Introduction
Malignant melanomas represent a refractory disease,
the incidence and mortality rates of which have been steadily
increasing worldwide (1,2). Melanomas, particularly advanced
melanomas, are not sensitive to traditional therapeutic regimens,
including surgery, radiation or chemotherapy, which are usually
accompanied by adverse side effects. Therefore, alternative
therapeutic regimens with improved effectiveness against malignant
melanomas are urgently required. Among novel developments,
immunotherapy-based approaches are promising and have become a
focus; they have emerged as an effective treatment option for
patients with malignant tumors (3,4). As
is already known, melanoma is one of the most immunogenic types of
cancer. Previous studies have indicated that numerous
melanoma-specific antibodies and lymphocytes are present in
patients with melanoma, which show responsiveness to
immune-stimulating agents (2,5,6).
However, immune evasion mechanisms are in existence in the tumor
microenvironment (7). Novel
immunotherapeutic methods may offer the potential to prevent tumor
recurrence, increase progression-free survival rates and improve
the quality of life of patients with melanoma.
In previous reports, the immunotherapeutic use of
dendritic cell (DC) vaccines, cytotoxic lymphocyte (CTL) cells and
cytokine-induced killer (CIK) cells have shown promising outcomes
in the improvement of cancer therapy (8,9).
Immunotherapy, particularly DCs co-cultured with CIK cells (DC-CIK)
therapy, has been widely investigated and applied as an important
option in the treatment of non-small-cell lung cancer (NSCLC)
(10). In addition, DC-CIK
cell-based immunotherapy is one of the most effective tools to
eliminate residual cancer cells, and is well-tolerated with a high
level of compliance (11).
Increasing evidence suggests that DC-CIK cell therapy is widely
used in advanced colorectal cancer (12), NSCLC (10) and liver cancer (13). DCs, major antigen-presenting cells,
capture and process tumor-associated antigens. DCs also activate
antigen-specific CTL cells and induce antitumor immune responses
(14). However, to the best of our
knowledge, there have been no reports on whether DC-CIK or DC-CTL
possess antitumor activity in melanomas.
In the present study, DC-CTL and DC-CIK cells were
cultured to examine their effects on phenotype, proliferation and
cytotoxicity against B16 melanoma tumor cells. In addition,
comparative investigations on the effects of specific
antigen-sensitized DC-CIK and DC-CTL cells against B16 melanoma
tumor cells were performed in vitro and in vivo.
Materials and methods
Animal tumor model and preparation of
DC-CTL cells and DC-CIK cells
Female C57BL/6 mice (8-week-old) were purchased from
Hebei Medical University (Shijiazhuang China). All animals (n=60,
15–20 g) were given free access to food and tap water and were
caged individually under controlled temperature (25±2°C) and
humidity (55±5%) with an artificial 12 h light/dark cycle) in
accordance with the animal care and use committee of Heibei Medical
University.
The present study was approved by the ethics
committee of Heibei Medical University.
B16 melanoma cells were purchased from the Chinese
Academy of Sciences Cell Bank (Shanghai, China) and cultured in
RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), which contained 10% fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.), 10% L-glutamine, 0.5% penicillin/streptomycin,
10% nonessential amino acids and 10% pyruvate, in a 5%
CO2 atmosphere and incubated at 37°C. The backs of the
mice were shaved completely, and a subcutaneous injection of
5×106 B16 melanoma cells was administered to the back.
Mice were selected for ablation when the diameter of the tumor
ranged between 5 and 10 mm, and was approximately round in shape.
Mice were sacrificed by intraperitoneal injection of sodium
pentobarbital (2%, 100 mg/kg, Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany), and blood samples were collected from heart
for separating PBMCs.
DC-CIK cells and DC-CTL cells were generated from
peripheral blood mononuclear cells (PBMCs) of mice. Briefly, PBMCs
were isolated from whole blood samples from mice using Ficoll
density gradient centrifugation at 2,000 × g for 20 min at
4°C using commercial lymphocyte separation medium (Sigma-Aldrich;
Merck Millipore). CIKs at a density of 2×105/well were
mixed and co-cultured with antigen-unpulsed DCs for 3 days.
Additionally, CIKs were cultured in 4X 40 ml serum-free medium
supplemented with 1,000 U/ml interleukin-2 (IL-2), 5 µg/ml CD3
monoclonal human anti-mouse antibodies (cat. no. GMP-A089; 1:500),
12.5 µg/ml RetroNectin (Novoprotein, Shanghai, China) and 1,000
U/ml interferon-γ (IFN-γ; Novoprotein), which had been induced and
cultured for 14 days at 37°C. Subsequently, ~2×106 DCs
were harvested and co-cultured with T cells (~2×107
cells) at a DC/T cell ratio of 1:10 for another 4 days to induce
antigen-specific CTL cells, which were stimulated with CD3
monoclonal antibody (50 ng/ml; Novoprotein), pre-coated onto
plastic plates and amplified by IL-2 (500 IU/ml; Novoprotein).
The C57BL/6 mice were randomly divided into three
groups, as mentioned above. In total, 106 DC-CIK cells
or DC-CTL cells in 0.2 ml PBS, or 0.2 ml PBS, were administered
intravenously into the tail of the mice in the respective
groups.
Morphologic observation and cellular
phenotype analysis
Morphological alterations of the DCs were observed
by scanning and transmission electron microscopy following culture
of the DCs for 7 days. Using flow cytometry (FCM), their phenotype
molecules, CD80+, CD86+ and HLA-DR+, were measured and recorded.
Subsequently, the DC-CIK and DC-CTL cells were collected following
14 days of cultivation, and the expression of surface markers,
CD3+CD56+ and CD3+CD8+, were examined and recorded.
Cytotoxicity towards tumor cells in
vitro
The cytotoxic activity of DC-CIK cells and DC-CTL
cells were assayed using calcein-AM (cat. no. 17783; Sigma-Aldrich;
Merck Millipore) according to the manufacturer's protocol. Briefly,
CAM media was prepared by diluting calcein-AM stock solution (1
mg/ml in DMSO) with PBS. Prewashed B16 melanoma cells were
resuspended in the CAM media (106 cells/ml) and incubated at 37°C
for 1 h with occasional shaking. The DC-CIK cells or DC-CTL cells
were resuspended with PBS at 1×106 cells/ml, and 200 µl of the
DC-CIK cells or DC-CTL cells were added into each well containing
B16 melanoma cells in a U-bottom 96-well plate. The effector to
target (E:T) ratio ranged between 10:1 and 40:1 (10:1, 20:1 and
40:1).
Measurements of CCL19 and CCL22
activity
The activities of CCL19 (cat. no. SBJ-M0271) and
CCL22 (cat. no. SBJ-M0267) were assessed ELISA kits (Nanjing
Senbeijia Biological Technology Co., Ltd., Nanjing, China). The
treated cells were collected at each time point and washed with
PBS. The supernatants were collected and measured to determine
protein concentration.
Detection of apoptosis using FCM
The apoptotic cells were differentiated from viable
or necrotic cells by the combined application of Annexin V-FITC and
propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA). The
samples were washed with PBS twice and adjusted to a concentration
of 1×106 cells/ml with 4°C PBS. Falcon tubes (12×75 mm; polystyrene
round-bottom) were used in the experiment, into each of which 100
µl of suspension was added. Subsequently, 10 µl of Annexin V-FITC
and 10 µl PI (20 µg/ml) were added into the labeled tubes and
incubated for at least 20 min at room temperature in the dark.
Following incubation, 400 µl of PBS binding buffer was added to
each tube without washing and analyzed using FCM (BD Biosciences)
within 30 min.
Detection of morphological alterations
in solid tumors using transmission electron microscopy
Uranyl acetate and lead citrate staining of the
cells were performed to detect morphological alterations. Briefly,
solid tumors were digested with pancreatin and fixed with 3%
glutaraldehyde precooled in 4°C for 2 h. To obtain ultrathin
sections of copper, the cells were washed once with PBS, fixed with
1% osmic acid for 1 h, dehydrated using acetone and embedded in
epoxide resin. Following staining with uranyl acetate and lead
citrate, the sections (100 nm) were examined under a Hitachi-800
transmission electron microscope (Hitachi, Ltd., Tokyo, Japan).
Western blot analysis
To investigate alterations in the expression levels
of caspase 3 and caspase 9 in the B16 melanoma cells and solid
tumors, the B16 melanoma cells samples were clarified by
centrifugation at 7,500 × g for 10 min at 4°C and protein
concentrations were determined using a BCA Protein Assay kit. The
B16 melanoma cells and solid tumors were homogenized and extracted
in NP-40 buffer, followed by 10 min boiling for denaturing and
centrifugation at 12,000 × g for 10 min at 4°C to obtain the
supernatant. The equal quantities of protein (50 µg/lane) were
loaded on 8% gels, followed by being blotted onto polyvinylidene
fluoride membranes using a wet transfer method. The membranes were
blocked with 5% non-fat milk in PBST for 4 h at room temperature
and then incubated with primary antibodies, caspase-3 (cat. no.
sc-1224, 1:1,000) and caspase-9 (cat. no. sc-133109, 1:1,000; all
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), in PBST overnight
at 4°C. The membranes were washed three times with PBST for 5 min
and then incubated in secondary antibody donkey anti-goat IgG
(1:10,000; cat. no. sc-2020) and goat anti-rabbit IgG (1:10,000;
cat. no. sc-2004; all Santa Cruz Biotechnology, Inc.) with 5% PBST
for 2 h at room temperature. The membranes were washed and detected
using ECL (GE Healthcare Life Sciences, Chalfont, UK), and were
exposed on Kodak radiographic film (Kodak, Rochester, NY, USA) in
the dark.
Statistical analysis
The results are expressed as the mean ± standard
deviation. All statistical analyses were performed using PRISM
version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Inter-group differences were analyzed using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
In vitro differentiation of DCs and
phenotypic analysis of cultured mature DC-CIK and DC-CTL cells
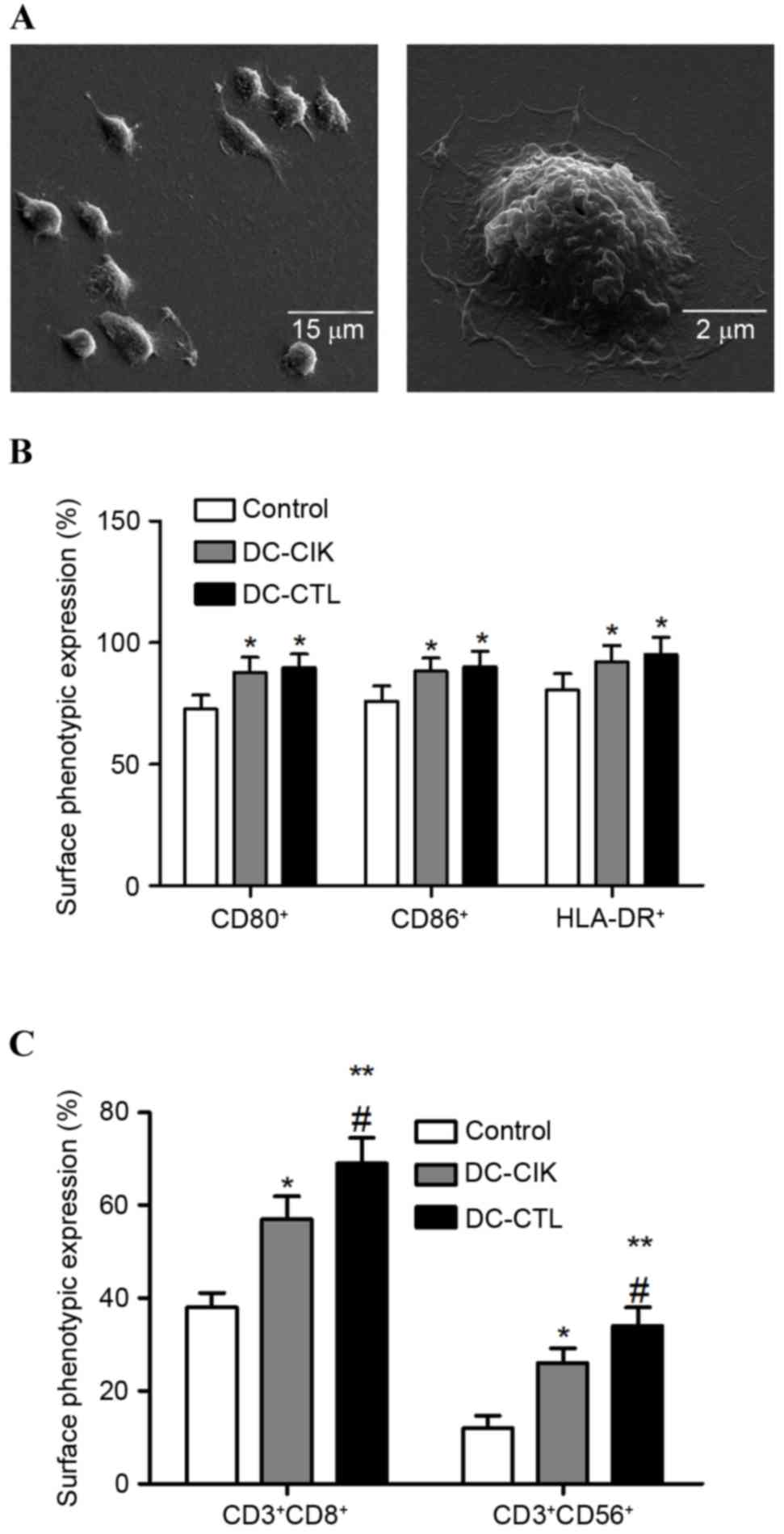
As shown in Fig.
1A, the DCs were generated from PBMCs obtained from 8-week-old
female C57BL/6 mice. The phenotypes of the co-cultured cells were
analyzed using FCM to detect whether co-culture affected the DC-CIK
and DC-CTL cell differentiation and maturation in vitro. The
results demonstrated that the expression levels of CD80+, CD86+ and
HLA-DR+ were significantly increased in the DC-CIK and DC-CTL
cells, compared with the control group. However, no significant
differences were observed in the expression levels of CD80+, CD86+
or HLA-DR+ between the DC-CIK cells group and the DC-CTL cells
group (Fig. 1B). Subsequently, the
proportions of CD3+CD8+ and CD3+CD56+ cells were found to be
significantly higher in the DC-CIK and DC-CTL cells, compared with
the control group. The proportions of CD3+CD8+ and CD3+CD56+ cells
were also significantly higher in the DC-CTL cells, compared with
the DC-CIK cells (Fig. 1C).
Cytotoxic activity of DC-CIK and
DC-CTL cells against B16 melanoma cells
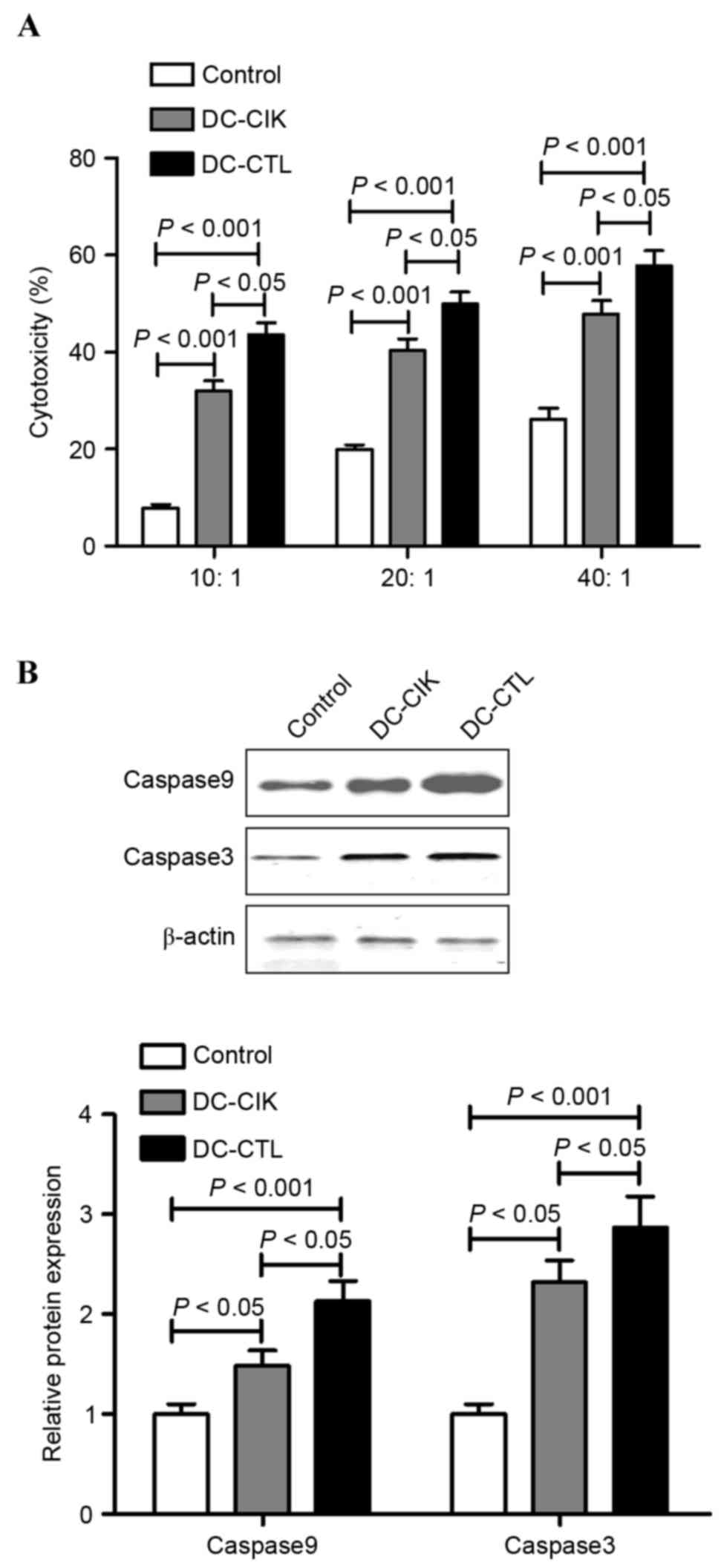
In the LDH cytotoxic analysis, B16 melanoma cells
were used as the target cells at various E:T ratios (10:1, 20:1 and
40:1) to evaluate the specific cytotoxic activity. The results
indicated that the cytotoxicity increased as the E:T ratio
increased between 10:1 and 40:1. However, the cytotoxic effect of
the DC-CIK group and DC-CTL group were significantly different
(P<0.05), with the cytotoxic effects on B16 melanoma cells by
DC-CTL cells being significantly higher, compared with that by
DC-CIK cells (Fig. 2A).
Subsequently, the present study examined whether DC-CIK cells and
DC-CTL cells regulated cell death in the B16 melanoma cell lines
through an apoptotic mechanism. Following incubation with the
DC-CIK cells of DC-CTL cells at the E:T ratio of 40:1, the protein
levels of caspase 3 and caspase 9 were measured using western blot
analysis. The results showed that the protein levels of caspase 3
and caspase 9 were increased in the B16 melanoma cells in the
presence of DC-CIK cells or DC-CTL cells, respectively. However,
the protein levels of caspase 3 and caspase 9 in the B16 melanoma
cells co-cultured with DC-CTL cells were significantly higher,
compared with those co-cultured with DC-CIK cells (Fig. 2B).
ELISA for chemokines CCL19 and
CCL22
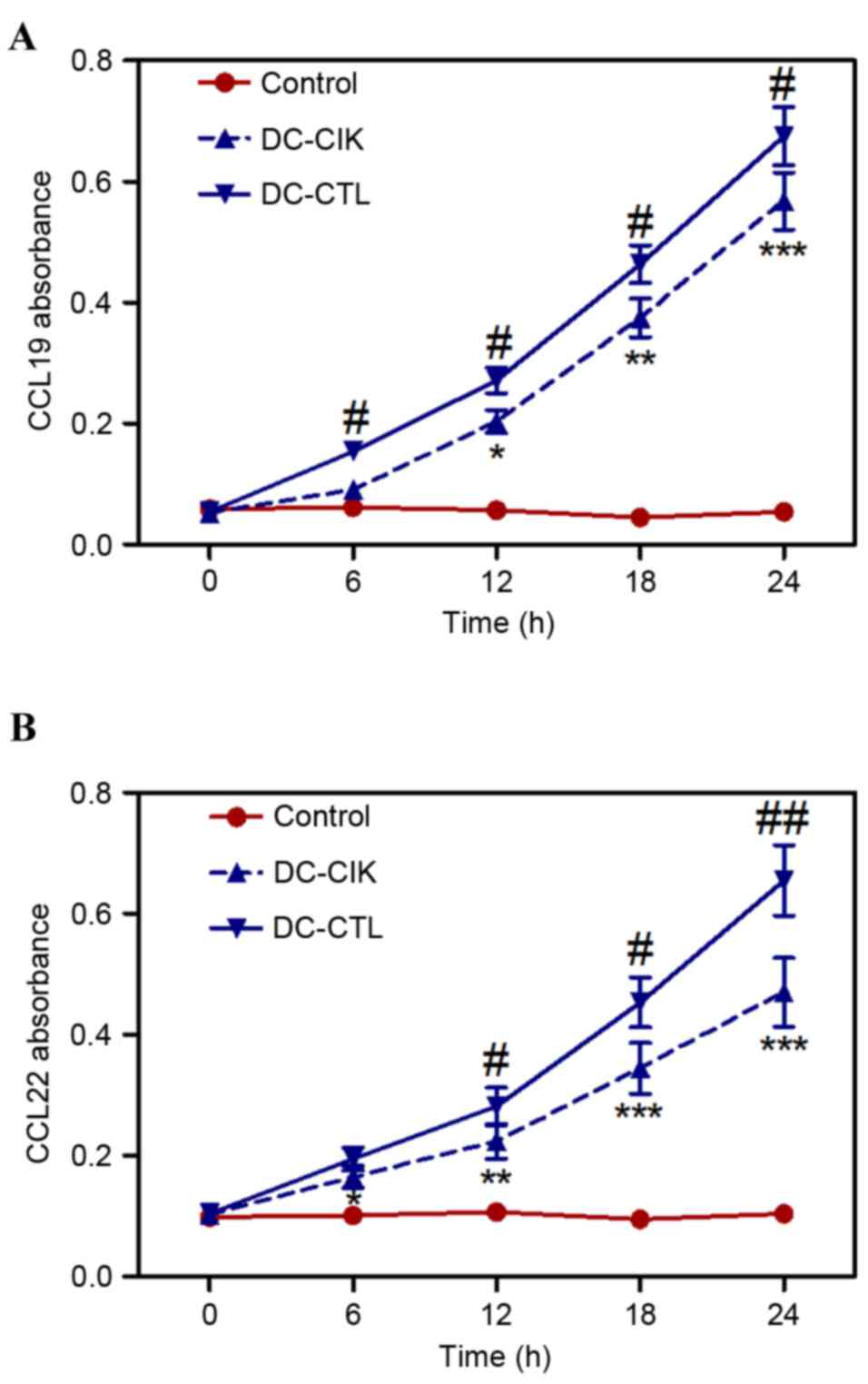
CCL19 is expressed in secondary lymphoid organs and
the thymus, and can induce DCs to migrate from the peripheral
region to areas of T cell accumulation in lymphoid organs, inducing
T helper 1 and T cells to form an immune response. CCL22 is
expressed in the spleen, peripheral blood T cells, and natural
killer cells. In the supernatant of the monocyte-derived DCs,
intact CCL22 become expressed at a high level and produces intense
chemotaxis for DCs (15). The
expression levels of CCL19 and CCL22 in the culture supernatants of
the co-cultured DC-CIK cells or DC-CTL cells were significantly
higher, compared with the control group following co-culture for 6
h (Fig. 3A and B). In addition,
the levels of CCL19 and CCL22 in B16 melanoma cells co-cultured
with DC-CTL cells were significantly higher, compared with those
co-cultured with DC-CIK cells (Fig. 3A
and B).
In vivo antineoplastic efficacy of
DC-CIK and DC-CTL cells
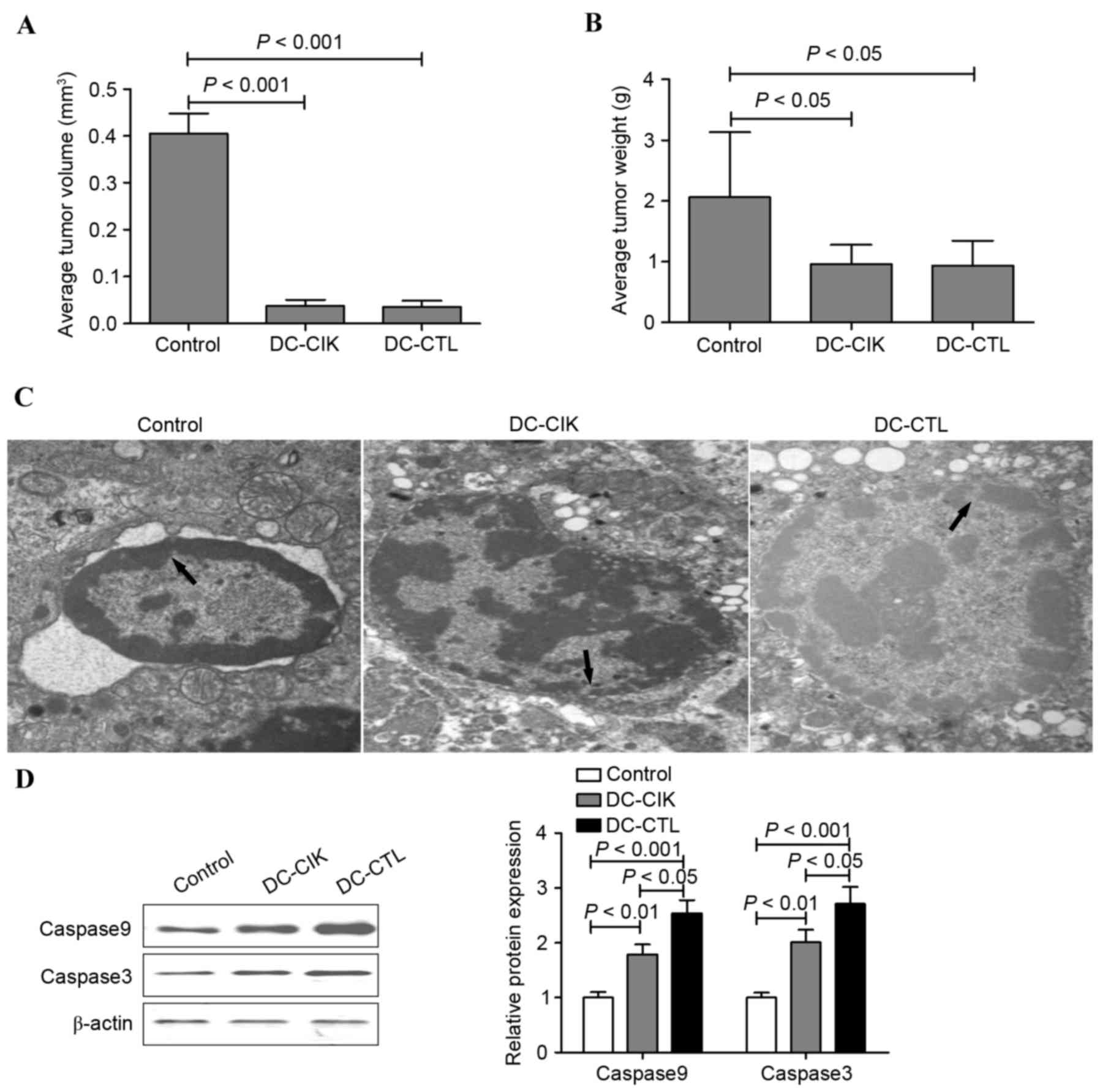
In order to examine whether DC-CIK cells or DC-CTL
cells can regulate B16 melanoma cell growth in vivo, the
present study performed a tumor xenograft experiment.
Tail-intravenous injection of DC-CIK cells and DC-CTL cells
attenuated B16 melanoma cell-engrafted tumor growth in vivo.
However, no significant differences between the antineoplastic
efficacy of the DC-CIK and DC-CTL cells were found in vivo
(Fig. 4A and B). To further
investigate the antineoplastic efficacy of DC-CIK and DC-CTL cells
in vivo, morphological alterations and apoptosis were
measured using a transmission electron microscope and FCM.
Following treatment with DC-CIK cells and DC-CTL cells, a region of
the nuclear membrane domed outward with a sharp angle, the nuclei
showed chromatin pyknosis and were clustered on the inner border of
karyotheca, cytoplasm condensation and swelling of mitochondria
were observed in the inner segment. By contrast, the nuclear
membrane appeared clear and complete in the normal B16 melanoma
cells (Fig. 4C). Following
treatment with the DC-CIK cells or DC-CTL cells, the effect on
cell-cycle distribution was determined using FCM. As shown in
Table I, an accumulation of cells
in the G0/G1 phase was observed followed
treatment of the B16 melanoma cell mouse model with DC-CIK cells
and DC-CTL cells. However, no significant differences in cell-cycle
distribution were found between the DC-CIK group and DC-CTL group.
The protein levels of caspase 3 and caspase 9 were also increased
in the B16 melanoma solid tumors treated with DC-CIK cells and
DC-CTL cells. However, the protein levels of caspase 3 and caspase
9 in the DC-CTL group were significantly higher, compared with
those in the DC-CIK group (Fig.
4D).
 | Table I.Effects of DC-CIK cells and DC-CTL
cells on the cell cycle distribution, AI and PI in B16 melanoma
cells. |
Table I.
Effects of DC-CIK cells and DC-CTL
cells on the cell cycle distribution, AI and PI in B16 melanoma
cells.
| Group |
G0/G1 | S | G2/M | AI | PI |
|---|
| DC-CIK |
81.65±2.39a |
14.27±1.49a |
8.16±0.86a |
14.32±3.01a |
20.86±1.25a |
| DC-CTL |
83.17±2.57a |
15.27±1.65a |
9.02±0.95a |
15.10±2.83a |
21.37±1.58a |
| Control | 59.47±1.62 | 14.82±0.71 | 21.63±1.76 | 3.58±1.26 | 41.75±1.88 |
Discussion
The use of personalized adoptive immunotherapy as a
potential novel approach is promising in the treatment of tumors
resistant to conventional therapies by providing precise and
optimal treatment to lower the rates of recurrence and metastasis
in malignant tumors (16,17). CIK cells are now considered a
primary candidate for personalized adoptive immunotherapy, which
have potent antiproliferative and cytotoxic capacities against
tumor cells (18,19). It is suggested that CIK cells are
heterogeneous in vitro-expanded T lymphocytes with mixed
natural killer (NK)-like T cells. The antitumor activity of CIK
cells is predominantly due to the high proliferative and cytolytic
potential of CD3+CD56+ NKT cells (20). TLC cells are a major component of
the cellular immune response and are essential cells required for
antitumor immunity (21). Previous
studies have indicated that DC-CTL/CIK therapy significantly
reduces several serological tumor markers, including AFP, CA199 and
CA242 in primary liver cancer, and CA724 in gastric cancer,
elevates the level of CD3+CD8+ T cells in
primary liver cancer and lung cancer, decreases the level of
CD3+CD4+ T cells in colon cancer, primary
liver cancer and lung cancer and decreases regulatory T cells in
all types of tumor (1,8–10).
These results indicate that DC-CTL/CIK can promote immune functions
in these patients (20). Based on
these studies, the present study aimed to perform a comparative
investigation of the effect of specific antigen-sensitized DC-CIK
and DC-CTL cells against B16 melanoma tumor cells.
The results of the present study showed that DC-CIK
cells and DC-CTL cells exhibited antineoplastic activities in
vitro and in vivo. In vitro, the cytotoxicity
increased as the E:T ratio increased between 10:1 and 40:1.
Comparison of the cytotoxic effect between the DC-CIK group and
DC-CTL group showed significant differences, in which the cytotoxic
effects on B16 melanoma cells were significantly higher when
exposed to DC-CTL cell co-culture, compared with DC-CIK cell
culture. In addition, DC-CIK cells and DC-CTL cells regulated B16
melanoma cell growth in vivo, as tail-intravenous injection
of DC-CIK cells and DC-CTL cells attenuated B16 melanoma
cell-engrafted tumor growth, induced G0/G1
cell cycle arrest and accelerated apoptosis.
CIK cells are a cell population obtained from PBMCs
stimulated with IFN-γ, IL-2 and CD3 monoclonal antibody (22). They can express the surface markers
of T cells and NK cells, CD3+CD56+ (23). In the present study, co-culture
appeared to affect DC-CIK cell and DC-CTL cell differentiation and
maturation in vitro, and the expression levels of
CD80+, CD86+ and HLA-DR+ were
significantly increased in the DC-CIK and DC-CTL cells. In
addition, the proportion of CD3+CD8+ and
CD3+CD56+ cells were found to be
significantly higher in the DC-CIK and DC-CTL cells, compared with
the control group. The proportion of CD3+CD8+
and CD3+CD56+ cells was also significantly
higher in the DC-CTL cells, compared with the DC-CIK cells. These
observations confirm and expand the findings of previous reports
that CD3+CD56+ cells show enhanced antitumor
activity in the presence of DC (8,24).
In conclusion, the results of the present study
demonstrated that the use of DC-CTL cell or DC-CIK cell therapy, as
a personalized adoptive immunotherapy, regulated the immune status
and inhibited tumor growth in vivo. In addition, the
experiments indicated that DC-CTL cells offer superior
antineoplastic activity, compared with DC-CIK cells against B16
melanoma tumor cells. These findings provide valuable insights into
the clinical curative effects of DC-CTL cell and DC-CIK cell
immunotherapy, and the design of immunotherapeutic strategies for
malignant tumors may be significant for the prevention of tumor
growth.
References
|
1
|
Wang Y, Xu Z, Zhou F, Sun Y, Chen J, Li L,
Jin H and Qian Q: The combination of dendritic cells-cytotoxic T
lymphocytes/cytokine-induced killer (DC-CTL/CIK) therapy exerts
immune and clinical responses in patients with malignant tumors.
Exp Hematol Oncol. 4:322015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ouyang Z, Wu H, Li L, Luo Y, Li X and
Huang G: Regulatory T cells in the immunotherapy of melanoma.
Tumour Biol. 37:77–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brower V: Combination immunotherapy
breakthrough for melanoma. Lancet Oncol. 16:e3182015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brower V: Immunotherapy combination
promising for melanomas. Lancet Oncol. 16:e2652015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boon T, Coulie PG, Van den Eynde BJ, van
der Bruggen P and Human T: cell responses against melanoma. Annual
Rev Immunol. 24:175–208. 2006. View Article : Google Scholar
|
|
6
|
Garbe C, Eigentler TK, Keilholz U,
Hauschild A and Kirkwood JM: Systematic review of medical treatment
in melanoma: Current status and future prospects. Oncologist.
16:5–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fourcade J and Zarour HM: Strategies to
reverse melanoma-induced T-cell dysfunction. Clin Dermatol.
31:251–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen R, Deng X, Wu H, Peng P, Wen B and Li
F and Li F: Combined immunotherapy with dendritic cells and
cytokine-induced killer cells for malignant tumors: A systematic
review and meta-analysis. Int Immunopharmacol. 22:451–464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El Ansary M, Mogawer S, Elhamid SA,
Alwakil S, Aboelkasem F, Sabaawy HE and Abdelhalim O: Immunotherapy
by autologous dendritic cell vaccine in patients with advanced HCC.
J Cancer Res Clin Oncol. 139:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S and Wang Z: Efficacy and safety of
dendritic cells co-cultured with cytokine-induced killer cells
immunotherapy for non-small-cell lung cancer. Int Immunopharmacol.
28:22–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin T, Song C, Chuo DY, Zhang H and Zhao
J: Clinical effects of autologous dendritic cells combined with
cytokine-induced killer cells followed by chemotherapy in treating
patients with advanced colorectal cancer: A prospective study.
Tumour Biol. 37:4367–4372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu X, Zhao H, Liu L, Cao S, Ren B, Zhang
N, An X, Yu J, Li H and Ren X: A randomized phase II study of
autologous cytokine-induced killer cells in treatment of
hepatocellular carcinoma. J Clin Immunol. 34:194–203. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan G, Zhang X, Feng H, Luo H and Wang Z:
The therapeutic effect of cytokine-induced killer cells on
pancreatic cancer enhanced by dendritic cells pulsed with K-ras
mutant peptide. Clin Dev Immunol. 2011:6493592011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wayteck L, Breckpot K, Demeester J, De
Smedt SC and Raemdonck K: A personalized view on cancer
immunotherapy. Cancer Lett. 352:113–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olioso P, Giancola R, Di Riti M, Contento
A, Accorsi P and Iacone A: Immunotherapy with cytokine induced
killer cells in solid and hematopoietic tumours: A pilot clinical
trial. Hematol Oncol. 27:130–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Y, Tao Q, Wang H, Xiong S, Zhang R,
Chen T, Tao L and Zhai Z: Dendritic cells decreased the concomitant
expanded Tregs and Tregs related IL-35 in cytokine-induced killer
cells and increased their cytotoxicity against leukemia cells. PLoS
One. 9:e935912014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jäkel CE and Schmidt-Wolf IG: An update on
new adoptive immunotherapy strategies for solid tumors with
cytokine-induced killer cells. Expert Opin Biol Ther. 14:905–916.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chávez-Galán L, Arenas-Del Angel MC,
Zenteno E, Chávez R and Lascurain R: Cell death mechanisms induced
by cytotoxic lymphocytes. Cell Mol Immunol. 6:15–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hishii M, Kurnick JT, Ramirez-Montagut T
and Pandolfi F: Studies of the mechanism of cytolysis by
tumour-infiltrating lymphocytes. Clin Exp Immunol. 116:388–394.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franceschetti M, Pievani A, Borleri G,
Vago L, Fleischhauer K, Golay J and Introna M: Cytokine-induced
killer cells are terminally differentiated activated CD8 cytotoxic
T-EMRA lymphocytes. Exp Hematol. 37:616–628.e2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wongkajornsilp A, Wamanuttajinda V,
Kasetsinsombat K, Duangsa-ard S, Sa-ngiamsuntorn K, Hongeng S and
Maneechotesuwan K: Sunitinib indirectly enhanced anti-tumor
cytotoxicity of cytokine-induced killer cells and CD3+CD56+ subset
through the co-culturing dendritic cells. PLoS One. 8:e789802013.
View Article : Google Scholar : PubMed/NCBI
|