Introduction
Pituitary adenoma (PA), the third most frequently
diagnosed intracranial tumor, accounts for ~10–25% of all primary
intracranial tumors (1). Although
the majority of PAs are benign, several of them exhibit an
aggressive behavior characterized by rapid cell growth and
involvement of the contiguous structures. Based on the signs and
symptoms secondary to the over-secretion of pituitary hormones by
the tumor, PA is classified into two major groups; functional
pituitary adenomas (FPAs) and non-FPAs (NFPAs). The latter accounts
for 80% of pituitary macroadenomas (2). Although surgery is the primary option
for treatment, NFPAs frequently have supra- or parasellar
extension, due to which total resection of tumor is often not
possible. The residual tumor regrows in 12–58% of patients within a
span of 5 years (3). The complex
tumor pathology of NFPAs has served as a barrier to the development
of effective therapeutic interventions. Identification of markers
that can predict aggressive characteristics of NFPAs may aid in the
treatment strategy and be of assistance in preventing
recurrence.
Wnt signaling is known to regulate cell
proliferation, polarity and cell death (4). Aberrant Wnt signal transduction
pathway has been implicated in tumorigenesis and tissue invasion
(5). Activation of the
Wnt-β-catenin pathway results in the abnormal accumulation of free
β-catenin in the nuclei of cancer cells. This in turn activates
transcription factors such as T-cell factor, to induce the
expression of target genes involved in cell proliferation,
including c-myc (6). In addition
to functioning as a transcriptional coactivator, β-catenin is known
for its role as a cell adhesion protein (7). β-catenin links E-cadherin molecules
to α-catenin leading to strong cadherin-mediated cell adhesion
(8). Aberrant expression of
β-catenin has a role in cellular transformation, tissue invasion
and metastasis (7).
c-myc is a potent oncogene that has been
demonstrated to promote tumorigenesis in a wide range of tissues
(9). Upregulated expression of
c-myc in tumor cells occurs through several mechanisms, including
gene amplification, chromosomal translocation, single nucleotide
polymorphism in regulatory regions, mutation of upstream signal
pathways and those that enhance the protein stability (10). This upregulation has a number of
consequences in cancer cell biology (11).
Previous studies (12,13)
have demonstrated the over-activation of the Wnt-signal pathway in
NFPAs. A previous study (14)
using gene expression microarrays indicated that β-catenin and
c-myc proteins were highly upregulated in NFPAs compared with
normal pituitary tissues. In a subsequent study (12), reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis were
used to confirm the overexpression of the two proteins in
aggressive NFPAs.
In the present study, the expression levels of
β-catenin and c-myc proteins in NFPAs were investigated using
immunohistochemical examination of tissue microarrays to assess
their relevance as markers for predicting aggressiveness and
recurrence of NFPAs.
Materials and methods
Subjects
A total of 212 NFPA specimens resected by
trans-sphenoidal surgery from patients (age range, 24–75 years)
between 2010 and 2012, were obtained from the Beijing Tiantan
Hospital (Beijing, China). The specimens were classified into two
groups; non-aggressive and aggressive. A total of 10 normal
pituitary glands were obtained from a donation program and served
as controls. The deceased donors comprised 6 males and 4 females,
aged 21–45 years (mean, 35 years) and had succumbed to
non-neurological diseases. NFPA and control samples were embedded
in paraffin wax to make archival blocks to be used for tissue
microarray (TMA). Prior to enrollment, written informed consent was
obtained from all NFPA subjects. The study was approved by the
Ethics Committee at Beijing Tiantan Hospital. All procedures
performed in studies involving human participants were in
accordance with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards.
All the cases met the following criteria: i) Each
paraffin block contained a sufficiently sized specimen to enable
the construction of TMAs; ii) no radiation therapy was administered
prior to surgery; iii) clinical information, including
endocrinological evaluation and imaging data, was available; and
iv) complete follow-up data for a minimum of 3 years.
The biological behavior was assessed according to
pre-operative magnetic resonance imaging (MRI)/computed tomography
scanning. Aggressive adenomas were defined as fulfilling any of the
following two conditions: i) Hardy classification grade III and IV
(15); ii) Knosp classification
grade III and IV (16).
Clinical and follow-up data
All patients were diagnosed based on pre-operative
sella MRI and postoperative pathology. Postoperative sella MRI
scans were performed within 72 h post-surgery to determine the
remaining residual tumor. These were evaluated by two
neuroradiologists and a neurosurgeon, all of whom were blinded to
the patient characteristics. The follow-up data for each patient
was obtained at 6-month intervals for the first 2 years. Tumor
recurrence was investigated by serial sella MRI scans performed at
one year following surgery, or in the event of recurrence of
clinical symptoms, whichever was the earlier. Recurrence was
defined as the presence of a new tumor in patients with total tumor
resection, based on the first post-operative MRI scan, or evidence
of new growth of an incompletely resected tumor on serial
post-operative MRI scans compared with the immediate post-operative
MRI scan. The follow-up data were collected for 3 years.
TMA construction
To check the quality of the tissues, 4-µm thick
sections of paraffin-embedded tissue from the 212 NFPA specimens
and the 10 control samples were stained with hematoxylin and eosin
(H&E). Three core biopsies of 2.0 mm diameter were selected
from each sample and transferred to tissue microarrays using the
Leica BOND-III fully automated arrayer (Leica Biosystems, Inc.,
Lincolnshire, IL, USA). The locations of the core samples were
randomly ordered and blinded to the pathologist with regard to the
identity on the TMA slides. In addition, 4-µm thick sections of
TMAs were obtained using a serial microtome and applied to
positively-charged glass slides. The slides were heated in a water
bath at 50°C and subsequently dewaxed and then rehydrated through
graded alcohols. The slides were dried at room temperature for
24–48 h and stored at −80°C for subsequent use. To minimize loss of
antigenicity, TMA slides were processed within a week.
Immunohistochemistry (IHC)
The tumor content and quality of all TMA slides were
evaluated in advance with H&E staining The TMAs were placed in
the Leica BOND-III, which is a fully automated, random and
continuous access slide-staining system that processes IHC tests
simultaneously. Primary antibodies for β-catenin (1:100, cat. no.
ab22656) and c-myc (1:100, cat. no. ab32072) (both from Abcam,
Cambridge, UK) were used. The optimal titers of the primary
antibodies for the remainder were determined on pre-experimental
results. The slides were scanned into digital images using Aperio
AT2 (Leica Biosystems, Inc.), then scored for staining positivity
and intensity. A total of two neuropathologists, who were blinded
to the clinical information of the patients, independently examined
and scored each case. Differences in interpretation were resolved
by a consensus.
Evaluation of immunohistochemical
examination
The β-catenin staining was observed in the nucleus
and c-myc staining in the cytoplasm and nucleus. The results were
calculated using Aperio AT2 (v12.1.0.5029; Leica Biosystems, Inc.)
with digital slide viewing software. The staining intensity for
β-catenin and c-myc was scored as follows: 0, no; 1, weak; 2,
moderate and 3, high intensity. The distribution of positively
stained cells was scored on a scale of 0–5 with 0 for no staining;
1, <20%; 2, 20–40%; 3, 40–60%; 4, 60–80% and 5, 80–100%. The
composite score was calculated as the staining intensity score ×
distribution score (score ≤6, weak expression; >6, strong
expression).
Statistical analysis
The chi-square test (χ2; P-value) was
used to determine the significance of the association between
β-catenin expression, c-myc expression and clinical parameters that
included aggressiveness and recurrence. Variables demonstrating a
significant association with NFPA recurrence on univariate analysis
were additionally subjected to multivariate analysis. Two-tailed
P<0.05 was considered to indicate a statistically significant
difference. Survival rates were determined using logistic
regression analysis. Analyses were performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical and pathological
features
All 212 NFPAs met the inclusion criteria. The
details of their clinicopathological parameters are presented in
Table I. At the time of the first
surgical treatment, the age of the patients ranged between 24–75
years (mean, 50 years; median, 52 years). There were more male
patients (n=124, 58.5%) compared with female patients (n=88,
41.5%). The aggressive group (n=97) comprised 41 (42.3%) females
and 56 (57.7%) males, while the non-aggressive group (n=115)
comprised 47 (40.9%) females and 68 (59.1%) males. Partial
resection or minimum residual tumor was identified in 130 (61.3%)
of the total patients.
 | Table I.Clinical and pathological
characteristics of study subjects. |
Table I.
Clinical and pathological
characteristics of study subjects.
| Characteristics | Patients (n=212) | Percentage |
|---|
| Gender |
|
|
| Male | 124 | 58.5 |
|
Female | 88 | 41.5 |
| Age (years) |
|
|
| Mean | 50 |
|
|
Median | 52 |
|
| Aggressiveness |
|
|
|
Aggressive | 97 | 45.8 |
|
Non-aggressive | 115 | 54.2 |
| Subtype |
|
|
| Null
cells | 148 | 69.8 |
|
Gonadotrophs | 26 | 12.3 |
| Silent
corticotrophs | 5 | 2.4 |
| Silent
3 | 33 | 15.5 |
| Surgical extent |
|
|
| Gross
total resection | 82 | 38.7 |
|
Residual | 130 | 61.3 |
| Recurrence (within 42
months) |
|
|
| Yes | 41 | 19.3 |
| No | 171 | 80.7 |
Tissue microarrays analysis;
correlation of β-catenin and c-myc expression with aggressive
behavior
TMA analysis to determine the correlation between
β-catenin and c-myc expression in NFPA samples was determined by
the immunohistochemical examination of TMA slides (Figs. 1 and 2). The score of β-catenin and c-myc
staining in the normal pituitary tissues was significantly low
compared with that in the non-aggressive (1.6±0.27 vs. 5.430.27;
P=0.001) and aggressive tumors (1.6±0.27 vs. 9.18±0.41,
P<0.001). Similar results were observed with c-myc staining,
where normal group demonstrated lower expression compared with the
non-aggressive (1.6±0.27 vs. 5.57±0.29, P=0.001) and aggressive
tumors (1.6±0.27 vs. 9.8±0.43, P<0.001), respectively. The
association of protein expression with aggressive behavior was
evaluated by the composite score of β-catenin and c-myc staining;
~71.1% of aggressive NFPAs displayed strong β-catenin staining
(P<0.01), and ~88.7% c-myc overexpression (P<0.001; Table II). However, of 115 non-aggressive
NFPAs 69 (60%) displayed weak β-catenin and 91 (70.1%) weak c-myc
staining.
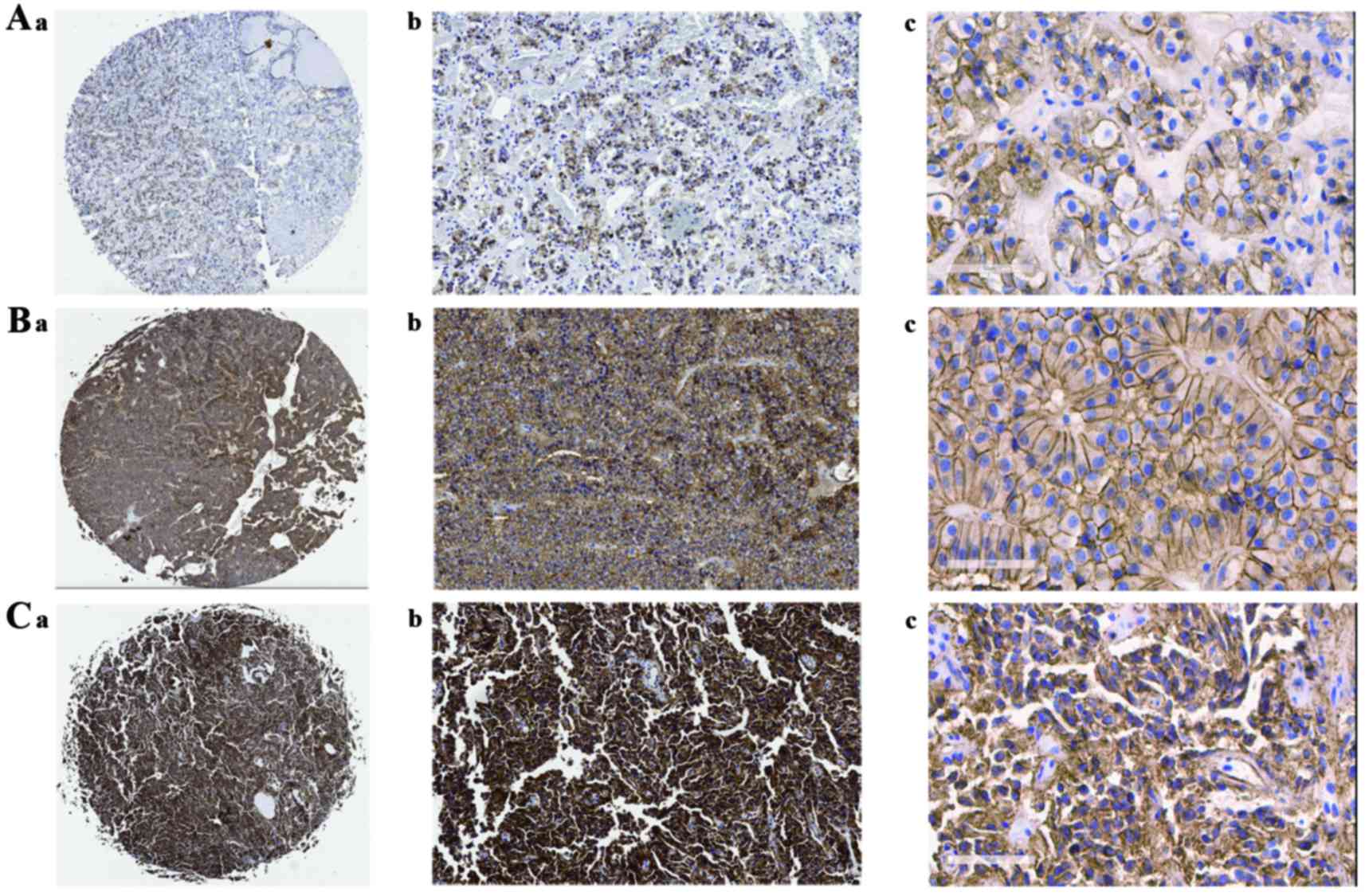 | Figure 1.Immunohistochemical study showing
expression of β-catenin tissue microarray. A-a (magnification, ×1),
A-b (magnification, ×100), A-c (magnification, ×400): Weak
β-catenin-positive cells representative of normal pituitary
tissues. B-a (magnification, ×1), B-b (magnification, ×100), B-c
(magnification, ×400): Weak β-catenin-positive cells representative
of non-aggressive NFPAs. C-a (magnification, ×1), C-b
(magnification, ×100), C-c (magnification, ×400): Strong
β-catenin-positive cells representative of aggressive NFPAs. NFPA,
non-functioning pituitary adenoma. |
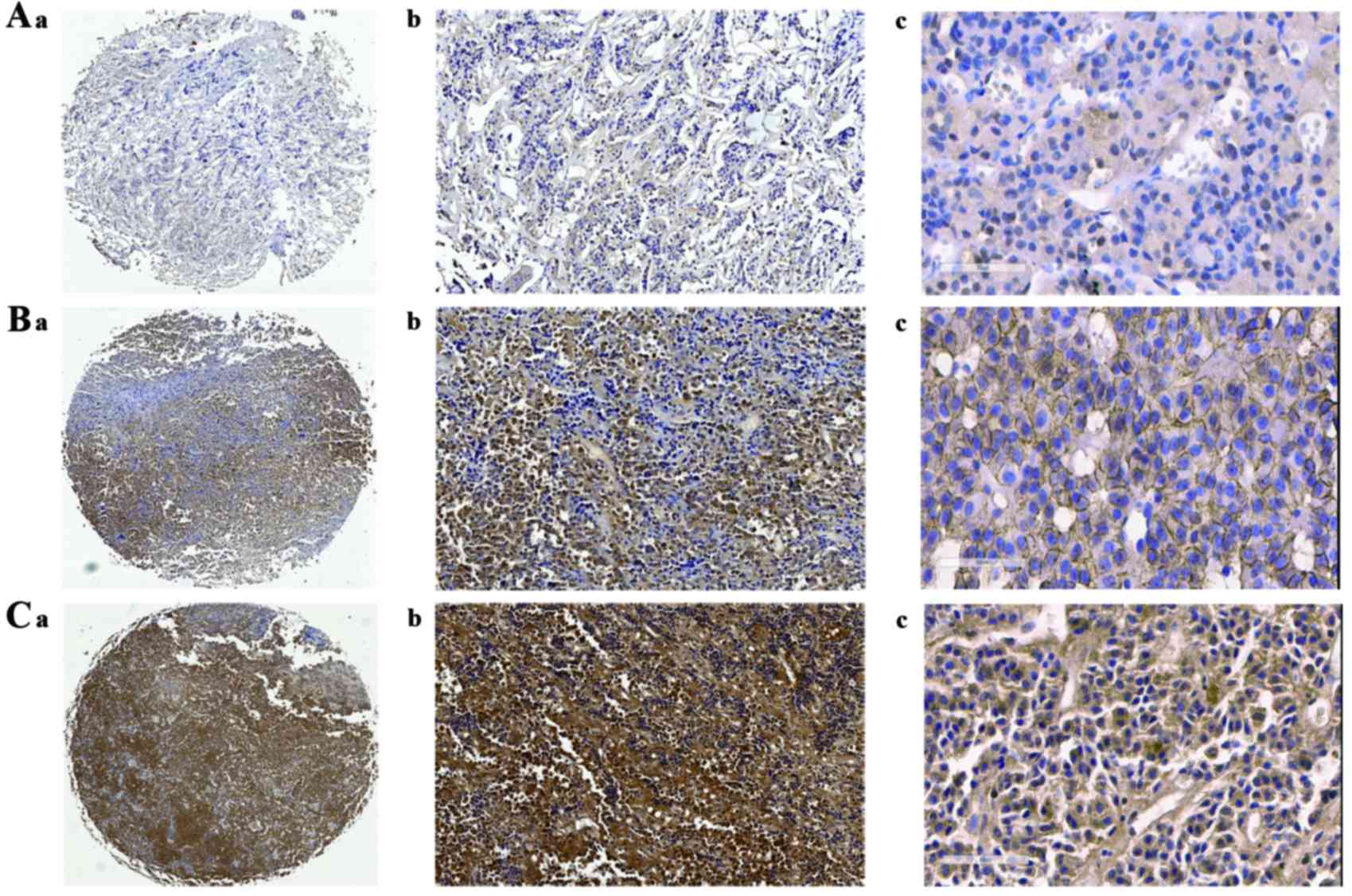 | Figure 2.Expression of c-myc assessed by
immunohistochemical examination on tissue microarray. A-a
(magnification, ×1), A-b (magnification, ×100), A-c (magnification,
×400): Weak c-myc -positive cells representative of normal
pituitary tissues. B-a (magnification, ×1), B-b (magnification,
×100), B-c (magnification, ×400): Weak c-myc-positive cells
representative of non-aggressive NFPAs. C-a (magnification, ×1),
C-b (magnification, ×100), C-c (magnification, ×400): Strong
c-myc-positive cells representative of aggressive pituitary
adenomas. NFPA, non-functioning pituitary adenoma. |
 | Table II.Univariate and multivariate analyses
for clinical and pathologic variables related to aggressive
non-functioning pituitary adenomas. |
Table II.
Univariate and multivariate analyses
for clinical and pathologic variables related to aggressive
non-functioning pituitary adenomas.
|
| Aggressiveness n
(%) | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Yes (n=97) | No (n=115) | χ2 | P-value | Odds ratio | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
Male | 56 (57.7%) | 68 (59.1%) | 0.042 | 0.837 |
|
|
|
|
Female | 41 (42.3%) | 47 (40.9%) |
|
|
|
|
|
| Age (years) |
|
|
|
|
|
|
|
|
<52 | 51 (52.6%) | 52 (45.2%) | 1.141 | 0.285 |
|
|
|
|
≥52 | 46 (47.4%) | 63 (54.8%) |
|
|
|
|
|
| Subtype |
|
|
|
|
|
|
|
| Null
cell | 69 (71.1%) | 79 (68.7%) | 3.708 | 0.295 |
|
|
|
|
Gonadotroph | 9 (9.3%) | 17 (14.8%) |
|
|
|
|
|
| Silent
corticotroph | 4 (4.1%) | 1 (0.9%) |
|
|
|
|
|
| Silent
3 | 15 (15.5%) | 18 (15.6%) |
|
|
|
|
|
| β-catenin |
|
|
|
|
|
|
|
|
Weak | 28 (28.9%) | 69 (60%) | 20.55 | <0.001 | 4.011 | 1.830–8.788 | 0.001 |
|
Strong | 69 (71.1%) | 46 (40%) |
|
|
|
|
|
| c-myc |
|
|
|
|
|
|
|
|
Weak | 11 (12.3%) | 91 (70.1%) | 96.861 | <0.001 | 30.833 | 13.613–69.839 | 0.001 |
|
Strong | 86 (88.7%) | 24 (20.9%) |
|
|
|
|
|
Univariate analysis of the clinical characteristics
indicated a significant correlation between β-catenin and c-myc
expression and aggressive behavior (P<0.001 and P<0.001;
Table II). There was, in
addition, a significant association between β-catenin and c-myc
expression (P=0.004; Table II).
However, the upregulation of the two proteins was not significantly
associated with age, gender or tumor subtype (data not shown;
Table II). Furthermore,
multivariate analysis indicated a significant association between
the overexpression of β-catenin alone with the aggressive behavior
of tumors (P=0.001; OR=4.011). There was no significant correlation
between the expression of β-catenin with c-myc (P<0.762;
OR=1.127).
Univariate analysis identified a significant
association between the expression of β-catenin and c-myc and
aggressive behavior (P<0.001; Table II). No significant association
with other clinicopathological parameters was observed (Table II). In the multivariate analysis,
c-myc (P<0.001, OR=30.833) and β-catenin (P=0.001, OR=4.011)
were significantly associated with aggressive NFPAs (Table II).
Recurrence-free survival analysis
Follow-up data were available for all 212 patients.
The patients were followed for 42 months. During follow-up, 41
(19.3%) patients experienced tumor recurrence (Table I). Univariate analysis indicated
positive β-catenin staining (P=0.002) and tumor aggressiveness
(P=0.004) to be significantly associated with recurrence, while
other clinicopathological parameters and positive c-myc staining
were not associated with NFPAs recurrence (Table III). Additional multivariate
analysis identified a significant association between recurrence
and β-catenin expression (P=0.021; OR=2.571) and with tumor
aggressiveness (P=0.043; OR=2.158; Table III).
 | Table III.Univariate and multivariate analyses
for clinical and pathological variables associated with
recurrence/progression-free survival. |
Table III.
Univariate and multivariate analyses
for clinical and pathological variables associated with
recurrence/progression-free survival.
|
| Recurrence (within
42 months) | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Yes (n=41) | No (n=171) | χ2 | P-value | Odds ratio | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
Male | 23 (56.1%) | 101 (59.1%) | 0.77 | 0.781 |
|
|
|
|
Female | 18 (43.9%) | 70 (40.9%) |
|
|
|
|
|
| Age |
|
|
|
|
|
|
|
|
<52 | 23 (56.1%) | 108 (63.2%) | 3.124 | 0.077 |
|
|
|
|
≥52 | 18 (43.9%) | 63 (36.8%) |
|
|
|
|
|
| Subtype |
|
|
|
|
|
|
|
| Null
cell | 29 (70.7%) | 119 (69.6%) | 2.709 | 0.439 |
|
|
|
|
Gonadotroph | 6 (14.6%) | 20 (11.7%) |
|
|
|
|
|
| Silent
corticotroph | 2 (4.9%) | 3 (1.8%) |
|
|
|
|
|
| Silent
3 | 4 (9.8%) | 29 (16.9%) |
|
|
|
|
|
| Aggressiveness |
|
|
|
|
|
|
|
|
Yes | 27 | 70 | 8.273 | 0.004 | 2.158 | 1.023–4.551 | 0.043 |
| No | 14 | 101 |
|
|
|
|
|
| β-catenin |
|
|
|
|
|
|
|
|
Weak | 10 (24.4%) | 87 (50.9%) | 9.348 | 0.002 | 2.571 | 1.150–5.747 | 0.021 |
|
Strong | 31 (75.6%) | 84 (49.1%) |
|
|
|
|
|
| C-myc |
|
|
|
|
|
|
|
|
Weak | 16 (39.1%) | 85 (49.7%) | 1.682 | 0.195 |
|
|
|
| Strong | 25 (60.9%) | 86 (50.3%) |
|
|
|
|
|
| Surgical
extent |
|
|
|
|
|
|
|
| Gross
total resection | 13 (31.7%) | 69 (40.4%) | 1.042 | 0.307 |
|
|
|
|
Residual | 28 (68.3%) | 102 (59.6%) |
|
|
|
|
|
Discussion
Aggressive behavior of tumors is the most important
prognostic factor in patients with NFPAs. Complications of surgery,
inability to achieve remission and inadequate therapy are problems
associated with aggressiveness (17). There is no effective medical
treatment for NFPAs, although post-operative radiotherapy is
recommended for treatment of residual tumor and to prevent
recurrence (18). Predicting the
aggressive behavior of NFPAs may be a key contribution for
therapeutic approaches and prognostic evaluation. In a previous
study (12), the Wnt signaling
pathway was demonstrated to be involved in tumorigenesis of
aggressive NFPAs; RT-qPCR and western blotting analysis
demonstrated overexpression of β-catenin and its downstream signal
target c-myc in aggressive NFPAs. The proteins β-catenin and c-myc
are considered to be useful markers of tumor progression in
different types of solid tumors (19–23).
Until now, there has been no reported correlation of
β-catenin and c-myc with different clinicopathological parameters
in NFPAs. The present study may be the first of its type to
investigate this association; it studied the correlation of
β-catenin and c-myc proteins with aggressiveness in NFPAs using IHC
on tissue microarrays. The data demonstrated that β-catenin and
c-myc expression was increased in the aggressive NFPAs compared
with the non-aggressive NFPAs and the normal pituitary tissues. On
univariate analysis of β-catenin and c-myc expression, no
statistical correlation was observed with respect to the
clinicopathological parameters. However, they demonstrated a
positive correlation with only aggressive behavior. Thus, β-catenin
and c-myc can be used as biological markers for the detection of
aggressive NFPAs. The results of the present study are consistent
with those reported for other types of cancer. β-catenin and c-myc
expression were upregulated in poor histological grade gastric
cancers (24) and β-catenin
expression was associated with higher grades in breast phyllodes
tumors (25). The increased
expression of c-myc was observed at a relatively late stage during
progression in prostate cancer and it was demonstrated to correlate
with tumor invasion and metastasis (26). Although a previous study reported
the correlation of invasion with silent subtype 3 adenomas
(26), no such clinicopathological
association was identified by the present study.
The reported recurrent rates vary between different
studies due to the difference in follow-up period and the number of
patients enrolled (27,28). In the present study, with a
follow-up period of 42 months, the mean of recurrence was 19.3%
(41/212) (Table I), which is
marginally lower than previously reported (20–30%). The follow-up
period in these studies ranged between 2 months and 24 years
(27,29). This difference could be associated
with the shorter follow-up in the present study and the variability
in the definition of recurrence/progression.
To the best of our knowledge, the present study is
the first investigation into the correlation of the expression
levels of β-catenin and c-myc proteins with tumor recurrence in a
large sample of NFPA specimens by using IHC on TMAs. The results
are in agreement with another published report (30), which suggested that the
aggressiveness of a tumor is an important pathological
characteristic for assessing the risk of tumor recurrence. There is
lack of data indicating association of β-catenin with recurrent
NFPAs and the present study provided the first such evidence of a
high β-catenin in immunohistochemical staining as being the most
important pathological feature for assessing the risk of
recurrence/progression of NFPAs.
The multivariate analysis demonstrated that an
increased IHC positivity of β-catenin is a strong predictive factor
of tumor recurrence. Although c-myc demonstrated a significant
correlation with aggressive NFPAs, no other association of c-myc
with recurrence was observed.
The observation that tumor resection is not
associated with tumor recurrence is in agreement with the results
reported by Brochier et al (3). They also reported that surgical
extent is not an independent factor of recurrence in pituitary
adenoma (31). This observation is
in contrast to the results of previous studies (30,31)
that indicated tumor resection was a predictive factor for the
recurrence of PA. The reason for this discrepancy in results can be
attributed to combination of different types of PA, the difference
in sample size and the length of follow-up duration in these
studies, whereas the present study analyzed a homogenous group of
NFPA samples. The follow-up data was analyzed using logistic
regression analysis instead of the classical Kalpan Meier survival
rate analysis. The survival time was not included to avoid
discrepancy caused by the low recurrence rate. These may be two
reasons for the inconsistency in results. Further studies are
required to validate the results of the present study.
In conclusion, the present study suggested that
β-catenin and c-myc can be a useful immunohistochemical marker for
identifying aggressive NFPAs. Only after validation in a large
number of samples can β-catenin be used for the prediction of
recurrence of NFPAs following surgical resection.
Acknowledgements
The present study was supported by the National
High-Tech Research and Development Program of China (grant no.
2014AA020610), the National Health and Family Planning Commission
for Public Welfare Industry Research Project (grant no. 201402008),
the National Natural Science Foundation of China (grant no.
3157060076) and the Natural Science Foundation of Beijing
Municipality (grant no. 7144198).
Glossary
Abbreviations
Abbreviations:
|
NFPA
|
nonfunctioning pituitary adenoma
|
|
MRI
|
magnetic resonance imaging
|
|
TMA
|
tissue microarray
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Scheithauer BW, Gaffey TA, Lloyd RV, Sebo
TJ, Kovacs KT, Horvath E, Yapicier O, Young WF Jr, Meyer FB, Kuroki
T, et al: Pathobiology of pituitary adenomas and carcinomas.
Neurosurgery. 59:341–353. 2006. View Article : Google Scholar
|
|
2
|
Saeger W, Lüdecke DK, Buchfelder M,
Fahlbusch R, Quabbe HJ and Petersenn S: Pathohistological
classification of pituitary tumors: 10 years of experience with the
german pituitary tumor registry. Eur J Endocrinol. 156:203–216.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brochier S, Galland F, Kujas M, Parker F,
Gaillard S, Raftopoulos C, Young J, Alexopoulou O, Maiter D and
Chanson P: Factors predicting relapse of nonfunctioning pituitary
macroadenomas after neurosurgery: A study of 142 patients. Eur J
Endocrinol. 163:193–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clevers H and Nusse R: Wnt/beta-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herr P, Hausmann G and Basler K: WNT
secretion and signalling in human disease. Trends Mol Med.
18:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin T, George Fantus I and Sun J: Wnt and
beyond Wnt: Multiple mechanisms control the transcriptional
property of beta-catenin. Cell Signal. 20:1697–1704. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michl P, Barth C, Buchholz M, Lerch MM,
Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Löhr M, et al:
Claudin-4 expression decreases invasiveness and metastatic
potential of pancreatic cancer. Cancer Res. 63:6265–6271.
2003.PubMed/NCBI
|
|
9
|
Sodir NM, Swigart LB, Karnezis AN, Hanahan
D, Evan GI and Soucek L: Endogenous myc maintains the tumor
microenvironment. Genes Dev. 25:907–916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wright JB, Brown SJ and Cole MD:
Upregulation of c-MYC in cis through a large chromatin loop linked
to a cancer risk-associated single-nucleotide polymorphism in
colorectal cancer cells. Mol Cell Biol. 30:1411–1420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin CY, Lovén J, Rahl PB, Paranal RM,
Burge CB, Bradner JE, Lee TI and Young RA: Transcriptional
amplification in tumor cells with elevated c-myc. Cell. 151:56–67.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Bai J, Li Z, Wang F, Cao L, Liu C,
Yu S, Yu G and Zhang Y: Low expression of secreted frizzled-related
protein 4 in aggressive pituitary adenoma. Pituitary. 18:335–342.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Formosa R, Gruppetta M, Falzon S, Santillo
G, DeGaetano J, Xuereb-Anastasi A and Vassallo J: Expression and
clinical significance of Wnt players and survivin in pituitary
tumours. Endocr Pathol. 23:123–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong L, Wu Y, Feng J, Yu S, Li C, Wu Y, Li
Z, Cao L, Wang F and Zhang Y: Overexpression of the cell adhesion
molecule claudin-9 is associated with invasion in pituitary
oncocytomas. Hum Pathol. 45:2423–2429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilson CB: A decade of pituitary
microsurgery. The Herbert Olivecrona lecture. J Neurosurg.
61:814–833. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: A
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–617. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenquist M: 14-3-3 proteins in
apoptosis. Braz J Med Biol Res. 36:403–408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kopp C, Theodorou M, Poullos N, Astner ST,
Geinitz H, Stalla GK, Meyer B, Molls M, Nieder C and Grosu AL:
Fractionated stereotactic radiotherapy in the treatment of
pituitary adenomas. Strahlenther Onkol. 189:932–937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monga SP: β-Catenin signaling and roles in
liver homeostasis, injury, and tumorigenesis. Gastroenterology.
148:1294–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen JB, Geyer SM, Lozanski G, Zhao W,
Heerema NA, Hall NC, Nagar VA, Hemminger JA, Jones JA, Porcu P, et
al: Complete response to induction therapy in patients with
myc-positive and double-hit non-Hodgkin lymphoma is associated with
prolonged progression-free survival. Cancer. 120:1677–1685. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prensner JR, Chen W, Han S, Iyer MK, Cao
Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, et al:
The long non-coding RNA PCAT-1 promotes prostate cancer cell
proliferation through cMyc. Neoplasia. 16:900–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin YU, Wu T, Yao Q, Zi S, Cui L, Yang M
and Li J: LGR5 promotes the proliferation of colorectal cancer
cells via the Wnt/β-catenin signaling pathway. Oncol Lett.
9:2859–2863. 2015.PubMed/NCBI
|
|
23
|
Chiurillo MA: Role of the Wnt/β-catenin
pathway in gastric cancer: An in-depth literature review. World J
Exp Med. 5:84–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soutto M, Romero-Gallo J, Krishna U,
Piazuelo MB, Washington MK, Belkhiri A, Peek RM Jr and El-Rifai W:
Loss of TFF1 promotes Helicobacter pylori-induced β-catenin
activation and gastric tumorigenesis. Oncotarget. 6:17911–17922.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho SK, Thike AA, Cheok PY, Tse GM and Tan
PH: Phyllodes tumours of the breast: The role of CD34, vascular
endothelial growth factor and β-catenin in histological grading and
clinical outcome. Histopathology. 63:393–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simpson DJ, Hibberts NA, McNicol AM,
Clayton RN and Farrell WE: Loss of pRb expression in pituitary
adenomas is associated with methylation of the RB1 CpG island.
Cancer Res. 60:1211–1216. 2000.PubMed/NCBI
|
|
27
|
Lee EH, Kim KH, Kwon JH, Kim HD and Kim
YZ: Results of immunohistochemical staining of cell-cycle
regulators: The prediction of recurrence of functioning pituitary
adenoma. World Neurosurg. 81:563–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Sullivan EP, Woods C, Glynn N, Behan LA,
Crowley R, O'Kelly P, Smith D, Thompson CJ and Agha A: The natural
history of surgically treated but radiotherapy-naïve nonfunctioning
pituitary adenomas. Clin Endocrinol (Oxf). 71:709–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang EL, Qian ZR, Rahman MM, Yoshimoto K,
Yamada S, Kudo E and Sano T: Increased expression of HMGA1
correlates with tumour invasiveness and proliferation in human
pituitary adenomas. Histopathology. 56:501–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dallapiazza RF, Grober Y, Starke RM, Laws
ER Jr and Jane JA Jr: Long-term results of endonasal endoscopic
transsphenoidal resection of nonfunctioning pituitary
macroadenomas. Neurosurgery. 76:42–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang EF, Zada G, Kim S, Lamborn KR,
Quinones-Hinojosa A, Tyrrell JB, Wilson CB and Kunwar S: Long-term
recurrence and mortality after surgery and adjuvant radiotherapy
for nonfunctional pituitary adenomas. J Neurosurg. 108:736–745.
2008. View Article : Google Scholar : PubMed/NCBI
|
















