Introduction
Intracerebral hemorrhage (ICH) is a condition
associated with poor prognosis and high mortality ranging from
25–50% (1). It is the most severe
subtype of stroke and accounts for 10–15% of all cases of stroke in
the majority of western populations (2), and up to 55% in China (3). This disease may occur at all ages,
however it affects younger people more frequently than ischemic
strokes. Although progress has been made in recent years in
understanding the complex pathogenesis of ICH, a major challenge
remains in the search for an effective therapeutic treatment for
ICH. Various novel treatment strategies, including stem cell
therapy, are experimental and have not proven successful in
clinical trials. Current therapeutic strategies for ICH remain
inadequate, therefore, a novel treatment approach based on the
pathogenic mechanism of ICH is of primary concern.
MicroRNAs (miRNAs) are a group of small short
sequence (18–25 nucleotides) non-coding RNAs that negatively
regulate target gene expression by translational inhibition and/or
mRNA degradation (4,5). It has previously been demonstrated
that miRNAs are important in numerous biological and pathological
processes, including cell proliferation, differentiation, apoptosis
and migration (6). The expression
of miRNAs is involved in the development of a variety of human
diseases including ICH, and multiple prior studies have
acknowledged the potential therapeutic uses of miRNAs in the
treatment of diseases (7–9). Of all the miRNAs, miRNA-126 (miR-126)
is a significant regulator of angiogenic signaling in endothelial
cells and is important in vascular integrity, cancer growth and
invasion and vascular inflammation (10–13).
It has been reported that miR-126 is involved in atherosclerosis
and exhibits an anti-atherogenic role by enhancing endothelial
repair (14). Regarding cerebral
ischemia, angiogenesis is regarded as a natural protective
mechanism and has been reported to be involved in
collagenase-induced ICH (15).
Therefore, targeting angiogenesis may be a feasible therapeutic
approach for the treatment of ICH.
The present study assessed miR-126 expression in a
rat model of ICH induced by collagenase to elucidate the distinct
underlying pathogenic mechanism of ICH. In addition, the study
aimed to identify potential therapeutic targets of ICH by miRNA
modulation.
Materials and methods
Animals and experimental designs
Adult male Wistar rats (n=12; Laboratory Animal
Center of Sun Yat-Sen University, Zhuhai, China) weighing between
320 and 350 g (12-weeks-old) were used in the present study. All
the animals were acclimated to the laboratory for ≥1 week prior to
testing. The rats were housed in separate cages with standard food
and water ad libitum under 12:12 h light-dark cycle with a
controlled temperature ranging between 20 and 22°C, and 50 and 65%
humidity. The animal experiments were performed in accordance with
the Principles of Laboratory Animal Care and approved by the Ethics
Committee of The Fifth Affiliated Hospital of Sun Yat-Sen
University (approval no. IACUC-15-083, Zhuhai, China). The rats
were randomly assigned to ICH model and sham groups.
ICH model
ICH was induced by intracerebral injection of
collagenase in accordance with the previously described protocol
(16,17). Briefly, the rats in the ICH model
group (n=6) were anesthetized with 2% isoflurane (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) and were placed into a
stereotaxic frame. Collagenase VIIS (0.075 U/500 nl saline;
Sigma-Aldrich, Merck Millipore, Darmstadt, Germany) was then
injected unilaterally into the caudate putamen for 5 min with a
glass syringe at the following stereotactic coordinates: 1.0 mm
posterior to and 2.2 mm lateral to the bregma, and 6.0 mm in depth
below the skull. Following injection, the needle was held in the
injection site for a further 10 min to prevent reflux. During the
surgery and the recovery periods, rectal temperature was maintained
at 37±0.5°C. The rats in the sham group (n=6) were administered an
equal volume of saline without collagenase VIIS. ICH was considered
to occur when the hematoma appeared in the caudate nucleus.
Vector construction and
transduction
The miR-126 lentivirus expression vector
(pWPXL-miR-126) and negative control vector (Shanghai GenePharma
Co., Ltd., Shanghai, China) was constructed by replacing the pWPXL
vector green fluorescence protein (GFP) fragment with the
pri-miR-126 sequence amplified from normal genomic DNA. The
oligonucleotide sequences for pri-miR-126 sequence were as follows:
Forward 5′-AATTATATCTCGAGGAGGGAGGATAGGAAT0AAT1-3′ and reverse
5′-GCTCGAATTCCAGAGGTCTCAGGGCTATGC-3′. The constructs were verified
by sequencing. Lentivirus expression plasmids were co-transfected
into HEK293T cells using Lipofectamine 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) following the
manufacturer's protocol. HEK293T cells were obtained from Cell Bank
of Chinese Academy of Sciences (Shanghai, China). These cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100
U/ml) and streptomycin (100 µg/ml). The titers of lentivirus vector
stocks were 0.4×109-2.0×109 particles/ml. The
lentivirus vectors were delivered via intraparenchymal injection of
rats as described previously (18), with slight modification (19). Briefly, the rats were anesthetized
by 400 mg/kg chloral hydrate (Sigma-Aldrich; Merck Millipore) and
the spine was held with two individual bars placed around the L3
vertebra. Subsequently, the thoracic T13 vertebra was drilled away
to provide access to the left side of the lumbar spinal cord with
the use of an operation microscope (Zeiss GmbH, Jena, Germany
Pentero). The dura mater and arachnoid mater were then exposed
intact, and lentivirus vectors [LV-Enhanced GFP or LV-miR-126] were
delivered by using an automatic microinjection device (KDS 310; KD
Scientific, Inc., Holliston, MA, USA) followed by suture of the
muscles and skin. Following the operation, the rats were housed in
individual cages for recovery.
Behavioral testing
Behavioral tests were performed at 1, 2 and 3 days
following transduction with LV-miR-126 to induce the overexpression
of miR-126, and evaluated by the rotarod and limb placement tests.
For the rotarod test, the rats were placed on an accelerating
rotarod cylinder and trained for 3 days prior to ICH surgery. The
speed was slowly increased (ranging from 10 to 40 rpm) within 2
min. Following transduction, the animals were put on the
accelerating rod again, and the duration of stay on the rotarod was
recorded. The duration was measured three times. The test ended if
the rats fell off the rungs or gripped the device and spun around
for 2 consecutive revolutions without attempting to walk on the
rungs. The limb placement test was performed to assess the
sensorimotor integration of forelimbs and hindlimb responses to
tactile and proprioceptive stimulation. The limb placement test had
three tasks, including ‘visual forward’, ‘visual lateral’ and
‘proprioception’. Visual forward was used to observe the forelimb
flexion. The stretch of the forelimbs was assessed as normal
stretch (0 point) and abnormal flexion (1 point). Visual lateral
was performed to observe the forelimb stretch by stimulating the
whiskers when the rat's trunk was held. The evaluations were
defined as normal lifting (0 point), abnormal lifting (1, 2, or 3
points). Proprioception was estimated by observation of the rat
stepping up on forelimbs and hindlimbs onto the table following a
pull-down of the forelimbs and hindlimbs below the table surface.
The score was classed to 0 point (normal lifting) 1, 2, or 3 points
(abnormal lifting) based on the number of normal stretches.
Evaluation of hemorrhage
The rats were sacrificed three days following
transduction under anesthesia with ketamine injection (100 mg/kg;
Sigma-Aldrich; Merck Millipore) via cervical dislocation. The
brains were immediately harvested and frozen. Coronal slices
(embedded in paraffin and cut into 20 µm thick sections) were
prepared according to Paxinos and Watson's stereotaxic atlas
(20). Hemorrhages were evaluated
by blind histological evaluation on three defined sections (+0.48,
−0.92 and −3.30 mm relative to the bregma) (21). The incidence of ICH was calculated
according to a previously described protocol (22). No hemorrhage was recorded as 0,
multiple, macroscopically visible hemorrhages were considered as 1
and hematoma was regarded as 2. Determination of ICH severity was
based on the number of petechial hemorrhages or hematoma per
infarct area.
Measurement of apoptotic cells
The apoptotic cells were evaluated by terminal
transferase deoxyuridine 5′-triphosphate nick end labeling (TUNEL)
assay using an in situ cell death detection kit (Roche
Diagnostics, Basel, Switzerland) according to the manufacturer's
protocol. Briefly, the brain tissue specimens were collected,
perfused with 4% paraformaldehyde, deparaffinized, dehydrated,
pretreated with proteinase K and peroxidase block (Dako North
America, Inc., Carpinteria, CA, USA) and incubated with TdT enzyme
at 37°C for 1 h. Subsequently, the sections were washed, incubated
with treptavidin-horseradish peroxidase for 15 min, washed again
and then incubated with diaminobenzidine (DAB). The number of
TUNEL-positive cells were counted using an Olympus microscope
(BX45-92P05; Olympus Corporation, Tokyo, Japan) at 10 randomly
selected fields.
Reverse transcription-quantitative
polymerase chain reaction. (RT-qPCR)
The mRNA levels of miR-126 in the cortical
homogenates were determined by RT-qPCR. Briefly, total RNA was
extracted with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) from the brain tissue specimens according
to the manufacturer's protocols. First-strand complementary DNA
(cDNA) was synthesized with the TaqMan microRNA Reverse
Transcription Kit (Applied Biosystems, Thermo Fisher Scientific,
Inc.) and Megaplex RT primers (Megaplex RT Rodent Pool A; Thermo
Fisher Scientific, Inc.). The primers were synthesized by Shanghai
Sangon Biotechnology Co., Ltd (Shanghai, China) and the sequences
were as follows: iR-126, forward
5′-TATAAGATCTGAGGATAGGTGGGTTCCCGAGAACT-3′ and reverse
5′-ATATGAATTCTCTCAGGGCTATGCCGCCTAAGTAC-3′; U6, forward
5′-ATCCGCAAAGACCTGT-3′ and reverse 5′-GGGTGTAACACTAAG-3′. The
relative expression levels were determined using the PrimeScript RT
Reagent kit (Takara Bio, Inc., Otsu, Japan) and the
2−ΔΔCq method (23).
PCR was run on a GeneAmp PCR System 9700 thermal cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
parameters: 1 predenaturation cycle of 5 min at 95°C, 40–50 cycles
of 95°C for 30 sec, 58–62°C for 30 sec, and 72°C for 30 sec, and a
final extension at 72°C for 5 min. U6 snRNA served as a reference
gene miRNA expression.
Western blotting
Total protein was extracted from the brain tissue
specimens using a protein extract kit (Cytoplasmic Protein
Extraction Kit; Wuhan Boster Biological Technology, Ltd., Wuhan,
China). The concentrations of protein were assessed using a BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc.). The samples (20
µg per lane) were then subjected to 10–12% SDS-PAGE electrophoresis
and transferred onto polyvinylidene fluoride or nitrocellulose
membranes. Subsequently, the membranes were blocked in 5% non-fat
dried milk for 2 h at room temperature, washed with phosphate
buffer saline (PBS), and incubated with rabbit anti-vascular
endothelial growth factor (VEGF)-A (1:1,000; cat. no. AB1876-I;
Sigma-Aldrich, Merck Millipore) or rabbit anti-caspase-3 (1:1,000;
cat. no. C8487; Sigma-Aldrich, Merck Millipore) antibodies
overnight at 4°C, followed by incubation with secondary anti-rabbit
IgG conjugated to horseradish peroxidase (1:5,000; cat. no. A0545;
Sigma-Aldrich; Merck Millipore) for 2 h at room temperature. The
intensity of protein bands was visualized by enhanced
chemiluminescence western blotting substrate (Pierce; Thermo Fisher
Scientific, Inc.) and was quantified by densitometry using Image J
software (National Institutes of Health, Bethesda, MD, USA).
Densitometric values were normalized to rabbit GAPDH (1:1,000; cat.
no. G9545; Sigma-Aldrich; Merck Millipore) internal control which
was incubated overnight at 4°C.
Statistical analysis
Data are presented as the mean ± standard deviation.
All the statistical analyses were performed with the use of
GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA,
USA). A paired Student's t-test or a one-way analysis of variance
with Tukey-Kramer's post hoc test was used to calculate P-values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
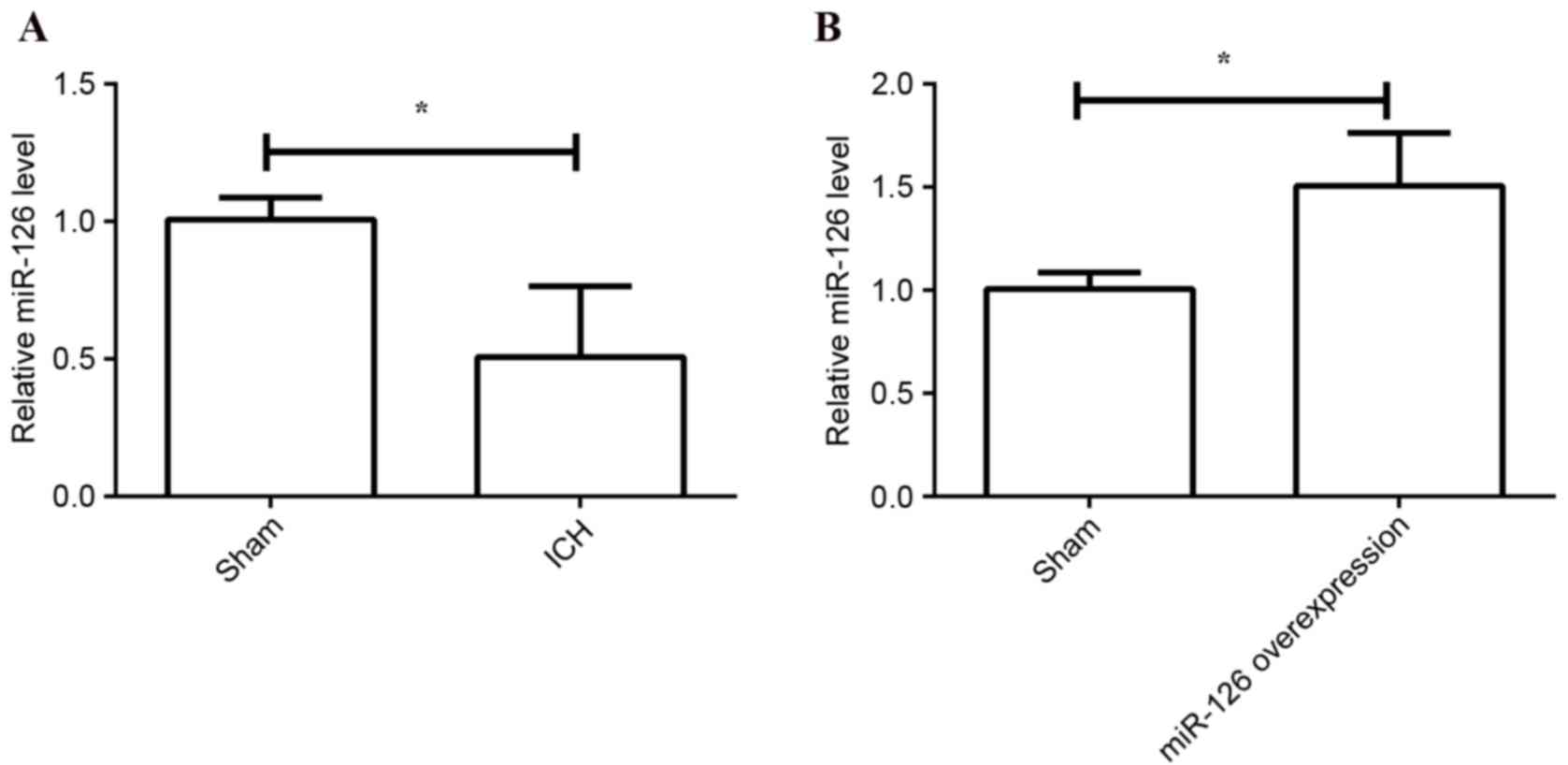
miR-126 levels decrease in ICH
To investigate the functional role of miR-126 in
ICH, the present study first determined the mRNA expression levels
of miR-126 in the model of ICH and the sham group by using RT-qPCR.
ICH was established by intracerebral injection of collagenase,
while the rats in the sham group received an equal volume of saline
without collagenase. U6 snRNA served as an internal control. The
results demonstrated that the relative expression levels of miR-126
were significantly decreased in the ICH group compared with the
sham group (P=0.026; Fig. 1A),
indicating that miR-126 may exhibit a protective role in ICH. To
further elucidate the protective role of miR-126 in ICH, the rats
in the ICH group were transiently transfected with LV-miR-126 or
negative control vector via intraparenchymal injection. The
expression of miR-126 was confirmed after 48 h of transfection
using RT-qPCR. The results demonstrated that the expression of
miR-126 was upregulated by transfection with LV-miR-126 compared
with transfection with negative control vector (P=0.013; Fig. 1B).
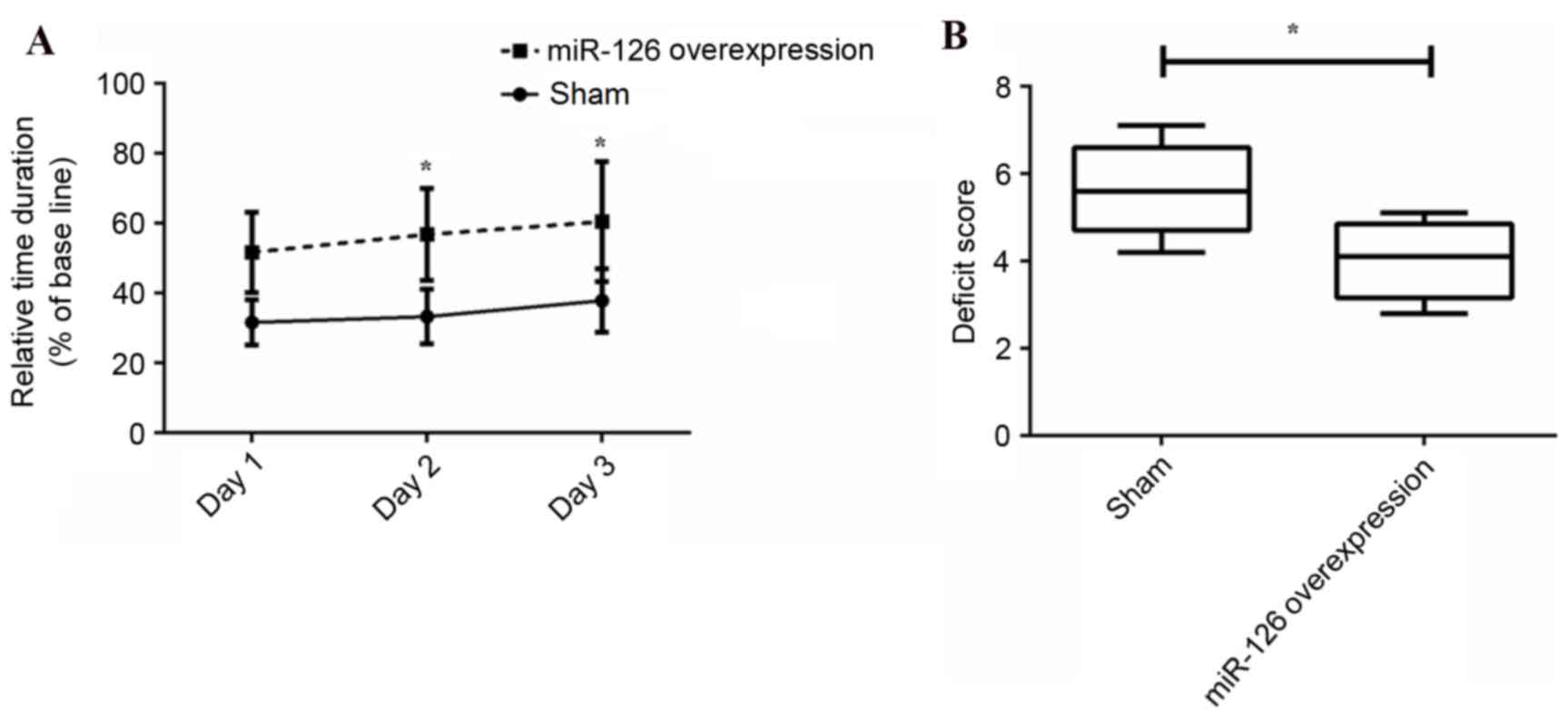
Overexpression of miR-126 improves
behavioral testing scores
The effect of overexpression of miR-126 on
behavioral testing (rotarod and limb placement tests) was
determined at 1, 2 and 3 days following transduction. As presented
in Fig. 2A, the relative duration
of stay on the rotarod was significantly improved at day 2
(P=0.029) and 3 (P=0.033) by overexpression of miR-126 compared
with the negative control group. In addition, the deficit score was
significantly reduced following overexpression of miR-126 (P=0.036;
Fig. 2B). The results suggested
that overexpression of miR-126 may significantly improve behavioral
performance in ICH.
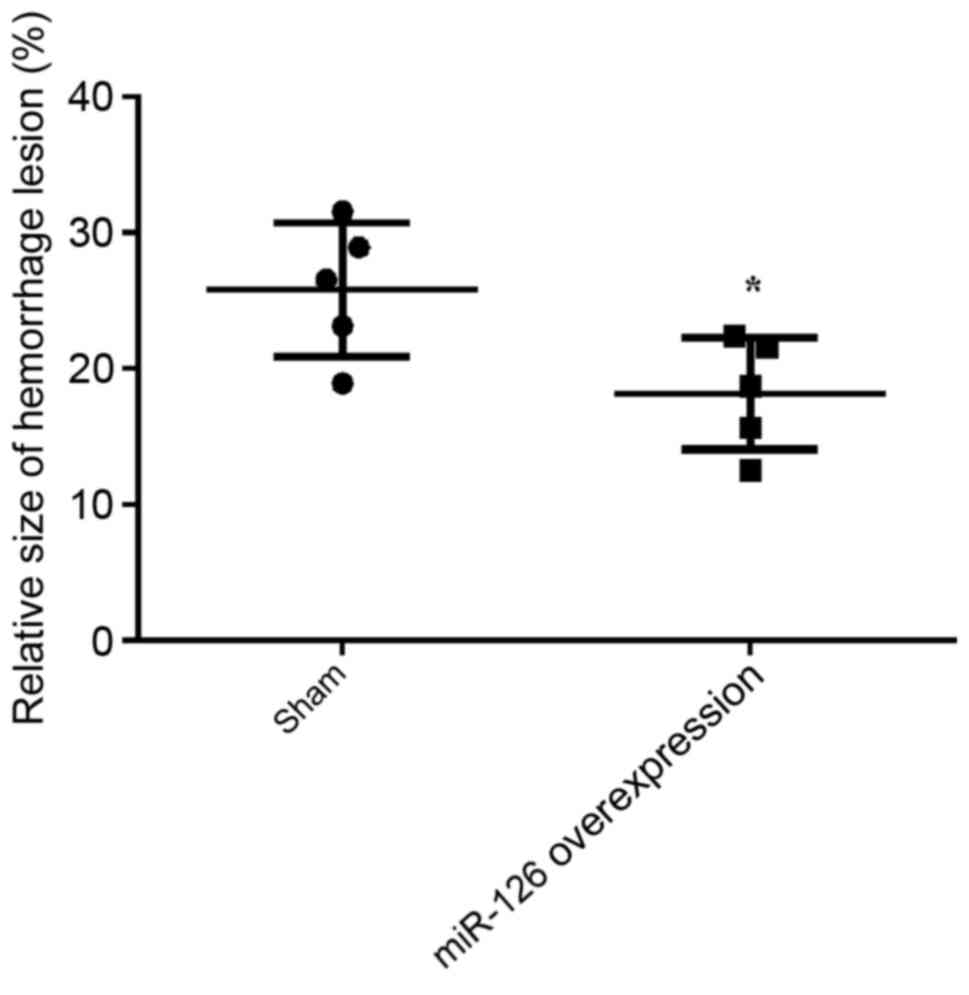
Overexpression of miR-126 decreases
hemorrhagic lesion size
The hemorrhagic lesion size following overexpression
of miR-126 was then measured. The rats were sacrificed 3 days after
transduction and brain specimens were collected. The hemorrhages
were evaluated by blind histological evaluation and the relative
size of hemorrhagic lesion was determined. As indicated in Fig. 3, the relative size of hemorrhagic
lesion was statistically reduced in the overexpression of miR-126
group compared with the negative control group (P=0.019),
demonstrating that overexpression of miR-126 may significantly
decrease the damage to the brain.
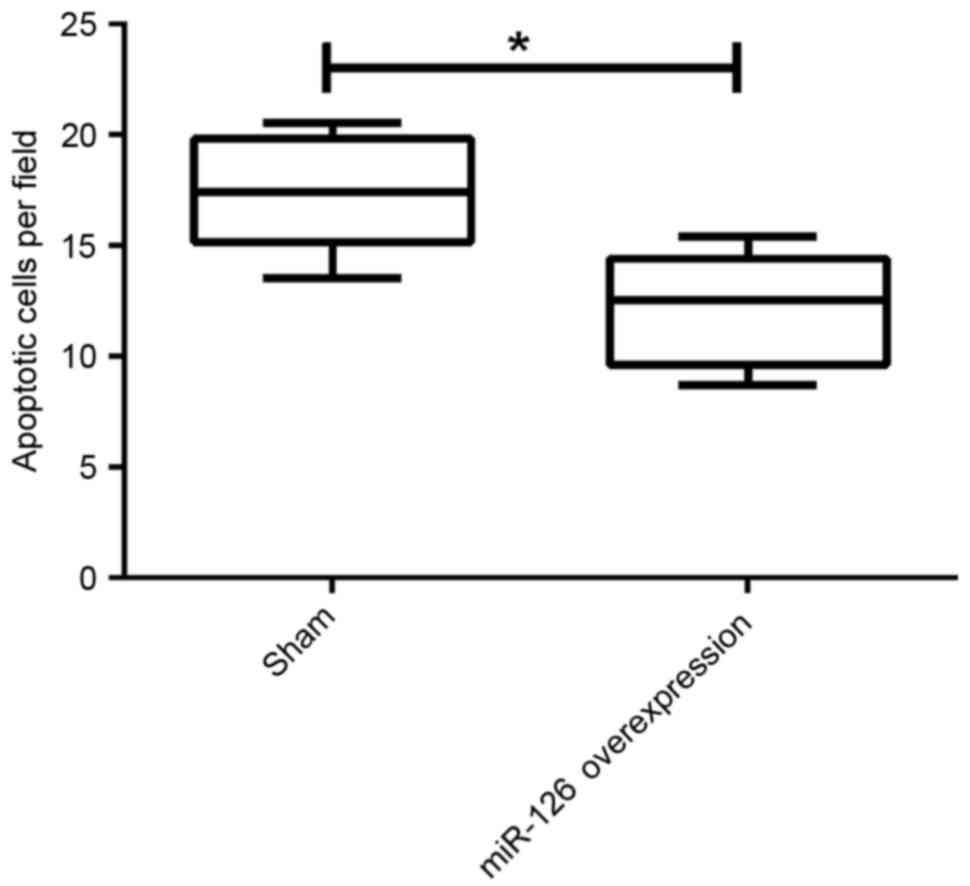
Overexpression of miR-126 decreases
apoptosis
Following transduction, the effects of
overexpression of miR-126 on apoptotic cells were evaluated by
TUNEL assay. The number of apoptotic cells was calculated at 10
randomly selected fields. The results demonstrated that the number
of apoptotic cells was statistically decreased by overexpression of
miR-126 compared with the negative control group (P=0.024; Fig. 4), indicating that overexpression of
miR-126 may significantly improve ICH by decreasing the number of
apoptotic cortical neurons.
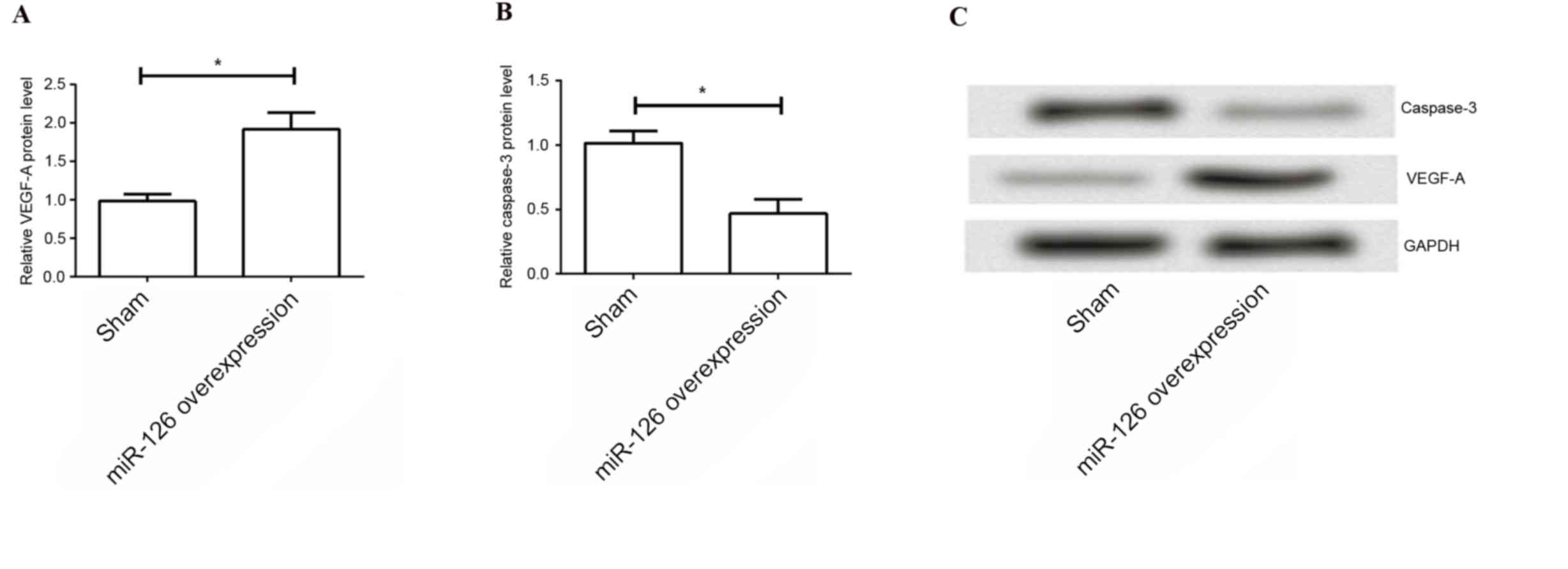
Overexpression of miR-126 increases
VEGF-A and decreases caspase-3
Furthermore, the present study evaluated the
underlying mechanism of the protective role of miR-126 in ICH. The
expression levels of VEGF-A and caspase-3 were determined by
western blotting. As indicated in Fig.
5, the results demonstrated that the expression levels of
VEGF-A were significantly higher in the overexpression of miR-126
group compared with those in the negative control group (P=0.031),
and the expression levels of caspase-3 were significantly reduced
by overexpression of miR-126 (P=0.016). These results suggested
that the protective role of overexpression of miR-126 on ICH may be
involved in the process of angiogenesis and cell apoptosis.
Discussion
miRNAs have previously been demonstrated to exhibit
an important role in various processes and pathways including cell
apoptosis, proliferation, metabolism and morphogenesis, and in
numerous human diseases including cerebrovascular disease (24). The present study, confirmed that
miR-126 was downregulated in the model of ICH induced by
collagenase in rats. Overexpression of miR-126 presented a
protective role in ICH. The behavioral performance of the animals
was significantly improved and the apoptotic cells were decreased.
The underlying mechanism may be associated with the upregulation of
VEGF-A and downregulation of caspase-3.
A series of pathophysiological processes have been
reported following acute ICH, including cell death (apoptosis and
necrosis), inflammation, disruption of neurovascular units
(cerebral endothelial cells, astrocytes, neurons and extracellular
matrix) and edema formation (25,26).
Apoptosis is characterized by the initiation of a series of
distinct morphological and biochemical alterations, leading to the
activation of caspases. Caspases are aspartate-specific cysteine
proteases that are constitutively expressed in brain tissue,
participating in the destruction of cells following activation by
intrinsic and extrinsic stimuli. Furthermore, disruption of
cerebral microvasculature that is formed by endothelial cell (ECs)
may be responsible for ICH. Angiogenesis following ICH is
considered to be a natural protective mechanism that regulates
brain recovery and repair. A previous study has suggested the
occurrence of cerebral angiogenesis in collagenase-induced ICH in
rats (15). Therefore, development
of novel treatment strategies based on apoptosis and angiogenesis
may be a possible target for IHC treatment.
The functional roles of miR-126, an endothelial
cell-specific miRNA, have been previously investigated. It is
located within intron 7 of epidermal growth factor-like domain 7
and is highly expressed in vascular ECs (11). It has been reported that miR-126 is
involved in various biological and pathological processes,
including angiogenic signaling and vascular integrity (10), cell proliferation and apoptosis
(27–29). Previous studies have confirmed that
miR-126 promotes angiogenesis in response to angiogenic growth
factors, including VEGF or basic fibroblast growth factor (30). VEGF is a heparin-binding growth
factor specific for vascular ECs that is associated with the
induction of angiogenesis (31).
Inhibiting the expression of VEGF prevents angiogenesis and has
been applied in different tumors in combination with chemotherapy
(32). In addition to the effects
on the vasculature, VEGF family members have been proposed as
potent modulators of neurogenesis and neural plasticity, indicating
their use in potential therapeutic strategies for neurodegenerative
disease and neural tissue repair (33). VEGF-A, one of the most important
members of VEGF family, is a potent mitogen, chemotactic factor and
EC survival factor (34) and is
the principle regulator of angiogenesis (35). In addition, it has been revealed
that miR-126 is a negative regulator of VEGF-A (36). Considering the functions of
miR-126, the present study hypothesized that miR-126 may be
involved in ICH and may exhibit a protective role in ICH.
To confirm the hypothesis, the present study
initially evaluated the expression levels of miR-126 in ICH
following intracerebral injection of collagenase. miR-126 was as
decreased in ICH as in atherosclerosis, and administration of
miR-126 may therefore be an effective potential method to protect
from ICH. Subsequently, the expression of miR-126 was upregulated
by transfection with an miR-126-expressing lentivirus. As expected,
overexpression of miR-126 significantly improved the behavioral
performance and reduced the hemorrhage size, indicating a
protective role of miR-126 in ICH. The apoptosis of cortical
neurons following overexpression of miR-126 was also observed. The
results indicated that apoptosis was statistically reduced by
overexpression of miR-126, demonstrating the anti-apoptotic effect
of miR-126 during ICH. Furthermore, the underlying mechanism of
apoptosis was investigated by determination of the expression of
caspase-3. Caspase-3 is a major cell death effector protease and is
important in apoptosis. It has been reported a neuroprotective
effect was observed in caspase-3-deficient mice following cerebral
ischemia (37). The present study
revealed that overexpression of miR-126 significantly decreased the
levels of caspase-3, demonstrating an anti-apoptotic effect in ICH.
The expression levels of VEGF-A in ICH were additionally measured
and in accordance with previous studies (38,39),
overexpression of miR-126 improved the levels of VEGF-A, promoting
angiogenesis in ICH.
In conclusion, the results of the present study
demonstrated that miR-126 protects against ICH. This
neuroprotection may occur due to an anti-apoptotic effect, or
angiogenesis induced by miR-126, however the underlying molecular
mechanism of its role in these processes remains to be fully
elucidated.
References
|
1
|
Hill MD, Silver FL, Austin PC and Tu JV:
Rate of stroke recurrence in patients with primary intracerebral
hemorrhage. Stroke. 31:123–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qureshi AI, Tuhrim S, Broderick JP, Batjer
HH, Hondo H and Hanley DF: Spontaneous intracerebral hemorrhage. N
Engl J Med. 344:1450–1460. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang QD, Niu Q, Zhou YH, Liu YH, Xu HW, Gu
WP, Tian FF, Xie YQ, Zhang L and Xia J: Incidence of cerebral
hemorrhage in the Changsha community. A prospective study from 1986
to 2000. Cerebrovasc Dis. 17:303–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qureshi IA and Mehler MF: The emerging
role of epigenetics in stroke: II. RNA regulatory circuitry. Arch
Neurol. 67:1435–1441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai Y, Wang L, Sun L, Ye P and Hui R:
Circulating microRNA-26a: Potential predictors and therapeutic
targets for non-hypertensive intracerebral hemorrhage. Med
Hypotheses. 77:488–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng HW, Wang YL, Lin JX, Li N, Zhao XQ,
Liu GF, Liu LP, Jiao Y, Gu WK, Wang DZ and Wang YJ: Circulating
MicroRNAs as potential risk biomarkers for hematoma enlargement
after intracerebral hemorrhage. CNS Neurosci Ther. 18:1003–1011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JM, Lee ST, Chu K, Jung KH, Kim JH, Yu
JS, Kim S, Kim SH, Park DK, Moon J, et al: Inhibition of Let7c
microRNA is neuroprotective in a rat intracerebral hemorrhage
model. PLoS One. 9:e979462014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuhnert F, Mancuso MR, Hampton J,
Stankunas K, Asano T, Chen CZ and Kuo CJ: Attribution of vascular
phenotypes of the murine Egfl7 locus to the microRNA miR-126.
Development. 135:3989–3993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S and Olson EN: AngiomiRs-key
regulators of angiogenesis. Curr Opin Genet Dev. 19:205–211. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei Y, Nazari-Jahantigh M, Neth P, Weber C
and Schober A: MicroRNA-126, −145 and −155: A therapeutic triad in
atherosclerosis? Arterioscler Thromb Vasc Biol. 33:449–454. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang T, Liu XJ, Zhang ZQ, Zhou HJ, Luo JK,
Huang JF, Yang QD and Li XQ: Cerebral angiogenesis after
collagenase-induced intracerebral hemorrhage in rats. Brain Res.
1175:134–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grossetete M and Rosenberg GA: Matrix
metalloproteinase inhibition facilitates cell death in
intracerebral hemorrhage in mouse. J Cereb Blood Flow Metab.
28:752–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Rogove AD, Tsirka AE and Tsirka
SE: Protective role of tuftsin fragment 1–3 in an animal model of
intracerebral hemorrhage. Ann Neurol. 54:655–664. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meunier A, Mauborgne A, Masson J, Mallet J
and Pohl M: Lentiviral-mediated targeted transgene expression in
dorsal spinal cord glia: Tool for the study of glial cell
implication in mechanisms underlying chronic pain development. J
Neurosci Methods. 167:148–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dominguez E, Mauborgne A, Mallet J,
Desclaux M and Pohl M: SOCS3-mediated blockade of JAK/STAT3
signaling pathway reveals its major contribution to spinal cord
neuroinflammation and mechanical allodynia after peripheral nerve
injury. J Neurosci. 30:5754–5766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paxinos G and Watson C: The rat brain in
stereotaxic coordinates. Academic Press; 6. pp. 1986
|
|
21
|
Gautier S, Ouk T, Pétrault M, Pétrault O,
Bérézowski V and Bordet R: PPAR-Alpha agonist used at the acute
phase of experimental ischemic stroke reduces occurrence of
thrombolysis-induced hemorrhage in rats. PPAR Res. 2015:2463292015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gautier S, Ouk T, Petrault O, Caron J and
Bordet R: Neutrophils contribute to intracerebral haemorrhages
after treatment with recombinant tissue plasminogen activator
following cerebral ischaemia. Br J Pharmacol. 156:673–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT
and Juo SH: Serum microRNA-21 and microRNA-221 as potential
biomarkers for cerebrovascular disease. J Vasc Res. 50:346–354.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kunz A, Dirnagl U and Mergenthaler P:
Acute pathophysiological processes after ischaemic and traumatic
brain injury. Best Pract Res Clin Anaesthesiol. 24:495–509. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan JR, Koo YX, Kaur P, Liu F, Armugam A,
Wong PT and Jeyaseelan K: microRNAs in stroke pathogenesis. Curr
Mol Med. 11:76–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamada S, Satoh K, Fujibuchi W, Hirota M,
Kanno A, Unno J, Masamune A, Kikuta K, Kume K and Shimosegawa T:
MiR-126 acts as a tumor suppressor in pancreatic cancer cells via
the regulation of ADAM9. Mol Cancer Res. 10:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Bai Y, Zhang F, Wang Y, Guo Y and
Guo L: miR-126 inhibits non-small cell lung cancer cells
proliferation by targeting EGFL7. Biochem Biophys Res Commun.
391:1483–1489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fish JE and Srivastava D: MicroRNAs:
Opening a new vein in angiogenesis research. Sci Signa. 2:pe12009.
View Article : Google Scholar
|
|
31
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wentink MQ, Timmerman P, van der Vliet HJ,
de Gruijl TD, Verheul HM and Griffioen AW: Preclinical testing of a
novel anti-angiogenic vaccine targeting human VEGF. Cancer Res.
75:2496. 2015. View Article : Google Scholar
|
|
33
|
Eichmann A and Simons M: VEGF signaling
inside vascular endothelial cells and beyond. Curr Opin Cell Biol.
24:188–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
Therapeutic implications. Semin Oncol. 29:(6 Suppl 16). S10–S14.
2002. View Article : Google Scholar
|
|
35
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasahira T, Kurihara M, Bhawal UK, Ueda N,
Shimomoto T, Yamamoto K, Kirita T and Kuniyasu H: Downregulation of
miR-126 induces angiogenesis and lymphangiogenesis by activation of
VEGF-A in oral cancer. Br J Cancer. 107:700–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le DA, Wu Y, Huang Z, Matsushita K,
Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA
and Moskowitz MA: Caspase activation and neuroprotection in
caspase-3-deficient mice after in vivo cerebral ischemia and
in vitro oxygen glucose deprivation. Proc Natl Acad Sci USA.
99:15188–15193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang D, Baumann JM, Sun YY, Tang M, Dunn
RS, Akeson AL, Kernie SG, Kallapur S, Lindquist DM, Huang EJ, et
al: Overexpression of vascular endothelial growth factor in the
germinal matrix induces neurovascular proteases and
intraventricular hemorrhage. Sci Transl Med. 5:193ra902013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui HJ, Liu QT, Zhou HJ and Tang T:
Abstract 3302: Vascular endothelial growth factor receptor
inhibition impairs intracerebral hemorrhage-induced angiogenesis.
Stroke. 43:A33022012.
|