Introduction
Oral squamous cell carcinoma (OSCC) accounts for
>90% of head and neck carcinomas; and, of these 90%, OSCCs of
the tongue (TSCC) are reported to occur with rates of up to 40–50%
(1). In order to enhance patient
survival following TSCC, surgical techniques and diagnostic
accuracy have improved; however, treatment failure persists due to
local recurrence, and regional lymph node and distant metastases
are common. It is well known that treatment failure may be
associated with cancer stem cells (CSCs) or ‘tumor-initiating
cells’ (2–4), which exhibit long-term self-renewal
and a high migratory capacity, as well as the ability to generate
phenotypically diverse tumor cells (5). This behavior is explained by the
‘cancer stem cell’ theory (6),
which is a basis for oncology research (4,7,8).
Previous studies in OSCC-derived cell lines have indicated that
cell subpopulations with phenotypic and behavioral characteristics
of normal epithelial stem cells may initiate tumorigenesis in
vivo (9,10). CSCs rarely divide; however, they
can produce fast-proliferating daughter cells. The majority of CSCs
in various types of cancer have been isolated from tumor cells
based on marker expression that characterizes stem cells in normal
tissues (11). However, few
studies have focused on the expression and function of a reliable
marker to identify TSCC stem cells; therefore, at present, there is
little understanding regarding their behavior and fate.
It has previously been reported that the p75
neurotrophin receptor (p75NTR) may be involved in the
invasion and poor prognosis of OSCC (12). As a member of the tumor necrosis
factor superfamily, p75NTR is a 75-kDa cell-surface
receptor glycoprotein (13,14),
which is involved in diverse cellular responses, including cell
proliferation and survival, and apoptosis in neural and non-neural
tissues (15,16) via unique pathways (17,18)
or activation of the intrinsic caspase pathway (19). Furthermore, the expression and
diverse function of p75NTR has previously been reported
in numerous types of cancer (20–22).
Okumura et al (23)
reported that p75NTR+ esophageal epithelial cells were
actually stem cells, since they were able to proliferate,
self-renew and undergo multidirectional differentiation. In
addition, p75NTR has been used to screen and identify
mouse testis peritubular smooth muscle precursors (24), rat adipose multipotent stem cells
(25) and human corneal epithelial
progenitor cells (26).
The present study detected p75NTR
expression in Tca-8113 and CAL-27 TSCC cell lines, and noted that
p75NTR+ TSCCs exhibited CSC properties, particularly
with regards to self-renewal and proliferation, multidirectional
differentiation, and strong in vivo tumorigenic
capacity.
Materials and methods
Cell source and culture
conditions
Tca-8113 and CAL-27 TSCC cell lines were kindly
provided by the Shanghai Key Laboratory of Stomatology (Department
of Oral and Maxillofacial-Head Neck Oncology, Ninth People's
Hospital, Shanghai Jiao Tong University School of Medicine,
Shanghai, China). The cell lines were originally purchased from the
Shanghai Cell Biology Institute of the Chinese Academy of Sciences
(Shanghai, China).
Tca-8113 cells were cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Chalfont, UK), 100 IU/ml penicillin and
100 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). CAL-27 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; HyClone; GE Healthcare Life Sciences) supplemented
with 10% (v/v) FBS, 100 IU/ml penicillin and 100 mg/ml
streptomycin. All cell cultures were maintained in a humidified
incubator containing 5% CO2/95% air at 37°C.
Flow cytometry and
fluorescence-activated cell sorting (FACS)
Tumor cells were harvested (final concentration,
1×106 cells/ml) with Buffer 1 (PBS containing 0.5%
bovine serum albumin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) and 2 mM EDTA). Cells were then incubated with the primary
antibody for 2 h at 4°C, washed twice in Buffer 1, and were
resuspended in 500 µl Buffer 1, to which phycoerythrin
(PE)-conjugated goat anti-mouse immunoglobulin G at a dilution of
1:100 (cat. no. 555749; BD Pharmingen, San Diego, CA, USA) was
added. Cells were incubated in the dark for 15 min at 4°C. After
staining, the samples were analyzed using a FACSCalibur flow
cytometer with CellQuest software (version 5.1; BD Biosciences, San
Jose, CA, USA). The primary antibody used was mouse anti-human
p75NTR at a dilution of 1:100 (cat. no. 557196; BD
Pharmingen). FACS of p75NTR+ cells was performed using a
Cytomation MoFlo® cytometer (Dako; Agilent Technologies,
Santa Clara, CA, USA). The top 25% most brightly stained cells were
isolated as p75NTR+ cells; cells incubated with
PE-conjugated antibodies only were used as controls.
Colony formation assay
p75NTR+ single cell suspensions were
prepared, diluted, and plated into a 96-well plate at various
densities (1×106/ml; 1×105/ml;
1×104/ml; 1×103/ml; 1×102/ml)
(27). Cells were allowed 2 weeks
to form colonies under standard conditions, and the rate at which
this occurred was recorded. To assess p75NTR+
differentiation, colonies formed by one cell type were collected
and incubated for another 2 weeks for p75NTR+ flow
cytometric analysis.
MTT cell viability assay
Briefly, sorted p75NTR+ cells, and
non-sorted Tca-8113 and CAL-27 cells (4×103 cells/well)
were seeded in 96-well plates. After 1, 2, 3, 4, 5, 6 or 7 days,
100 µl MTT (5 mg/ml) was added, followed by incubation for a
further 4 h at 37°C. The reaction was terminated by replacing
MTT-containing medium with 100 µl acidic isopropanol (10% SDS, 5%
isopropanol, 0.01 mol/l HCl); the resulting formazan crystals were
dissolved by gentle agitation for ~10 min at room temperature. For
colorimetric analysis, absorbance (490 nm) was measured on a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The optical density (OD) values were analyzed using Quantity One
analysis software (version 29.0; Bio-Rad Laboratories, Inc.). Each
assay was repeated at least three times. Relative cell viability
was compared to untreated (blanks) cells.
Scratch assay
Live tumor cells (Tca-8113 and CAL-27) were
harvested from standard cultures and grown to confluence on 6-well
Permanox™ plates. Consistently shaped wounds were made using a
sterile 200 µl pipette tip across each well, creating a cell-free
area, according to previously described methods (28). Cultures were gently washed with PBS
to remove loose cells and adherent cells were maintained in culture
medium supplemented with 1% FBS. Non sorted control cells were also
scratched, washed, and maintained in culture medium supplemented
with 1% FBS after scratching. Immediately after scratching and at
12 h, 3, 4, 5 and 8 d, images of the scraped areas were captured
under phase contrast microscopy. The wounded areas and scratch
widths were measured at six different points per image, and the
same scratched area was used for every assessment.
Xenograft tumorigenicity assay
Throughout experiments, animals were maintained
under the Guidelines for Animal Experimentation of the School and
Hospital of Stomatology, Shandong University (Jinan, China).
Experiments were conducted according to the National Institute of
Health Guidelines for Research Involving Recombinant or Synthetic
Nucleic Acid Molecules (November, 2013) the (regarding the care and
use of animals for experimental procedures. In addition, the
present study was independently reviewed and approved by the Animal
Experimentation Committee of Shandong University, and was approved
by the Medical Ethics Committee of the School of Medicine, Shandong
University.
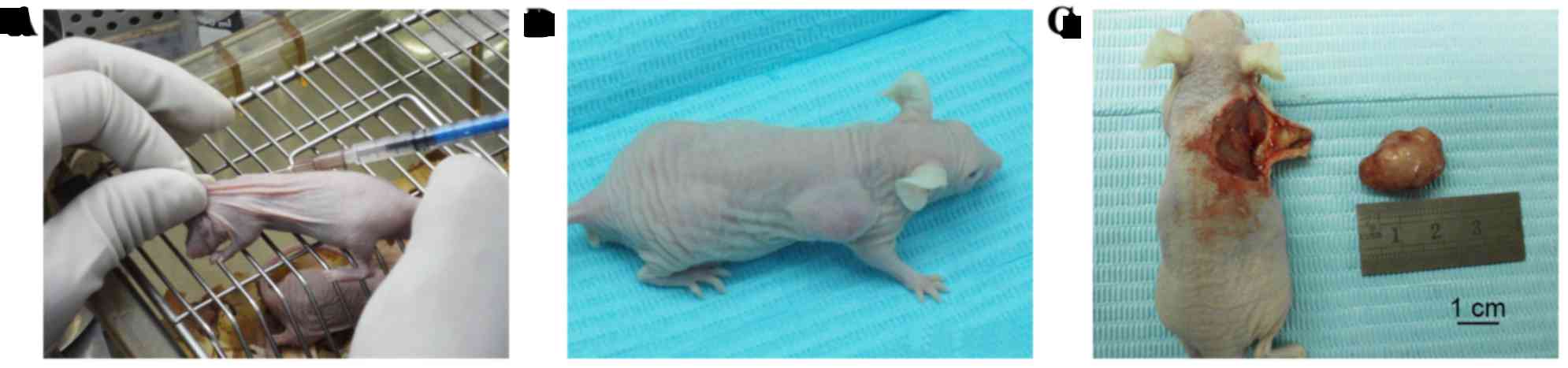
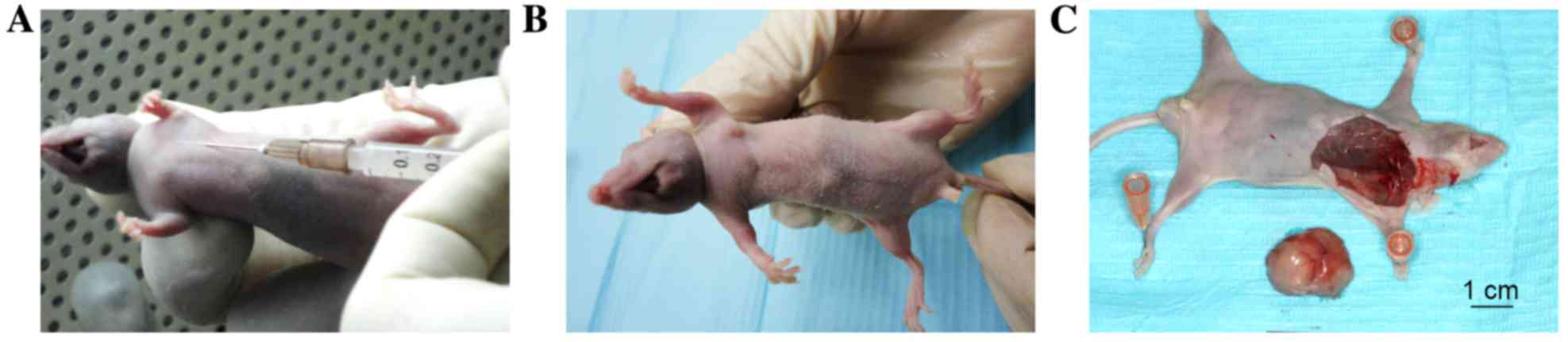
Briefly, 5–6 week-old male BALB/c nude mice (~18±1.2
g; n=106) were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China), and were maintained in
plastic cages (n=3–5/cage) under standard laboratory conditions
with a 12 h dark, 12 h light cycle and a constant temperature of
23°C and humidity of 48%. All mice were fed a standard rodent diet
ad libitum. After 1 week of acclimation, mice were randomly
divided into four groups (named as follows: 75NTR+,
Tca-8113 cells, non-sorted Tca-8113 cells, p75NTR+
CAL-27 cells and non-sorted CAL-27 cells injection; n=24/group) and
each group was subdivided into four subgroups (n=6/subgroup). Four
cell suspensions of p75NTR+ and non-sorted cells were
prepared (1.0×103/ml, 1.0×104/ml,
1.0×105/ml and 1.0×106/ml) in 200 µl
serum-free DMEM. These suspensions were injected subcutaneously
into BALB/c nude mice, following anesthetization with 10% chloral
hydrate (400 mg/100 g body weight) at room temperature. Tca-8113
cells were injected into the backs of the mice (Fig. 1), whereas CAL-27 cells were
injected into the axilla of the mice (Fig. 2). Sterile PBS was injected into the
contralateral side as a control.
At the end of the experiment, the mice were
anesthetized as aforementioned (10% chloral hydrate, 400 mg/100 g)
and subjected to transcardial perfusion with a fixative of 4%
paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) 6 weeks
following tumor cell inoculation. Subsequently, tumors were removed
and immersed in the same fixative for an additional 24 h at 4°C.
Samples were dehydrated with graded ethanol and were embedded in
paraffin according to standard procedures. Subsequently, 5-µm
serial sections were prepared for histological hematoxylin and
eosin (H&E) staining (LEICA SM 2010R; Leica Microsystems GmbH,
Wetzlar, Germany).
Histological examination and image
analysis
H&E staining was performed to investigate the
morphology. The prepared sections were immersed in Erthlich's
haematoxylin for 15 min. Then the sections were washed with
distilled water and differentiated in 1% HCl in 70% alcohol for 1
min and washed again for 2 min. After that, the sections were
stained with 1% eosin for 10 min and washed with distilled water.
Finally, all sections were dehydrated and mounted. The stained
sections were observed and then digital images were taken with a
light microscope (Olympus BX-53; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
For comparing distant metastases between
p75NTR+ and negative groups, a χ2 test was
performed. Fisher's exact test was used to compare tumorigenicity
between p75NTR+ and negative groups. Other experimental
data are presented as the mean ± standard deviation and were
analyzed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were conducted using SPSS 19.0 software (SPSS Inc.,
Chicago, IL, USA).
Results
Flow cytometry
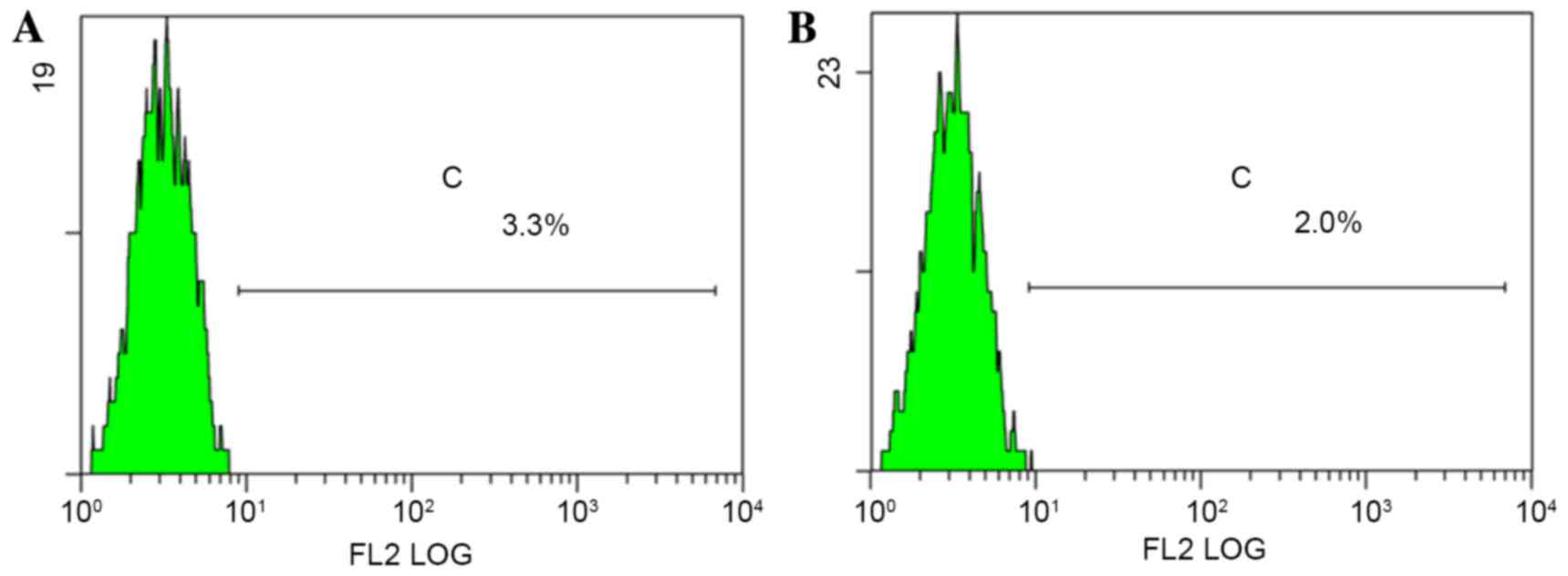
p75NTR+ cells were detected in both TSCC
cell lines (Fig. 3).
p75NTR+ cells accounted for 3.1 and 1.9% of Tca-8113 and
CAL-27 cells, respectively (an average of three experiments). After
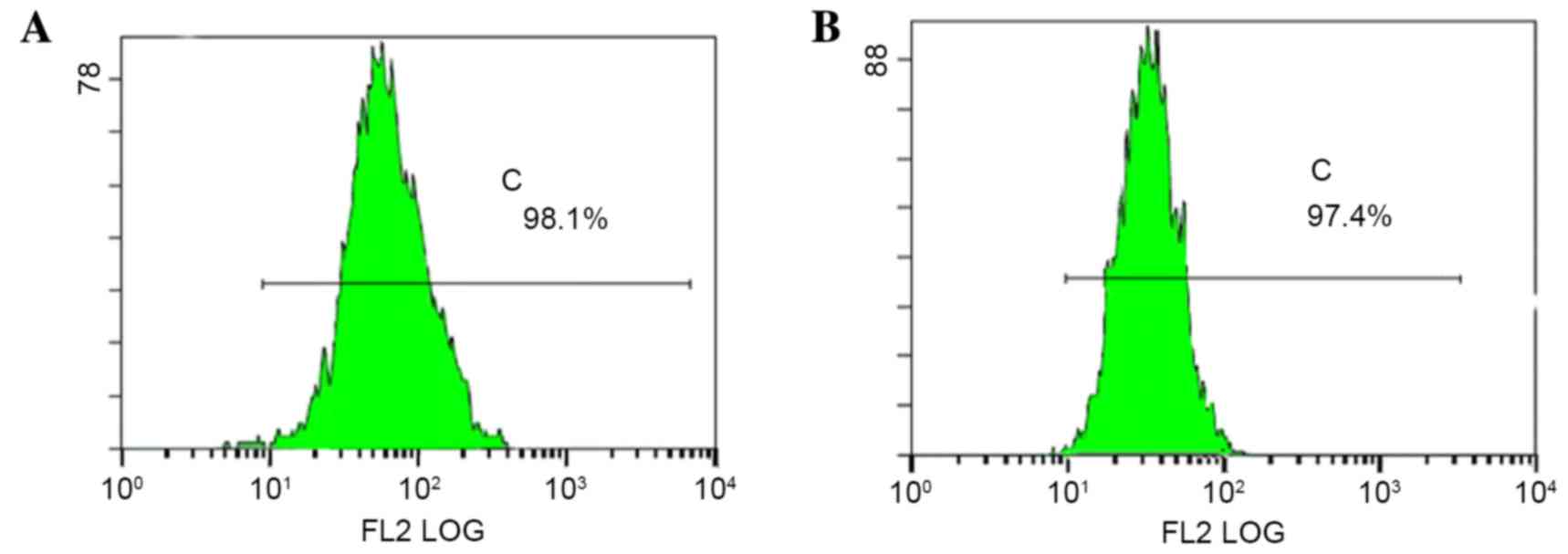
cell sorting, p75NTR+ cells accounted for 98.1
(Tca-8113) and 97.4% (CAL-27) of all sorted cells (Fig. 4).
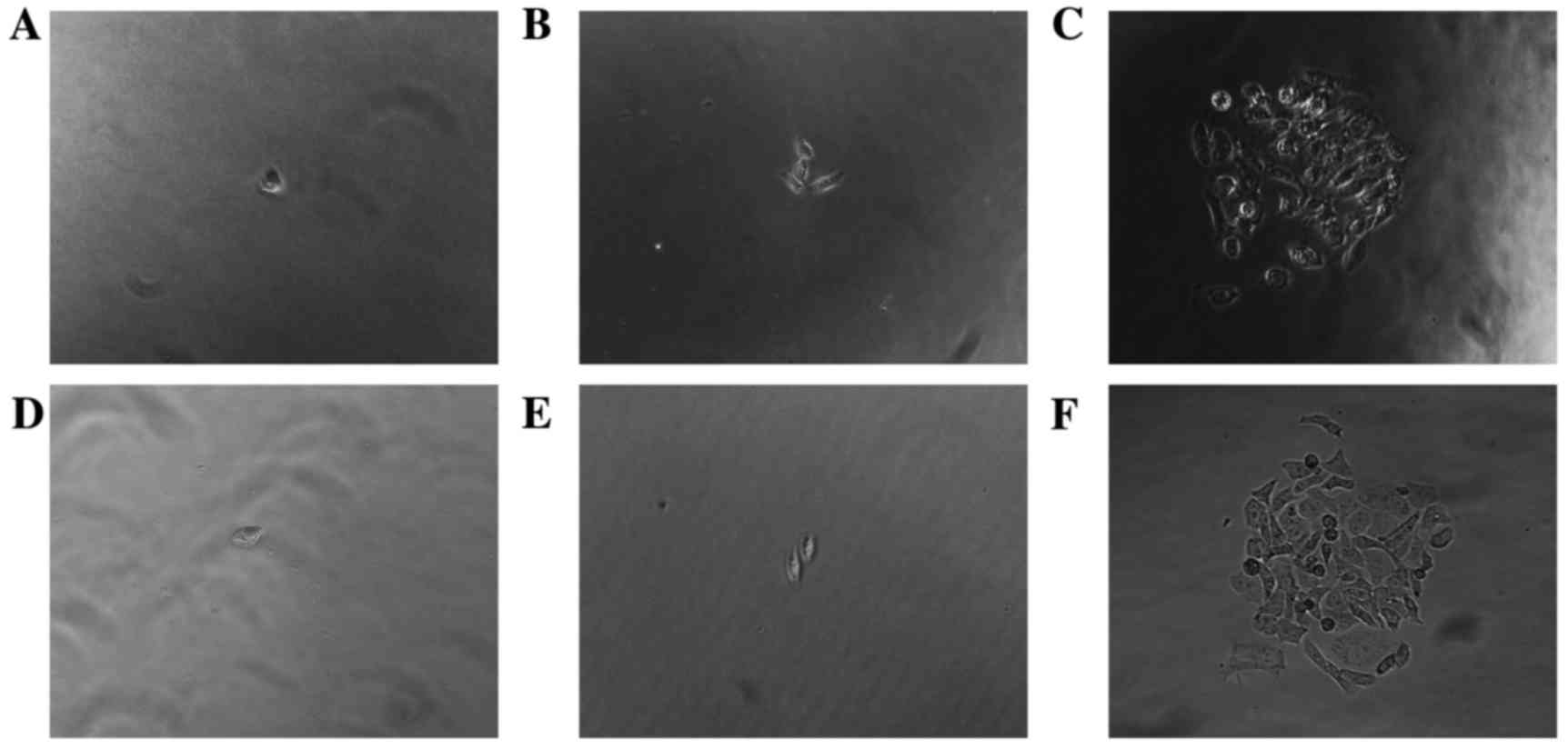
Colony formation assay
The colony-forming ability of p75NTR+,
and non-sorted Tca-8113 and CAL-27 TSCC cells (Fig. 5) was assessed. The number of
colonies formed by p75NTR+ cells from each cell line was
greater compared with the number formed by non-sorted TSCC cells
(Table I). Following 2 weeks of
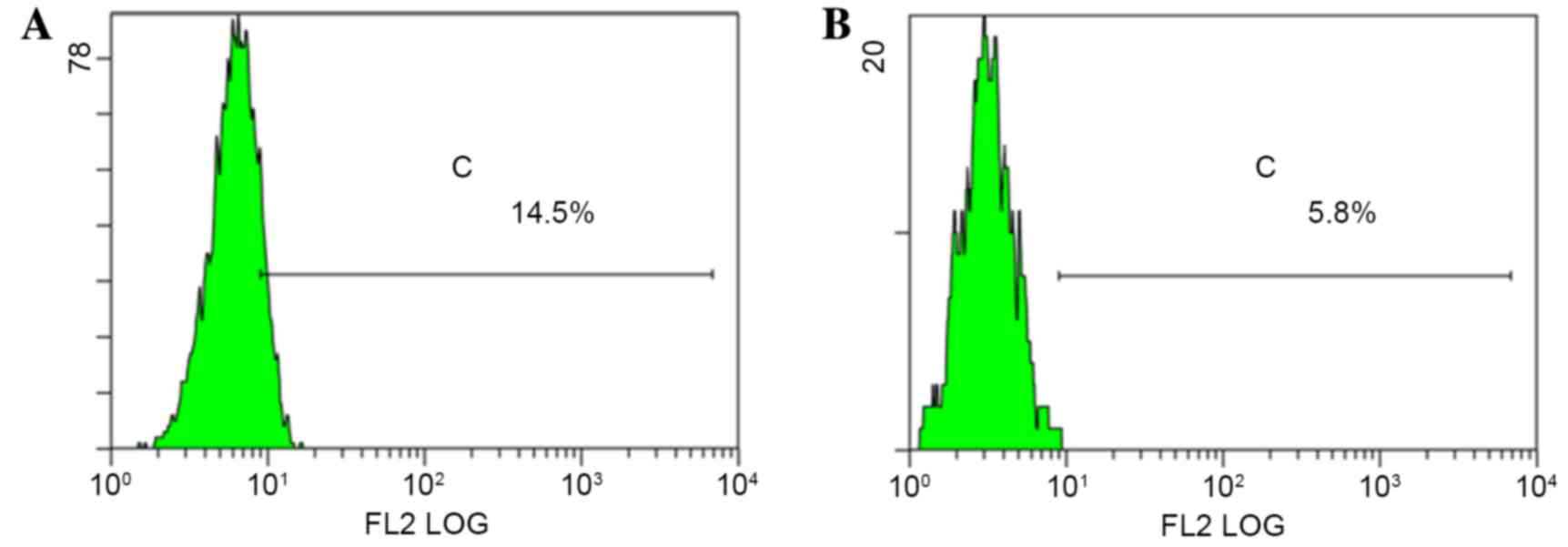
culture, monoclonal p75NTR+ cells were collected and
cultured for a further 2 weeks, after which they were analyzed by
flow cytometry. Data indicated that the colonies contained both
p75NTR+ and p75NTR− cells. The proportion of
p75NTR+ cells in the Tca-8113 and CAL-27 cell
populations was 14.5 and 5.8%, respectively (Fig. 6). These results indicated that p75
NTR+ cells exhibit self-renewing and multidirectional
differentiation properties.
 | Table I.Colony formation between
p75NTR+ and non-sorted cells. |
Table I.
Colony formation between
p75NTR+ and non-sorted cells.
| Cell line | p75NTR+
cell colony formation (%) | Non-sorted cell
colony formation (%) | P-value |
|---|
| Tca-8113 | 32.92±1.91 | 7.09±1.20 | P=0.0001 |
| CAL-27 | 34.96±2.03 | 5.54±2.16 | P=0.0001 |
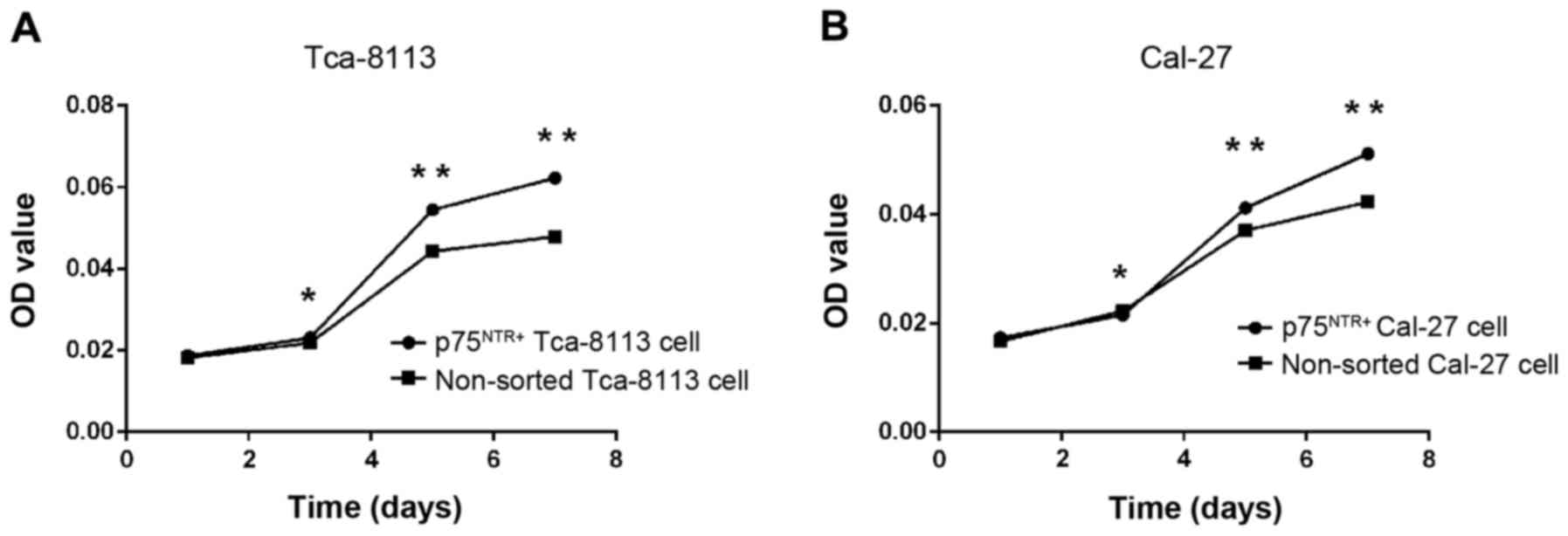
MTT cell viability assay
To determine the proliferative ability of
p75NTR+ and non-sorted cells in vitro, an MTT
assay was conducted. The results revealed no differences in
proliferative ability, according to OD values, on the first day
(Fig. 7). After 3 days of culture,
OD values for the p75NTR+ cells were greater compared
with the non-sorted cells (P<0.05), indicating a stronger
proliferative ability of p75NTR+ cells in vitro.
Furthermore, proliferation was greater on days 5 and 7 (P<0.01;
Fig. 7).
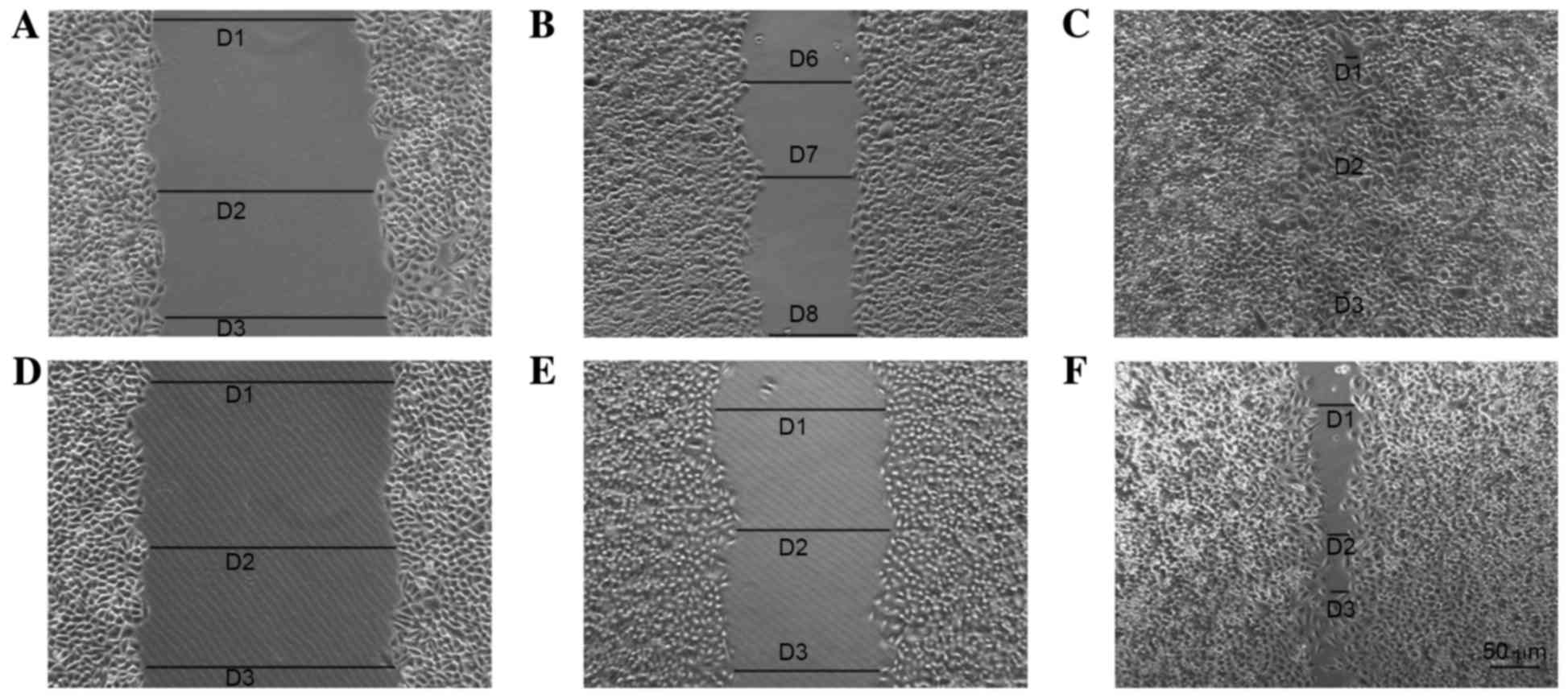
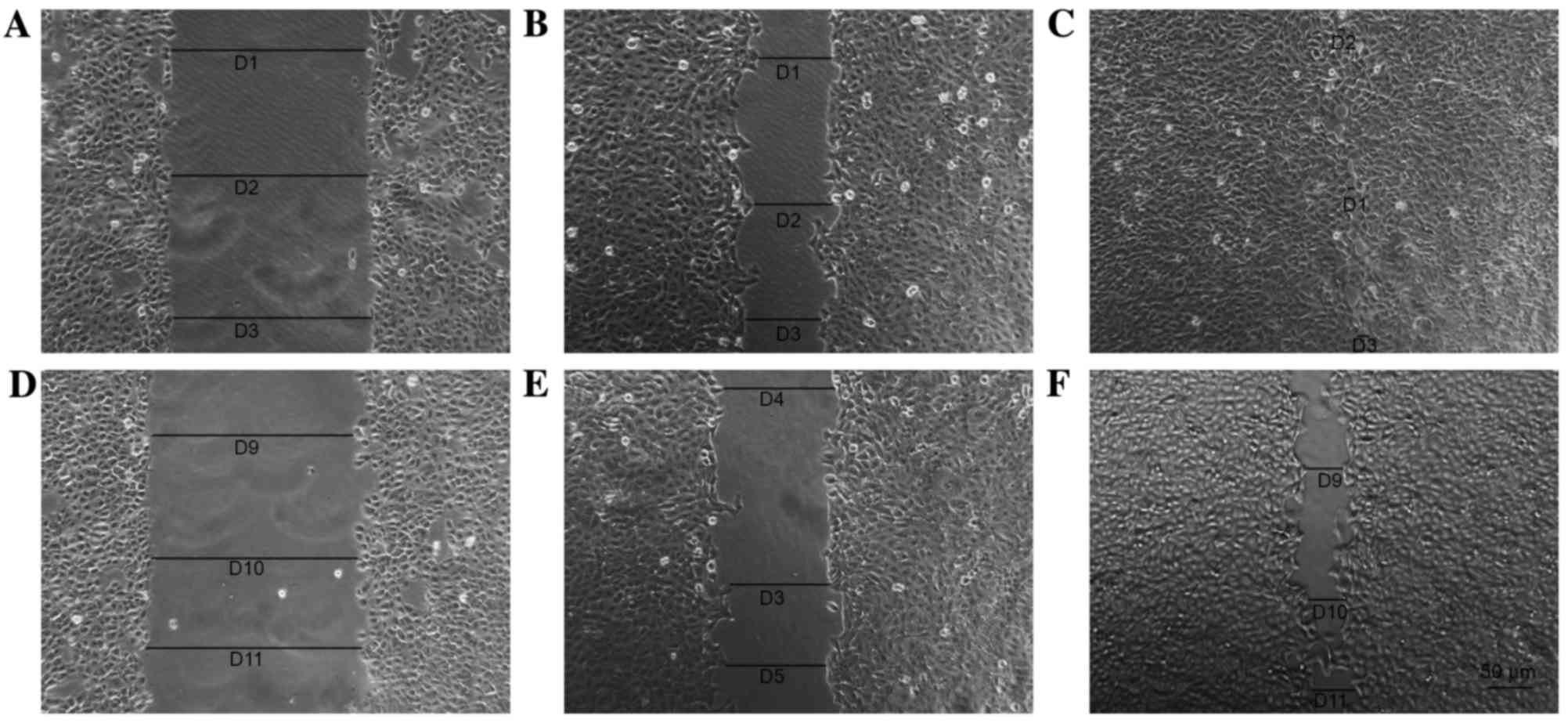
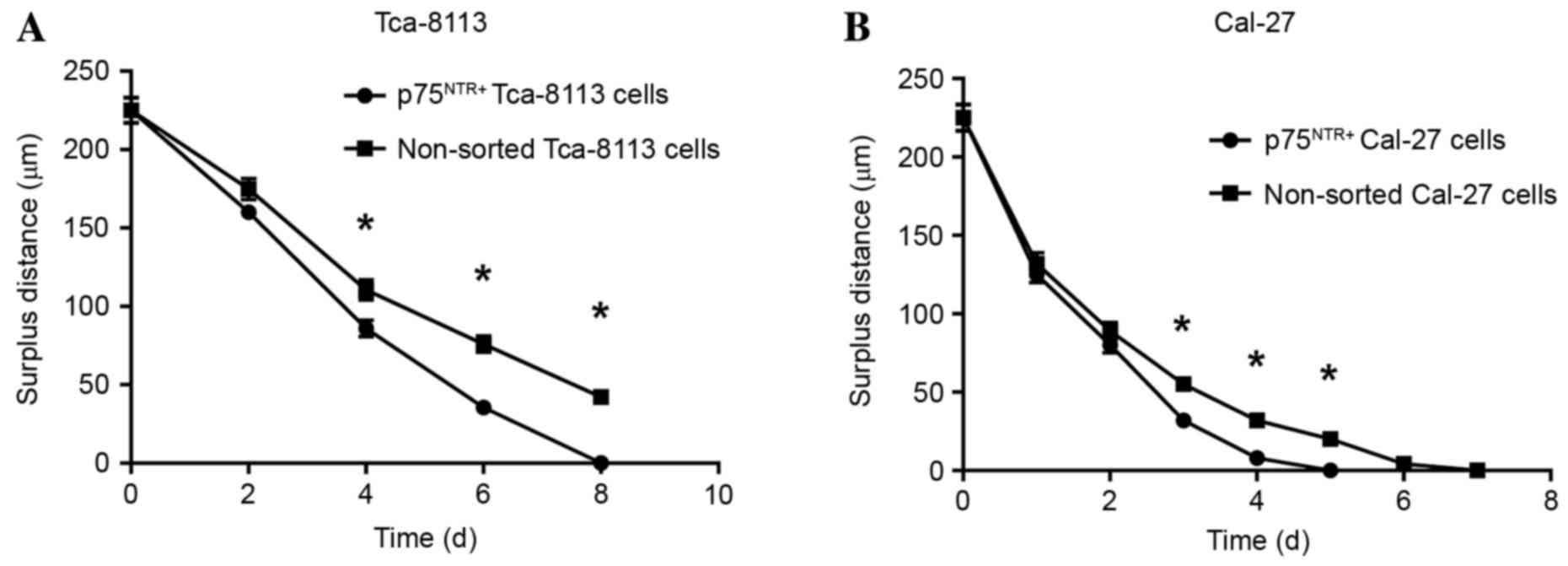
Scratch assay
Cells in each group were observed 8 days after the
scratch assay and the data indicated that p75NTR+ cells
exhibited better wound healing compared with the controls (Figs. 8 and 9). By day 8, the wounded area in the
Tca-8113 p75NTR+ cell group was covered, whereas
non-sorted cells still retained a denuded area (Fig. 8). In the CAL-27 p75NTR+
cell group, cells covered the wounded area by day 5 (Fig. 9). These results suggested that
p75NTR+ cells exhibit better migratory and invasive
characteristics compared with non-sorted cells (Tca-8113,
P<0.05; CAL-27, P<0.05; Fig.
10).
Xenograft tumorigenicity assay
Various numbers of sorted p75NTR+ and
non-sorted TSCC cells were injected into nude mice, as
aforementioned. The results indicated that at least
5×103 p75NTR+ cells or 5×104
non-sorted cells were required to generate tumors. Tumorigenicity
of the p75NTR+ and non-sorted cell groups are presented
in Table II. Tumorigenicity of
p75NTR+ cells was significantly higher compared with
non-sorted cells according to Fisher's exact test.
 | Table II.Tumorigenicity of p75NTR+
and non-sorted tongue squamous cell carcinoma cells in a nude mouse
xenograft model. |
Table II.
Tumorigenicity of p75NTR+
and non-sorted tongue squamous cell carcinoma cells in a nude mouse
xenograft model.
|
| Cell orders of
magnitude/tumor numbers |
|
|---|
|
|
|
|
|---|
| Cell group |
1.0×103 |
1.0×104 |
1.0×105 |
1.0×106 | P-value |
|---|
| p75NTR+
Tca-8113 cells | 1/6 | 5/6 | 6/6 | 6/6 |
|
| Non-sorted Tca-8113
cells | 0/6 | 2/6 | 2/6 | 6/6 | P=0.0176 |
| p75NTR+
CAL-27 cells | 2/6 | 5/6 | 5/6 | 6/6 |
|
| Non-sorted CAL-27
cells | 0/6 | 1/6 | 2/6 | 5/6 | P=0.0084 |
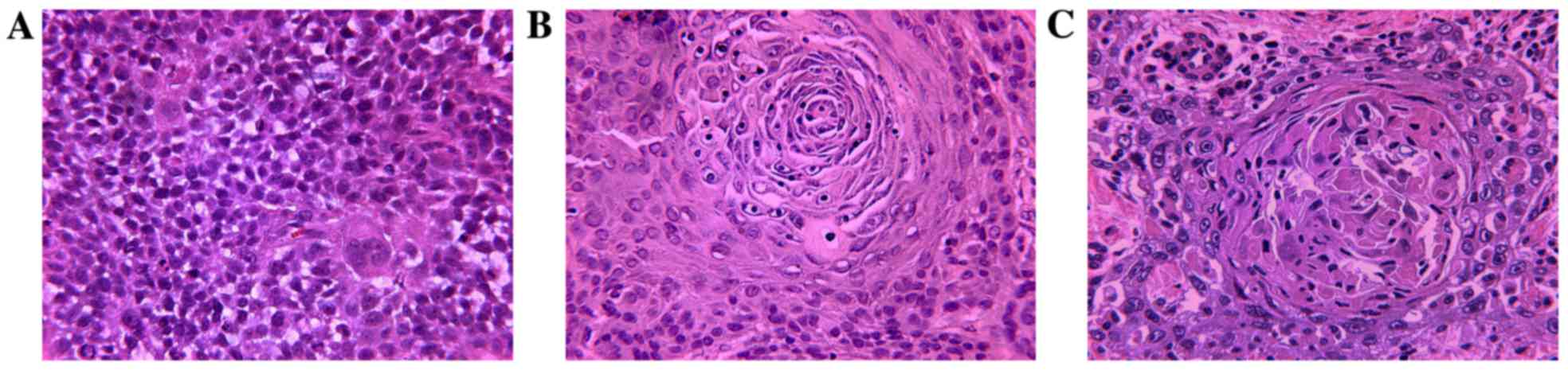
H&E staining was used to identify morphological
differences between the treatment groups. Stained sections were
observed and digital images were captured under a light microscope
(Olympus BX-53; Olympus Corporation). Both tumor types appeared to
be squamous cell carcinomas with no intercellular bridges and
undetectable mitosis. Tumors originating from Tca-8113 cells had
larger necrotic areas compared with those generated from CAL-27
cells. The tumor cell lines generated abundant cancer cell nests,
thus resembling normal human, moderately differentiated TSCC
(Fig. 11).
Discussion
p75NTR expression was present in Tca-8113
and CAL-27 TSCC cell lines, as indicated in the results of the
present study; these data were similar to those of previous
studies, which detected the presence of cancer stem cells (CSCs)
(29,30). p75NTR+ cells isolated
from TSCC cell lines exhibited characteristics of CSCs, and were
able to self-renew, proliferate and undergo multidirectional
differentiation. Furthermore, p75NTR+ cells exhibited
strong tumorigenic capacity in vivo. These results indicated
that p75NTR may be considered a useful surface marker of
TSCC stem cells.
Previous studies have reported on the existence of
CSCs in brain tumors (4,6), liver (31,32),
lung (33), breast (34), colorectal (35) and pancreatic carcinoma (36), and thyroid tumors (37). Bao et al (38) reported that cluster of
differentiation (CD)133+ CSCs contributed to glioma
radioresistance via preferential activation of the DNA damage
checkpoint response and increased DNA repair capacity. In addition,
the proportion of CD133+ cells in these tumors was 2–3%.
Liu et al (39) reported
that CD133+ CSCs, which accounted for 10.2% of brain
glioblastoma cells, exhibited chemoresistance due to their
increased expression of ATP-binding cassette sub-family G member 2
and O-6-methylguanine-DNA methyltransferase.
The results of the present study are consistent with
those of previous reports, which demonstrated that the expression
of p75NTR is associated with the occurrence and
development of numerous types of cancer, including stomach cancer
(40), retinal neuroblastoma
(41), prostatic carcinoma
(42), pancreatic cancer (43) and melanoma (44). The present results suggested that
the CSC theory is valid; and a small population of CSCs stimulate
tumor recurrence and metastases.
Self-renewal is a hallmark of CSCs, since they are
able to form new, identical stem cells that can proliferate, expand
and differentiate. Colony-forming ability reflects self-renewal
capacity, and previous studies regard colony-forming cells as CSCs
(34,45). In the present study, a colony
formation assay confirmed that p75NTR+ cells exhibited a
better colony-forming ability compared with non-sorted cells, and
these data agree with the fact that CSCs are capable of
self-renewal. In addition, flow cytometry confirmed that the
proportion of p75NTR+ cells was significantly decreased
after 4 weeks of colonization in vitro. Therefore,
p75NTR+ cells may generate p75NTR+ and
p75NTR− progenies, thus suggesting they possess
multidirectional differentiation ability.
The results of an MTT assay revealed that the
viability of p75NTR+ cells was significantly higher
compared with non-sorted cells. Invasion and metastasis are
important biological characteristics of malignant tumors, which are
associated with patient mortality and chemoresistance. According to
the results of a scratch assay, p75NTR+ cells possess
migratory ability. Kiyosue et al (12) reported a significant association
between the magnitude of p75NTR positivity and the mode
of tumor invasion. Furthermore, Soland et al (46) reported that the average risk of
local recurrence was increased by ~17-fold in OSCC when
p75NTR expression was positive, and marked tumor cell
dissociation was detected at the invasive front. Therefore,
p75NTR+ may be involved in the invasive pattern of
cancer cells.
In the present study nude mice were inoculated with
various numbers of p75NTR+ cells isolated from Tca-8113
and CAL-27 TSCC cell lines, or non-sorted cells, in order to
determine whether p75NTR+ cells exhibit greater
tumorigenicity compared with non-sorted cells. The results
indicated that that p75NTR+ cells possess stronger
tumorigenicity. H&E staining revealed that the inoculated mice
possessed squamous cell carcinoma with no intercellular bridges and
undetectable mitosis. In addition, Tca-8113 cell tumors manifested
larger necrotic areas compared with those derived from CAL-27
cells. Both tumor types possessed abundant cancer cell nests, which
resembled normal human, moderately differentiated TSCC. Therefore,
the present study suggested that p75NTR+ cells may be
useful for the surface marker identification of TSCC stem
cells.
In conclusion, p75 neurotrophin receptor
(p75NTR) expression was detected in TSCC cell lines and
found that p75NTR+ cells isolated from TSCC cell lines
possess the characteristics of cancer stem cells. These findings
suggest that p75NTR may be considered a useful surface
marker for the identification of TSCC stem cells, providing a
potential target for novel therapies.
Acknowledgements
The present study was supported by the Shandong
Province Science and Technique Foundation, China (grant no.
ZR2012HM055) to Professor Fenghe Zhang.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamburger AW and Salmon SE: Primary
bioassay of human tumor stem cells. Science. 197:461–463. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costea DE, Tsinkalovsky O, Vintermyr OK,
Johannessen AC and Mackenzie IC: Cancer stem cells - new and
potentially important targets for the therapy of oral squamous cell
carcinoma. Oral Dis. 12:443–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
7
|
Seigel GM, Campbell LM, Narayan M and
Gonzalez-Fernandez F: Cancer stem cell characteristics in
retinoblastoma. Mol Vis. 11:729–737. 2005.PubMed/NCBI
|
|
8
|
Chumsri S, Phatak P, Edelman MJ, Khakpour
N, Hamburger AW and Burger AM: Cancer stem cells and individualized
therapy. Cancer Genomics Proteomics. 4:165–74. 2007.PubMed/NCBI
|
|
9
|
Locke M, Heywood M, Fawell S and Mackenzie
IC: Retention of intrinsic stem cell hierarchies in
carcinoma-derived cell lines. Cancer Res. 65:8944–8950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tudor D, Locke M, Owen-Jones E and
Mackenzie IC: Intrinsic patterns of behavior of epithelial stem
cells. J Investig Dermatol Symp Proc. 9:208–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiyosue T, Kawano S, Matsubara R, Goto Y,
Hirano M, Jinno T, Toyoshima T, Kitamura R, Oobu K and Nakamura S:
Immunohistochemical location of the p75 neurotrophin receptor
(p75NTR) in oral leukoplakia and oral squamous cell carcinoma. Int
J Clin Oncol. 18:154–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rabizadeh S and Bredesen DE: Ten years on:
Mediation of cell death by the common neurotrophin receptor
p75(NTR). Cytokine Growth Factor Rev. 14:225–239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liepinsh E, Ilag LL, Otting G and Ibáñez
CF: NMR structure of the death domain of the p75 neurotrophin
receptor. EMBO J. 16:4999–5005. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodríguez-Tébar A, Dechant G, Götz R and
Barde YA: Binding of neurotrophin-3 to its neuronal receptors and
interactions with nerve growth factor and brain-derived
neurotrophic factor. EMBO J. 11:917–922. 1992.PubMed/NCBI
|
|
16
|
Wang X, Bauer JH, Li Y, Shao ZH, Zetoune
FS, Cattaneo E and Vincenz C: Characterization of a p75(NTR)
apoptotic signaling pathway using a novel cellular model. J Biol
Chem. 276:33812–33820. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harding TC, Xue L, Bienemann A, Haywood D,
Dickens M, Tolkovsky AM and Uney JB: Inhibition of JNK by
overexpression of the JNL binding domain of JIP-1 prevents
apoptosis in sympathetic neurons. J Biol Chem. 276:4531–4534. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park DS, Morris EJ, Bremner R, Keramaris
E, Padmanabhan J, Rosenbaum M, Shelanski ML, Geller HM and Greene
LA: Involvement of retinoblastoma family members and E2F/DP
complexes in the death of neurons evoked by DNA damage. J Neurosci.
20:3104–3114. 2000.PubMed/NCBI
|
|
19
|
Salehi AH, Xanthoudakis S and Barker PA:
NRAGE, a p75 neurotrophin receptor-interacting protein, induces
caspase activation and cell death through a JNK-dependent
mitochondrial pathway. J Biol Chem. 277:48043–48050. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krygier S and Djakiew D: Neurotrophin
receptor p75(NTR) suppresses growth and nerve growth
factor-mediated metastasis of human prostate cancer cells. Int J
Cancer. 98:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khwaja F and Djakiew D: Inhibition of
cell-cycle effectors of proliferation in bladder tumor epithelial
cells by the p75NTR tumor suppressor. Mol Carcinog. 36:153–160.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pflug BR, Onoda M, Lynch JH and Djakiew D:
Reduced expression of the low affinity nerve growth factor receptor
in benign and malignant human prostate tissue and loss of
expression in four human metastatic prostate tumor cell lines.
Cancer Res. 52:5403–5406. 1992.PubMed/NCBI
|
|
23
|
Okumura T, Shimada Y, Imamura M and
Yasumoto S: Neurotrophin receptor p75(NTR) characterizes human
esophageal keratinocyte stem cells in vitro. Oncogene.
22:4017–4026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campagnolo L, Russo MA, Puglianiello A,
Favale A and Siracusa G: Mesenchymal cell precursors of peritubular
smooth muscle cells of the mouse testis can be identified by the
presence of the p75 neurotrophin receptor. Biol Reprod. 64:464–472.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto N, Akamatsu H, Hasegawa S, Yamada
T, Nakata S, Ohkuma M, Miyachi E, Marunouchi T and Matsunaga K:
Isolation of multipotent stem cells from mouse adipose tissue. J
Dermatol Sci. 48:43–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi H, Li DQ, Shine HD, Chen Z, Yoon KC,
Jones DB and Pflugfelder SC: Nerve growth factor and its receptor
TrkA serve as potential markers for human corneal epithelial
progenitor cells. Exp Eye Res. 86:34–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rota LM, Lazzarino DA, Ziegler AN, LeRoith
D and Wood TL: Determining mammosphere-forming potential:
Application of the limiting dilution analysis. J Mammary Gland Biol
Neoplasia. 17:119–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zen Y, Fujii T, Yoshikawa S, Takamura H,
Tani T, Ohta T and Nakanuma Y: Histological and culture studies
with respect to ABCG2 expression support the existence of a cancer
cell hierarchy in human hepatocellular carcinoma. Am J Pathol.
170:1750–1762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seo DC, Sung JM, Cho HJ, Yi H, Seo KH,
Choi IS, Kim DK, Kim JS, El-Aty AMA and Shin HC: Gene expression
profiling of cancer stem cell in human lung adenocarcinoma A549
cells. Mol Cancer. 6:752007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu SL, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mitsutake N, Iwao A, Nagai K, Namba H,
Ohtsuru A, Saenko V and Yamashita S: Characterization of side
population in thyroid cancer cell lines: Cancer stem-like cells are
enriched partly but not exclusively. Endocrinology. 148:1797–1803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin H, Pan Y, Zhao L, Zhai H, Li X, Sun L,
He L, Chen Y, Hong L, Du Y and Fan D: p75 neurotrophin receptor
suppresses the proliferation of human gastric cancer cells.
Neoplasia. 9:471–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dimaras H and Gallie BL: The p75 (NTR)
neurotrophin receptor is a tumor suppressor in human and murine
retinoblastoma development. Int J Cancer. 122:2023–2029. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khwaja F, Tabassum A, Allen J and Djakiew
D: The p75(NTR) tumor suppressor induces cell cycle arrest
facilitating caspase mediated apoptosis in prostate tumor cells.
Biochem Biophys Res Commun. 341:1184–1192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dang CX, Zhang Y, Ma QY and Shimahara Y:
Expression of nerve growth factor receptors is correlated with
progression and prognosis of human pancreatic cancer. J Gastroen
Hepatol. 21:850–858. 2006. View Article : Google Scholar
|
|
44
|
Marchetti D, Aucoin R, Blust J, Murry B
and Greiter-Wilke A: p75 neurotrophin receptor functions as a
survival receptor in brain-metastatic melanoma cells. J Cell
Biochem. 91:206–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diamandis P, Wildenhain J, Clarke ID,
Sacher AG, Graham J, Bellows DS, Ling EK, Ward RJ, Jamieson LG,
Tyers M and Dirks PB: Chemical genetics reveals a complex
functional ground state of neural stem cells. Nat Chem Biol.
3:268–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Soland TM, Brusevold IJ, Koppang HS,
Schenck K and Bryne M: Nerve growth factor receptor (p75 NTR) and
pattern of invasion predict poor prognosis in oral squamous cell
carcinoma. Histopathology. 53:62–72. 2008. View Article : Google Scholar : PubMed/NCBI
|