Introduction
Glucocorticoid has been widely used for treating
connective tissue diseases, including systemic lupus erythematosus,
nephrotic syndrome and complications following organ
transplantation (1–3). However, glucocorticoid treatment
always induces femoral head necrosis, which, if not properly
treated, may cause femoral head collapse and osteoarthritis that
consequently requires artificial joint replacement (4,5). At
present, the incidence of steroid-induced necrosis of the femoral
head (SINFH) exceeds trauma-induced femoral head necrosis (6). However, the pathogenesis of SINFH
remains controversial, and the various theories explaining SINFH
pathogenesis are predominantly classified into the osteoporosis
theory, the lipid metabolism disorders theory, the vasculopathy
theory, the intraosseous hypertension theory and the glucocorticoid
cytotoxicity theory (7–12).
Accumulating evidence indicates that steroids can
cause osteocyte, osteoblast and chondrocyte apoptosis, which is the
cytological basis for femoral head necrosis (13). In addition, oxidative stress also
serves an important role in SINFH pathogenesis (14). When cells are stimulated by the
external environment, reactive oxygen species (ROS) increase, which
alters the balance between the oxidative system and
anti-oxidization system of the body, and consequently causes
oxidative stress (15).
Furthermore, oxidative stress can cause DNA peroxidation, which
results in DNA oxidative damage (16). It has been reported that steroids
can increase ROS and induce oxidative stress (14).
Vitamin E is an important anti-oxidant substance and
can regulate oxidative stress and control the generation of free
radicals (17). It has been
reported that vitamin E can markedly reduce the incidence of SINFH
in rabbits and may be used for the prevention of SINFH (18). However, the underlying mechanism of
the effect of vitamin E in SINFH has, to the best of our knowledge,
never been thoroughly investigated. Therefore, the present study
aimed to investigate the effects of vitamin E on osteocyte
apoptosis and DNA oxidative damage in bone marrow hemopoietic cells
at the early stage of SINFH.
Materials and methods
Animals, grouping and treatment
Healthy Japanese white rabbits at 4 months old
(weight, 2.5±0.5 kg) were selected, fed with a standard diet and
raised separately. All the animals were supplied by the Test Animal
Center of the Inner Mongolia Medical University (Huhhot, China) and
the entire experimental procedure was approved by the Ethics
Committee of the Inner Mongolia Medical University. The animals
were randomly divided into three groups. Based on the modeling
method of Yamamoto et al (19), the Steroid group (group S, n=12)
was treated by injecting twice with Escherichia coli (E.
coli) endotoxin (100 µg/kg; The National Institute for the
Control of Pharmaceutical and Biological Products, Beijing, China)
through a marginal ear vein, with a 24 h-interval between the two
injections. On the occasion of the second injection with E.
coli endotoxin, methylprednisolone (20 mg/kg; Pfizer, Puurs,
Belgium) was injected into the right gluteus of the rabbits three
times in total, and at intervals of 24 h. The vitamin E treated
group (group E, n=12) was treated the same way as the group S, and
was fed daily with vitamin E (0.6 g/kg/d; Zhejiang Medicine Co.,
Ltd., Xinchang Pharmaceutical Factory, Xinchang, China) from the
beginning of the experiment. The control group (group C, n=12) was
treated with the equivalent volume of normal saline at the same
time as the aforementioned two groups. All the experimental animals
were administered penicillin by intramuscular injection, 800,000
units per rabbit, twice a week, to prevent infection.
Tissue treatment
Animals in all groups were sacrificed with an
intravenous injection dose of 3% sodium pentobarbital (30 mg/kg;
Beijing Propbs Biotechnology Co., Ltd., Beijing, China). The
animals were euthanized at two time points: Weeks 4 and 6. Left
femoral heads were fixed in 5% glutaraldehyde for 1 week, and
decalcified for 2 h with 5% nitric acid. The femoral head
weight-bearing area was opened along the coronal plane, and
1.0×1.5×1.0 mm3 tissue samples were obtained from the subchondral
area, embedded in paraffin wax and then cut along the coronal plane
into 4 µm thick sections. Hematoxylin and eosin (H&E) staining
was performed and vacant bone lacunae were counted to establish
femoral head necrosis. Right femoral heads were fixed in 10%
formaldehyde for 1 week and decalcified for 45 days with 15% EDTA.
Then the appearance and color of the bone specimens were noted and
the degree of decalcification was established physically.
Histopathology
The pathological change of osteocyte necrosis was
always indicated by the percentage of vacant bone lacunae. More
specifically, five visual fields were selected and 50 bone lacunae
were randomly selected from each to count vacant bone lacunae in
each field via optical microscopy, at magnification, ×200. These
observations were used to estimate the percentage of vacant bone
lacunae in the bone lacunae, that is, the vacant bone lacunae rate.
The vacant bone lacunae rate in the femoral head subchondral area
of a normal grown-up rabbit ranged between 8 and 12%, a higher
vacant bone lacunae rate in the femoral head subchondral area in
comparable sections indicated osteonecrosis (20).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay apoptosis detection
A TUNEL cell apoptosis test kit was used to test
osteocyte apoptosis. TUNEL staining was performed using an in
situ cell-death detection kit AP (Roche Diagnostics GmbH,
Mannheim, Germany) in accordance with the manufacturer's protocol.
Particles stained yellow in a cell nucleus indicated an apoptotic
cell. A total of 10 visual fields were randomly selected from each
section and imaged via light microscopy, magnification, ×200, and
50 osteocytes were counted in each field to calculate apoptotic
cell index (number of apoptotic cells/total number of cells in the
visual field).
Immunohistochemistry
A caspase-3 colorimetric kit (Wuhan Boster
Biological Technology Ltd., Wuhan, China) and B-cell lymphoma 2
(Bcl-2) immunohistochemistry kit (Wuhan Boster Biological
Technology Ltd.) were used. For the Bcl-2 assay, the femoral head
tissues of each group at week 4 were sectioned, and
streptavidin-peroxidase immunohistochemical staining was performed
to observe Bcl-2 expression under a light microscope. Positive
Bcl-2 expression appeared as intracytoplasmic yellow particles. A
known positively stained section was used as a positive control,
and PBS instead of primary antibodies was used as a negative
control. A total of 10 visual fields were randomly selected under a
light microscope at magnification, ×200, to calculate the
Bcl-2-positive cell percentage. For caspase-3 analysis, femoral
head tissues of each group at week 4 were sectioned, and observed
with a light microscope. A known positively stained section was
used as a positive control, and PBS instead of primary antibodies
was used as a negative control. Particles stained a tan color in
the cytoplasm indicated a positive result and the scoring method of
Cakir et al (21) was used
as follows: i) Cytoplasm staining degree; 1, weak staining, stained
light yellow or only a very small number of yellow or brown cells;
3, strong staining, the majority of cells are stained yellow or
tan; and, 2, medium staining, a staining degree between 1 and 2;
and ii) Percentage of stained cells in the cells counted; 0,
<1%; 1, 1–5%; 2, 6–50%; 3, 51–75%; and 4, >75%. Finally, the
total score of each section was calculated as follows: Total
score=staining degree score × stained cell percentage. A total
score of 36 indicated strongly positive (+++), 3–4
medium positive (++), l-2 weakly positive (+) and 0 negative (−)
staining.
Assessment of DNA oxidative
damage
Accumulation of ROS can cause DNA oxidative damage
and 8-hydroxy-2′-deoxyguanosine (8-OHdG) is recognized as a marker
for DNA oxidative damage. The monoclonal antibody N45.1
specifically binds with 8-OHdG. Sections were placed into
H2O2 with a volume fraction of 0.3% for 30
min incubation at 37°C, digested with 0.1% trypsin for 15 min, and
then reacted with the monoclonal antibody N45.1 (catalog no.
ab48508; 1:20; Abcam, Cambridge, UK). The sections were incubated
for 2 h at 37°C, then 3,3′-diaminobenzidine was used for color
development for 5 min, and finally H&E staining was performed.
Marrow bone hematopoietic cells included hematopoietic stem cells,
myeloid stem cells, lymphoid stem cells and their differentiated
cells. Five visual fields were randomly selected to count marrow
bone hematopoietic cells with DNA oxidative damage, 50 marrow bone
hematopoietic cells were counted in each field to calculate the
percentage of count marrow bone hematopoietic cells with DNA
oxidative damage in total marrow bone hematopoietic cells, that is,
the DNA oxidative damage rate of marrow bone hematopoietic
cells.
Statistical analysis
All the data were in the form of mean ± standard
deviation, and SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. The statistical test of
vacant bone lacunae rate and osteocyte apoptosis index was carried
out by the variance analysis method for completely randomized
design. Pairwise comparison of several specimens' averages was
carried out by least significant difference. Correlation analysis
was carried out using Person correlation analysis and linear
regression analysis methods. P<0.05 was considered to indicate a
statistically significant difference. An independent sample t-test
was carried out for comparing all Bcl-2 test data, and P<0.01
was considered to indicate a statistically significant difference.
For caspase-3 test results, Fisher's exact probability in a 2×2
table was adopted for pairwise specimen rate difference test. A
t-test was carried to the resulting quantitative data, or t-test in
case of heterogeneity of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Histopathological changes
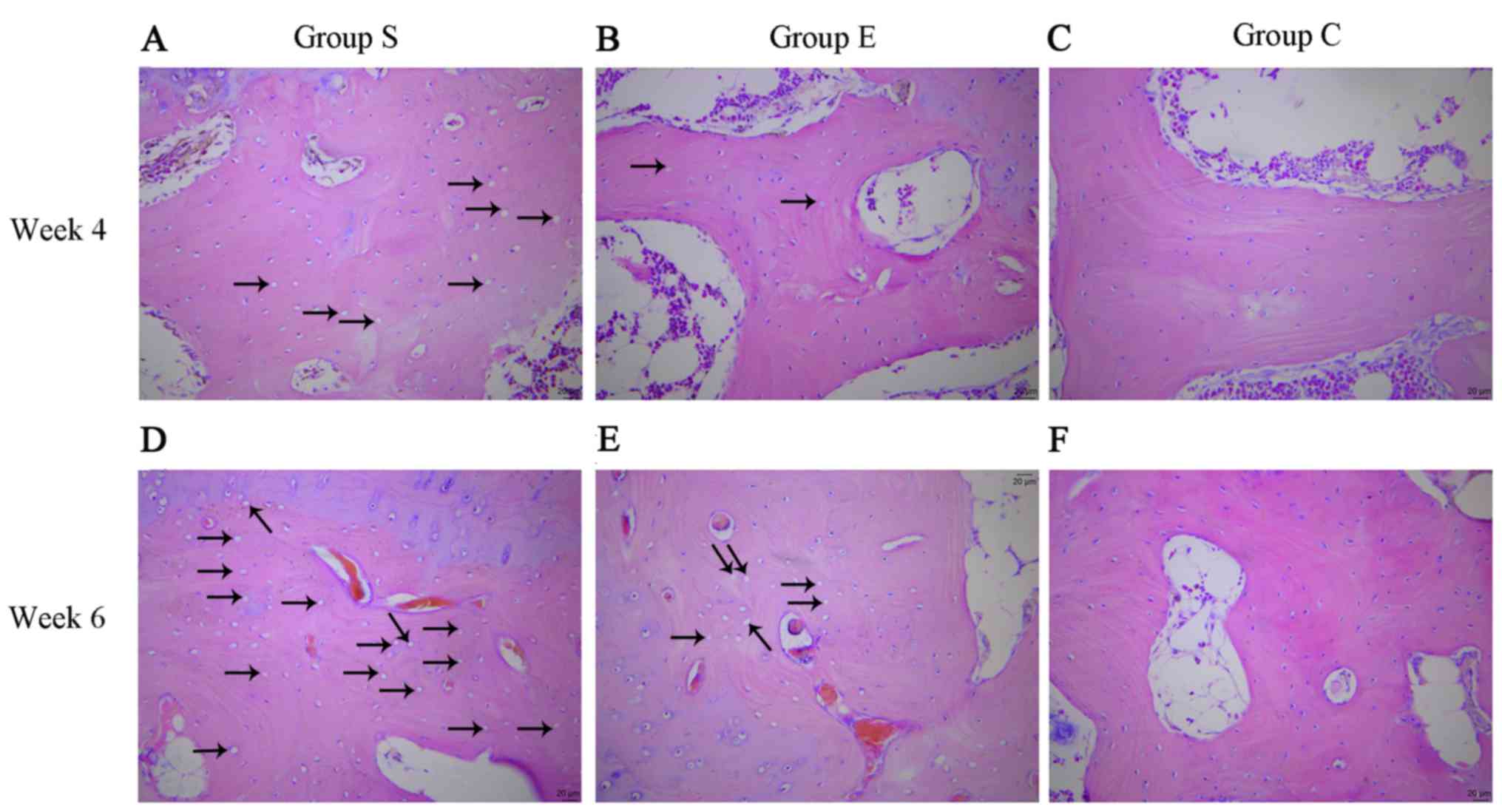
At week 4, group S femoral head pathological
sections exhibited typical osteonecrosis; bone trabeculae were
fewer and thinner, became broken, fragmented and structurally
disordered, intraosseous adipose cells became enlarged, the
arrangement of the osteoblasts became irregular and vacant bone
lacunae increased (Fig. 1A). In
group E the bone trabeculae in the subchondral area of femoral head
were fewer and thinner, a number bone lacunae became empty and
empty bone lacunae were distributed locally, abundant hematopoietic
cells existed in the medullary space and there was an increase in
the numbers of mast adipose cells (Fig. 1B). In group C the bone trabeculae
remained intact, well organized, dense and complete, and the
osteocytes had big and central cell nuclei (Fig. 1C).
At week 6, group S sections demonstrated osteocyte
necrosis, including karyopyknosis, karyolysis and karyorrhexis;
more bone lacunae vacuoles occurred, intramedullary vessels were
compressed, vascular lumens became narrow and fat embolus and
thrombus were observed (Fig. 1D).
In group E, bone trabeculae in the femoral head subchondral area
were thin and fragile, hematopoietic cells in the medullary space
became scarcer, the number of mast adipose cells increased and the
number of soft bone lacunae vacuoles increased (Fig. 1E). In the group C sections, bone
trabeculae remained intact, well organized, dense and complete, and
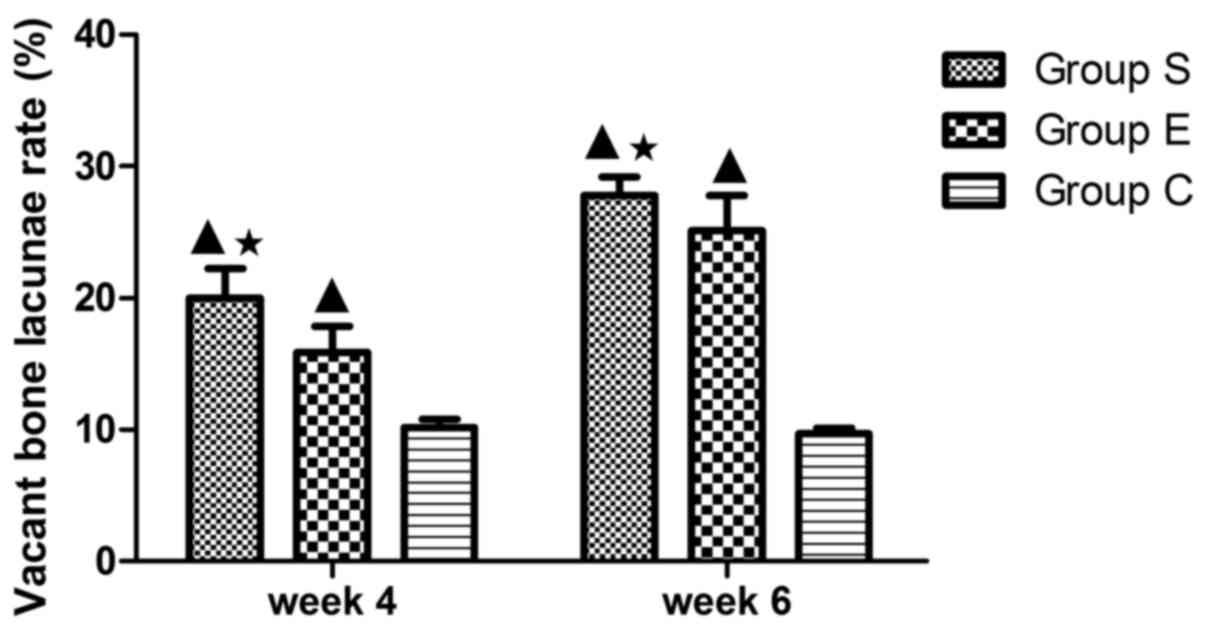
the osteocytes had big and central cell nuclei (Fig. 1F). In group S, the rate of vacant
bone lacunae demonstrated significant differences compared with
group C at weeks 4 and 6 (P<0.01); compared with the group E,
there was a statistical significance at weeks 4 and 6 (P<0.05).
The average vacant bone lacunae rates of each group at weeks 4 and
6 are presented in Fig. 2.
TUNEL apoptosis
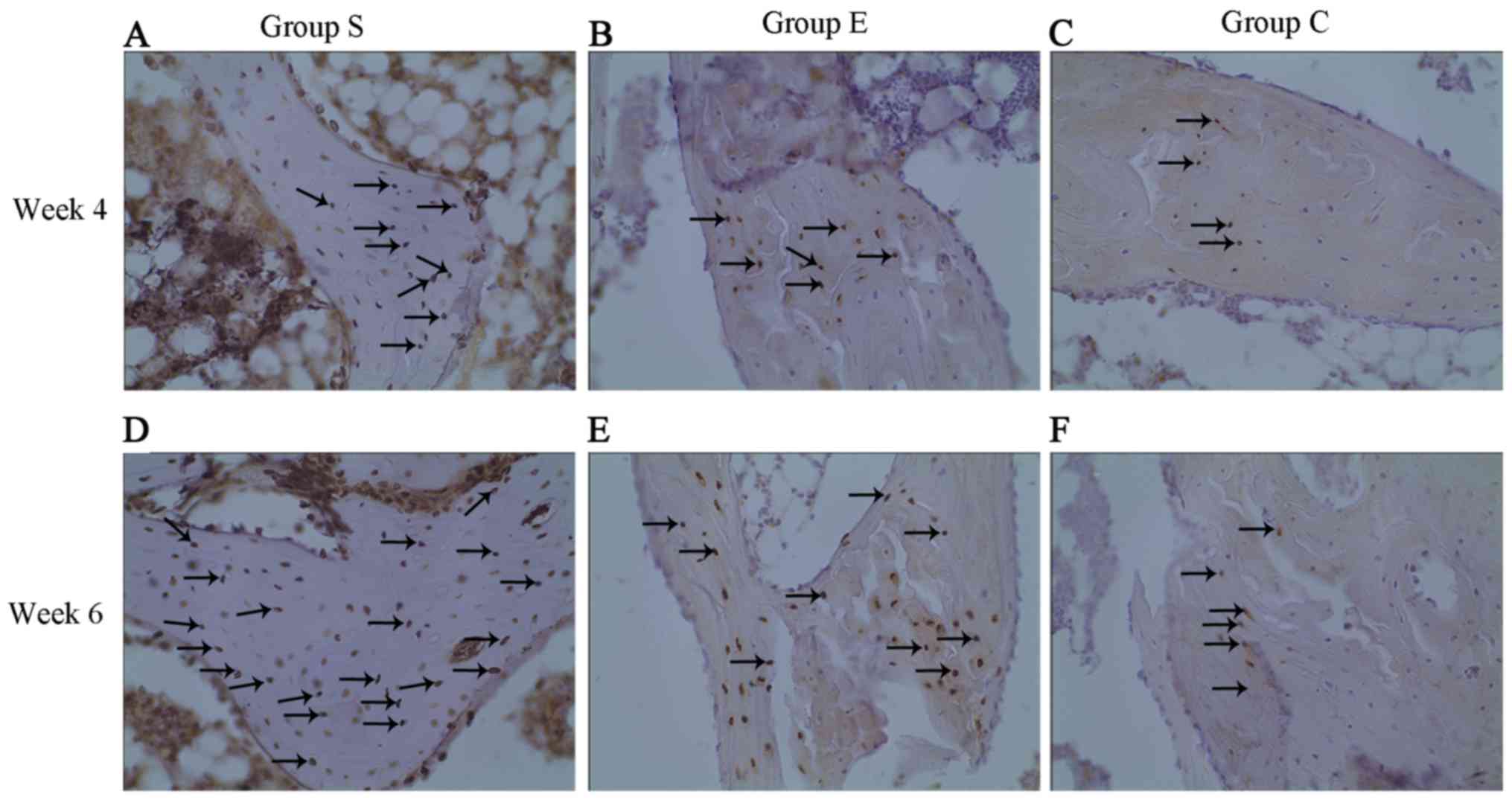
Osteocyte apoptosis was examined in each group at
weeks 4 and 6 following modeling (Fig.
3). As presented in Fig. 3,
apoptotic osteocytes (cells with yellow particles in the bone
lacunae were apoptotic) were mainly in group S (Fig. 3A and D) and a large number of
apoptotic osteocytes were observed in the bone trabeculae; in group
E (Fig. 3B and E), apoptotic
osteocytes were fewer compared with group S; and a small number of
apoptotic osteocytes were identified in group C (Fig. 3C and F). The apoptosis index of
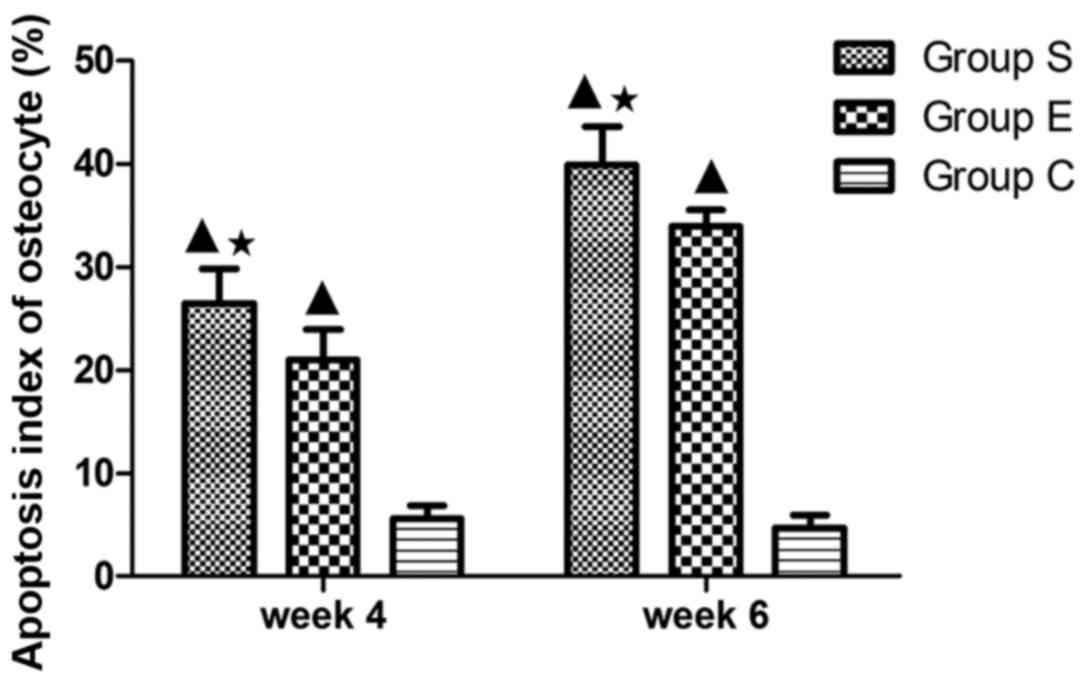
osteocytes in each group at weeks 4 and 6 are presented in Fig. 4. These findings indicated that
treatment with vitamin E inhibited steroid-induced osteocyte
apoptosis.
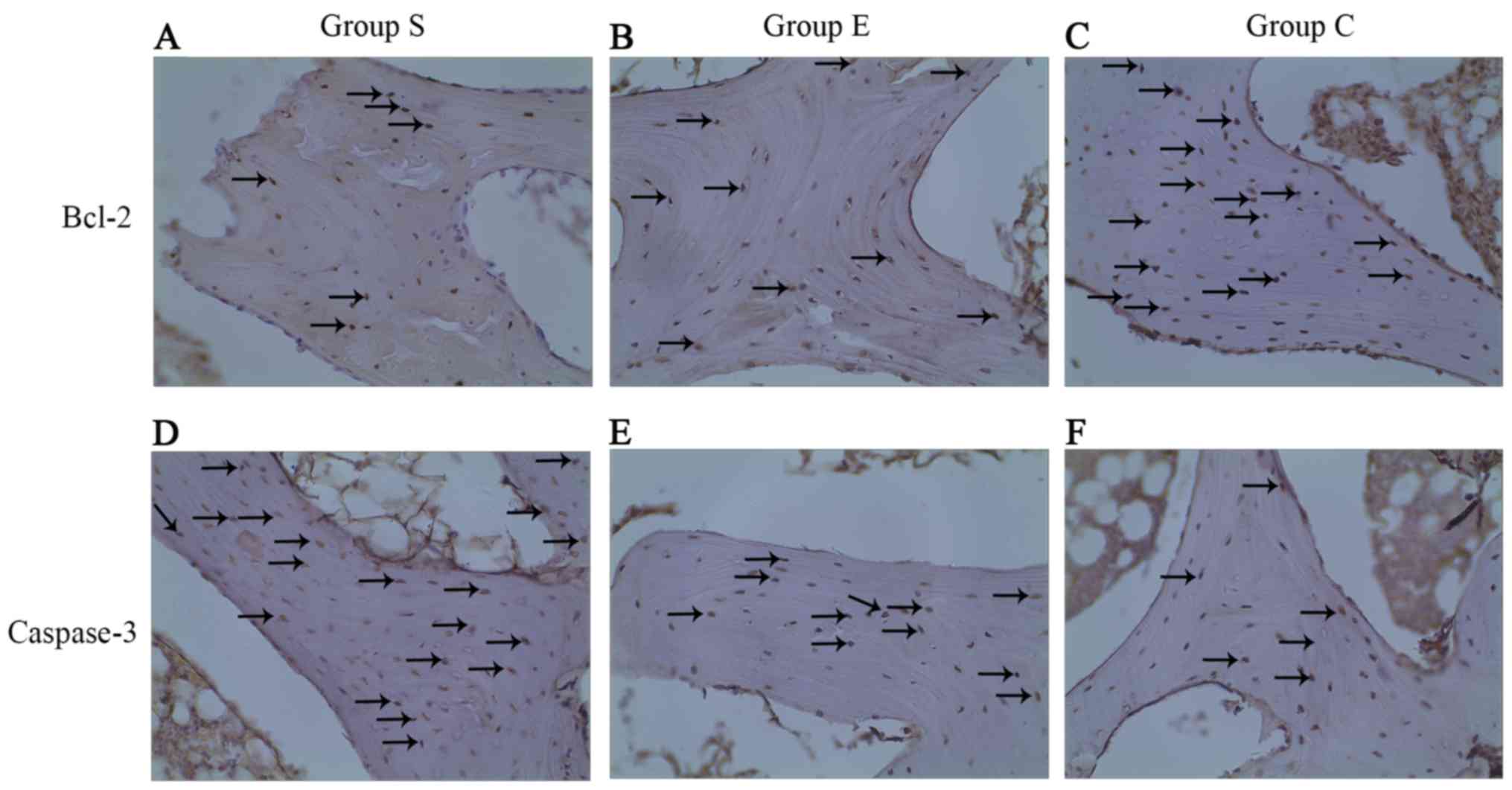
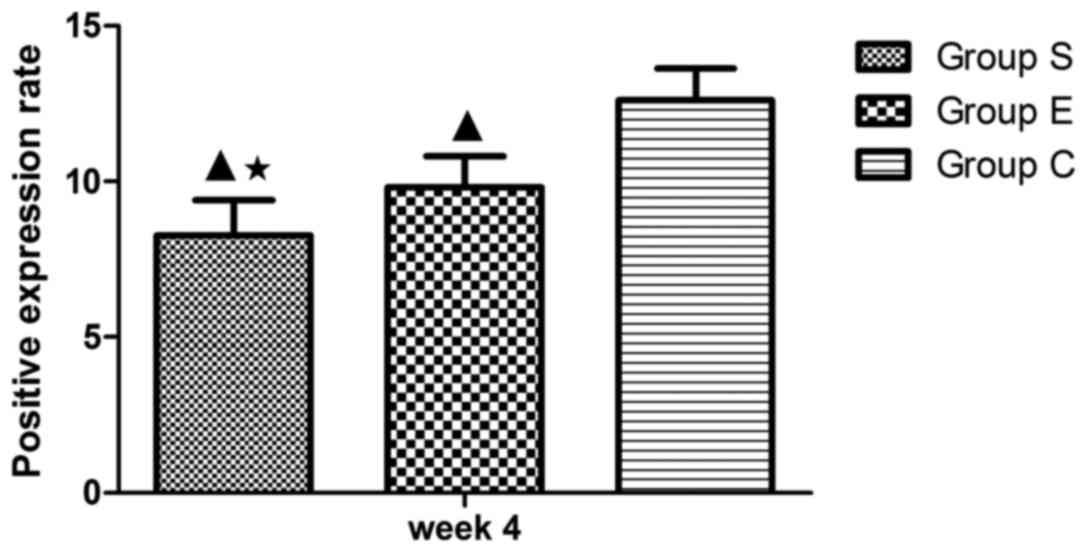
Expression of caspase-3 and Bcl-2
The expression of two apoptosis-associated proteins,
Bcl-2 and caspase-3, was examined in each group at week 4 of the
model (Fig. 5) and the proportion
of Bcl-2 positive cells was counted (Fig. 6). Bcl-2 is an inhibitor of cell
apoptosis. In group S, numerous cells demonstrated positive
expression of Bcl-2 (Fig. 5A), the
proportion of positive cells was 8.26±1.13% (Fig. 6). In group E, there were more cells
expressing Bcl-2 (Fig. 5B); the
proportion of positive cells was 9.81±1.01% (Fig. 6). In group C, cells expressing
Bcl-2 were the majority (Fig. 5C);
the proportion of positive cells was 12.60±1.03% (Fig. 6). Subsequently, the expression of
caspase-3 was examined in each group at week 4 of the model. As
presented in Table I, in the 12
femoral heads (bilateral femoral heads, 6×2) of group S, four were
negative (32%), and eight were positive (68%), of which two were
weakly positive (18%), five were medium positive (38%), and one was
strongly positive (12%; Fig. 5D).
In the 12 femoral heads (bilateral femoral heads: 6×2) of group E,
five were negative (45%), and seven were positive (55%), of which
three were weakly positive (18%), three were medium positive (27%),
and one was strongly positive (10%; Fig. 5E). In the 12 femoral heads
(bilateral femoral heads: 6×2) of group C, 11 were negative (95%),
and one was weakly positive (5%); none was medium or strongly
positive (Fig. 5F). Accordingly,
the data from the present study suggest that vitamin E suppresses
the steroid-induced downregulation of anti-apoptotic Bcl-2 in
addition to the upregulation of pro-apoptotic caspase-3 in
osteocytes, and thus inhibits steroid-induced osteocyte
apoptosis.
 | Table I.Positive expression of caspase-3 at
week 4. |
Table I.
Positive expression of caspase-3 at
week 4.
|
| Number of
samples |
|---|
|
|
|
|---|
| Group | − (%) | + (%) | ++ (%) | +++ (%) |
|---|
| Group S | 4 (32) | 2 (18) | 5 (38) | 1 (12) |
| Group E | 5 (45) | 3 (18) | 3 (27) | 1 (10) |
| Group C | 11 (95) | 1 (5) | 0 (0) | 0 (0) |
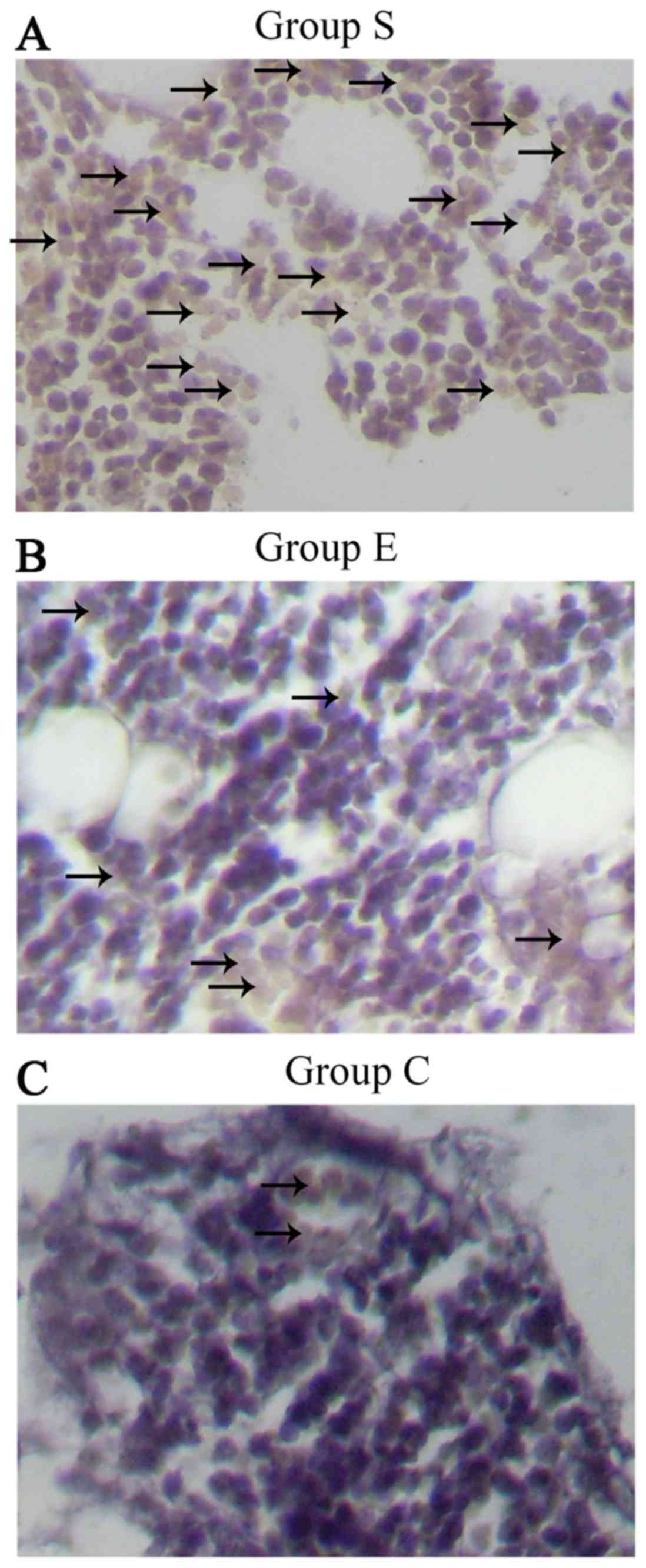
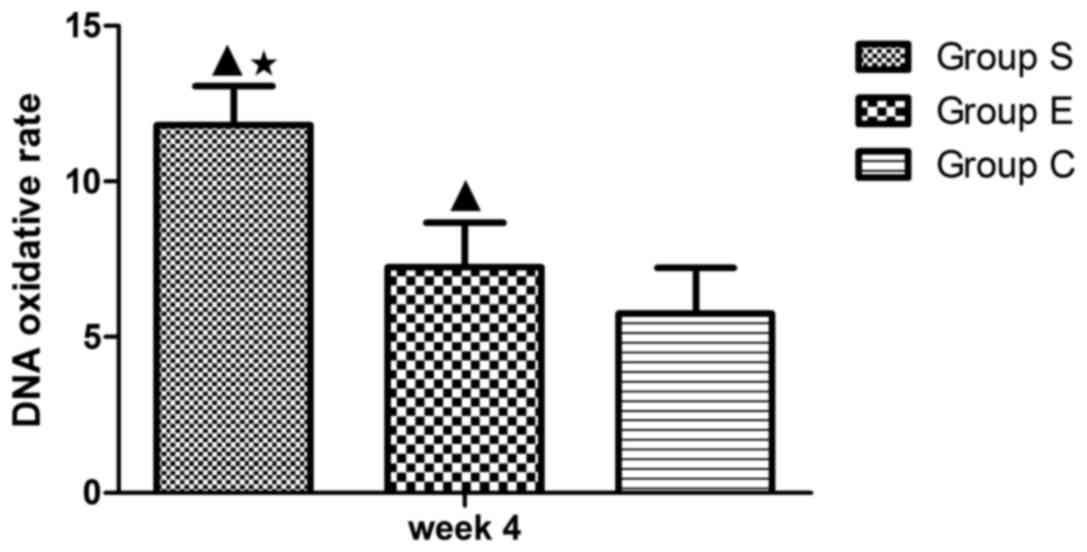
Assessment of DNA oxidative damage in
marrow bone hematopoietic cells
The DNA oxidative damage of bone marrow hemopoietic
cells was investigated in groups S (Fig. 7A), E (Fig. 7B) and C (Fig. 7C) at week 4 following the modeling.
As presented in Fig. 8, the DNA
oxidative damage rate of marrow bone hematopoietic cells of group E
was lower (7.24±1.44%) compared with group S (11.80±1.26%), while
higher compared with group C (5.75±1.47%). These data suggested an
inhibitory effect of vitamin E on the DNA oxidative damage in bone
marrow hemopoietic cells at an early stage of SINFH.
Discussion
Oxidative stress is caused by ROS accumulation or
the dysfunction of ROS clearance and/or oxidative damage repair
capability. ROS can activate the JNK pathway in osteoblasts,
inactivate the ERK-l/2 and Akt pathways, and induce osteoblast
apoptosis (22). Reduced
glutathione (GSH) is necessary for maintaining the oxidative
balance in the body, while the oxidative stress inducer buthionine
sulfoximine can cause femoral head necrosis by inhibiting GSH
synthesis (23), thus indicating
that steroids can increase ROS. In addition, ROS can cause protein
structure mutation or biological deactivation, DNA strand breakage,
DNA locus mutation, DNA double-strand aberration, and finally cause
oxidative damage (24). In the
present study, it was demonstrated that the DNA oxidative damage of
marrow bone hematopoietic cells following exposure to steroids was
markedly greater compared with the control group.
A previous study (25) indicated that anti-oxidants can
effectively eliminate free radicals or maintain the dynamic
equilibrium of free radicals, and thus contribute to maintaining
normal cell metabolism and preventing DNA damage. Vitamin E is a
powerful free radical scavenger, interacting with active free
radicals and changing lipid peroxide into hydroxyl lipid (26). Furthermore, vitamin E can inhibit
oxidative enzymes and activate the anti-oxidase system (27). Therefore, administration of vitamin
E can facilitate anti-oxidant capacity and protect DNA from free
radical attack. A previous study (18) identified that vitamin E may inhibit
the occurrence of SINFH in rabbits. However, the underlying
mechanism remains to be elucidated. In the present study, the DNA
oxidative damage in marrow bone hematopoietic cells following
treatment with vitamin E was significantly decreased compared with
the model group, suggesting that vitamin E may suppress the
oxidative stress reaction and alleviate steroid-induced bone injury
via the inhibition of DNA oxidative damage in marrow bone
hematopoietic cells.
Glucocorticoid is recognized as an inducer of cell
apoptosis (28). Long-term
exposure to glucocorticoid or exposure to high levels of
glucocorticoid directly leads to the apoptosis of osteoblasts,
osteocytes and osteoclasts (29).
In addition, Weinstein et al (30) suggested that glucocorticoid can
also cause femoral head necrosis. Kogianni et al (31) demonstrated that cell apoptosis
occurred in steroid-induced femoral head necrosis from the
perspective of Fas cell surface death receptor/CD95. Kabata et
al (32) demonstrated that in
SINFH, a large number of osteoblasts and osteoclasts become
apoptotic, and, as apoptosis continues, osteoporosis is caused, the
mechanical bearing structure of the bone is damaged, the femoral
heads in the weight-bearing area become flattened and collapse, and
ultimately femoral head necrosis is caused. The present study
indicated that the number of apoptotic cells was directly
proportional to the duration of exposure to steroids. In addition,
steroid treatment was associated with the downregulation of Bcl-2,
an inhibitor of cell apoptosis (33).
The core mechanism of cell apoptosis is the
activation of the caspase family, which is an important molecule
group for regulating cell apoptosis, and serves a crucial executor
role in cell apoptosis (34). The
caspase family can directly split cell structure, prevent DNA
repair and interfere with DNA and RNA assembly by degrading nuclear
lamina and the connection between apoptotic and surrounding cells;
furthermore, caspases can break the nuclear structure and generate
apoptotic bodies (35). For
example, activated caspase-3 affects DNA replication and
transcription, and damages DNA repair (36). In addition, caspase-3 and Bcl-2
control cell apoptosis separately without mutual interference. In
normal osteocytes, Bcl-2 inhibits apoptosis by preventing apoptosis
signaling (37). The present study
demonstrated that Bcl-2 expression in the osteocytes of group S was
lower compared with group C. In animal models with SINFH, treatment
with steroids reduced the expression of Bcl-2 and increased the
expression of caspase-3, and thus the apoptosis of osteocytes is
upregulated, which consequently causes femoral head necrosis.
A previous study suggested that vitamin E has the
potential to be used as an anti-oxidant for inhibition of
thioacetamide-induced caspase activation and hepatic cell apoptosis
(38). Vitamin E also suppresses
pulmonary epithelial cell apoptosis via decreasing tumor necrosis
factor and myeloperoxidase (39).
When used jointly with vitamin C, vitamin E increases the Bcl-2
level in mitochondria and decreases the level of Bcl-2-like protein
4, suppresses the release of cytochrome C, activates caspase-9 and
caspase-3, and thus, inhibits myocardial cell apoptosis (40). In the present study, it is
suggested that vitamin E may inhibit steroid-induced osteocyte
apoptosis and DNA oxidative damage in bone marrow hemopoietic
cells, potentially via upregulation of Bcl-2 and downregulation of
caspase-3. Therefore, vitamin E may be used for the prevention of
steroid-induced bone damage.
Acknowledgements
This study was supported by The Inner Mongolia
Autonomous Region Natural Funded Projects (grant no.
2015MS0895).
References
|
1
|
Tanaka Y, Yoshikawa N, Hattori S, Sasaki
S, Ando T, Ikesa M and Honda M: Japanese Study Group for Renal
Disease in Children: Combination therapy with steroids and
mizoribine in juvenile SLE: A randomized controlled trial. Pediatr
Nephrol. 25:877–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodson EM and Alexander SI: Evaluation and
management of steroid-sensitive nephrotic syndrome. Curr Opin
Pediatr. 20:145–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tönshoff B, Höcker B and Weber LT: Steroid
withdrawal in pediatric and adult renal transplant recipients.
Pediatr Nephrol. 20:409–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simon JP, Berger P and Bellemans J: Total
hip arthroplasty in patients less than 40 years old with avascular
necrosis of the femoral head. A 5 to 19-year follow-up study. Acta
Orthop Belg. 77:53–60. 2011.PubMed/NCBI
|
|
5
|
Motomura G, Yamamoto T, Yamaguchi R,
Ikemura S, Nakashima Y, Mawatari T and Iwamoto Y: Morphological
analysis of collapsed regions in osteonecrosis of the femoral head.
J Bone Joint Surg Br. 93:184–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagasawa K, Tada Y, Koarada S, Tsukamoto
H, Horiuchi T, Yoshizawa S, Murai K, Ueda A, Haruta Y and Ohta A:
Prevention of steroid-induced osteonecrosis of femoral head in
systemic lupus erythematosus by anti-coagulant. Lupus. 15:354–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin L, Li YB and Wang YS:
Dexamethasone-induced adipogenesis in primary marrow stromal cell
cultures: Mechanism of steroid-induced osteonecrosis. Chin Med J
(Engl). 119:581–588. 2006.PubMed/NCBI
|
|
8
|
Drescher W, Schlieper G, Floege J and
Eitner F: Steroid-related osteonecrosis-an update. Nephrol Dial
Transplant. 26:2728–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takano-Murakami R, Tokunaga K, Kondo N,
Ito T, Kitahara H, Ito M and Endo N: Glucocorticoid inhibits bone
regeneration after osteonecrosis of the femoral head in aged female
rats. Tohoku J Exp Med. 217:51–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siler U, Rousselle P, Müller CA and Klein
G: Laminin gamma2 chain as a stromal cell marker of the human bone
marrow microenvironment. Br J Haematol. 119:212–220. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyanishi K, Yamamoto T, Irisa T,
Yamashita A, Jingushi S, Noguchi Y and Iwamoto Y: Bone marrow fat
cell enlargement and a rise in intraosseous pressure in
steroid-treated rabbits with osteonecrosis. Bone. 30:185–190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu YF, Chen WM, Lin YF, Yang RC, Lin MW,
Li LH, Chang YH, Jou YS, Lin PY, Su JS, et al: Type II collagen
gene variants and inherited osteonecrosis of the femoral head. N
Engl J Med. 352:2294–2301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zalavras C, Shah S, Birnbaum MJ and
Frenkel B: Role of apoptosis in glucocorticoid-induced osteoporosis
and osteonecrosis. Crit Rev Eukaryot Gene Expr. 13:221–235. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ichiseki T, Kaneuji A, Katsuda S, Ueda Y,
Sugimori T and Matsumoto T: DNA oxidation injury in bone early
after steroid administration is involved in the pathogenesis of
steroid-induced osteonecrosis. Rheumatology (Oxford). 44:456–460.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jørgensen A: Oxidatively generated DNA/RNA
damage in psychological stress states. Dan Med J.
60:B46852013.PubMed/NCBI
|
|
17
|
Traber MG and Atkinson J: Vitamin E,
antioxidant and nothing more. Free Radic Biol Med. 43:4–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuribayashi M, Fujioka M, Takahashi KA,
Arai Y, Ishida M, Goto T and Kubo T: Vitamin E prevents
steroid-induced osteonecrosis in rabbits. Acta Orthop. 81:154–160.
2010. View Article : Google Scholar :
|
|
19
|
Yamamoto T, Miyanishi K, Motomura G,
Nishida K, Iwamoto Y and Sueishi K: Animal models for
steroid-induced osteonecrosis. Clin Calcium. 17:879–886. 2007.(In
Japanese). PubMed/NCBI
|
|
20
|
Conradie MM, De Wet H, Kotze DD, Burrin
JM, Hough FS and Hulley PA: Vanadate prevents
glucocorticoid-induced apoptosis of osteoblasts in vitro and
osteocytes in vivo. J Endocrinol. 195:229–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cakir E, Yilmaz A, Demirag F, Oguztuzun S,
Sahin S, Yazici UE and Aydin M: Prognostic significance of
micropapillary pattern in lung adenocarcinoma and expression of
apoptosis-related markers: Caspase-3, bcl-2 and p53. APMIS.
119:574–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu PZ, Lai CY and Chan WH: Caffeine
induces cell death via activation of apoptotic signal and
inactivation of survival signal in human osteoblasts. Int J Mol
Sci. 9:698–718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ichiseki T, Ueda Y, Katsuda S, Kitamura K,
Kaneuji A and Matsumoto T: Oxidative stress by glutathione
depletion induces osteonecrosis in rats. Rheumatology (Oxford).
45:287–290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meira LB, Bugni JM, Green SL, Lee CW, Pang
B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline
JL, et al: DNA damage induced by chronic inflammation contributes
to colon carcinogenesis in mice. J Clin Invest. 118:2516–2525.
2008.PubMed/NCBI
|
|
25
|
Dizdaroglu M, Jaruga P, Birincioglu M and
Rodriguez H: Free radical-induced damage to DNA: Mechanisms and
measurement. Free Radic Biol Med. 32:1102–1115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Constantinou C, Neophytou CM, Vraka P,
Hyatt JA, Papas KA and Constantinou AI: Induction of DNA damage and
caspase-independent programmed cell death by vitamin E. Nutr
Cancer. 64:136–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Itoh H, Ohkuwa T, Yamazaki Y, Shimoda T,
Wakayama A, Tamura S, Yamamoto T, Sato Y and Miyamura M: Vitamin E
supplementation attenuates leakage of enzymes following 6
successive days of running training. Int J Sports Med. 21:369–374.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Canalis E and Delany AM: Mechanisms of
glucocorticoid action in bone. Ann N Y Acad Sci. 966:73–81. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato M, Sugano N, Ohzono K, Nomura S,
Kitamura Y, Tsukamoto Y and Ogawa S: Apoptosis and expression of
stress protein (ORP150, HO1) during development of ischaemic
osteonecrosis in the rat. J Bone Joint Surg Br. 83:751–759. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weinstein RS, Nicholas RW and Manolagas
SC: Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis
of the hip. J Clin Endocrinol Metab. 85:2907–2912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kogianni G, Mann V, Ebetino F, Nuttall M,
Nijweide P, Simpson H and Noble B: Fas/CD95 is associated with
glucocorticoid-induced osteocyte apoptosis. Life Sci. 75:2879–2895.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kabata T, Kubo T, Matsumoto T, Nishino M,
Tomita K, Katsuda S, Horii T, Uto N and Kitajima I: Apoptotic cell
death in steroid induced osteonecrosis: An experimental study in
rabbits. J Rheumatol. 27:2166–2171. 2000.PubMed/NCBI
|
|
33
|
Haÿ E, Lemonnier J, Fromigué O and Marie
PJ: Bone morphogenetic protein-2 promotes osteoblast apoptosis
through a Smad-independent, protein kinase C-dependent signaling
pathway. J Biol Chem. 276:29028–29036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
35
|
Poreba M, Strózyk A, Salvesen GS and Drag
M: Caspase Substrates and Inhibitors. Cold Spring Harb Perspect
Biol. 5:a0086802013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Végran F, Boidot R, Solary E and
Lizard-Nacol S: A short caspase-3 isoform inhibits
chemotherapy-induced apoptosis by blocking apoptosome assembly.
PLoS One. 6:e290582011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Byrd JC, Shinn C, Waselenko JK, Fuchs EJ,
Lehman TA, Nguyen PL, Flinn IW, Diehl LF, Sausville E and Grever
MR: Flavopiridol induces apoptosis in chronic lymphocytic leukemia
cells via activation of caspase-3 without evidence of bcl-2
modulation or dependence on functional p53. Blood. 92:3804–3816.
1998.PubMed/NCBI
|
|
38
|
Sun F, Hayami S, Ogiri Y, Haruna S, Tanaka
K, Yamada Y, Tokumaru S and Kojo S: Evaluation of oxidative stress
based on lipid hydroperoxide, vitamin C and vitamin E during
apoptosis and necrosis caused by thioacetamide in rat liver.
Biochim Biophys Acta. 1500:181–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Demiralay R, Gürsan N and Erdem H: The
effects of erdosteine, N-acetylcysteine, and vitamin E on
nicotine-induced apoptosis of pulmonary cells. Toxicology.
219:197–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin F, Yan C, Patel R, Liu W and Dong E:
Vitamins C and E attenuate apoptosis, beta-adrenergic receptor
desensitization, and sarcoplasmic reticular Ca2+ ATPase
downregulation after myocardial infarction. Free Radic Biol Med.
40:1827–1842. 2006. View Article : Google Scholar : PubMed/NCBI
|