Introduction
Hand, foot and mouth disease (HFMD) is a common
acute infectious disease caused by various human intestinal
viruses. HFMD usually affects preschool children (age, <3 years)
and has caused widespread epidemics in this population (1). It is commonly caused by enterovirus
71 (EV71) and coxsackievirus A16 (1) and EV71 leads to more HFMD compared
with coxsackievirus A16. Meanwhile, EV71 is the most neurovirulent
of enteroviruses and is responsible for fatal outcomes. EV71 is a
member of an enterovirus group that belongs to the Picornaviridae
family. Similar to other members of Picornaviridae, it is a
non-enveloped, positive, non-segmented RNA virus, with a diameter
of ~30 nm. Its genomic RNA is packaged within the capsid composed
of 60 copies of 4 structural proteins, VP1, VP2, VP3 and VP4. It
was first isolated in California, in 1969. Since the 1960s, EV71
has led to numerous epidemic outbreaks worldwide. Presently, it has
become a major public health issue, especially in Asia-Pacific
region (2).
The severity of HFMD varies; patients with mild HFMD
present with mild fever and a self-limiting herpetic rash on the
hands, feet and mouth. However, in patients with severe HFMD, the
disease progresses rapidly and may involve meningitis, brainstem
encephalitis, pulmonary angioneurotic edema, pulmonary hemorrhage,
myocarditis, and even cardiopulmonary failure and mortality
(3,4). Good oral and skin hygiene is crucial
for patients with mild HFMD. Additionally, quarantine of infected
individuals is also effective in order to limit the spread of the
virus. Symptomatic supportive treatments should be conducted when
required. No established antiviral treatments are available for
severe HFMD. Intravenous immunoglobulin treatment has been reported
to be effective and is used in various Asian countries; however,
sufficient evidence for its effectiveness is lacking.
Glucocorticoid may also be used to reduce brain and pulmonary
edema. A comprehensive life support including fluid management,
inotrope and respiratory support therapy is regularly conducted
(5–7).
The pathogenesis of severe HFMD caused by EV71
infection is not fully understood. Numerous studies have
demonstrated that cellular and humoral immune dysfunction in
patients with HFMD, in particular the abnormal expression of
inflammatory cytokines and chemokines and an imbalance in the
expression of anti- and proinflammatory cytokines, is involved in
the exacerbation of HFMD (8–11).
In the present study, the expression levels of
inflammatory cytokines and chemokines were detected in peripheral
blood samples obtained from children with EV71-induced HFMD. The
relationship between abnormal cytokine/chemokine expression and
various clinical presentations of HFMD was analyzed in order to
clarify the mechanisms underlying the exacerbation of HFMD. The
clinical significance of the results is discussed.
Materials and methods
Patient selection
The present study involved 72 children (39 male, 33
female) with mild or severe HFMD who were treated at Hangzhou Sixth
People's Hospital (Hangzhou, China) between March 2011 and June
2013. All diagnoses were made using the Foot and Mouth Disease
Treatment Guidelines (2010) issued by the Ministry of Health and
the Expert Consensus on the Clinical Treatment of Severe EV71
Infection (6,7). Patients who received
immunomodulators, or who had other viral or bacterial infections or
other immune disorders, were excluded.
In addition, 26 healthy children (control group),
who had normal findings on physical examination from Hangzhou Sixth
People's Hospital, were recruited. Verbal consent was obtained from
all participants. Ethical approval (no. 2017-11) for the present
study was obtained from the Ethics Committee of the First
Affiliated Hospital, College of Medicine, Zhejiang University
(Hangzhou, China).
Sample collection
Venous blood samples (3 ml) were collected from all
children in the HFMD and control groups within 24 h of admission.
The blood samples were collected in EDTA tubes and centrifuged at
3,000 × g for 15 min at room temperature to obtain plasma samples,
which were subsequently stored at −80°C until further analysis. In
addition, stool samples were collected from the children with HFMD,
and these were subjected to reverse transcription-polymerase chain
reaction (RT-PCR) analysis for the detection of EV71 RNA.
EV71 detection
A diagnostic PCR-fluorescence probing kit (cat. no.
DA-BT109) for enterovirus RNA (Da An Gene Co., Ltd., Guangzhou,
China) was used for the detection of EV71. The RNA extraction
reagent was conducted in the PCR-fluorescence probing kit. All
forward and reverse primer sequences used were contained within the
kit. The procedure was conducted according to the manufacturer's
protocol. The thermal cycling parameters were as follows: cDNA
synthesis at 42°C for 25 min; denaturation at 94°C for 3 min; and
40 cycles at 93°C for 15 sec and 55°C for 45 sec. RT-PCR was
performed on the CFX96™ Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). If test sample had a
typical S-shaped amplification curve and the Cq value (12) was lower than 34.9, it was deemed to
be positive, otherwise it was negative.
Cytokine and chemokine detection
Plasma expression levels of the cytokines
interleukin (IL)-4, IL-12, IL-18, tumor necrosis factor-α (TNF-α)
and interferon-γ (IFN-γ), and the chemokines IL-8, regulated on
activation, normal T cell expressed and secreted (RANTES), monocyte
chemoattractant protein-1 (MCP-1) and IFN-γ-inducible protein-10
(IP-10) were determined using double-antibody sandwich
enzyme-linked immunosorbent assays (ELISA; R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocols.
The catalogue numbers for all ELISA kits used were as follows:
IL-4, D4050; IL-12, D1200; IL-18, 7620; TNF-α, DTA00C; IFN-γ,
DIF50; IL-8, D8000C; RANTES, DRN00B; MCP-1, DCP00; IP-10,
DIP100.
Statistical analysis
Data were analyzed using SPSS 13.0 statistical
software (SPSS, Inc., Chicago, IL, USA) and were representative of
three independent experiments expressed as the mean ± standard
deviation. Multi-sample means were detected using one-way ANOVA
analysis of variance and the Student's t-test. The multiple
comparisons test was adjusted by Bonferroni test. Correlations were
analyzed using Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic data
Of the 72 study participants (39 male, 33 female),
28 (17 male, 11 female; average age, 2.3±0.8 years) had mild HFMD
and 44 (22 male, 22 female; average age, 2.1±0.7 years) had severe
HFMD. The control group included 16 males and 10 females, with an
average age of 2±0.6 years. No significant differences in age or
gender were present between the mild HFMD, severe HFMD and control
groups.
Cytokine and chemokine expression
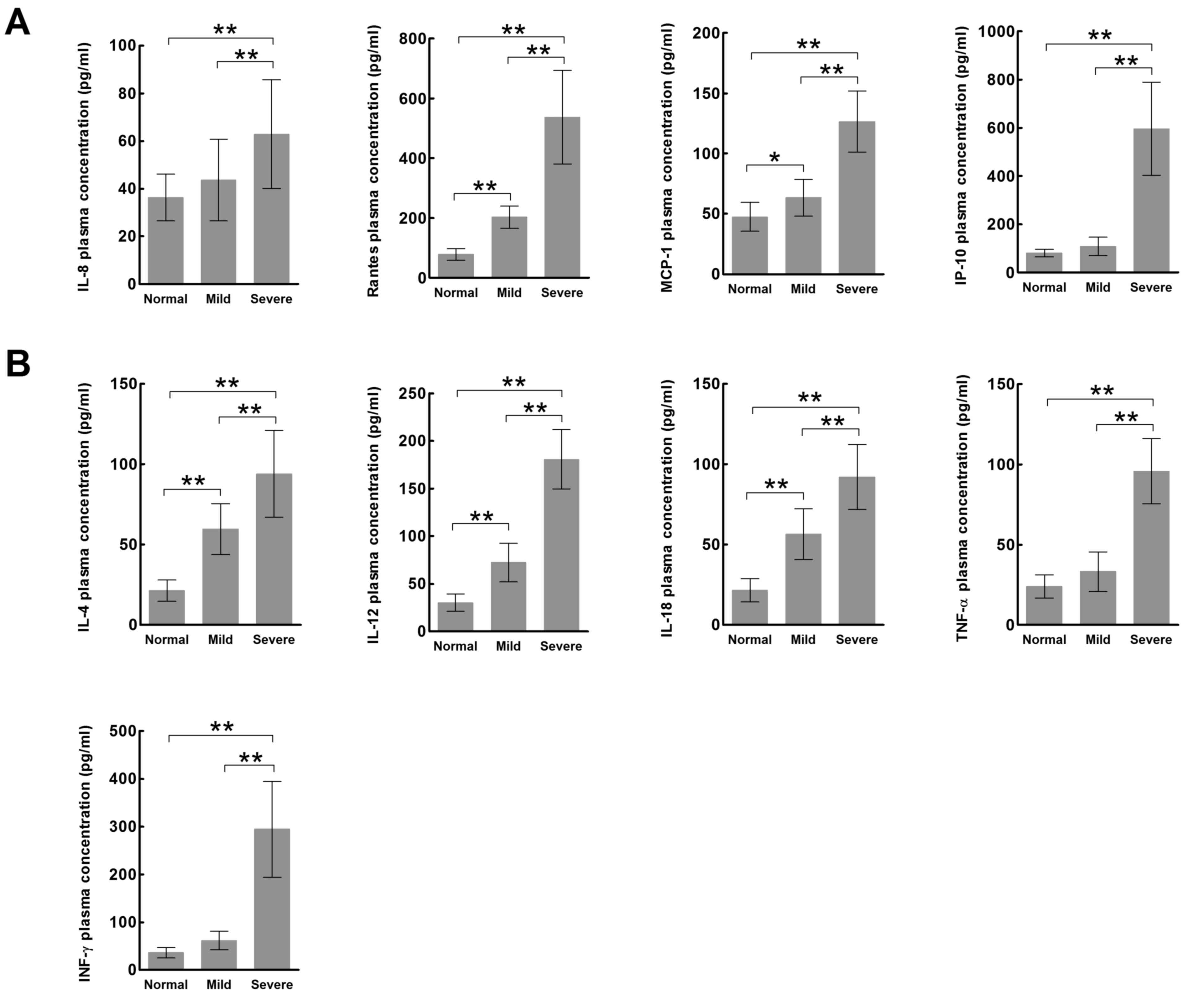
The plasma expression levels of all chemokines
(IL-8, RANTES, MCP-1, IP-10; Fig.
1A) and cytokines (IL-4, IL-12, IL-18, TNF-α, IFN-γ; Fig. 1B) were significantly increased in
the severe HFMD group compared with the mild HFMD and normal
control groups (P<0.01). In addition, the expression levels of
the chemokines RANTES and MCP-1 (Fig.
1A) and the cytokines IL-4, IL-12 and IL-18 (Fig. 1B) were significantly increased in
the mild HFMD group compared with the normal control group
(P<0.05).
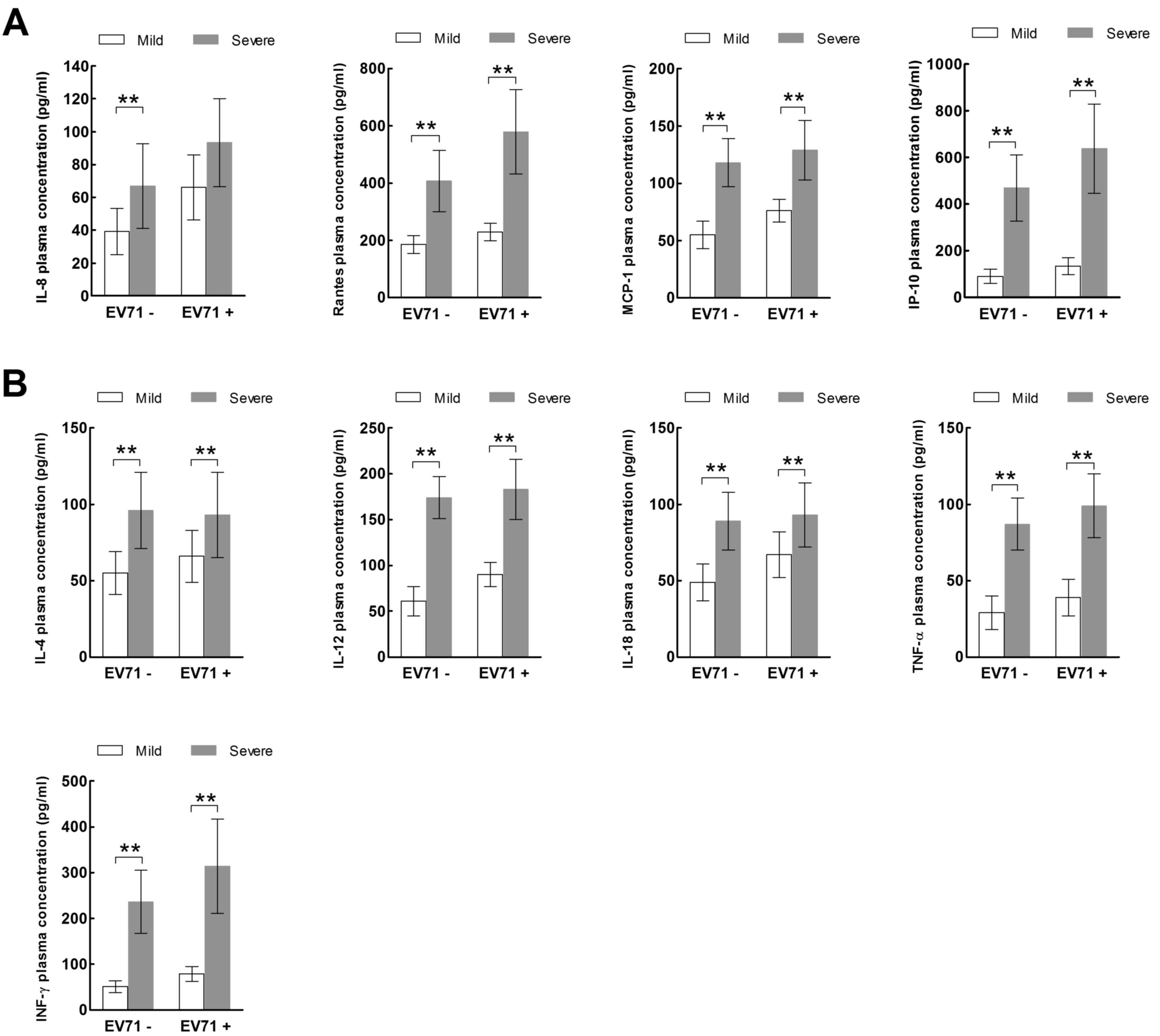
The children in the severe HFMD group were further
divided into those with encephalitis (E group; n=26; 14 male, 12
female) and those with both encephalitis and pulmonary edema (E+P
group; n=18; 8 male, 10 female). No significant differences in age
or gender were present between the E and E+P groups. The plasma
expression levels of all chemokines, with the exception of IL-8
(Fig. 2A), and all cytokines, with
the exception of IL-4 (Fig. 2B)
were significantly increased in the E+P group compared with the E
group (P<0.05).
 | Figure 2.Expression of inflammatory (A)
chemokines and (B) cytokines in children with severe hand, foot and
mouth disease, in the E and E+P groups. *P<0.05, **P<0.01. E,
encephalitis group; E+P, encephalitis with pulmonary edema group;
IL, interleukin; RANTES, regulated on activation, normal T cell
expressed and secreted; MCP-1, monocyte chemoattractant protein-1;
IP-10, IFN-γ-inducible protein-10; TNF-α, tumor necrosis factor-α;
IFN-γ, interferon-γ. |
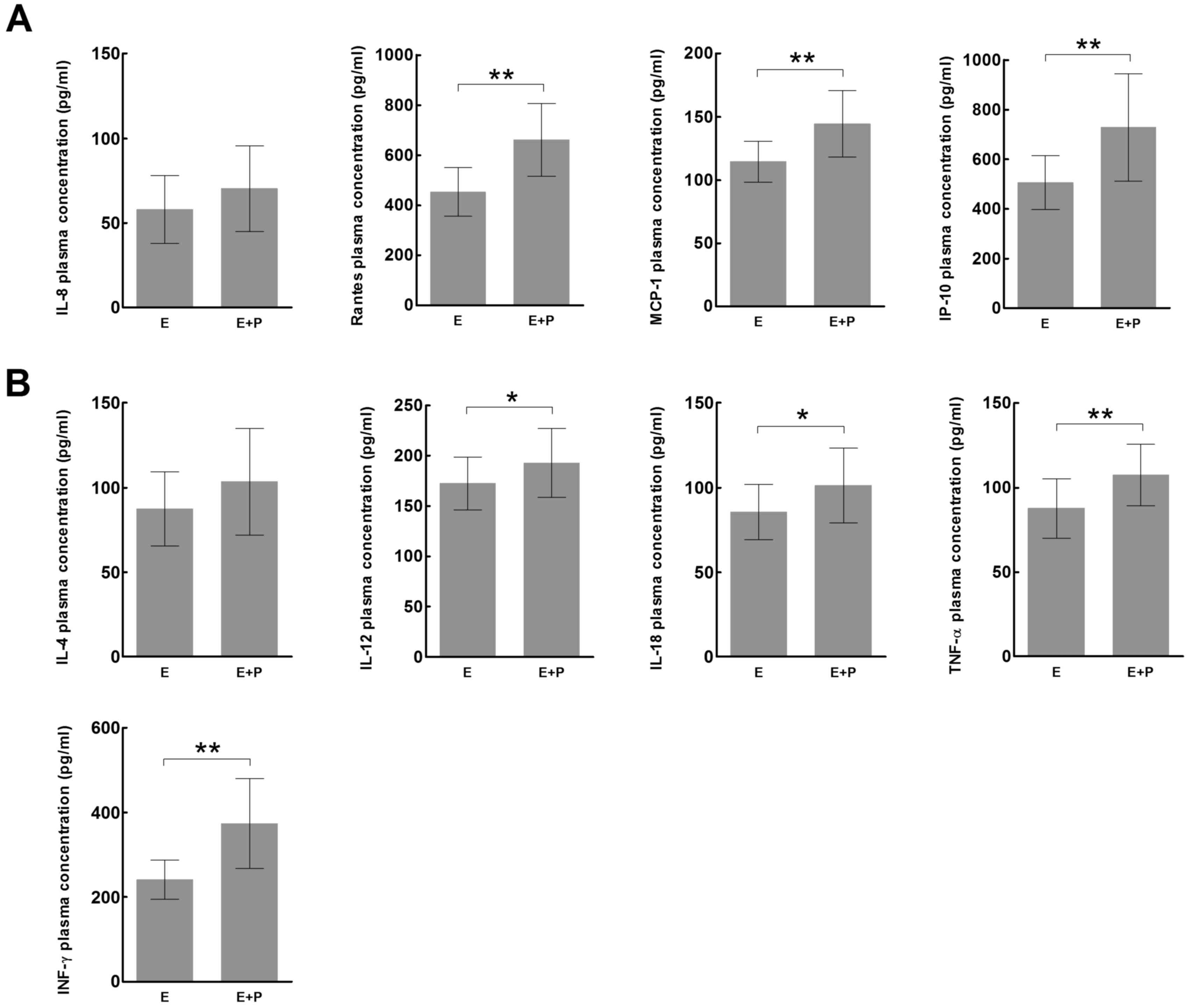
EV71 RNA detection in stool samples
and its correlation with plasma cytokine and chemokine
expression
Stool samples from the children with HFMD were
analyzed for the presence of EV71 RNA using RT-PCR (Table I). The plasma expression levels of
all chemokines, with the exception of IL-8 (Fig. 3A), and all cytokines (Fig. 3B) were significantly increased in
children whose stool samples tested positive for EV71 RNA compared
with children whose stool samples were negative for EV71 RNA
(P<0.05). In the mild HFMD group, the plasma expression levels
of all chemokines, with the exception of IL-8 (Fig. 4A), and all cytokines, with the
exception of IL-4 (Fig. 4B) were
significantly increased in the EV71-positive patients compared with
the EV71-negative patients (P<0.05). However, in the severe HFMD
group, only the expression levels of RANTES (Fig. 4A), IP-10 and IFN-γ (Fig. 4B) were significantly increased in
the EV71-positive patients compared with the EV71-negative patients
(P<0.05).
 | Figure 3.Expression of inflammatory (A)
chemokines and (B) cytokines in patients with hand, foot and mouth
disease whose stool samples tested positive or negative for EV71
RNA. *P<0.05, **P<0.01es. EV71, enterovirus 71; IL,
interleukin; RANTES, regulated on activation, normal T cell
expressed and secreted; MCP-1, monocyte chemoattractant protein-1;
IP-10, IFN-γ-inducible protein-10; TNF-α, tumor necrosis factor-α;
IFN-γ, interferon-γ. |
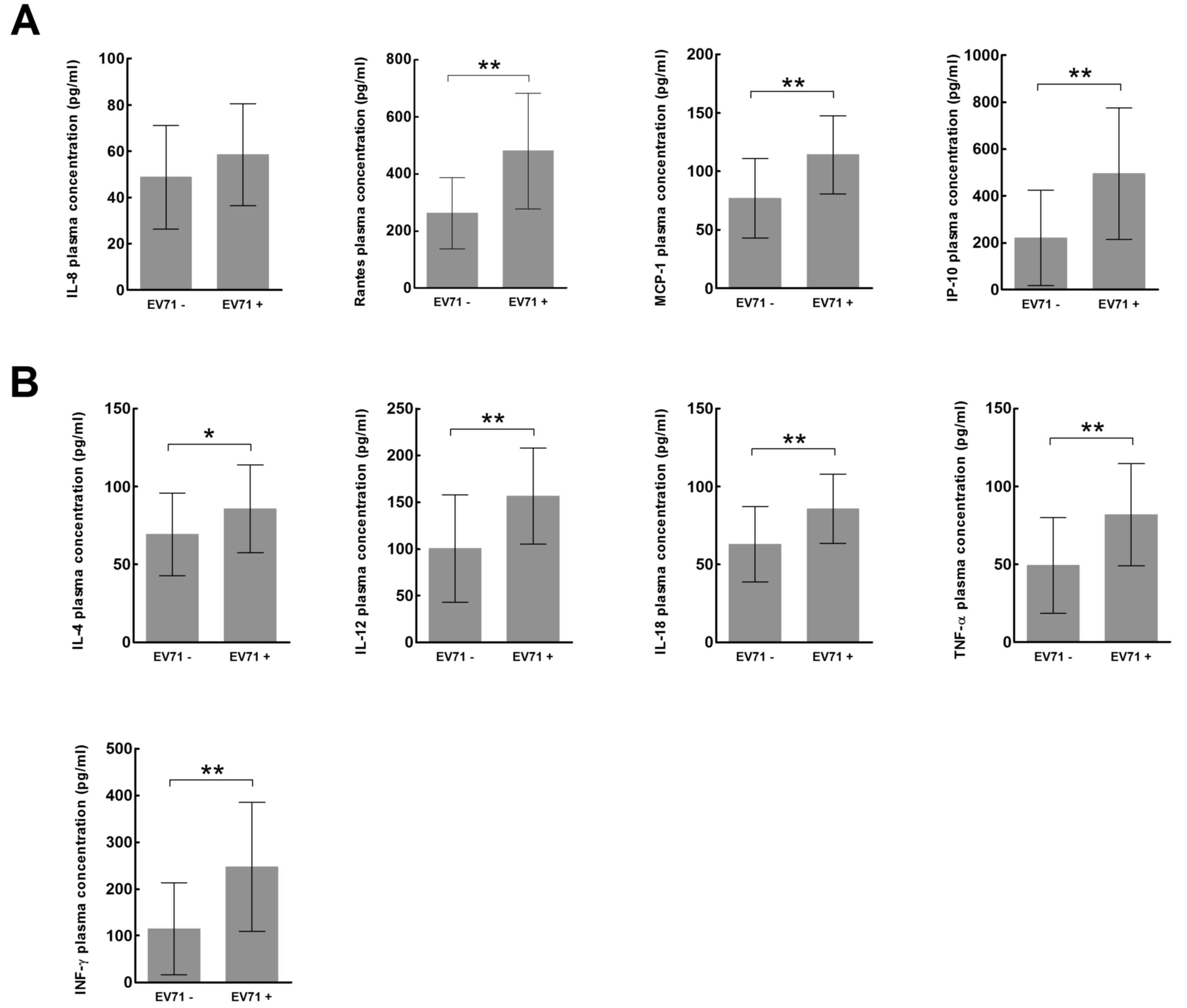
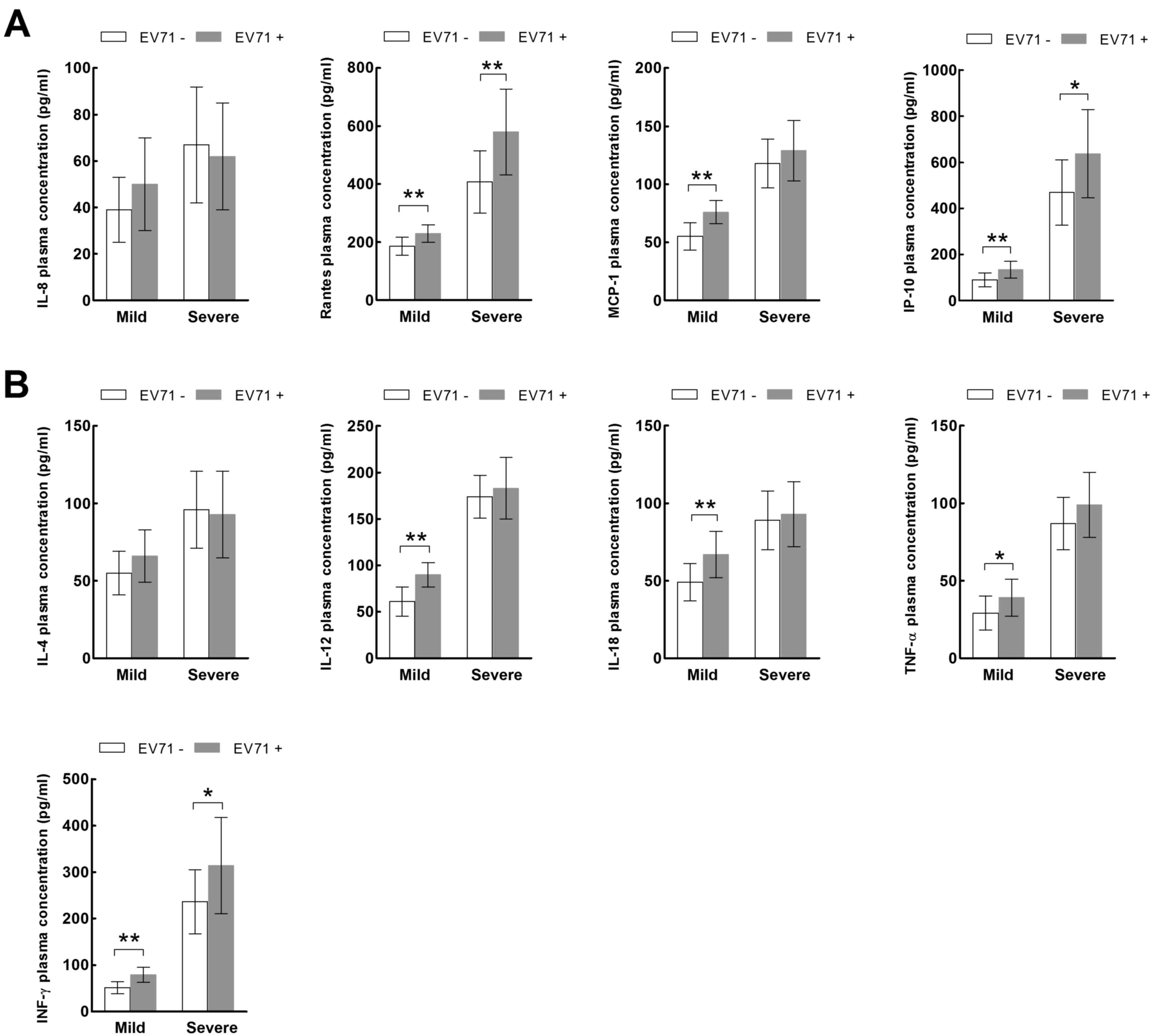 | Figure 4.Expression of inflammatory (A)
chemokines and (B) cytokines in the mild and severe hand, foot and
mouth disease groups according to EV71 status. *P<0.05,
**P<0.01. EV71, enterovirus 71; IL, interleukin; RANTES,
regulated on activation, normal T cell expressed and secreted;
MCP-1, monocyte chemoattractant protein-1; IP-10, IFN-γ-inducible
protein-10; TNF-α, tumor necrosis factor-α; IFN-γ,
interferon-γ. |
 | Table I.Hand, foot and mouth disease groups
and EV71 status. |
Table I.
Hand, foot and mouth disease groups
and EV71 status.
|
| EV71-PCR (+) | EV71-PCR (−) |
|---|
|
|
|
|
|---|
| Group | No. (M/F) | Average age (y) | No. (M/F) | Average age (y) |
|---|
| Mild | 11 (7/4) | 2.3±0.8 | 17 (10/7) | 2.2±0.9 |
| Severe (E) | 17 (8/9) | 1.9±0.7 | 9 (6/3) | 2.1±0.6 |
| Severe (E+P) | 11 (6/5) | 2.1±0.8 | 7 (2/5) | 2.2±0.6 |
The children with HFMD were regrouped according to
their EV71 status. Of the 72 study patients, 33 (18 male, 15
female) were EV71 negative, and 39 (21 male, 18 female) were EV71
positive. Of the 33 EV71-negative patients, 17 (10 male, 7 female)
had mild HFMD and 16 (8 male, 8 female) had severe HFMD. Of the 39
EV71-positive patients, 11 (7 male, 4 female) had mild HFMD and 28
(14 male, 14 female) had severe HFMD (Table I).
In the EV71-negative group, the plasma expression
levels of all cytokines (Fig. 5A)
and chemokines (Fig. 5B) were
significantly increased in children with severe HFMD compared with
children with mild HFMD (P<0.01). In the EV71-positive group,
the plasma expression levels of all cytokines, with the exception
of IL-8 (Fig. 5A), and all
chemokines (Fig. 5B) were
significantly increased in children with severe HFMD compared with
children with mild HFMD (P<0.01).
Discussion
The immune system is a complex, sophisticated and
coordinated network, and its dynamic equilibrium sustains health.
Upon invasion by microorganisms, inflammatory mediators are
generated in ‘cascades’ through a series of pathways, which results
in systemic inflammatory response syndrome. In addition, the
excessive generation of inflammatory mediators may lead to tissue
and organ damage; therefore, various anti-inflammatory mediators
are produced to inhibit excessive inflammatory responses and
protect the body from immune damage (13). Pro- and anti-inflammatory mediators
coexist in the infection-induced immunological stress response, and
the growth, decline and equilibrium of the two determine the
evolution, development and prognosis of infectious disease
(14).
In children, the immune system is not fully
developed, which facilitates the invasion of pathogenic
microorganisms. As a result, children, particularly preschoolers,
exist in a physiological immunocompromised state, and their
specific and non-specific immune responses are poor compared with
those of adults. This may partially explain why preschoolers are
susceptible to HFMD.
Following EV71 infection, susceptible cells and
non-specific immune cells are stimulated to produce cytokines
including IFNs, TNF-α and IL-12. These cytokines are involved in
the early control of viral replication and infection. The virus
further induces the production of chemokines, including IL-8, MCP-1
and IP-10, which contribute to the recruitment of non-specific
immune cells, including natural killer cells, monocytes and
macrophages, to the infection site. Furthermore, the activation of
these cells by cytokines results in the secretion of inflammatory
mediators and cytokines, interference with viral replication and
the death of virus-infected host cells. These responses expand and
enhance anti-infection immunity and accelerate recovery from HFMD,
leading to a self-limiting disease (10,11,15–17).
However, the pathogenic mechanisms that lead to the exacerbation of
EV71 infection and to abnormal cytokine expression remain
unknown.
In the present study, the relationship between
plasma cytokines (IL-4, IL-12, IL-18, TNF-α, IFN-γ) and chemokines
(IL-8, RANTES, MCP-1, IP-10), and disease severity (mild vs. severe
groups), complications (E vs. E+P groups) and fecal EV71 status
(EV71-positive vs. EV71-negative groups) was examined in children
with HFMD. The results demonstrated that cytokine and chemokine
expression levels were i) significantly increased in the severe
HFMD group compared with the mild HFMD and normal control groups;
ii) significantly increased in the E+P group compared with the E
group; and iii) significantly increased in the EV71-positive group
compared with the EV71-negative group, regardless of disease
severity. These results suggested that EV71 infection-induced
abnormal expression of cytokines and chemokines was involved in the
exacerbation of HFMD.
It has previously been demonstrated that cytokines,
including IL-1β, IL-6, IL-18, TNF-α and IFN-γ, have inhibitory
effects on the replication and infective ability of numerous
viruses, and that these compounds are major components of the early
non-specific immune response to viral infections (18). Conversely, chemokines, including
IL-8, RANTES, MCP-1 and IP-10, are involved in the recruitment and
activation of immune cells (19),
thereby further enhancing antiviral immunity. Overexpression or
imbalances in the expression of these immune factors aggravates the
inflammatory response to infection, causing damage to infected
tissues and leading to loss of or disordered organ function. Under
these circumstances, the vulnerable immune system of children is
unable to effectively eliminate the virus and control inflammation,
which results in further spread of the virus and eventually causes
severe HFMD.
Lin et al (10) confirmed the aforementioned
mechanism by demonstrating that the peripheral expression levels of
IL-1β and several other inflammatory cytokines were significantly
increased in patients with EV71-induced HFMD with both encephalitis
and pulmonary edema, as compared with in those with only
encephalitis, those with mild symptoms or normal controls. A
previous study reported similar results; demonstrating that the
expression levels of inflammatory cytokines, including IFN-γ, MCP-1
and IP-10 in the peripheral blood and cerebrospinal fluid were
significantly increased in patients with pulmonary edema compared
with patients with encephalitis or patients with mild disease
(11). Similar results have been
reported for the inflammatory cytokine TNF-α, which was reported to
have an abnormally high expression in patients with HFMD displaying
pulmonary edema and encephalitis (20).
These results indicated that in the early stages of
infection the increase in the levels of various cytokines and
chemokines may contribute to the early activation of specific and
non-specific immunity. This maximizes the mobilization of various
types of immune cells to participate in anti-EV71 infection
immunity for the alleviation of or recovery from the disease. When
the immune system fails to effectively clear the virus and control
inflammation, the disease is exacerbated. Overexpression of
inflammatory cytokines and chemokines in infected tissues induces
excessive immune responses and results in cytokine cascade reaction
and cytokine storm through autocrine and paracrine mechanisms,
resulting in severe local and systemic inflammation, and eventually
tissue damage and organ dysfunction.
In conclusion, during the development and
progression of HFMD, various cytokines and chemokines are involved
in the occurrence of inflammation. Some of these compounds clear
the virus, whereas others exacerbate the disease. However, at
present, the immune pathogenesis of HFMD, its cytokine expression
patterns and the changes in these patterns are not clear. Further
investigation into the involvement of HFMD-associated cytokines and
chemokines in the pathogenesis of the disease is required to search
for cytokine indices to be used to monitor disease progression,
determine prognosis, and guide clinical diagnosis and
treatment.
Acknowledgements
The present study was supported in part by grants
(grant no. 2014ZZ01) from the State Key Laboratory for Diagnosis
and Treatment of Infectious Diseases, China.
References
|
1
|
Huang X, Wei H, Wu S, Du Y, Liu L, Su J,
Xu Y, Wang H, Li X, Wang Y, et al: Epidemiological and etiological
characteristics of hand, foot and mouth disease in Henan, China,
2008–2013. Sci Rep. 5:89042015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solomon T, Lewthwaite P, Perera D, Cardosa
MJ, McMinn P and Ooi MH: Virology, epidemiology, pathogenesis, and
control of enterovirus 71. Lancet Infect Dis. 10:778–790. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Huo X, Dai Y, Yang Z, Lei Y, Jiang
Y, Li G, Zhan J and Zhan F: Evidences for intertypic and intratypic
recombinant events in EV71 of hand, foot and mouth disease during
an epidemic in Hubei Province, China, 2011. Virus Res. 169:195–202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L, Hu J and Zhou Z: Central nervous
system damage associated with hand-foot-mouth disease induced by
enterovirus 71. J Appl Clin Pediatr. 23:1782–1785. 2008.
|
|
5
|
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ
and Solomon T: Clinical features, diagnosis, and management of
enterovirus 71. Lancet Neurol. 9:1097–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
The Ministry of Health of the People's
Republic of China: Guideline for the diagnosis and treatment of
hand foot and mouth disease, Beijing, China, 2010. Int J Respir.
30:1473–1475. 2010.
|
|
7
|
Clinical Experts Group of the Ministry of
Health for Hand, Foot and Mouth Disease: Experts consensus on
rescue and treatment of severe cases with enterovirus 71 (EV71)
infection. Zhonghua Er Ke Za Zhi. 49:675–678. 2011.(In Chinese).
PubMed/NCBI
|
|
8
|
Lin JY and Shih SR: Cell and tissue
tropism of enterovirus 71 and other enteroviruses infections. J
Biomed Sci. 21:182014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang LY, Hsiung CA, Lu CY, Lin TY, Huang
FY, Lai YH, Chiang YP, Chiang BL, Lee CY and Huang LM: Status of
cellular rather than humoral immunity is correlated with clinical
outcome of enterovirus 71. Pediatric Res. 60:466–471. 2006.
View Article : Google Scholar
|
|
10
|
Lin TY, Chang LY, Huang YC, Hsu KH, Chiu
CH and Yang KD: Different proinflammatory reactions in fatal and
non-fatal enterovirus 71 infections: Implications for early
recognition and therapy. Acta Paediatr. 91:632–635. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SM, Lei HY, Yu CK, Wang JR, Su IJ and
Liu CC: Acute chemokine response in the blood and cerebrospinal
fluid of children with enterovirus 71-associated brainstem
encephalitis. J Infect Dis. 198:1002–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR,
Yu CK, Su IJ and Liu CC: Pathogenesis of enterovirus 71 brainstem
encephalitis in pediatric patients: Roles of cytokines and cellular
immune activation in patients with pulmonary edema. J Infect Dis.
188:564–570. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Napolitano LM: Immune stimulation in
sepsis: To be or Not to be? Chest. 127:1882–1885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van den Berghe G, Wouters P, Weekers F,
Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P,
Lauwers P and Bouillon R: Intensive insulin therapy in the
critically ill patients. N Engl J Med. 345:1359–1367. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lekkou A, Karakantza M, Mouzaki A,
Kalfarentzos F and Gogos CA: Cytokine production and monocyte
HLA-DR expression as predictors of outcome for patients with
community-acquired severe infections. Clin Diagn Lab Immunol.
11:161–167. 2004.PubMed/NCBI
|
|
17
|
Wan S, Xia C and Morel L: IL-6 produced by
dendritic cell from lupus-prone mice inhibits CD4+CD25+ T cell
regulatory functions. J Immunol. 178:271–279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woods PS, Tazi MF, Chesarino NM, Amer AO
and Davis IC: TGF-β-induced IL-6 prevents development of acute lung
injury in influenza A virus-infected F508del CFTR-heterozygous
mice. Am J Physiol Lung Cell Mol Physiol. 308:L1136–L1144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SM, Lei HY, Huang MC, Su LY, Lin HC,
Yu CK, Wang JL and Liu CC: Modulation of cytokine production by
intravenous immunoglobnlin in patients with enterovirus
71-associated brainstem encephalitis. J Clin Viral. 37:47–52. 2006.
View Article : Google Scholar
|
|
20
|
Wang SM, Lei HY, Su LY, Wu JM, Yu CK, Wang
JR and Liu CC: Cerebrospinal fluid cytokines in enterovirus 71
brain stem encephalitis and echovirus meningitis infections of
varying severity. Clin Microbiol Infect. 13:677–682. 2007.
View Article : Google Scholar : PubMed/NCBI
|