then the eyes
were removed and were fixed in 4% paraformaldehyde with
phosphate-buffered saline (PBS; pH 7.4) for 2 h at 4°C. The eyes
were then dehydrated in a graded alcohol series and embedded in
paraffin. The paraffin-embedded tissues were cut into 5 µm
sections. Subsequently, the sections were stained with hematoxylin
and eosin (H&E) by fluorescence microscope (Leica DMI4000B;
Leica Microsystems GmbH, Düren, Germany). For immunohistochemical
analysis, sections (5 µm) were prepared from paraffin-embedded
tissues and were incubated overnight at 4°C with antibodies against
tumor necrosis factor-α (TNF-α; polyclonal rabbit anti-rat; cat.
no. 74120; 1:100; GeneTex, Inc., Irvine, CA, USA). The sections
were then stained with biotinylated anti-rabbit immunoglobulin G
secondary antibody (cat. no. BA-1000; 1:200; Vector Laboratories,
Inc., Burlingame, CA, USA) for 2 h (room temperature) followed by
incubation with horseradish peroxidase streptavidin (cat. no.
SA-5704; Vector Laboratories, Inc.) for 1 h (room temperature).
Specific labeling was visualized by incubation with
diaminobenzidine (DAB; cat. no. ZLI-9017; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). Finally, the sections
were counterstained with hematoxylin (cat. no. G1080; Solarbio
Science & Technology Co., Ltd., Beijing, China). Images were
captured using a Leica DMI4000B (Leica Microsystems GmbH, Wetzlar,
Germany) and the results were quantified using Image-Pro Plus 6.0
(Media Cybernetics, Inc., Rockville, MD, USA). Retinal cell numbers
in the ganglion cell layer (GCL) were counted in the region within
a fixed 100-µm column.
Western blotting
Western blotting was performed using standard
methods. Retinal protein was extracted using a
radioimmunoprecipitation assay buffer (Beijing Zhongshan Golden
Bridge Biotechnology; OriGene Technologies, Inc., Rockville, MD,
USA) and were quantified using a protein assay (Bradford Protein
Assay; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amounts of protein (800 µmol/l) were separated by 8–12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
electroblotted onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked in 5%
skim milk for 2 h (room temperature) and were incubated with
antibodies against TNF-α (polyclonal rabbit anti-rat; cat. no.
74120; 1:1,000; GeneTex, Inc.), NF-κB (polyclonal rabbit anti-rat;
cat. no. 54672; 1:1,000; GeneTex, Inc.), inducible nitric oxide
synthase (iNOS; polyclonal rabbit anti-rat; cat. no. ab15323;
1:500; Abcam, Cambridge, UK) and intercellular adhesion molecule-1
(ICAM-1; polyclonal rabbit anti-rat; cat. no. 16174-1-AP; 1:1,000;
Proteintech Group, Inc., Rosemont, IL, USA) overnight at 4°C.
Subsequently, the membranes were washed in 0.1% TBS-Tween-20 and
incubated with anti-rabbit IgG secondary antibody (cat. no.
ZDR-5306; 1:5,000, Beijing Zhongshan Golden Bridge Biotechnology;
OriGene Technologies, Inc.) at room temperature for 1 h. Monoclonal
mouse anti-β-actin (cat. no. TA-09; 1:1,000; Zhongshan Golden
Bridge Biotechnology Co., Ltd.) was used as an internal reference.
Finally, the blots were scanned with a ChemiDoc™ MP system (Bio-Rad
Laboratories, Inc.) and the bands were semi-quantified using ImageJ
1.51 software (National Institutes of Health, Bethesda, MA,
USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from retinas (taken from 3
rats for each group) using TRIzol® reagent (cat. no.
15596; Thermo Fisher Scientific, Inc., Waltham, MA, USA), RNA was
reverse transcribed into cDNA using the TransScript First-Strand
cDNA Synthesis SuperMix (cat. no. AT301; TransGen Biotech Co.,
Ltd., Beijing, China). The primer sequences were as follows:
β-actin, forward 5′-AGCCATGTACGTAGCCATCC-3′, reverse
5′-ACCCTCATAGATGGGCACAG-3′; NF-κB, forward
5′-TGAGGCTGTTTGGTTTGAGA-3′, reverse 5′-TTATGGCTGAGGTCTGGTCTG-3′;
iNOS, forward 5′-TATCTGCAGACACATACTTTACGC-3′, reverse
5′-TCCTGGAACCACTCGTACTTG-3′; and ICAM-1, forward
5′-GGCCTCAGTCAGTGTGA-3′ and reverse 5′-AACCCCATTCAGCGTCA-3′. The
relative mRNA expression levels of NF-κB, iNOS and ICAM-1 were
detected by RT-qPCR with TransStart Top Green qPCR SuperMix (cat.
no. AQ131; TransGen Biotech Co., Ltd.). β-actin mRNA was used as an
internal control. All procedures were performed according to the
manufacturers' protocols. The relative mRNA expression levels were
determined using the 2-ΔΔCq method (10).
Measurement of BRB breakdown using
Evans blue
2% Evans blue dye (Sigma-Aldrich; Merck Millipore)
in saline was administered via the tail vein of rats (n=4/group) as
a BRB permeability tracer 2 h prior to sacrifice. Rats were
sacrificed via injection of chloral hydrate (concentration:10%; 600
mg/kg) into the tail vein. for 10 sec at a dose of 45 mg/kg, and
after the dye had circulated for 120 min, the eyes were immediately
fixed in 4% paraformaldehyde for 2 h. Subsequently, the anterior
segments were removed and the retinas were dissected and washed in
cold PBS. The retinas were then spread on glass slides, vitreous
side up, and mounted with mounting medium. Images were captured
using a confocal scanning laser imaging system fitted with
krypton-argon lasers (FV1000; Olympus Corporation, Tokyo,
Japan).
Quantitative evaluation of Evans blue
dye extravasation
Evans blue dye (Sigma-Aldrich; Merck Millipore) was
dissolved in normal saline (30 mg/ml), and was injected into the
tail vein of rats (n=3/group) for 10 sec at a dose of 45 mg/kg.
After the dye had circulated for 120 min, the chest cavity was
opened and the left heart ventricle was cannulated. Each rat was
perfused with PBS (37°C) for 2 min to clear the dye, ensuring the
physiological pressure was maintained at 120 mmHg. Immediately
after perfusion, the eyes were enucleated and retinas were
carefully dissected. The weight of each retina was measured after
thorough drying in a Speed-Vac. Albumin leakage into the retinal
tissue was estimated via the measurement of extravasated Evans blue
dye. Evans blue was extracted by incubating each retina in 0.3 ml
formamide for 18 h at 70°C. The extract was filtered through a
30,000 MW filter at a speed of 300 × g for 45 min at 4°C.
The absorbance of the filtrate was measured using a
spectrophotometer at 620 and 740 nm, the absorption maximum for
Evans blue in formamide. Calculations were based on the external
standards dissolved in the same solvent. The concentration of dye
in the extracts was calculated from a standard curve of Evans blue
in formamide and normalized to the dry retinal weight and the
time-averaged concentration of Evans Blue in the plasma.
Terminal deoxynucleotidyl transferase
biotin-dUTP nick end labeling (TUNEL)
Apoptosis was examined by TUNEL assay.
TUNEL-positive nuclei in the GCL of the retina were counted.
Briefly, following 8 min fixation with ice-cold acetone solution,
cryopreserved tissue sections were washed three times with PBS. The
sections were incubated with 1 ml blocking buffer (3% normal goat
serum (Sigma-Aldrich; Merck Millipore) in PBS for 1 h at room
temperature. Following incubation, the sections were washed in a
permeabilization solution (0.1% Triton X-100 in 0.1% sodium
citrate) for 2 min on ice. After washing, the sections were
incubated in 50 µl TUNEL reaction mixture (cat. no. 12156792910;
Roche Diagnostics GmbH, Mannheim, Germany) for 60 min at 37°C in
the dark. Subsequently, the sections were counterstained with
4′,6-diamidino-2-phenylindole. The sections were washed and
observed under a fluorescence microscope (Olympus Corporation). The
retinal cell numbers in the GCL were counted in the region within a
1-mm column.
Statistical analysis
Data are presented as the mean ± standard deviation
(each experiment was repeated 3 times) and were analyzed by SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Results were analyzed
by one-way analysis of variance followed by a least significant
difference procedure. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Niaspan treatment on
DR
To determine whether diabetes induces DR and whether
Niaspan treatment regulates DR recovery, H&E staining was
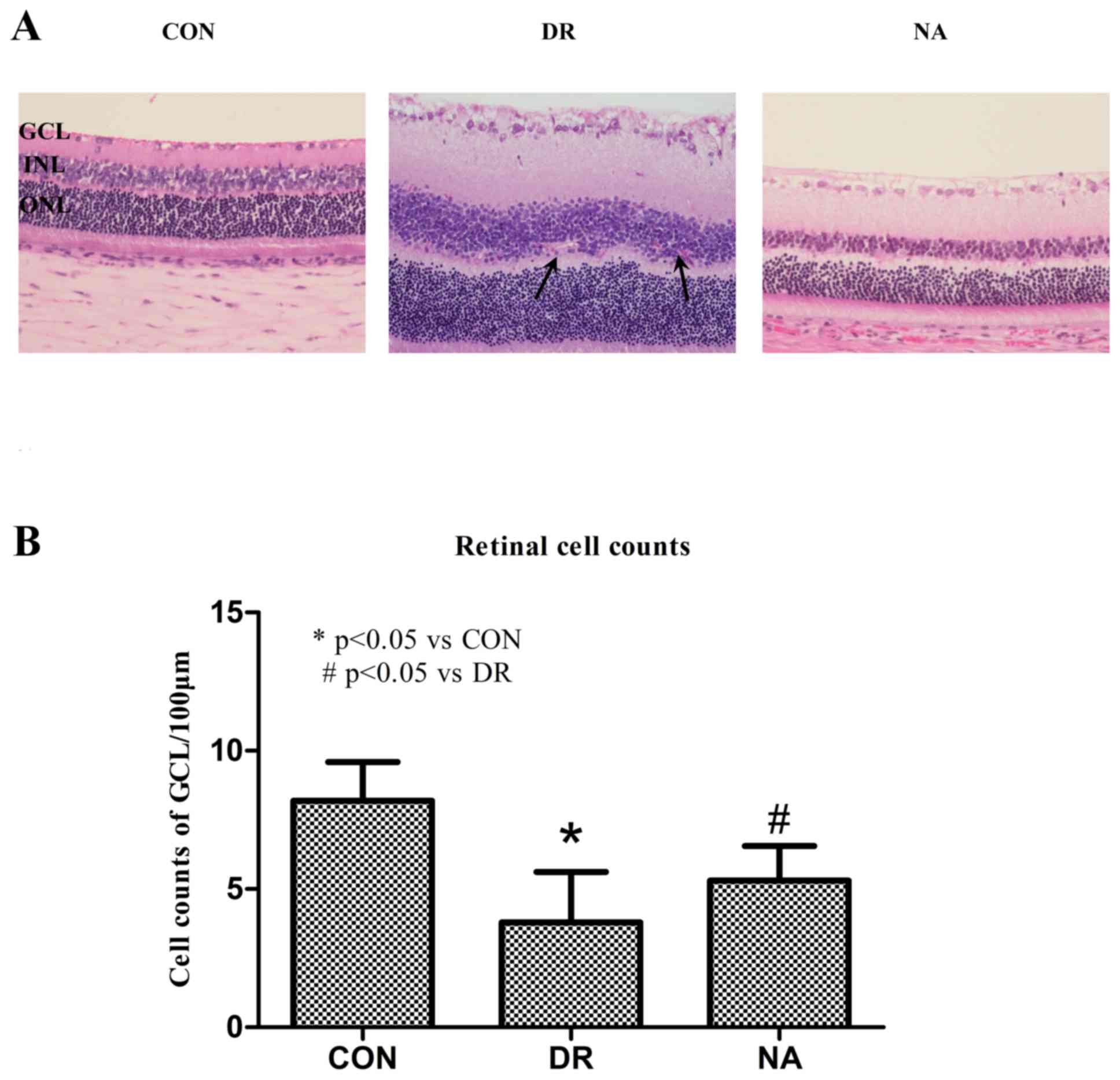
performed. As presented in Fig. 1,
non-diabetic rats exhibited normal retinas; all cell layers of the
retina were clear and neatly arranged. In the DR group, cells were
disorganized after diabetic modeling. Obvious inflammatory cell
infiltration in the GCL, hemorrhage, and neovascularization in the
inner nuclear layer (INL) were observed. In the Niaspan-treated
retina, retinal edema and hemorrhage were markedly attenuated, and
the ganglionic layer was neatly arranged (Fig. 1A). Furthermore, cell number in the
retinal GCL was significantly reduced in the DR group (P<0.05)
compared with the control group. Treatment with Niaspan was able to
significantly reverse the reduction in retinal cell numbers
(P<0.05) compared with in the diabetic retinas (Fig. 1B).
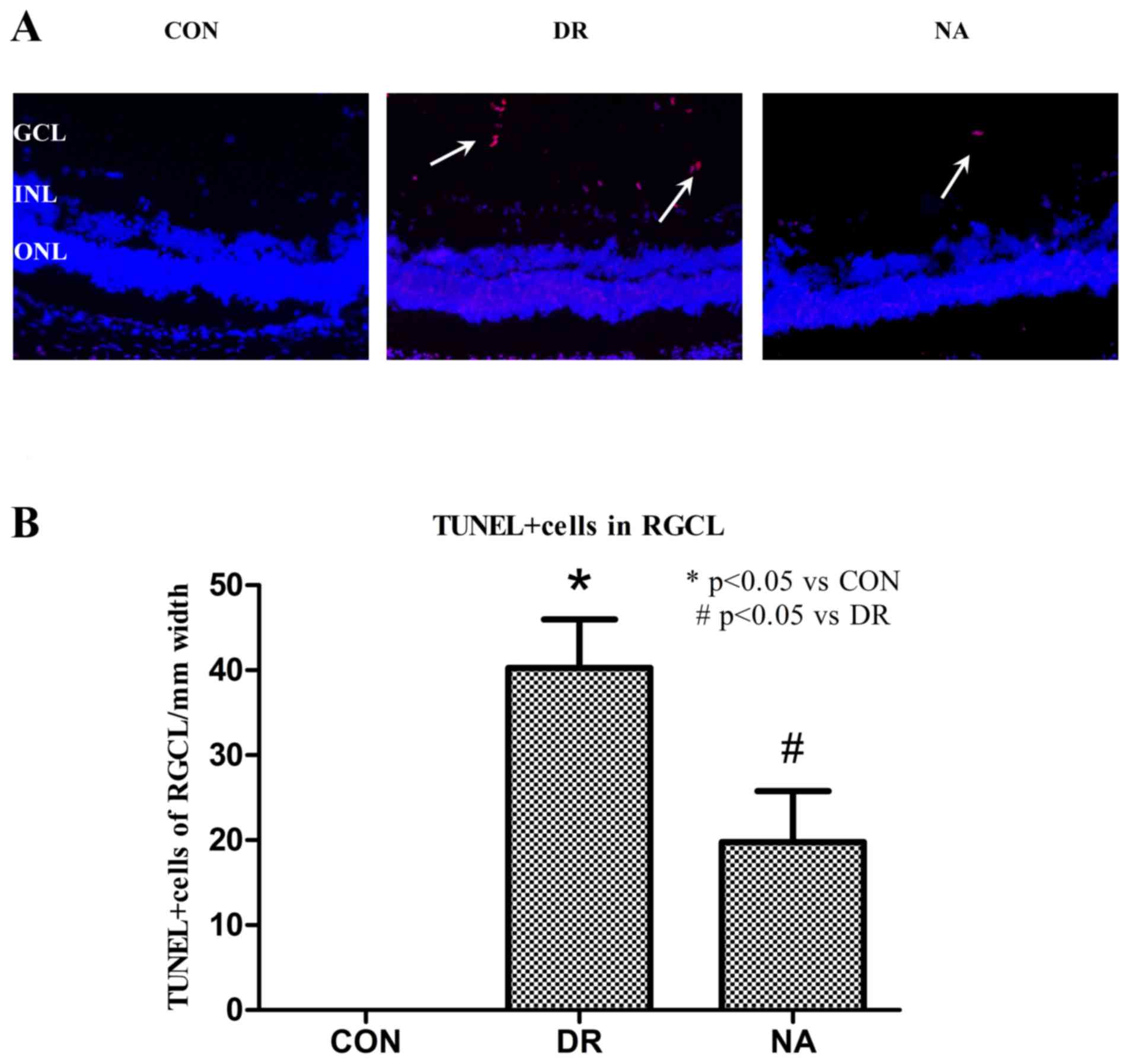
Niaspan reduces DR-induced apoptosis
of retinal cells in the GCL
Niaspan inhibits retinal cell apoptosis. Abundant
numbers of TUNEL+ cells were detected in the GCL of
diabetic retina (40.25±5.7373; P<0.05) compared with in the
control retina. Conversely, fewer apoptotic cells were detected in
the GCL of the Niaspan-treated group (19.75±6.0208; P<0.05)
compared with in the diabetic retina. No TUNEL+ cells
were observed in the control group retinas (Fig. 2).
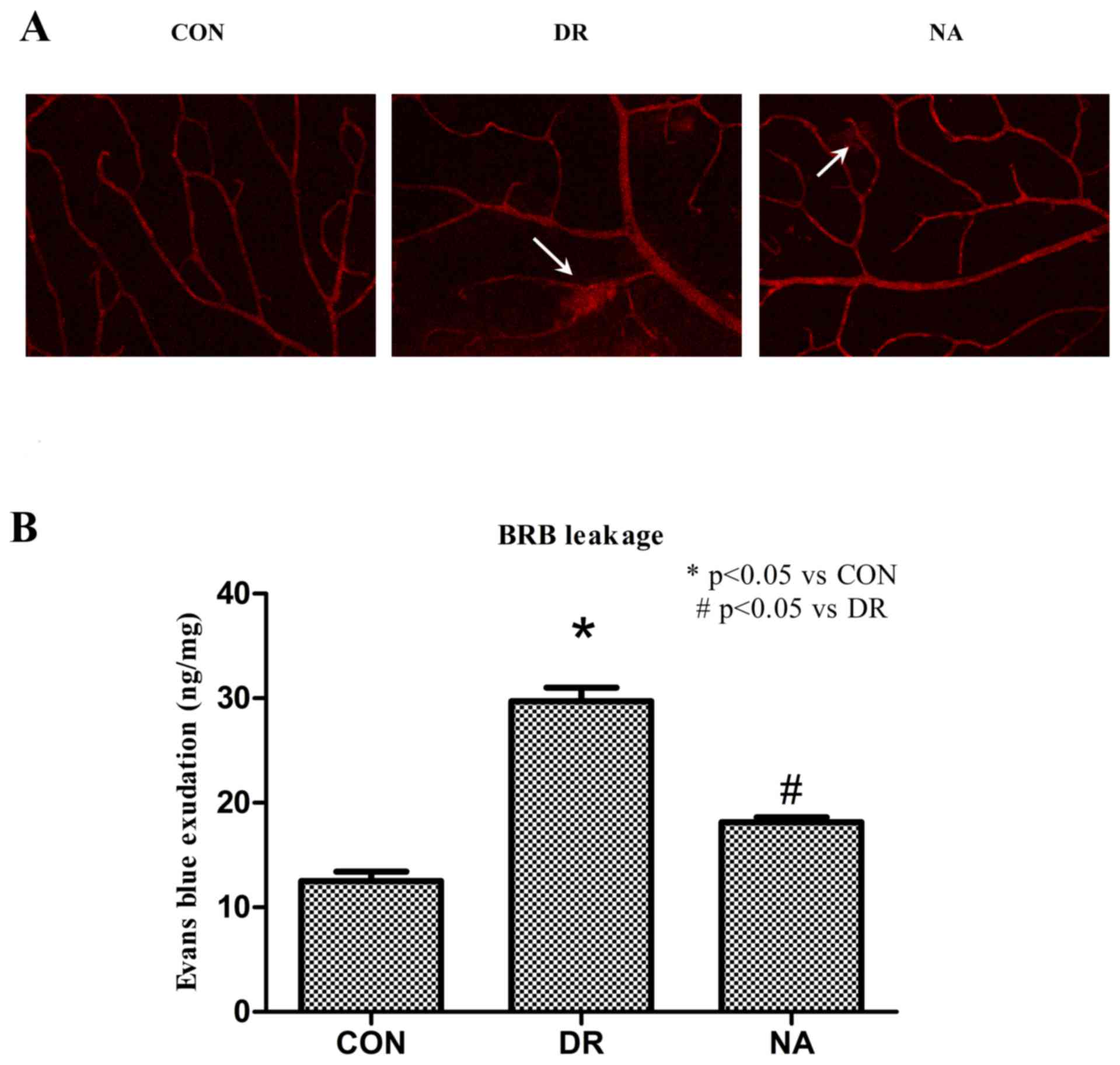
Niaspan prevents DR-induced BRB
breakdown
DR-induced breakdown of the BRB was assessed by
Evans blue extravasation from retinal vessels. As an initial
approach, retinal blood vessel integrity was analyzed in flat mount
retinas. Evans blue was observed as being confined to the retinal
blood vessels without any leakage occurring in control rats.
Conversely, the dye was shown to leak from the vessels to the
surrounding tissue in DR rats. Niaspan treatment of diabetic rats
was able to prevent this effect (Fig.
3A). Quantitative detection of Evans blue dye from the retinal
tissue confirmed the results obtained by fluorescence microscopy.
Diabetes increased BRB permeability in diabetic rats (29.71±1.3214
ng Evans blue/mg dry weight retina; P<0.05) compared with the
control rats (12.5±0.91591 ng Evans blue/mg dry weight retina).
Treatment with Niaspan significantly prevented BRB breakdown in
diabetic rats (18.15±0.45211 ng Evans blue/mg dry weight retina;
P<0.05) compared with untreated diabetic rats (Fig. 3B).
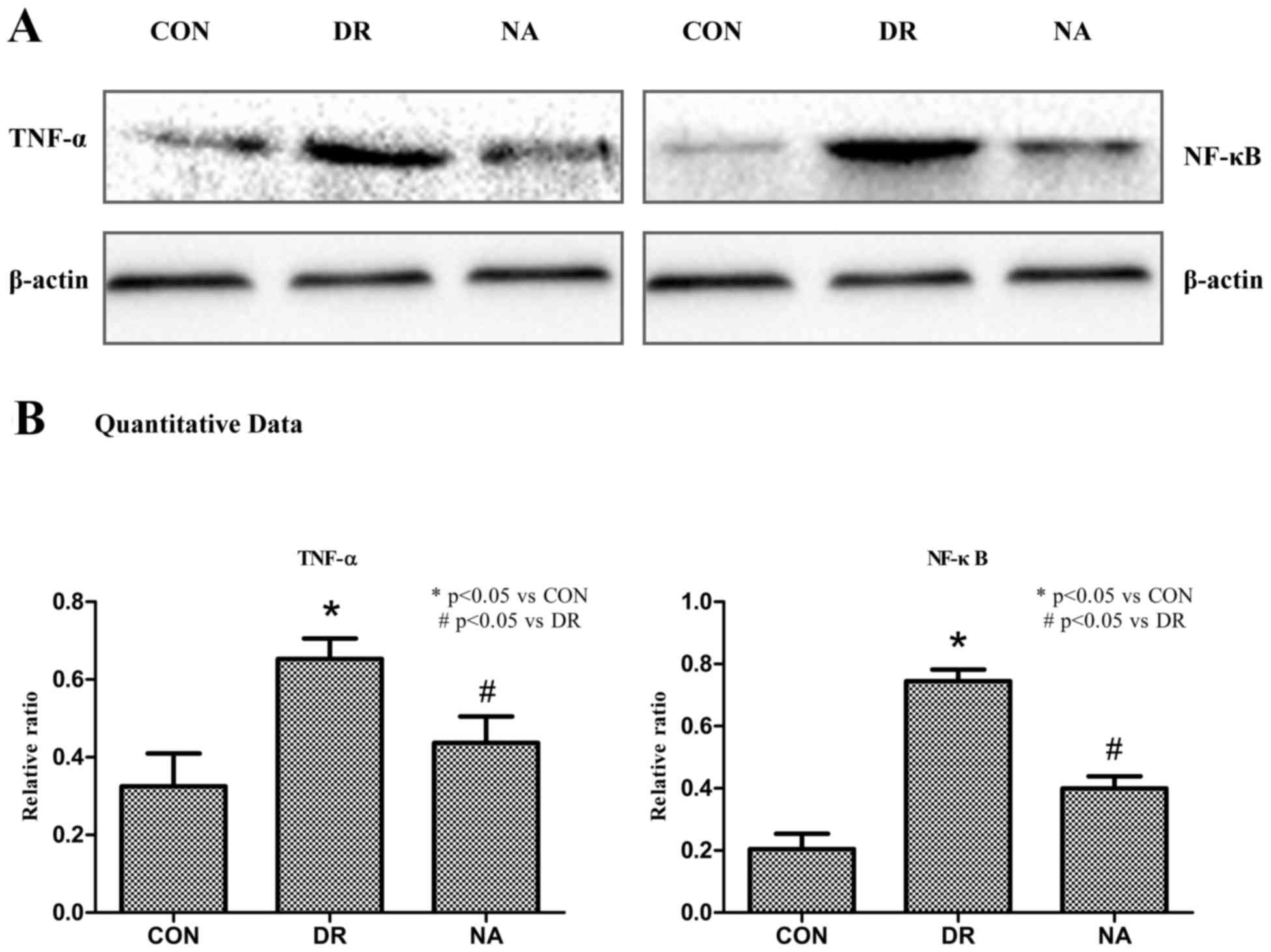
Niaspan reduces DR-induced TNF-α and
NF-κB retinal expression
To determine whether treatment with Niaspan
regulates TNF-α and NF-κB expression TNF-α and NF-κB expression
levels were detected. As presented in Fig. 4, western blotting indicated that DR
markedly increased the expression levels of TNF-α and NF-κB
(Fig. 4A). A quantitative analysis
revealed that there was a significant increase in TNF-α and NF-κB
in diabetic rats (P<0.05) compared with in the control rats.
Treatment with Niaspan was able to significantly prevent the
increase in TNF-α and NF-κB (P<0.05) compared with in diabetic
rats (Fig. 4B).
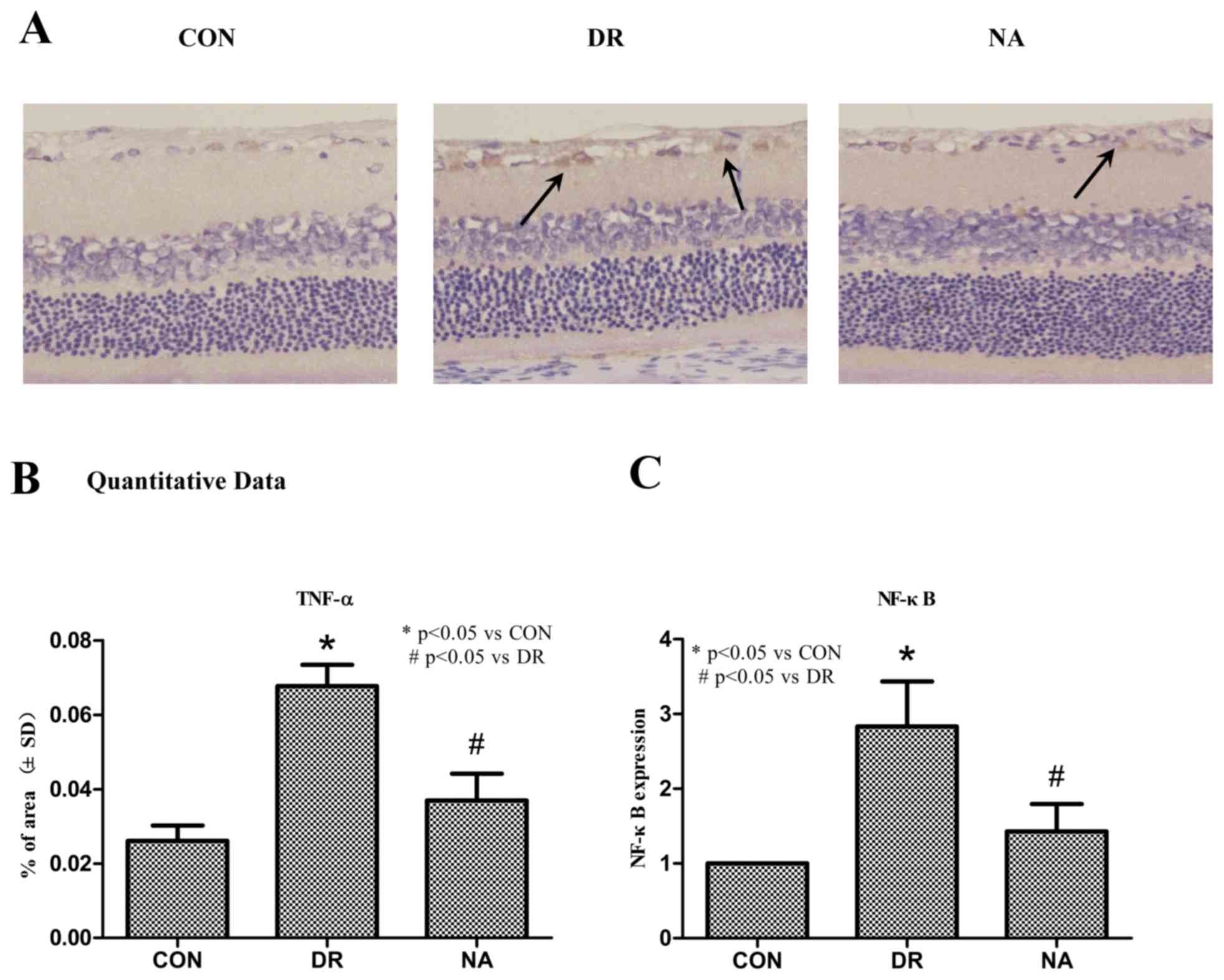
Immunohistochemistry indicated that treatment with
Niaspan significantly decreased the expression levels of TNF-α
(Fig. 5A). A quantitative analysis
revealed that there was a significant increase in TNF-α expression
in diabetic rats (P<0.05) compared with control rats. Treatment
with Niaspan was able to significantly prevent the increase in
TNF-α (P<0.05) compared with in diabetic rats (Fig. 5B). PCR analysis detected a
significant increase in NF-κB expression in diabetic rats
(P<0.05) compared with control rats. Treatment with Niaspan was
able to significantly prevent the increase in NF-κB expression
(P<0.05) compared with in diabetic rats (Fig. 5C).
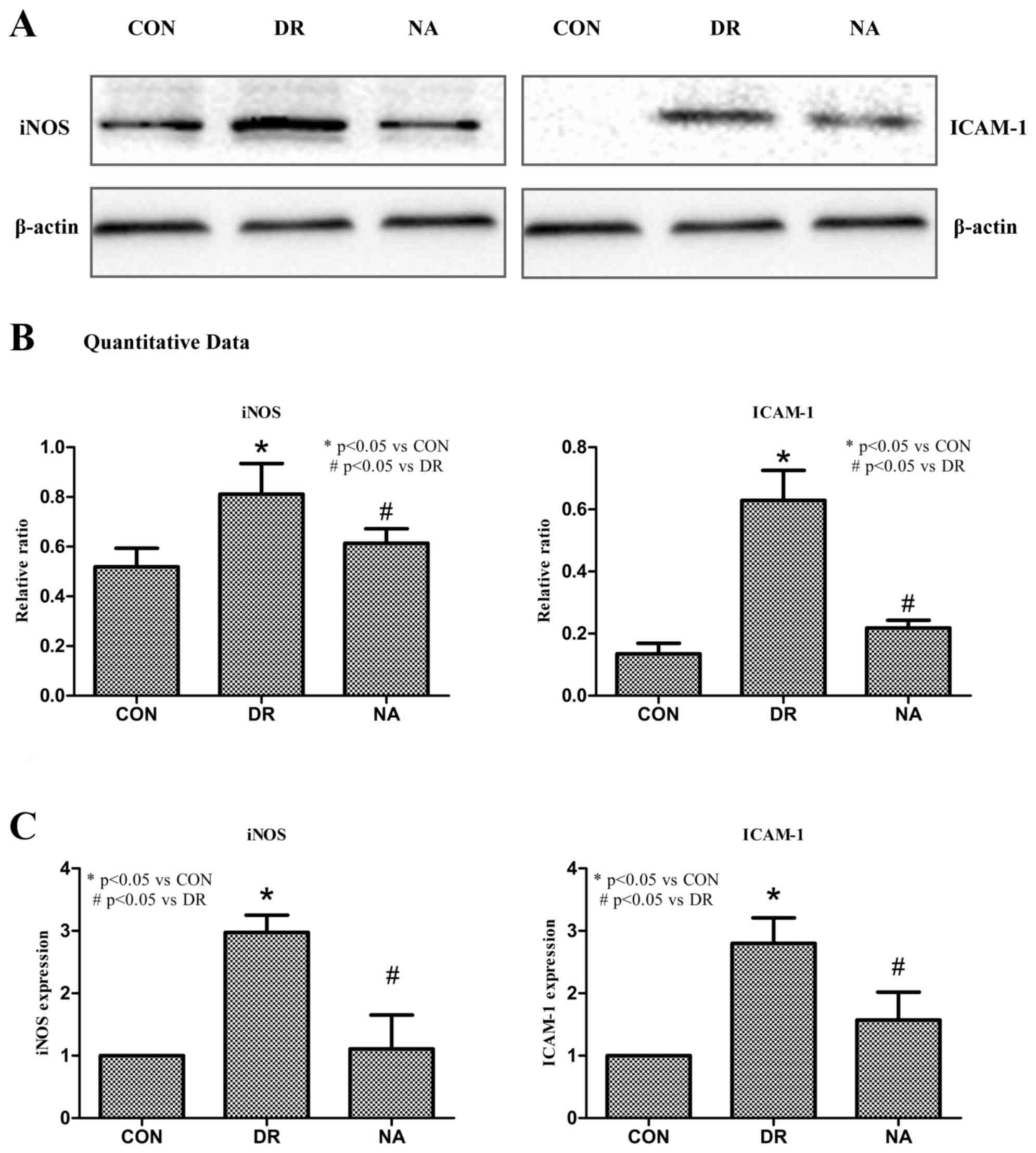
Niaspan reduces DR-induced iNOS and
ICAM-1 retinal expression
To determine whether Niaspan treatment regulates
iNOS and ICAM-1 target gene expression ICAM-1 and iNOS expression
levels were detected. As presented in Fig. 6, western blotting indicated that
Niaspan treatment markedly decreased the expression levels of
ICAM-1 and iNOS (Fig. 6A). A
quantitative analysis revealed that there was a significant
increase in iNOS and ICAM-1 expression in diabetic rats (P<0.05)
compared with in control rats. Treatment with Niaspan was able to
significantly prevent the increase in iNOS and ICAM-1 expression
(P<0.05) compared with in diabetic rats (Fig. 6B). PCR analysis detected a
significant increase in iNOS and ICAM-1 expression in diabetic rats
(P<0.05) compared with in control rats. Treatment with Niaspan
was able to significantly prevent the increase in iNOS and ICAM-1
(P<0.05) expression compared with in diabetic rats (Fig. 6C).
Discussion
The present study used the common animal model of
STZ-induced DR; the results confirmed that STZ injection resulted
in diabetes and significantly induced DR; however, long-term
Niaspan treatment reduced the formation and development of DR, and
inhibited the development of inflammation. This conclusion is based
on several lines of experimental evidence. Initially, the present
study indicated that treatment of diabetic rats with Niaspan
markedly decreased hemorrhage, leukocyte infiltration and apoptosis
in the GCL of the diabetic retina. STZ has previously been
demonstrated to induce hyperglycemia and lead to the generation of
oxidative stress (11), which is a
typical characteristic of DR in rats, which may promote the
destruction of endothelial integrity and breakdown of the BRB.
These alterations in endothelial integrity were detected by cell
apoptosis analysis and retinal vascular permeability assay.
Thirdly, treatment of DR with Niaspan significantly decreased the
expression of inflammatory mediators, including TNF-α, NF-κB, iNOS
and ICAM-1 compared with in diabetic retinas.
DR is a vascular, neuroinflammatory disease that is
characterized by cell apoptosis and neuroinflammation. Apoptosis,
which is a type of programmed cell death, has been detected in the
retina following ischemia-induced injury. A previous study reported
that during the course of diabetes, apoptosis occurs early in
endothelial cells and retinal ganglion cells (12). In addition, endothelial cell and
pericyte loss is one of the earliest and key manifestations of DR,
which may lead to BRB breakdown (13). An early sign of DR in an
experimental model of diabetes is vascular inflammation due to
oxidative stress; furthermore, proinflammatory cytokines (14) and inflammatory mediators have been
reported to promote increased vascular permeability, leukocyte
adhesion and retinal cell death (15). As a consequence, Niaspan may
mitigate cell apoptosis and BRB breakdown by downregulating
inflammatory factors.
TNF-α is a major proinflammatory cytokine that is
involved in numerous inflammatory pathologies, and is predominantly
produced by macrophages (16).
Increased levels of TNF-α have been detected in the vitreous of
diabetic patients with proliferative DR (17) and in diabetic rat retinas (18). TNF-α is a potent mediator of
leukostasis induced by vascular endothelial growth factor,
interleukin-1 α, and platelet-activating factor in the retinal
vasculature (19), and also
mediates the cell death/apoptosis of retinal neurons and vascular
endothelial cells in DR (13). The
involvement of the inflammatory cytokine TNF-α in the apoptotic
cell death of retinal endothelial cells during the early and late
stages of DR in a rat model of STZ-induced diabetes has previously
been investigated (18,20). The present study suggested that
Niaspan markedly decreases TNF-α in the diabetic retina, which may
contribute to the beneficial effects of Niaspan treatment.
Activation of NF-κB induces the expression of
numerous inflammatory cytokines, including TNF-α (21), which are crucial factors in
inflammation. However, TNF-α is not only induced by NF-κB, but is
also a strong activator of NF-κB (22). In addition, inhibition of TNF-α may
inhibit the activity of NF-κB (23), which is a widely expressed
inducible transcription factor that is an important regulator of
several genes involved in mammalian inflammatory and immune
responses, proliferation and apoptosis (24). The present study demonstrated that
Niaspan may significantly prevent the increase in TNF-α and NF-κB
expression, which was induced by DR. These findings suggested that
NF-κB, under the regulation of TNF-α, may be associated with
diabetes-induced inflammation in the retina.
Upregulation of iNOS has been detected in the
retinas of experimental diabetic rodents and human patients in
previous studies (25,26). In addition, iNOS serves an
important role in leukostasis, apoptosis and BRB breakdown
(27,28). Concurrently, white blood cells
interact with, and bind to, ICAM-1 on the surface of endothelial
cells in a multi-step process leading to adherence of the blood
cells to the endothelial wall. Notably, suppression of ICAM-1
attenuates retinal leukostasis in animal models of DR (29). TNF-α regulates the expression of
adhesion molecules, including ICAM-1, which is correlated with the
increase in leukostasis and BRB breakdown in diabetic rat retinas
(18). Furthermore, a previous
study demonstrated that suppression of NF-κB (11 activation in the
retinas of diabetic rats inhibited the expression of inflammatory
mediators, including iNOS and ICAM-1, and capillary degeneration
and pericyte loss in these animals (24).
In conclusion, the present study indicated that DR
leads to the generation of important inflammatory cytokines, which
may lead to endothelial cell apoptosis and BRB breakdown. These
findings suggested that Niaspan-induced downregulation of TNF-α may
contribute to amelioration of the inflammatory reaction in diabetic
rats. Furthermore, TNF-α may induce reactive oxygen species
formation, NF-κB activation and iNOS expression in inflammatory
cells, and rapidly upregulate the expression of ICAM-1 at the
endothelial surface (30). In
accordance with these findings, the reduction of TNF-α may reduce
apoptosis of endothelial cells and BRB breakdown. The results of
the present study strongly indicated that Niaspan may be considered
a potential therapeutic agent for the treatment of DR via
inhibition of the inflammatory process.
Acknowledgements
The present study was supported by the National
Science Foundation of Tianjin (grant nos. 12JCYBJC33900,
14JCYBJC28000 and 2013KZ119) and the National Natural Science
Foundation of China (grant nos. 81371038 and 91442124).
Glossary
Abbreviations
Abbreviations:
|
DR
|
diabetic retinopathy
|
|
TNF-α
|
tumor necrosis factor-α
|
|
NF-κB
|
nuclear factor-κB
|
|
STZ
|
streptozotocin
|
|
BRB
|
blood-retinal barrier
|
|
ICAM-1
|
intercellular cell adhesion
molecule-1
|
|
iNOS
|
inducible nitric oxide synthase
|
|
H&E
|
hematoxylin and eosin
|
|
GCL
|
ganglion cell layer
|
|
INL
|
inner nuclear layer
|
|
ONL
|
outer nuclear layer
|
References
|
1
|
Aiello LP, Gardner TW, King GL,
Blankenship G, Cavallerano JD, Ferris FL III and Klein R: Diabetic
retinopathy. Diabetes Care. 21:143–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stem MS and Gardner TW: Neurodegeneration
in the pathogenesis of diabetic retinopathy: Molecular mechanisms
and therapeutic implications. Curr Med Chem. 20:3241–3250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abcouwer SF: Angiogenic factors and
cytokines in diabetic retinopathy. J Clin Cell Immunol. (Suppl 1).
2013.PubMed/NCBI
|
|
4
|
Chapman MJ, Assmann G, Fruchart JC,
Shepherd J and Sirtori C: European Consensus Panel on HDL-C:
Raising high-density lipoprotein cholesterol with reduction of
cardiovascular risk: The role of nicotinic acid-a position paper
developed by the European Consensus Panel on HDL-C. Curr Med Res
Opin. 20:1253–1268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elam MB, Hunninghake DB, Davis KB, Garg R,
Johnson C, Egan D, Kostis JB, Sheps DS and Brinton EA: Effect of
niacin on lipid and lipoprotein levels and glycemic control in
patients with diabetes and peripheral arterial disease: The ADMIT
study: A randomized trial. Arterial Disease Multiple Intervention
Trial. JAMA. 284:1263–1270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heemskerk MM, Dharuri HK, van den Berg SA,
Jónasdóttir HS, Kloos DP, Giera M, van Dijk KW and van Harmelen V:
Prolonged niacin treatment leads to increased adipose tissue PUFA
synthesis and anti-inflammatory lipid and oxylipin plasma profile.
J Lipid Res. 55:2532–2540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Si Y, Zhang Y, Zhao J, Guo S, Zhai L, Yao
S, Sang H, Yang N, Song G, Gu J and Qin S: Niacin inhibits vascular
inflammation via downregulating nuclear transcription factor-κB
signaling pathway. Mediators Inflamm. 2014:2637862014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W and Yan H: Simvastatin increases
circulating endothelial progenitor cells and reduces the formation
and progression of diabetic retinopathy in rats. Exp Eye Res.
105:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei S, Li H, Xu J, Liu Y, Gao X, Wang J,
Ng KF, Lau WB, Ma XL, Rodrigues B, et al: Hyperglycemia-induced
protein kinase C β2 activation induces diastolic cardiac
dysfunction in diabetic rats by impairing caveolin-3 expression and
Akt/eNOS signaling. Diabetes. 62:2318–2328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arden GB and Sivaprasad S: The
pathogenesis of early retinal changes of diabetic retinopathy. Doc
Ophthalmol. 124:15–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barber AJ, Lieth E, Khin SA, Antonetti DA,
Buchanan AG and Gardner TW: Neural apoptosis in the retina during
experimental and human diabetes. Early onset and effect of insulin.
J Clin Invest. 102:783–791. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joussen AM, Doehmen S, Le ML, Koizumi K,
Radetzky S, Krohne TU, Poulaki V, Semkova I and Kociok N: TNF-alpha
mediated apoptosis plays an important role in the development of
early diabetic retinopathy and long-term histopathological
alterations. Mol Vis. 15:1418–1428. 2009.PubMed/NCBI
|
|
14
|
Joussen AM, Poulaki V, Le ML, Koizumi K,
Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B,
et al: A central role for inflammation in the pathogenesis of
diabetic retinopathy. FASEB J. 18:1450–1452. 2004.PubMed/NCBI
|
|
15
|
Krady JK, Basu A, Allen CM, Xu Y, LaNoue
KF, Gardner TW and Levison SW: Minocycline reduces proinflammatory
cytokine expression, microglial activation, and caspase-3
activation in a rodent model of diabetic retinopathy. Diabetes.
54:1559–1565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
el Abu Asrar AM, Maimone D, Morse PH,
Gregory S and Reder AT: Cytokines in the vitreous of patients with
proliferative diabetic retinopathy. Am J Ophthalmol. 114:731–736.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joussen AM, Poulaki V, Mitsiades N,
Kirchhof B, Koizumi K, Döhmen S and Adamis AP: Nonsteroidal
anti-inflammatory drugs prevent early diabetic retinopathy via
TNF-alpha suppression. FASEB J. 16:438–440. 2002.PubMed/NCBI
|
|
19
|
Vinores SA, Xiao WH, Shen J and
Campochiaro PA: TNF-alpha is critical for ischemia-induced
leukostasis, but not retinal neovascularization nor VEGF-induced
leakage. J Neuroimmunol. 182:73–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Zhang Q, Soderland C and Steinle
JJ: TNFα and SOCS3 regulate IRS-1 to increase retinal endothelial
cell apoptosis. Cell Signal. 24:1086–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun X, Han F, Yi J, Han L and Wang B:
Effect of aspirin on the expression of hepatocyte NF-κB and serum
TNF-α in streptozotocin-induced type 2 diabetic rats. J Korean Med
Sci. 26:765–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeda K, Kermani P, Anastasia A, Obinata
Y, Hempstead BL and Kurihara H: BDNF protects human vascular
endothelial cells from TNFα-induced apoptosis. Biochem Cell Biol.
91:341–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao JJ, Hu YW, Wang YC, Sha YH, Ma X, Li
SF, Zhao JY, Lu JB, Huang C, Zhao JJ, et al: ApoM suppresses
TNF-α-induced expression of ICAM-1 and VCAM-1 through inhibiting
the activity of NF-κB. DNA Cell Biol. 34:550–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kern TS: Contributions of inflammatory
processes to the development of the early stages of diabetic
retinopathy. Exp Diabetes Res. 2007:951032007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ellis EA, Guberski DL, Hutson B and Grant
MB: Time course of NADH oxidase, inducible nitric oxide synthase
and peroxynitrite in diabetic retinopathy in the BBZ/WOR rat.
Nitric Oxide. 6:295–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Asrar Abu AM, Desmet S, Meersschaert A,
Dralands L, Missotten L and Geboes K: Expression of the inducible
isoform of nitric oxide synthase in the retinas of human subjects
with diabetes mellitus. Am J Ophthalmol. 132:551–556. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leal EC, Manivannan A, Hosoya K, Terasaki
T, Cunha-Vaz J, Ambrósio AF and Forrester JV: Inducible nitric
oxide synthase isoform is a key mediator of leukostasis and
blood-retinal barrier breakdown in diabetic retinopathy. Invest
Ophthalmol Vis Sci. 48:5257–5265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosales MA, Silva KC, Duarte DA, de
Oliveira MG, de Souza GF, Catharino RR, Ferreira MS, de Lopes Faria
JB and de Lopes Faria JM: S-nitrosoglutathione inhibits inducible
nitric oxide synthase upregulation by redox posttranslational
modification in experimental diabetic retinopathy. Invest
Ophthalmol Vis Sci. 55:2921–2932. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyamoto K, Khosrof S, Bursell SE, Rohan
R, Murata T, Clermont AC, Aiello LP, Ogura Y and Adamis AP:
Prevention of leukostasis and vascular leakage in
streptozotocin-induced diabetic retinopathy via intercellular
adhesion molecule-1 inhibition. Proc Natl Acad Sci USA.
96:10836–10841. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Liu H, Al-Shabrawey M, Caldwell
RW and Caldwell RB: Inflammation and diabetic retinal microvascular
complications. J Cardiovasc Dis Res. 2:96–103. 2011. View Article : Google Scholar : PubMed/NCBI
|