Introduction
Stem cells (SCs) offer a promising approach to
regenerative medicine due to an unlimited capacity for self-renewal
and differentiation into derivatives of 3 germ layers. For this
reason, SCs may be useful as a cell replacement therapy in numerous
diseases (1). However, questions
have been raised concerning the consequences of SC therapy given
that available evidence indicates that certain pluripotent SC lines
are genetically unstable, a trait that may lead to ineffective
differentiation and, more importantly, uncontrolled proliferation
(2). The genomic integrity of SCs
is, therefore, an important issue, particularly considering that
such cells are expected to be applied in clinical practice in the
near future (3,4). Concerns also exist about the response
of SCs to treatment with ionizing radiation (IR).
SCs used in regenerative medicine will, inevitably,
be exposed to IR (5,6) administered for diagnosis and
treatment (7). This is important
because undifferentiated cells differ greatly from differentiated
cells in numerous aspects, including the course of the cell cycle,
metabolic profile, initial level of reactive oxygen species,
capacity for DNA repair, apoptosis and the frequency of de
novo mutations (8). In
addition, hESCs and hiPSCs differ in terms of genotypes and
phenotypes (9). All of these
factors affect the radiosensitivity of SCs and differentiated
cells. SCs possess a unique, short cell cycle, which has an effect
on the DNA damage response (DDR) and the DNA repair mechanisms in
pluripotent SCs are more efficient compared with those in
differentiated cells. Homologous recombination (HR) is the primary
repair mechanism for DNA double strand breaks (DSBs). Unrepaired
DNA damage in pluripotent SCs directs cells to programmed cell
death or differentiation, a response that prevents the accumulation
of mutations and contributes to the genetic instability of SC
populations (10). Despite the
intensive research into SCs in recent years, an understanding of
the response of these cells to IR remains limited (11). Furthermore, Long et al
demonstrated the importance of early inducible expression of
specific genes on the sensitivity of cancer and normal cells to IR
(12).
The present study had the following aims: i) To
determine the early IR-induced response of hESCs and hiPSCs by
measuring phosphorylated H2A histone family member X
(γH2AX) and the expression of DNA repair genes; and ii) to
compare the early DDR mechanisms in hESCs and hiPSCs. The current
study demonstrated that hiPSCs, during the primary response, are
more resistant to IR exposure compared with hESCs, exhibiting a
response that is similar to that observed in primary human dermal
fibroblasts (PHDFs). This indicates that although PHDFs were
successfully reprogrammed to hiPSCs, the hiPSCs retain features
that are characteristic of the parental differentiated cells.
Furthermore, hiPSCs and hESCs promote high activation of different
repair genes; breast cancer 2 (BRCA2), X-ray
repair complementing defective repair in Chinese hamster cells
4 (XRCC4) and DNA-dependent protein kinase catalytic
subunit (PRKCD) in hiPSCs, and tumor suppressor
protein P53 (P53), RAD51 recombinase
(RAD51) and PRKDC in hESCs. The results of the
present study contribute to an improved understanding of the
changes in the early IR-induced response of pluripotent SCs and
consequently provide important information for the safe application
of pluripotent SCs in regenerative medicine.
Materials and methods
Culture of hiPSCs
The PHDFs were obtained by full thickness punch
biopsy of patients' skin, diagnosed in Greater Poland Cancer Centre
(Poznan, Poland), following signing of informed consent. PHDFs were
reprogrammed as previously described (13). The pluripotent nature of hiPSCs
obtained following reprogramming of PHDFs was confirmed and is
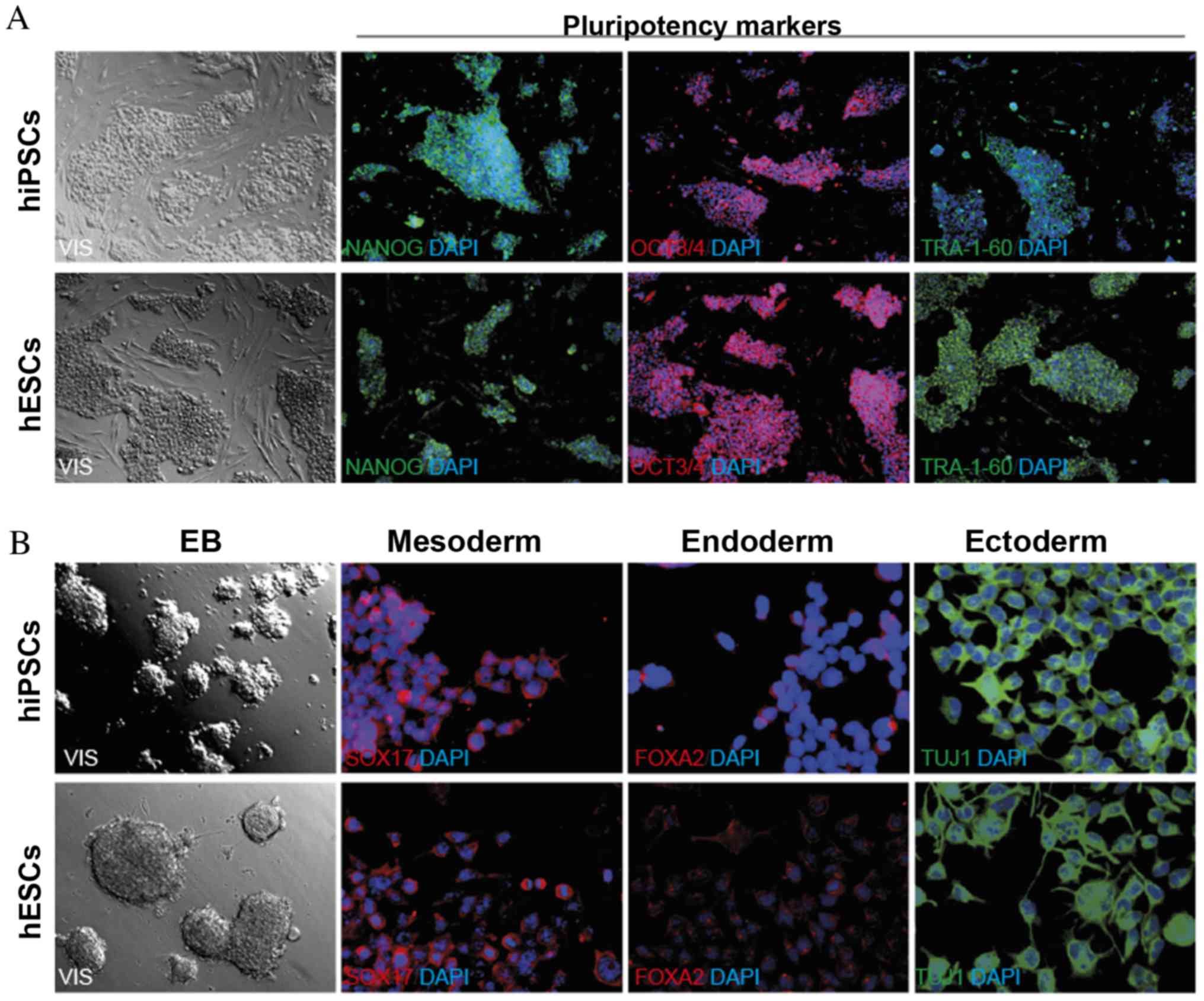
presented in Fig. 1. hiPSCs
obtained following reprogramming from PHDFs were seeded onto 10 cm
Petri dishes in Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
that had been previously coated with inactivated murine embryonic
fibroblasts (MEFs) as a feeder layer (1×106). Following
24 h preparation of the feeder layer, hiPSCs were seeded at
2×106 in standard hiPSC growth medium containing the
following: Dulbecco's modified Eagle's medium (DMEM) F12 with
L-glutamine (Merck KGaA, Darmstadt, Germany); 20% KnockOut Serum
Replacement (Thermo Fisher Scientific, Inc., Waltham, MA, USA); 1%
non-essential amino acid solution (Sigma-Aldrich; Merck KGaA); 0.1
mM β-mercaptoethanol (Merck KGaA); and 0.5% penicillin-streptomycin
(Merck KGaA). Prior to use, the medium was supplemented with
fibroblast growth factor 2 (10 ng/ml; Thermo Fisher Scientific,
Inc.) (13). The culture medium
was changed daily. Cells were cultured in a humidified atmosphere
of 5% CO2 at 37°C.
Culture of hESCs
The culturing process for hESCs (BGV01; American
Type Culture Collection, Manassas, VA, USA) was almost identical to
that described for hiPSCs, except that the standard hESC growth
medium consisted of 5% KSR and 15% FBS (Biowest USA, Riverside, MO,
USA) instead of 20% KSR (12). The
culture medium was changed daily. Cells were cultured at 37°C, in a
humidified atmosphere containing 5% CO2.
Culture of PHDFs
Isolated PHDFs were seeded on 10 cm Petri dishes at
2.5×106 in human fibroblast growth medium that contained
the following: DMEM-High Glucose (Biowest USA); 10% FBS (Biowest
USA) and 1% penicillin-streptomycin (Merck KGaA). The medium was
changed every 2–3 days. Cells were cultured at 37°C, in a
humidified atmosphere containing 5% CO2.
Embryoid body (EB) formation and
immunofluorescence staining
To verify the ability of the hiPSCs to differentiate
into three primary germ layers, EB formation and immunofluorescence
staining (NANOG, OCT3/4, TRA-1-60 and SOX17, FOXA2, TUJ1) was
performed (Fig. 1) (1).
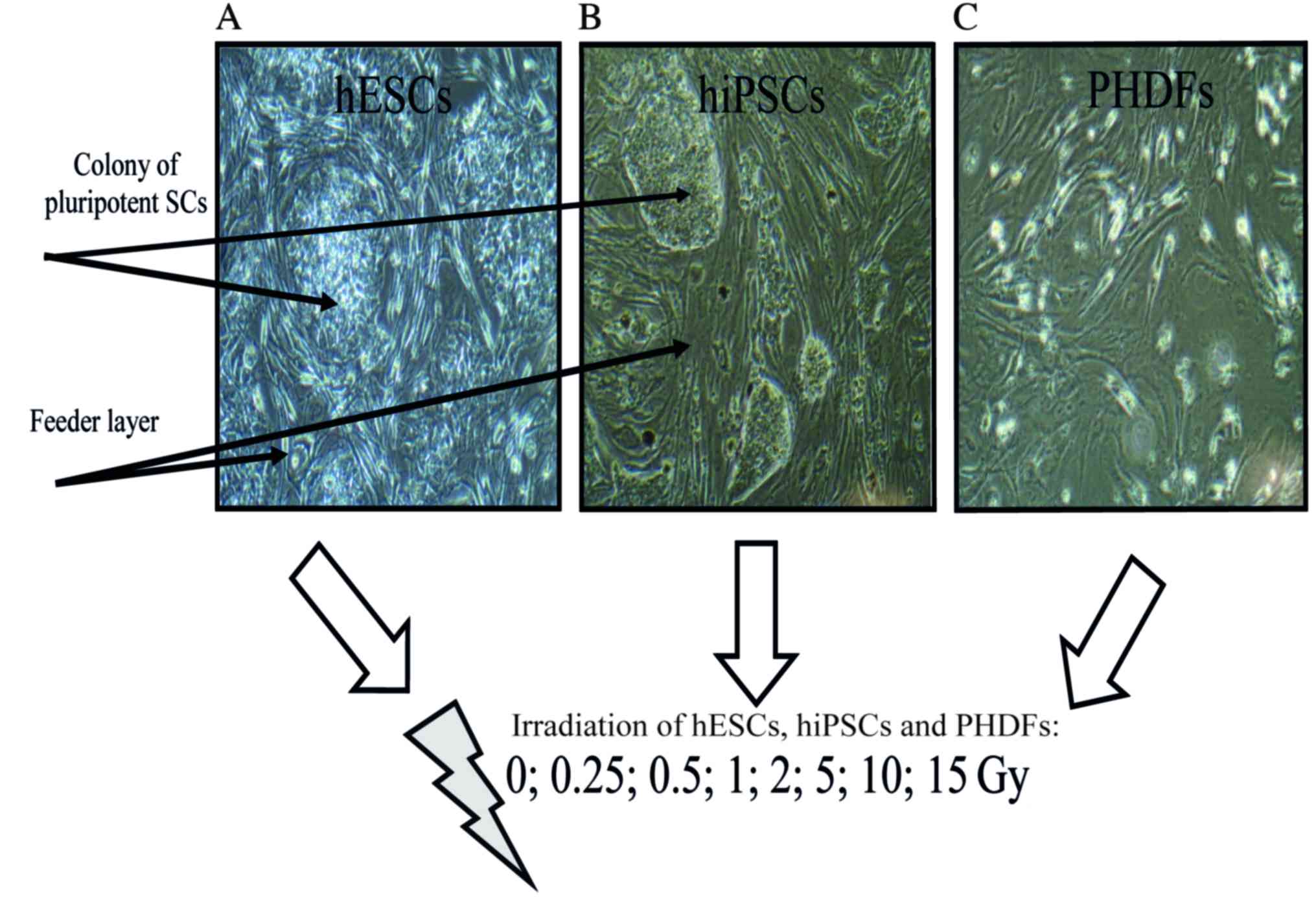
Irradiation
Confluent hiPSCS, hESCs and PHDFs were irradiated at
room temperature in the aforementioned growth medium, using a
Clinac 2300C/D linear accelerator (Varian Medical Systems, Inc.,
Palo Alto, CA, USA) at the following dose levels: Low dose (0,
0.25, 0.5 and 1 Gy); and high dose (2, 5, 10 and 15 Gy).
Immediately following irradiation, cells were incubated for 1 h in
a humidified atmosphere of 5% CO2 at 37°C. The selection
of 1 h as the first time-point following treatment with a
DNA-damaging agent, to ensure the most visible changes, was
selected based on data from the existing literature (14–17)
(Fig. 2).
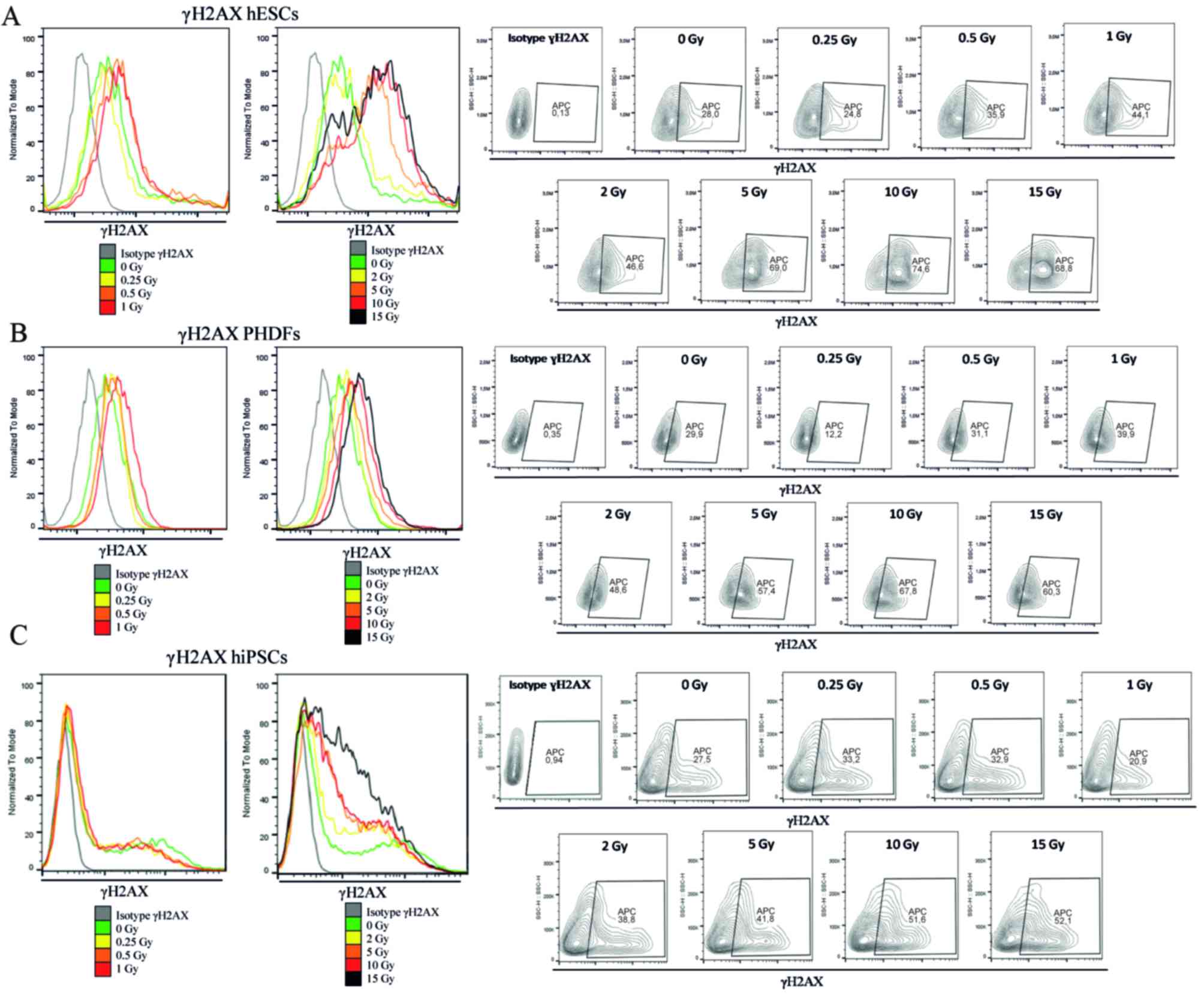
Flow cytometry analysis of γH2AX
Cells (hESCs, hiPSCs and PHDFs) were stained for
γH2AX with the Alexa Fluor® 647 Mouse H2AX
(pS139) antibody (catalog no. 560447, BD Biosciences) according to
the manufacturer's instructions. Briefly, ~5×105
IR-treated and untreated cells were collected. Fixation and
permeabilization were performed simultaneously for all cells using
BD Cytofix/Cytoperm™ Fixation/Permeabilization solution for 20 min
(BD Biosciences) at room temperature. Fixed cells were rinsed and
subsequently stained with H2AX antibody (5 µl/test) in 20 µl BD
Perm/Wash™ buffer for 20 min at room temperature. Cells were
resuspended in 1 ml staining buffer and analyzed with a flow
cytometer (BD Accuri™ C6) using a 675/25 FL4 filter within 1 h. For
isotype control, the Alexa Fluor® 647 Mouse IgG1 κ
Isotype Control (catalog no. 557714; 5 µl/test; BD Biosciences) was
used. Fluorescence intensity in arbitrary units was plotted in
histograms and contour plots, and the mean fluorescence intensity
was calculated. Data were analyzed using FlowJo software (FlowJo
v10; LLC, Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRI Reagent®
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Total RNA (1 µg per 20 µl reaction volume) was
reverse-transcribed using iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol (25°C for 5 min, 42°C for 30 min, 85°C for
5 min). Amplification products of individual gene transcripts were
detected via fluorescent probes (Universal Probe Library; Roche
Diagnostics, Basel, Switzerland) and Probe Master (LightCycler 480
Probes Master; Roche Diagnostics). The appropriate primers
(Sigma-Aldrich; Merck KGaA) were designed with Universal Probe
Library software (Roche Diagnostics), with sequences presented in
Table I.
 | Table I.Forward and reverse primer
sequences. |
Table I.
Forward and reverse primer
sequences.
| Gene | Primer
sequence | Probe |
|---|
| P53 | Forward:
ctttccacgacggtgaca | 71 |
|
| Reverse:
tcctccatggcagtgacc |
| RAD51 | Forward:
atcactaatcaggtggtagctcaa | 58 |
|
| Reverse:
cccctcttcctttcctcaga |
| BRCA2 | Forward:
cctgatgcctgtacacctctt | 45 |
|
| Reverse:
gcaggccgagtactgttagc |
| XRCC4 | Forward:
tggtgaactgagaaaagcattg | 68 |
|
| Reverse:
tgaaggaaccaagtctgaatga |
| PRKDC | Forward:
agaggctgggagcatcact | 31 |
The reaction conditions for all amplicons were as
follows: initially 95°C for 10 min, followed by 45 cycles at 94°C
for 10 sec, 60°C for 15 sec and 72°C for 1 sec. All reactions were
performed in the presence of 3.2 mM MgCl2. cDNA samples (2.5 µl for
total volume of 10 µl) were analyzed for genes of interest and for
the reference gene GAPDH. The average cycle threshold (Cq)
value was used to calculate the mRNA expression levels of genes of
interest, relative to the expression level of the reference
gene-GAPDH. This was conducted via use of the comparative
cycle time (ΔCq) method. Furthermore, the Cq method was applied to
calculate the relative amount of target gene expression and the
expression level of each target gene was normalized to GAPDH
(05–190-541-001; Roche Diagnostics) expression
using-2ΔΔCq (18). The
reaction was carried out in triplicate for the gene of
interest.
Statistical analysis
All experiments were performed at least 3 times.
Results are presented as the mean ± standard deviation. Comparisons
between the study groups and controls were performed using one-way
analysis of variance followed by post-hoc analysis using Tukey's
multiple comparison test with a single pooled variance. Comparisons
between the study groups and controls were performed with GraphPad
Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Obtaining hiPSCs from PHDFs
The microscopic evaluation of hiPSCs indicated that
hiPSC colonies composed of homogenous cells with a high nucleus to
cytoplasm ratio, demonstrated characteristics of pluripotent SCs.
The expression of pluripotency markers (NANOG, OCT3/4, TRA-1-60) in
hiPSCs was confirmed by immunofluorescence analysis. To prove the
ability of hiPSCs to differentiate into three primary germ layers
(meso-, endo- and ectoderm), spontaneous differentiation via EB
formation was performed. In the two-week EB formation, the presence
of specific markers: SOX17, FOXA2 and TUJ1 was assessed by
immunofluorescence staining (Fig.
1).
The irradiation of cells
In order to investigate and compare the early
responses of pluripotent SCs, the analyzed cells (feeder- dependent
hESCs and hiPSCs; PHDFs) were irradiated at the following range of
doses: 0, 0.25, 0.5, 1, 2, 5, 10 and 15 Gy (Fig. 2).
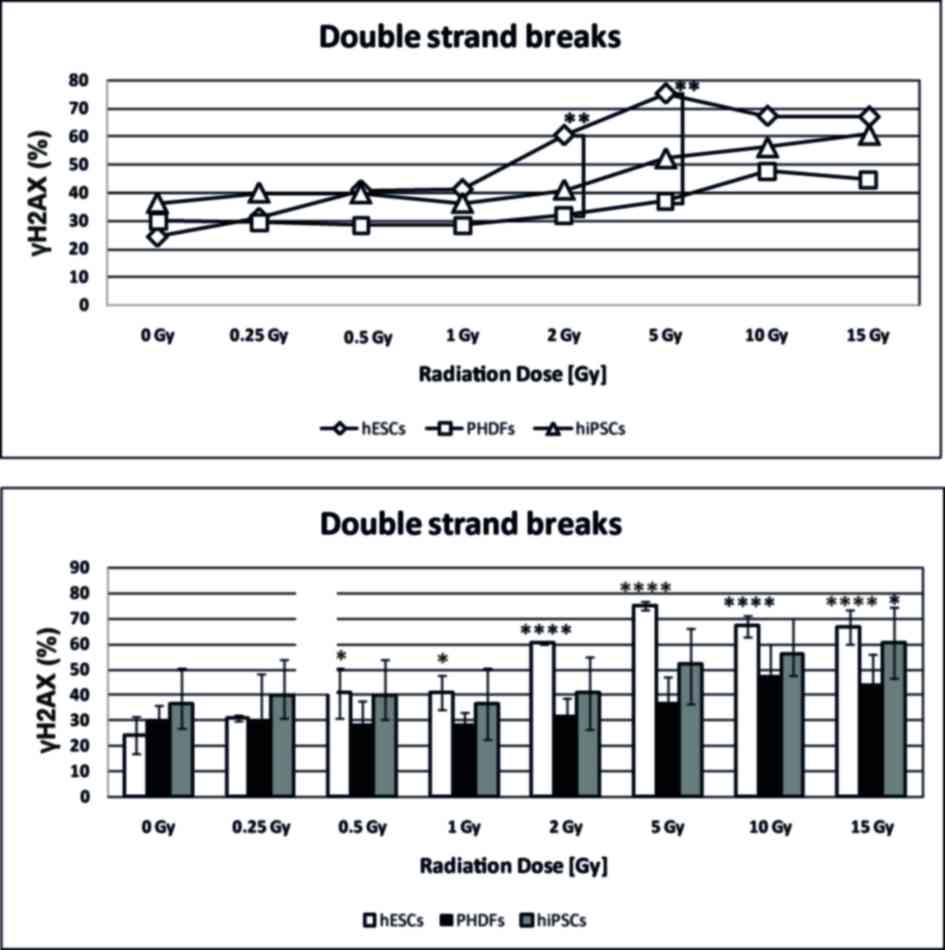
Analysis of DSBs in irradiated
cells
DSBs, as a percentage of γH2AX, were more
common in SCs compared with PHDFs. The number of DSBs observed in
hESCs that were irradiated at 2 and 5 Gy was significantly higher
compared with the number of DSBs in PHDFs (P<0.01; Fig. 3). Both SC cell lines exhibited,
statistically significant in the case of hESCs particularly above 2
Gy (P<0.0001), increases in DSBs at high doses of γ-radiation
compared with the control at 0 GyIrradiated PHDFs exhibited a
noticeable but not statistically significant increase in DSBs
(Fig. 3). Representative
histograms and contour plots for each cell line are presented in
Fig. 4.
P53 expression
Based on relative abundance and normalized fold
expression (Fig. 5A), P53
expression was highest in hESCs. However, P53 expression was
only significantly different compared with 0 Gy at 10 Gy in hESCs
(P<0.05).
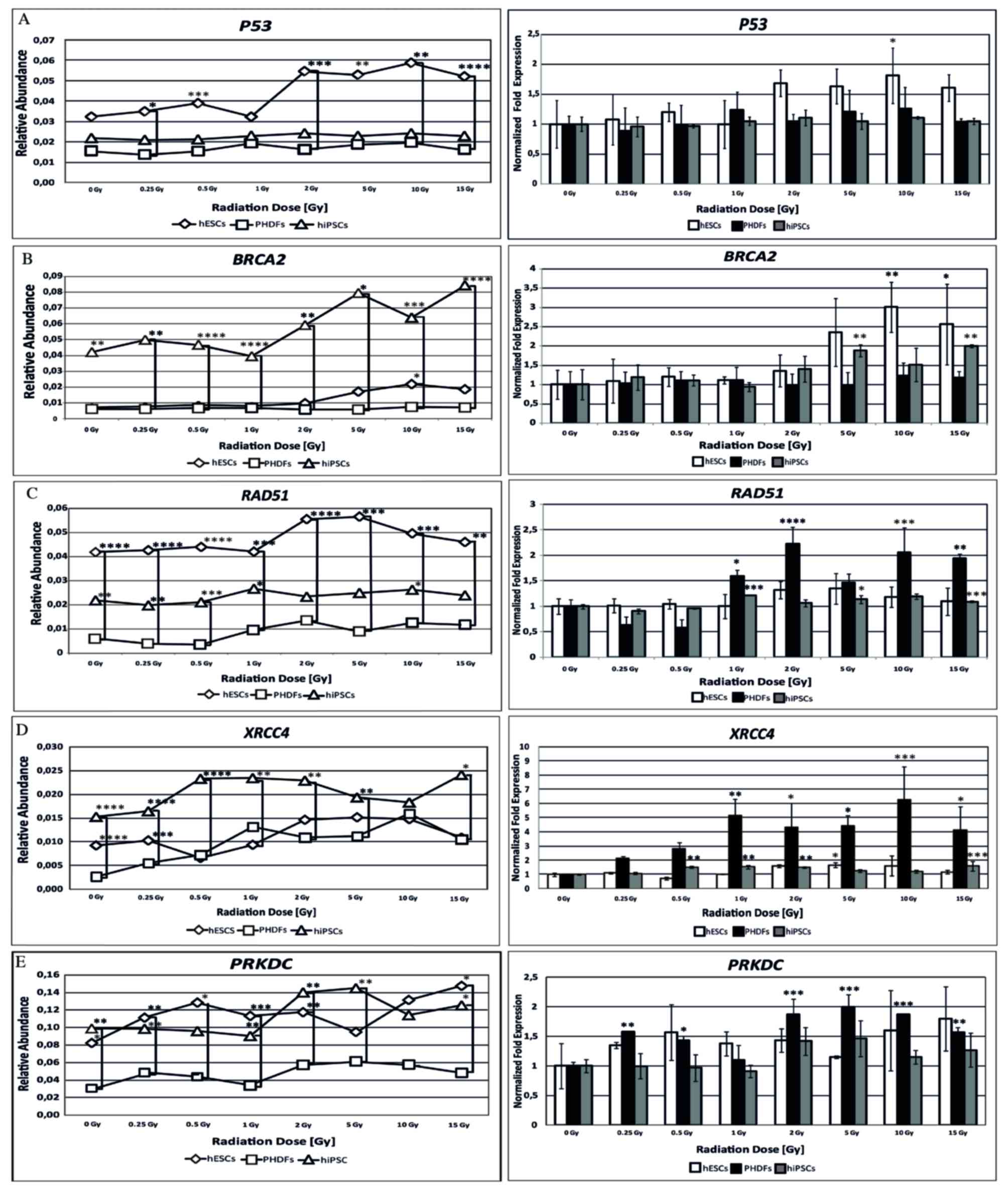 | Figure 5.Expression of activated genes
involved in DNA repair in irradiated cells. Expression of (A)
P53 (B) BRCA2 and (C) RAD51 genes involved in
homologous recombination method of DNA repair. Expression of (D)
XRCC4 and (E) PRKDC genes involved in the
non-homologous end joining method of DNA repair. *P<0.05;
**P<0.01; ***P<0.001; and ****P<0.0001 vs. cells at 0 Gy.
Significant differences between different cell lines at the same
radiation doses are indicated by brackets. P53, tumor suppressor
protein P53; BRCA2, breast cancer 2; RAD51, RAD51 recombinase;
XRCC4, X-ray repair complementing defective repair in Chinese
hamster cells 4; PRKDC, DNA-dependent protein kinase catalytic
subunit; SCs, stem cells; hESCs, human embryonic SCs; PHDFs,
primary human dermal fibroblasts; hiPSCs, human induced pluripotent
SCs; Gy, gray. |
BRCA2 expression
Of the 3 cell lines, the present study demonstrated
that the relative abundance of BRCA2 gene expression was
highest in hiPSCs. However, an increase in the relative abundance
of BRCA2 transcript was also observed in hESCs. Normalized
fold expression of the BRCA2 gene was statistically
significant at certain radiation doses in hESCs and hiPSCs; at 10
(P<0.01) and 15 Gy (P<0.05) in hESCs the expression was
double that observed in cells at 0 Gy, and significant differences
were observed in hiPSCs at 5 and 15 Gy (P<0.01; Fig. 5B).
RAD51 expression
The relative abundance of RAD51 mRNA differed
among cell lines; the highest level was observed in hESCs, followed
by hiPSCs and PHDFs. Although PHDFs exhibited the lowest level of
relative abundance of the RAD51 transcript, they also
demonstrated the largest increase in RAD51 normalized fold
expression, significantly higher compared with SC lines, reaching a
two-fold increase at 2 Gy compared with 0 Gy (P<0.0001; Fig. 5C).
XRCC4 expression
hiPSCs had the highest relative abundance of
XRCC4 mRNA. By contrast, lower levels were observed in hESCs
and PHDFs. However, based on normalized fold expression,
XRCC4 expression was highest in PHDFs; 5 times higher at 1
Gy compared with 0 Gy (P<0.01). XRCC4 expression was also
high in hiPSCs, with significantly higher levels compared with
controls at certain doses (P<0.01; Fig. 5D).
PRKDC expression
Both SC lines exhibited similar relative abundance
of PRKDC gene expression, which was significantly higher
compared with differentiated cells (P<0.05). Compared with cells
at 0 Gy in each cell line, the relative PRKDC expression was
highest in PHDFs, with statistically significant increases between
2 and 15 Gy doses (P<0.01; Fig.
5E).
Gene expression profile of individual
cell lines
Due to the relative abundance of the aforementioned
gene transcripts, the gene expression profiles for each cell line
was distinct. hESCs had the highest expression of the PRKCD
gene, exhibiting the highest initial and final relative abundance.
RAD51 and BRCA2 expression were also visible in this
cell line. In hESCs, the expression of XRCC4 and P53
was notably lower compared with RAD51, BRCA2 and
particularly PRKCD expression (Fig. 6). In hiPSCs, the most prominent
gene was PRKDC. Expression of the BRCA2 gene in this
cell line was lower but observable. The lowest level of gene
expression was observed for RAD51, P53 and
XRCC4 genes (Fig 6). In
PHDFs, the relative abundance of the PRKDC transcript was
high compared with BRCA2, RAD51, P53 and
XRCC4 genes (Fig. 6).
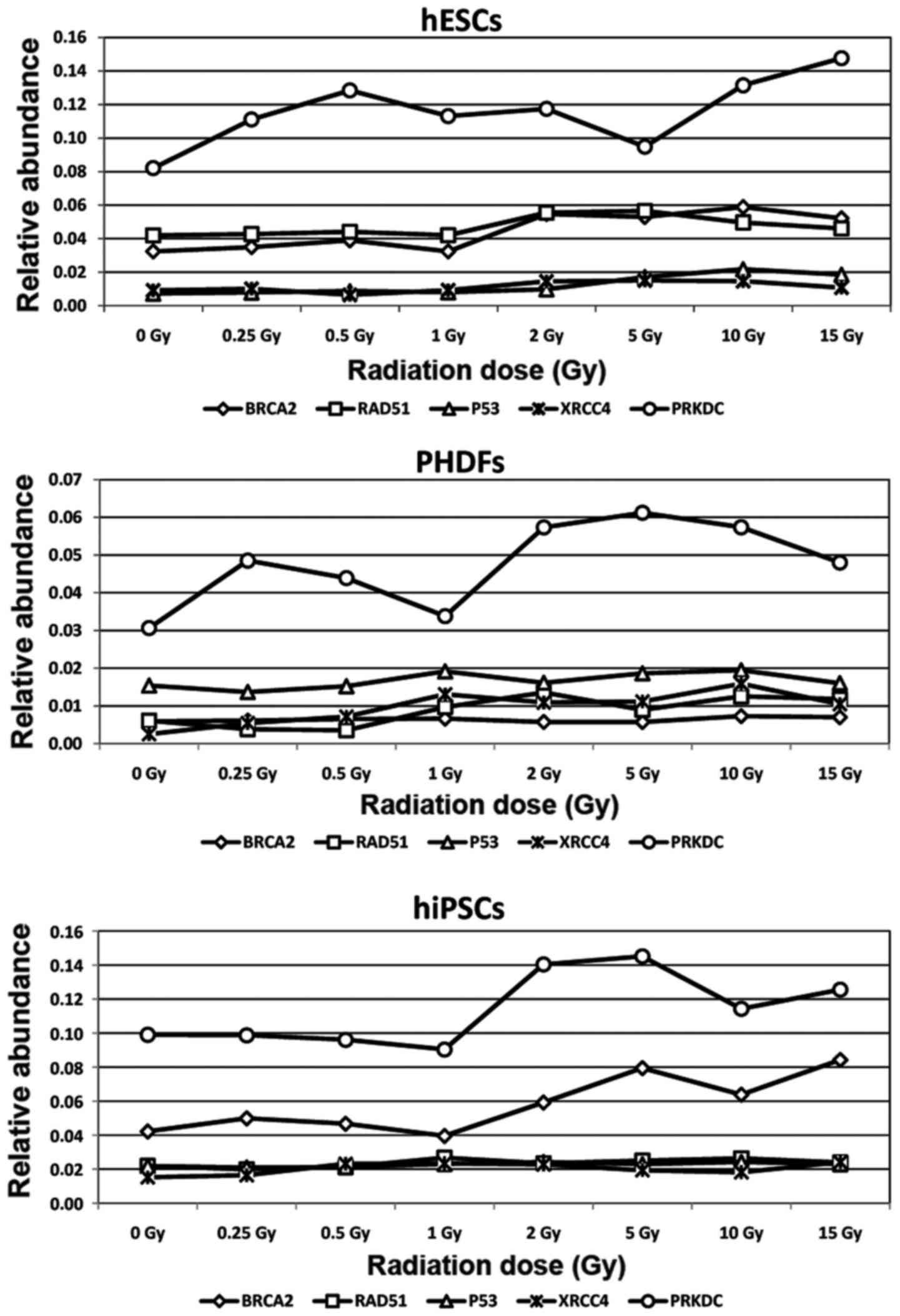 | Figure 6.Gene expression profile of activated
genes in cell lines following ionizing radiation treatment. hESCs,
PHDFs and hiPSCs were characterized by distinct DNA repair gene
expression profiles, indicating the existence of cell
line-dependent DNA damage response mechanisms. SCs, stem cells;
hESCs, human embryonic SCs; PHDFs, primary human dermal
fibroblasts; hiPSCs, human induced pluripotent SCs; Gy, gray;
BRCA2, breast cancer 2; RAD51, RAD51 recombinase; P53, tumor
suppressor protein P53; XRCC4, X-ray repair complementing defective
repair in Chinese hamster cells 4; PRKDC, DNA-dependent protein
kinase catalytic subunit. |
Discussion
Human pluripotent stem cells present a promising
approach for numerous disorders. However, pluripotent SCs used in
regenerative medicine will inevitably be exposed to IR for
diagnostic purposes and/or radiotherapy treatment. For this reason,
it is important to broaden our understanding of the radioresistance
properties in these cells. Firstly, hiPSCs were generated from
PHDFs, with pluripotent nature and capacity to differentiate into
three primary germ layers, verified. The present study evaluated
and compared the early response of hESCs and hiPSCs to IR
administered at doses ranging from low to high (0–15 Gy). The
primary aim of this study was to determine DSB formation and the
activation of the principal DNA repair genes following IR
treatment. Notably, the results of the current study demonstrated
that hiPSCs, in the initial response, are less sensitive to IR
compared with hESCs. Furthermore, hiPSCs have a different
expression profile of DNA damage response associated genes
following IR, which has an impact on radioresistance and makes them
less resistant to radiation compared with hESCs.
The present study identified that DSB formation in
hiPSCs is similar to PHDFs, but differs compared with hESCs. Mouse
and human pluripotent SCs exhibit important differences that should
be considered in any analysis. The majority of previous studies
have been conducted with mouse ESCs (mESCs) (19–21).
However, these results cannot be directly applied to human cells.
Although mESCs and hESCs are well-understood, little is known about
miPSCs, and information about hiPSCs is limited. mESCs have
increased genetic stability compared with differentiated cells
because, in response to DSBs, the recruitment of the stemness
factor spalt like transcription factor 4 is required to activate
the critical ataxia telaniectasia mutated (ATM)-dependent cellular
pathway (19). In somatic cells,
cyclin-dependent kinase 2 (CDK2), the major regulator of the
G1/S transition, is impaired by cell division cycle 25A
(CDC25A) phosphatase, which initiates G1
checkpoint. In mESCs, CDK2 is protected from regulation by
nuclear CDC25A, indicating that high CDK2 activity
may be responsible for the instant escape of mESCs from the
G1 phase and the following G1 checkpoint
following DNA damage (20).
Compared with hESCs, mESCs have a higher level of strand breaks and
endogenous repair foci, potentially due to global chromatin
decondensation rather than pre-existing DNA damage, indicating
differences in chromatin organization between mESCs and hESCs. The
differentiation process leads to a reduction in histone
acetylation, γH2AX foci formation and sensitivity of
replicating cell chromatin (21).
Sokolov et al (22) provided evidence that doses of up to
1 Gy of IR did not influence the pluripotency of hESCs. They
demonstrated that the surviving hESCs maintained a high expression
of genes responsible for pluripotency, including octamer binding
transcription factor 4 (OCT4), NANOG, sex determining region
Y-box 2, stage-specific embryonic antigen 4, telomerase
reverse transcriptase and T cell receptor alpha locus
(TRA-1-60 and TRA1-81). Momčilovic et al (23) first reported that, following 2 Gy
of γ-radiation, hESCs temporarily arrested the cell cycle in the
G2 stage, however, not in the G1 stage. For
complete induction of G2 arrest, ATM phosphorylation and
localization at the sites of DNA DSBs is required. It was also
observed that, ~16 h following irradiation, hESCs restarted the
cell cycle with a high proportion of aberrant mitotic figures.
Filion et al (24) also
demonstrated that hESCs, in contrast with somatic cells, lack a
G1 checkpoint. In response to IR, hESCs rapidly induced
phosphorylation of H2AX and P53, and stopped dividing
in the G2 phase. This phenomenon is enhanced by the
activation of ATM, checkpoint kinase 2 (CHK2)
and survivin pathways without cyclin dependent kinase
inhibitor 1A (P21) recruitment. However, strong activation of
survivin does not guarantee increased cell survival; the opposite
occurs and the hESCs undergo apoptosis, thus, ensuring the genomic
integrity of the surviving hESCs. Neganova et al (25) reported that downregulation of
CDK2 in hESCs leads to G1/S checkpoint activation
via the ATM-CHK2-P53-P21 pathway. Decreased production of
CDK2 also triggers DSBs and high levels of apoptosis.
miPSCs are less sensitive to low and high dose IR
compared with mouse hematopoietic stem/progenitor cells. Hayashi
et al (26) reported that
irradiated miPSCs retained the ability to form embryoid bodies
(EBs) with 3 germ layers. The diameter of formed EBs decreased in a
radiation dose-dependent manner. However, the further/more
comprehensive response of iPSCs to IR was not evaluated. However,
the results of that study indicated that different types of human
pluripotent SCs cannot be treated equally, a result that was
confirmed in the present study; hESCs exhibited the highest
radiosensitivity to all IR doses. This indicates that hESCs possess
a high DNA repair capacity, however also undergo mass cell death
when damaged, thus avoiding genetic instability in irradiated cell
populations. Consistent with previous studies (27), the present study demonstrated that
hiPSCs exhibit a level of radioresistance, which results in the low
formation of γH2AX (Figs. 3 and
4). This is notable given that
hiPSCs are difficult to maintain in culture and readily undergo
apoptosis. Spontaneous SC differentiation or dedifferentiation were
ruled out as pluripotency of these cells was maintained at a high
and stable level throughout the experiment. The results indicate
that hiPSCs may partially preserve a transcriptional memory of
their parent somatic cells (PHDFs).
The current study demonstrated that, following IR,
P53 was more highly expressed by hESCs compared with hiPSCs
and PHDFs. Depending on stress severity, nuclear P53
transactivates proapoptotic or antioxidant genes, while cytoplasmic
P53 has an important role in inducing
mitochondrial-dependent apoptosis (28). In mESCs, P53 is scarcely
phosphorylated in response to IR, as evidenced by the low
expression of the P53-target P21 gene. However,
despite a dysfunctional P21 pathway, mESCs are capable of
inducing ATM and γH2AX foci, which is indispensable
for the activation of DDR (29).
P53 regulates pro- and anti-differentiation pathways in
mESCs (30), and in mESCs
P53 has an anti-differentiation role through direct
regulation of the Wnt signaling pathway. In mESCs,
anti-proliferative P53 is localized primarily in the
cytoplasm, thus, preventing the cell from activating its target
genes. In response to IR, P53 accumulates in the cell
nucleus, where induction of P53 target genes subsequently
occurs. Target genes that are induced include mouse double
minute 2 homolog, P21, phorbol-12-myristate-13-acetate-induced
protein 1, and P53 upregulated modulator of apoptosis;
inhibition of NANOG is also important (31,32).
Previous studies have demonstrated that mESCs and hESCs possess a
nonfunctional P53-P21 axis of the G1/S checkpoint
pathway, which is regulated by specific DDR mechanisms. Dolezalova
et al (33) demonstrated
that, although hESCs have the ability to stabilize and activate the
P53 protein, these cells do not produce the P21 protein even in the
presence of P21 mRNA. This mechanism is regulated by the
miRNA family, specifically miR-302, which post-transcriptionally
regulates the expression of the P21 protein. Solozoba and Blattner
(34) demonstrated that P53
is more abundant in mESCs compared with somatic cells, primarily
due to the enhanced stability of P53 RNA and protein de
novo synthesis. During the differentiation process, the level
of P53 mRNA decreases and, consequently, so does P53 protein
synthesis. Furthermore, in differentiated ESCs, miR-125a and
miR-125b expression, well established repressors of p53, was
increased.
Unlike mESCs, hESCs are difficult to maintain in
culture as they readily undergo spontaneous differentiation and
apoptosis. In apoptotic hESCs, P53 accumulates and is subsequently
followed by initiation of the apoptotic mitochondrial pathway.
Although P53 accumulation in mESCs and hESCs was demonstrated by
Qin et al (35), activation
of the transcription of P53 target genes was significantly
lower in hESCs compared with mESCs. Lower P53 levels are associated
with the promotion of cell survival and reduced spontaneous
differentiation. The first investigation of hESC genome-wide
transcriptional changes following IR was performed by Wilson et
al (36), which demonstrated
that although some genes involved in developmental pathways were
altered with doses up to 4 Gy of IR, expression of pluripotency
genes remained unchanged. Furthermore, surviving cells retained the
capacity to recover and form teratomas, the definitive test of
pluripotency. In hESCs, the majority of P53-binding sites are
unique to each pluripotency- and differentiation-dependent state
and define stimulus-specific P53 responses. During early
differentiation, activated P53 targets influence core pluripotency
factors, including OCT4 and NANOG in chromatin
enriched with H3K27me3 and H3K4me3. When DNA damage occurs, P53
specifically binds to genes that are associated with cell migration
and motility (37). Sokolov and
Neumann (38) and Sokolov et
al (39) investigated the
effects of low doses (up to 0.1 Gy) of IR exposure on
stress-responsive gene expression in hESCs. The results indicated
that this type of gene expression in hESCs is temporal and cell
line dependent, however, there was no linear dose-dependent
association within the lowest doses of IR (38,39).
Knockout of P53 in reprogrammed human cells
leads to genomic instability. Transient suppression of P53
facilitates reprogramming due to an increased number of iPSC
colonies with high expression of pluripotency genes, however,
apoptotic and DNA damage mechanisms are not affected, thus,
resulting in the generation of a stable iPSC cell line (40). Notably, in hiPSCs, genome
surveillance is achieved via hypersensitivity to apoptosis and a
low accumulation of DNA lesions (21), this was also confirmed in the
present study. In hiPSCs, apoptosis is mediated by constitutive
P53 expression and upregulation of pro-apoptotic P53
target genes belonging to the BCL-2 family. Although apoptosis
sensitivity in hiPSCs is elevated, DNA lesions following genotoxic
treatment have been demonstrated to be less common in hiPSCs
compared with PHDFs, partially due to increased expression of genes
that code for antioxidant proteins (41). Momcilovic et al (42) performed a comprehensive
investigation to assess the radiosensitivity of hESCs and hiPSCs,
which confirmed that both irradiated pluripotent SC lines exhibited
robust expression of pluripotency markers. Subsequently, it was
demonstrated that the activation level of ATM and its target
proteins in hiPSCs are similar to results obtained in hESCs.
Similar to hESCs, hiPSC differentiation was arrested in the
G2 phase, confirming that the reprogramming process
leads to loss of G1/S arrest, thus, preserving cells
from differentiation. Finally, it was demonstrated that hESCs and
hiPSCs repair DNA lesions predominantly via HR. However, in
addition to elevated expression of genes engaged in HR, hESCs and
hiPSCs also exhibited increased expression of genes involved in
non-homologous end joining (NHEJ), which is the opposite to what
occurs in mESCs. The present study partially expands and updates
the results reported by Momcilovic et al (42) as a wider range of IR doses (0–15
Gy) were employed. The present study demonstrated that, although
P53 was highly expressed by hESCs, its expression was
significantly lower in hiPSCs and PHDFs. These results are
consistent with data from hESCs, which exhibited the largest number
of DSBs (Fig. 5A). This is
consistent with previously published data, indicating that
P53 is highly activated in response to DNA damage. This
phenomenon may also reflect a more effective pluripotency state of
hESCs compared with hiPSCs, demonstrating the important role of
P53 in maintaining pluripotency.
Homology-dependent and independent DNA repair
pathways are involved in the protection of cells against IR-induced
DNA damage and consequent chromosomal changes (43). For mESCs, the homology-dependent
mechanism is particularly important. Unlike MEFs, ESCs have the
capacity to repair endogenous DNA damage without the activation of
NHEJ. Tichy et al (44)
demonstrated that mESCs translate RAD51 mRNA, a major
component of HR, with higher efficacy compared with MEFs. Excessive
RAD51 expression is likely to be a major mechanism by which
cells respond to DSBs or delay the replication fork without
affecting cell proliferation rate. Sioftanos et al (45) demonstrated that, in mESCs,
BRCA1 and BRCA2 heterozygosity is associated with the
formation of low numbers of RAD51 foci per nucleus, however,
the general radiosensitivity of these cells was not altered.
However, the authors, do not rule out the potential emergence of
late effects such as enhanced mutagenesis.
Notably, expression levels of genes involved in the
NHEJ mechanism vary substantially between mESCs and hESCs.
Ku70/80 is expressed at higher levels in mESCs
compared with hESCs and somatic cells. However, mESCs are also
characterized by a deficiency in PRKDC. Therefore, following IR,
mESCs and hESCs differ in the extent of DNA damage response; hESCs
rejoin breaks rapidly, via the NHEJ mechanism, thus, expressing a
high level of PRKDC, whereas mESCs favor HR for DSB repair
(46). The pathways that maintain
genetic stability exhibit enhanced activity in hESCs compared with
PHDFs and formed EBs. In response to DNA-damaging agents, which
includes IR (47), the mRNA levels
of certain DNA repair genes are elevated, including BRCA1,
xeroderma pigmentosum type B, XRCC4 and Ku80.
However, the mRNA levels of xeroderma pigmentosum type A,
xeroderma pigmentosum type C and xeroderma pigmentosum
type G are notably decreased. Felgentreff et al
(48) reported that the NHEJ
pathway has a critical role in somatic cell reprogramming and in
maintaining the genomic stability of hiPSCs. Particularly, it was
demonstrated that DNA ligase 4 and PRKDC are required for
the correct functioning of the NHEJ mechanism and high
reprogramming efficiency (48).
The results of the present study confirm previous reports that
indicated that pluripotent SCs more effectively activate certain
DNA repair genes (RAD51, BRCA2, XRCC4 and
PRKDC) following IR compared with PHDFs. hiPSCs are
characterized by high BRCA2, XRCC4 and PRKCD
gene expression, whereas hESCs exhibit higher expression of
RAD51 and PRKDC (Fig.
5). Although HR is characteristic for pluripotent SCs as a
major DSB repair mechanism, SCs exhibit a capacity to induce
components of NHEJ. Differences between cell lines in terms of
expression of DNA repair genes also resulted in a distinct gene
expression profile for each line (Fig
6). The initial response of cells changed in a dose-dependent
manner, however, in the majority of cases, significant changes were
not observed until 2 Gy, indicating that this dose may be the
threshold dose for initiating DDR. The results presented in this
study indicate that, although pluripotent SCs possess similar
features, including self-renewal and a capacity to differentiate
into derivatives of 3 germ layers, they also possess highly
dissimilar radiosensitivity and, consequently, genome integrity
machinery.
In conclusion, the present study has described the
early IR-induced response of hESCs, hiPSCs and PHDFs. Pluripotent
SCs form an increased number of DSBs following IR treatment
compared with PHDFs. Notably, hESCs are extremely sensitive to IR,
which was indicated by a high number of DSBs. The level of DSB
formation in hiPSCs in response to IR resembles that observed in
PHDFs rather than in hESCs. The accumulation of DSBs results in a
distinct gene expression profile of activated DNA repair genes in
each cell line. Irradiated pluripotent SCs readily activate genes
belonging to HR and NHEJ, indicating that they possess effective
DNA repair mechanisms, in contrast to the genes that are activated
by fully differentiated cells. The present study contributes to an
improved understanding of the induction of DNA damage repair
mechanisms in human pluripotent SCs in response to IR. The results
of the current study may contribute to safer clinical application
of pluripotent SCs in patients with a high risk of developing
cancer. In addition, the present study emphasizes the questions
surrounding the reprogramming process in the evaluation of
activated DDR mechanisms. However, given the preliminary nature of
the current study, more research is required to reach definitive
conclusions.
Acknowledgements
Funding for the present study was supported by the
National Science Centre (grant no. 2012/07/E/NZ3/01819) and by The
Greater Cancer Centre (grant no. 19/02/2016/PRB/WCO/010). The
authors would like to thank Bradley Londres for his assistance in
editing this study.
Glossary
Abbreviations
Abbreviations:
|
SCs
|
stem cells
|
|
hESCs
|
human embryonic stem cells
|
|
hiPSCs
|
human induced pluripotent stem
cells
|
|
PHDFs
|
primary human dermal fibroblasts
|
|
IR
|
ionizing radiation
|
|
DSBs
|
double strand breaks
|
|
P53
|
tumor suppressor protein P53
|
|
RAD51
|
RAD51 recombinase
|
|
BRCA2
|
breast cancer 2
|
|
XRCC4
|
X-ray repair complementing defective
repair in Chinese hamster cells 4
|
|
PRKDC
|
DNA-dependent protein kinase
catalytic subunit
|
References
|
1
|
Suchorska WM, Lach MS, Richter M,
Kaczmarczyk J and Trzeciak T: Bioimaging: An useful tool to monitor
differentiation of human embryonic stem cells into chondrocytes.
Ann Biomed Eng. 44:1845–1859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Augustyniak E, Trzeciak T, Richter M,
Kaczmarczyk J and Suchorska W: The role of growth factors in stem
cell-directed chondrogenesis: A real hope for damaged cartilage
regeneration. Int Orthop. 39:995–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen MF, Lin CT, Chen WC, Yang CT, Chen
CC, Liao SK, Liu JM, Lu CH and Lee KD: The sensitivity of human
mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol
Biol Phys. 66:244–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin YW, Na YJ, Lee YJ, An S, Lee JE, Jung
M, Kim H, Nam SY, Kim CS, Yang KH, et al: Comprehensive analysis of
time- and dose-dependent patterns of gene expression in a human
mesenchymal stem cell line exposed to low-dose ionizing radiation.
Oncol Rep. 19:135–144. 2008.PubMed/NCBI
|
|
5
|
Zaman MH: The role of engineering
aproaches in analysis cancer invasion and metastasis. Nat Rev
Cancer. 13:596–603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Magalhães JP: How ageing processess
influence cancer. Nat Rev Cancer. 13:357–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sokolov MV and Neumann RD: Human embryonic
stem cell responses to ionizing radiation exposures: Current state
of knowledge and future challenges. Stem Cells Int.
2012:5791042012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suchorska WM, Augustyniak E and Łukjanow
M: Genetic stability of pluripotent stem cells during anti-cancer
therapies. Exp Ther Med. 11:695–702. 2016.PubMed/NCBI
|
|
9
|
Soldner F, Hockemeyer D, Beard C, Gao Q,
Bell GW, Cook EG, Hargus G, Blak A, Cooper O and Mitalipova M:
Parkinson's disease patient-derived induced pluripotent stem cells
free of viral reprogramming factor. Cell. 136:964–977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fung H and Weinstock DM: Repair at single
targeted DNA double-strand breaks in pluripotent and differentiated
human cells. PLoS One. 6:e205142011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rocha CR, Lerner LK and Okamoto OK: The
role of DNA repair in the pluripotency and differentiation of human
stem cells. Mutat Res. 752:25–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long XH, Zhao ZQ, He XP, Wang HP, Xu QZ,
An J, Bai B, Sui JL and Zhou PK: Dose-dependent expression changes
of early response genes to ionizing radiation in human
lymphoblastoid cells. Int J Mol Med. 19:607–615. 2007.PubMed/NCBI
|
|
13
|
Wróblewska J: A new method to generate
human induced pluripotent stem cells (iPS) and the role of the
protein KAP1 in epigenetic regulation of self-renewal. Doctoral
dissertation. http://www.wbc.poznan.pl/Content/373798/index.pdf2015.
|
|
14
|
Banáth JP, MacPhail SH and Olive PL:
Radiation sensitivity, H2AX phosphorylation and kinetics of repair
of DNA strand breaks in irradiated cervical cancer cell lines.
Cancer Res. 64:7144–7149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An J, Huang YC, Xu QZ, Zhou LJ, Shang ZF,
Huang B, Wang Y, Liu XD, Wu DC and Zhou PK: DNA-PKcs plays a
dominant role in the regulation of H2AX phosphorylation in response
to DNA damage and cell cycle progression. BMC Mol Biol. 11:182010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scarpanto E, Castagna S, Aliotta R, Azzarà
A, Ghetti F, Filomeni E, Giovannini C, Pirillo C, Testi S, Lombardi
S and Tomei A: Kinetics of nuclear phosphorylation (γ-H2AX) in
human lymphocytes treated in vitro with UVB, bleomycin and
mitomycin C. Mutagenesis. 28:465–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mah LJ, Vasireddy RS, Tang MM, Georgiadis
GT, El-Osta A and Karagiannis TC: Quantification of gammaH2AX foci
in response to ionising radiation. J Vis Exp. pii:19572010.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong J, Todorova D, Su NY, Kim J, Lee PJ,
Shen Z, Briggs SP and Xu Y: Stemness factor Sall4 is required for
DNA damage response in embryonic stem cells. J Cell Biol.
208:513–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koledova Z, Kafkova LR, Krämer A and
Divoky V: DNA damage-induced degradation of Cdc25A does not lead to
inhibition of Cdk2 activity in mouse embryonic stem cells. Stem
Cells. 28:450–461. 2010.PubMed/NCBI
|
|
21
|
Banáth JP, Bañuelos CA, Klokov D, MacPhail
SM, Lansdorp PM and Olive PL: Explanation for excessive DNA
single-strand breaks and endogenous repair foci in pluripotent
mouse embryonic stem cells. Exp Cell Res. 315:1505–1520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sokolov MV, Panyutin IV, Onyshchenko MI,
Panyutin IG and Neumann RD: Expression of pluripotency-associated
genes in the surviving fraction of cultured human embryonic stem
cells is not significantly affected by ionizing radiation. Gene.
455:8–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Momčilovic O, Choi S, Varum S, Bakkenist
C, Schatten G and Navara C: Ionizing radiation induces ataxia
telangiectasia mutated-dependent checkpoint signaling and G(2) but
not G(1) cell cycle arrest in pluripotent human embryonic stem
cells. Stem Cells. 27:1822–1835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filion TM, Qiao M, Ghule PN, Mandeville M,
van Wijnen AJ, Stein JL, Lian JB, Altieri DC and Stein GS: Survival
responses of human embryonic stem cells to DNA damage. J Cell
Physiol. 220:586–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neganova I, Vilella F, Atkinson SP, Lloret
M, Passos JF, von Zglinicki T, O'Connor JE, Burks D, Jones R,
Armstrong L and Lako M: An important role for CDK2 in G1 to S
checkpoint activation and DNA damage response in human embryonic
stem cells. Stem Cells. 29:651–659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi N, Monzen S, Ito K, Fujioka T,
Nakamura Y and Kashiwakura I: Effects of ionizing radiation on
proliferation and differentiation of mouse induced pluripotent stem
cells. J Radiat Res. 53:195–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagaria PK, Robert C, Park TS, Huo JS,
Zambidis ET and Rassool FV: High-fidelity reprogrammed human IPSCs
have a high efficacy of DNA repair and resemble hESCs in their MYC
transcriptional signature. Stem Cell Int. 2016:38262492016.
|
|
28
|
Han MK, Song EK, Guo Y, Ou X, Mantel C and
Broxmeyer HE: SIRT1 regulates apoptosis and Nanog expression in
mouse embryonic stem cells by controlling p53 subcellular
localization. Cell Stem Cell. 2:241–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chuykin IA, Lianguzova MS, Pospelova TV
and Pospelov VA: Activation of DNA damage response signaling in
mouse embryonic stem cells. Cell Cycle. 7:2922–2928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KH, Li M, Michalowski AM, Zhang X,
Liao H, Chen L, Xu Y, Wu X and Huang J: A genomwide study
identifies the Wnt signaling pathway as a major target of p53 in
murine embryonic stem cells. Proc Natl Acad Sci USA. 107:69–74.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abdelalim EM and Tooyama I: Knockdown of
p53 suppressess Nanog expression in embryonic stem cells. Biochem
Biophys Res Commun. 443:652–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Solozobova V, Rolletschek A and Blattner
C: Nuclear accumulation and activation of p53 in embryonic stem
cells after DNA damage. BMC Cell Biol. 10:462009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dolezalova D, Mraz M, Barta T, Plevova K,
Vinarsky V, Holubcova Z, Jaros J, Dvorak P, Pospisilova S and Hampl
A: MicroRNAs regulate p21(Waf1/Cip1) protein expression and the DNA
damage response in human ebryonic stem cells. Stem Cells.
30:1362–1372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Solozobova V and Blattner C: Regulation of
p53 in embryonic stem cells. Exp Cell Res. 316:2434–2446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J,
Li J, Song Z, Qu X, Zhou P, et al: Regulation of apoptosis and
differentiation by p53 in human embryonic stem cells. J Biol Chem.
282:5842–5852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilson KD, Sun H, Huang M, Zhang WY, Lee
AS, Li Z, Wang SX and Wu JC: Effects of ionizing radiation on
self-renewal and pluripotency of human embryonic stem cells. Cancer
Res. 70:5539–5558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akdemir KC, Jain AK, Allton K, Aronow B,
Xu X, Cooney AJ, Li W and Barton MC: Genome-wide profiling reveals
stimulus-specific functions of p53 during differentiation and DNA
damage of human embryonic stem cells. Nucleic Acid Research.
42:205–223. 2014. View Article : Google Scholar
|
|
38
|
Sokolov M and Neumann R: Effects of low
doses of ionizing radiation exposures on stress-responsive gene
expression in human embryonic stem cells. Int J Mol Sci.
15:588–604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sokolov M, Nguyen V and Neumann R:
Comparative analysis of whole-genome gene expression changes in
cultured human embryonic stem cells in response to low, clinical
diagnostic relevant and high doses of ionizing radiation exposure.
Int J Mol Sci. 16:14737–14748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rasmussen MA, Holst B, Tümer Z, Johnsen
MG, Zhou S, Stummann TC, Hyttel P and Clausen C: Transient p53
suppression increases reprogramming of human fibroblasts without
affecting apoptosis and DNA damage. Stem Cell Reports. 3:404–413.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dannenmann B, Lehle S, Hildebrand DG,
Kübler A, Grondona P, Schmid V, Holzer K, Fröschl M, Essmann F,
Rothfuss O and Schulze-Osthoff K: High glutathione and glutathione
preoxidase-2 levels mediate cell-type-specific DNA damage
protection in human induced pluripotent stem cells. Stem Cell
Reports. 4:886–898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Momcilovic O, Knobloch L, Fornsaqlio J,
Varum S, Easley C and Schatten G: DNA damage responses in human
induced pluripotent stem cells and embryonic stem cells. PLoS One.
5:e134102010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Griffin C, Waard Hd, Deans B and Thacker
J: The involvement of key DNA repair pathways in the formation of
chromosome rearrangements in embryonic stem cells. DNA Repair
(Amst). 4:1019–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tichy ED, Pillai R, Deng L, Tischfield JA,
Hexley P, Babcock GF and Stambrook PJ: The abundance of Rad51
protein in mouse embryonic stem cells is regulated at multiple
levels. Stem Cell Res. 9:124–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sioftanos G, Ismail A, Föhse L, Shanley S,
Worku M and Short SC: BRCA1 and BRCA2 heterozygosity in embryonic
stem cells reduces radiation-induced Rad51 focus formation but is
not associated with radiosensitivity. Int J Radiat Biol.
86:1095–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bañuelos CA, Banáth JP, MacPhail SH, Zhao
J, Eaves CA, O'Connor MD, Lansdorp PM and Olive PL: Mouse but not
human embryonic stem cells are deficient in rejoining of ionizing
radiation- induced DNA double-strand breaks. DNA Repair (Amst).
7:1471–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maynard S, Swistowska AM, Lee JW, Liu Y,
Liu ST, Da Cruz AB, Rao M, de S, ouza-Pinto NC, Zeng X and Bohr VA:
Human embryonic stem cells have enhanced of multiple forms of DNA
damage. Stem Cells. 26:2266–2274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Felgentreff K, Du L, Weinacht KG, Dobbs K,
Bartish M, Giliani S, Schlaeger T, DeVine A, Schambach A, Woodbine
LJ, et al: Differential role of nonhomologous end joining factors
in the generation, DNA damage response and myeloid differentiation
of human induced pluripotent stem cells. Proc Natl Acad Sci USA.
111:8889–8894. 2014. View Article : Google Scholar : PubMed/NCBI
|