Introduction
Asthma is characterized by airway inflammation,
bronchial hyper-responsiveness and airway remodeling. Airway
remodeling entails a wide array of pathophysiological events,
including epithelial damage, mucus gland and goblet cell
hyperplasia, subepithelial fibrosis, smooth muscle hypertrophy and
hyperplasia, vascular changes and disturbances in the homeostasis
of the extracellular matrix (ECM) (1). These factors contribute to persistent
bronchial hyper-responsiveness and airway remodeling and may
negatively impact pulmonary function (1). However, despite extensive previous
studies aiming to elucidate the pathological processes responsible
for airway remodeling, few therapeutic strategies have proved to be
effective in reversing the remodeling (1–3).
Therefore, novel therapeutic strategies targeting airway remodeling
are required.
Genetic and environmental factors may influence
development and clinical presentation of asthma and airway
remodeling. Previous genome-wide association studies reported that
the orosomucoid-like 3 (ORMDL3) gene was strongly associated with
development asthma and recurrent wheezing (3–5).
However, the biological role of ORMDL3 in asthma remains to be
elucidated. In 2007 the role of ORMDL3 in asthma development was
confirmed and abnormal expression of ORMDL3 was detected in over a
third of asthmatic children under the age of 7 years (6). In particular, the rs7216389 site was
closely correlated with childhood asthma susceptibility.
As in human asthma patients, ORMDL3 gene expression
in bronchial epithelial cells was identified to be substantially
elevated in wild-type mice exposed to allergens (by 127-fold)
(7). Additionally, ORMDL3
expression was induced by allergen and cytokine [interleukin (IL)-4
and IL-13] stimulation and was reported to promote matrix
metalloproteinase-9 (MMP-9) expression in asthmatic mice (2,8–10).
MMPs are proteolytic enzymes that degrade ECM components under
physiological conditions and during pathological processes
(3). Upregulation or inappropriate
secretion of MMP-9 by structural or inflammatory cells has been
reported to contribute to the pathophysiology of asthma,
particularly airway remodeling (11). Activation of the ROS proto-oncogene
1, receptor tyrosine kinase (ROS)-extracellular signal-regulated
kinase (ERK)-MMP-9 signaling pathway was also reported to induce
cell migration, proliferation and extracellular matrix collagen
synthesis (12–14). Monocyte migration was associated
with MMP-9 activity; however, the activation of ERK1/2 signaling
pathways and overexpression of ORMDL3 in eosinophils was reported
to increase ERK1/2 phosphorylation in vivo (11). The present study used a mouse model
of asthma to determine the role of ORMDL3 in asthmatic airway
remodeling and investigate the potential association between ORMDL3
and ROS-ERK-MMP-9 signaling pathway in this process.
Materials and methods
Animals
BALB/c female mice, 8-weeks old weighing 20±2 g were
purchased from the Experimental Animal Center of Shandong
University Medical College (Jinan, China). The mice were housed in
a specific pathogen-free animal facility at the animal center. All
animals were reared under relative conditions at 20 to 26°C, a
humidity of 60 to 70% (cage pressure difference between inside and
outside, +15 Pa; ventilation speed, 55 times/h) and were housed in
a room with a 12 h light/dark cycle. In addition, the animals had
access to chow and water ad libitum. All experimental procedures
described in this work were performed in accordance with the
guidance suggestions for the care and use of laboratory animals,
formulated by the Animal Ethics Committee of Shandong Medical
University.
Mouse model of asthma
A total of 28 mice were maintained in the absence of
environmental pathogens for 1 week prior to the experiments in the
present study. They were divided into three groups: i) An asthmatic
model group (n=10); ii) budesonide-treated group (n=10); and iii)
control group (n=8). The mice in the asthmatic and budesonide
treatment groups were sensitized with 100 µg ovalbumin (OVA; grade
V; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 4 mg aluminum
hydroxide (Sigma-Aldrich; Merck KGaA) emulsified in
phosphate-buffered saline (PBS) on days 1, 7 and 14, then exposed
to 1% OVA 3 times a week for 30 min from day 28. Mice in the
budesonide treatment group were exposed to aerosol budesonide (100
µg/kg) 3 times per week for 30 min from day 21. Mice in the control
group were sensitized and challenged with PBS instead. Aerosol
budesonide was generated using a Germany Berry nebulizer (Inqua Neb
PLUS). Following 6 weeks, the mice were anesthetized by
intraperitoneal injection with 1% pentobarbital sodium (50 mg/kg;
Wuhan Yitai Technology Co., Ltd., Wuhan, Hubei, China) and then
sacrificed to collect the lung tissues samples. The mouse asthma
model was established as previously described (8).
Hematoxylin and eosin staining
The lungs were harvested and tissue sections were
prepared as previously described (15). Standard hematoxylin and eosin
staining techniques were applied, and each specimen with a complete
tracheal cross was observed via microscope (DM4000B; Leica
Microsystems, Inc., Buffalo Grove, IL, USA), and Leica IM 50 Image
Manager software (DM4000B; Leica Microsystems, Inc.) was used to
measure the bronchial wall inner perimeter (Pi), bronchial wall
peripheral area (Wat1), the bronchial wall area (Wat2). The total
bronchial wall area (Wat) and bronchial wall thickness were
calculated as follows: Wat=Wat1+Wat2; bronchial wall
thickness=Wat/Pi. These measurements were used to evaluate airway
remodeling (10).
Masson staining
Masson staining was performed as previously
described (15). To estimate the
extent of collagen fiber deposition, staining was categorized into
four levels: i) Level 0, no collagen or only a small quantity of
filamentous collagen; ii) level 1, few slender and fasciculate
collagen fibers; iii) level 2, collagen fiber fusion into a thin
strip; and iv) level 3, broad collagen fibers or collagen fibers
molded into small flakes. A total of 5 high-powered fields of each
specimen were reviewed and the indices of collagen deposition in
the 3 groups were calculated.
Immunohistochemistry
Expression levels of ORMDL3, MMP-9 and ERK in the
formalin-fixed, paraffin-embedded lung tissue sections were
assessed by immunohistochemical staining. Samples were incubated
with antibodies specific to ORMDL3 (dilution, 1:200; cat. no.
ab107639; Abcam, Cambridge, UK), ERK (dilution, 1:200; cat. no.
9102; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated (p)-ERK (dilution, 1:200; cat. no. 9103; Cell
Signaling Technology, Inc.) and MMP-9 (dilution, 1:200; cat. no.
AB19016; Merck KGaA) overnight, at 4°C, then staining was detected
with biotinylated secondary antibodies (goat anti-rabbit
horseradish peroxidase-IgG secondary antibodies; dilution, 1:2,000;
cat. no. SP-9001; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China) and DAB reagent (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) at 37°C in the incubator for 30 min. A
total of 10 fields of each specimen were reviewed, in 10 random
visual fields, and staining was assessed using ImageJ software
v.10.2 (https://imagej.nih.gov/ij/).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from lung tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). mRNA was transcribed into cDNA using the
PrimeScript RT Master Mix Perfect Real-Time kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was performed with
the Step One Plus Real-Time PCR system (Biosystems 7500; Takara
Biotechnology Co., Ltd.), using cDNA and SYBR-Green (Takara
Biotechnology Co., Ltd.). Expression levels of ORMDL3 and MMP-9
were normalized to GAPDH. The primers for qPCR were as follows:
ORMDL3, sense 5′-GGGGGTGGTCAGGAAAGAGGCT-3, antisense
5′-GGGTTGCCAGGAAGCCCACAAA-3; GAP DH, sense
5′-CCAGGTGGTCTCCTCTGACTT-3, antisense
5′-GTTGCTCGTAGCCAAATTCGTTGT-3; and MMP-9 sense
5′-CCTCTGGAGGTCGACGTGA-3; antisense 5′-TAGGCTTTCTCTCGGTACTGGAA-3.
PCR amplification conditions were as follows: initial denaturation
95°C for 10 min, followed by 95°C for 30 sec and 60°C for 1 min,
for a total of 40 cycles.
Western blotting
Lung tissue protein content was assessed using a
Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) to which proteinase inhibitors were added. Subsequently, 4xSDS
sample buffer was added and 40 µg protein was loaded on to a 15%
SDS-PAGE, which was probed with primary antibodies against ORMDL3
(dilution, 1:500; cat. no. ab107639; Abcam), ERK (dilution, 1:500;
cat. no. 9102; Cell Signaling Technology, Inc.), p-ERK (dilution,
1:500; cat. no. 9106; Cell Signaling Technology, Inc.), MMP-9
(dilution, 1:500; cat. no. AB19016; Merck KGaA) and β-actin
(dilution, 1:2,000; dilution, 1:5,000; cat. no. SC-130300; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C on a shaker for12
h. It was then probed with the secondary antibody, goat anti rabbit
horseradish peroxidase-IgG (dilution, 1:5,000; cat. no. ZB230;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.), at 24°C for 1
h. The scanned intensities were quantified using an
electrochemiluminescence kit (cat. no. WBKLS0050; EMD Millipore,
Billerica, MA, USA) and scanning the gels using the Step One
Software v2.1 (Bio-Rad Laboratories, Inc.). The band intensity was
analyzed by Image J software v 10.2 (https://imagej.nih.gov/ij/). Relative absorbance
values of each target protein were normalized relative to β-actin
expression and experiments were repeated in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
version 20.0 (IBM SPSS, Armonk, NY, USA). Data are presented as the
mean ± standard deviation. Multiple groups were compared using
one-way analysis of variance and the Kruskal-Wallis test. Pearson
correlation analysis was used to analyze the correlation between
various factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
Asthma mouse model
A mouse model of asthma was established as
previously described (8). Briefly,
animals were pre-sensitized with OVA and aluminum hydroxide, then
exposed to OVA 3 times per week for 6 weeks. To investigate the
capacity of budesonide to modify airway inflammation some mice were
treated with aerosol budesonide. After 6 weeks, the mice were
euthanized and the lungs were collected to assess airway
inflammation and damage.
Airway inflammation
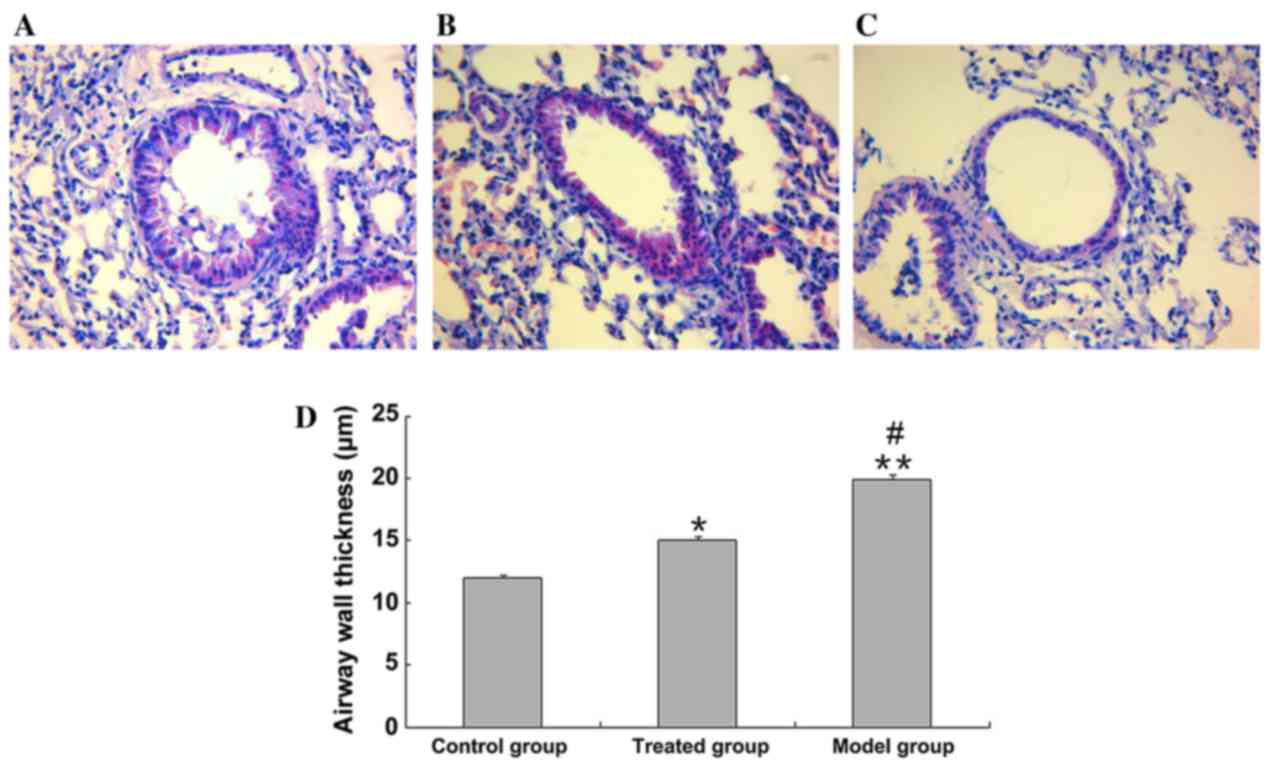
In the asthmatic model group, tracheal epithelial
cells were shed, the small airway layer was doubled, smooth muscle
layer was thickened and a large number of lymphocytes and
macrophages had infiltrated the bronchoalveolar walls (Fig. 1A-C). Airway structures in the lungs
of healthy control mice were intact and there were no signs of
epithelial cell proliferation, tube wall edema or inflammatory cell
infiltration. As indicated in Fig.
1D, bronchial hyperplasia and airway wall thickness was
significantly increased in the model group compared with the
control group (19.9±0.4 vs. 12.1±0.2 µm; P<0.01). In the
budesonide-treated group bronchial hyperplasia and airway wall
thickness was significantly reduced compared with the model groups
(15.1±0.3 vs. 19.9±0.4 µm; P<0.01). However, budesonide
treatment did not entirely ameliorate asthma-induced airway wall
thickening to the same level as the control group (15.1±0.3 vs.
12.1±0.2 µm; P<0.05; P=0.011; Fig.
1D); however, infiltration of inflammatory cells and damage to
airway structures was substantially alleviated following
administration of budesonide (Fig.
1).
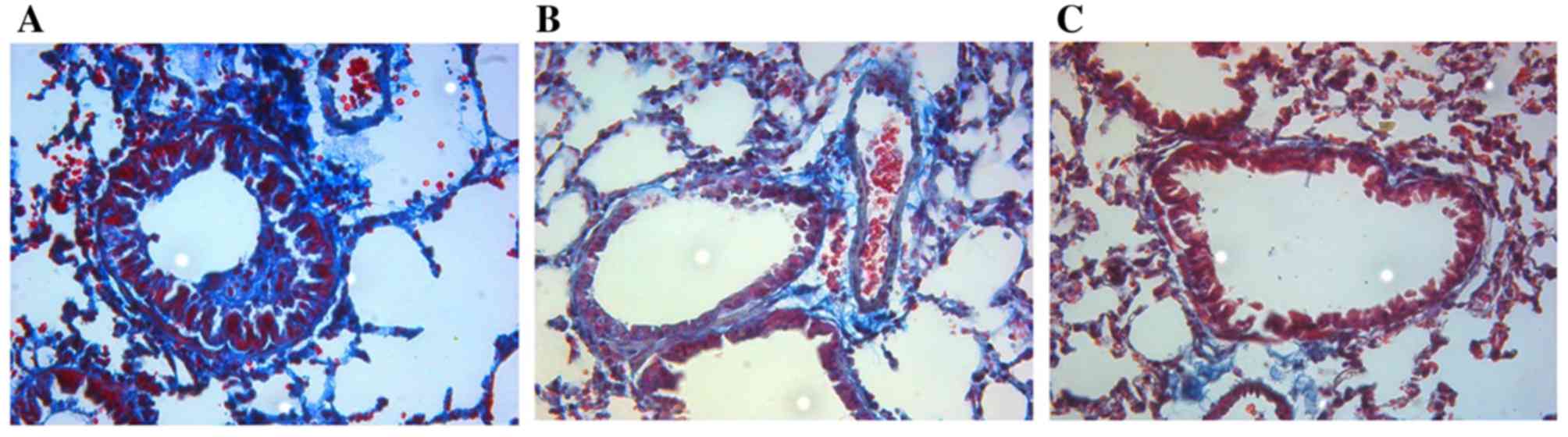
Airway remodeling
The airway walls were thin and mucosal structures
were intact, without obvious proliferation of smooth muscle
hyperplasia or collagen deposition in the control group. In the
asthmatic model group, the airway walls were thickened compared
with the control group and smooth muscle hyperplasia and
hypertrophy were observed. Large deposits of collagen surrounded
the vessels and alveolar interstitium, and the collagen-staining
index was significantly higher compared with the control group
(3.50±0.21 and 0.8±0.24; P<0.01). Following budesonide
treatment, the tracheal wall hyperplasia was reduced and the
collagen-deposition index was significantly reduced compared with
the asthmatic model group (1.90±0.23 and 3.50±0.21; P<0.01);
however, it was still higher than the control group (1.90±0.23 and
0.8±0.24, respectively; P<0.01; Fig. 2).
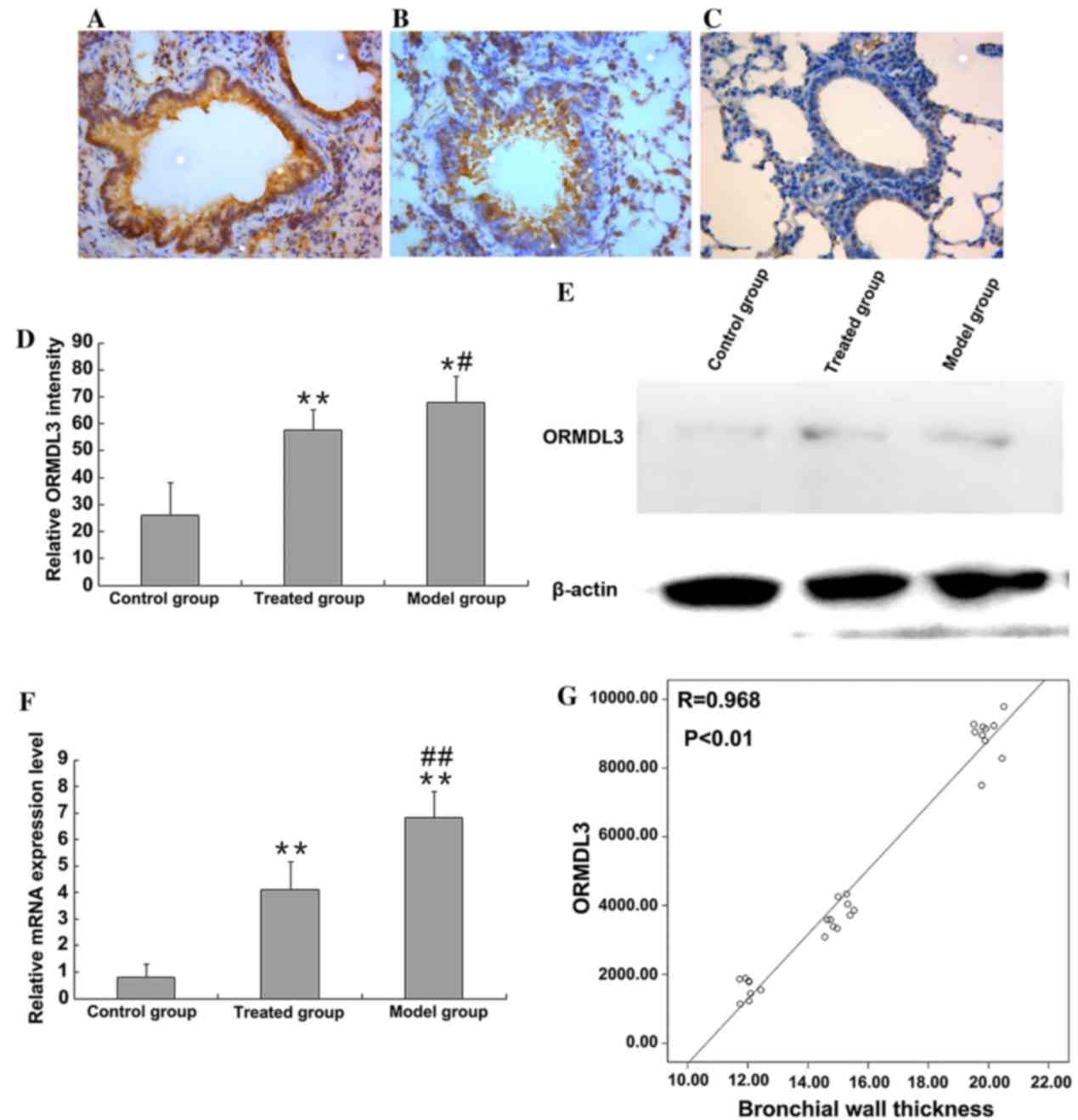
ORMDL3 expression level
ORMDL3 expression level in lung tissues was detected
by immunohistochemical staining. ORMDL3 was primarily located in
the nucleus and cytoplasm of inflammatory and epithelial cells, as
indicated in Fig. 3A-C. The level
of ORMDL3 immunohistochemical staining was quantified using ImageJ
(Fig. 3D). ORMDL3 expression level
was low in the control group; however, ORMDL3 expression increased
in asthmatic mice (67.98±9.85 and 26.08±12.18%; P<0.05,
P=0.026). Compared with the asthmatic model group, administration
of budesonide significantly reduced ORMDL3 expression (67.98±9.85
and 57.56±7.57%; P<0.05, P=0.021); however, the treatment did
not entirely reduce the level of ORMDL3 expression to healthy
control levels (57.56±7.57 and 26.08±12.18%; P<0.01; Fig 3D). Western blotting confirmed these
observations (Fig. 3E), and to
further confirm these results, the level of ORMDL3 mRNA in lung
tissues was assessed by RT-PCR (Fig.
3F). The ORMDL3 mRNA level in the lung tissues of asthmatic
model mice was significantly elevated when compared with healthy
control mice (P<0.01) and administration of budesonide to
asthmatic mice significantly reduced the mRNA expression of ORMDL3
(P<0.01), however, not to healthy control levels (P<0.01).
Upregulation of ORMDL3 protein was significantly positively
correlated with bronchial wall thickness (r=0.968, P<0.01;
Fig. 3G). As bronchial wall
thickening is a characteristic feature of asthma airway remodeling,
these data indicated that ORMDL3 may serve a key role in airway
remodeling.
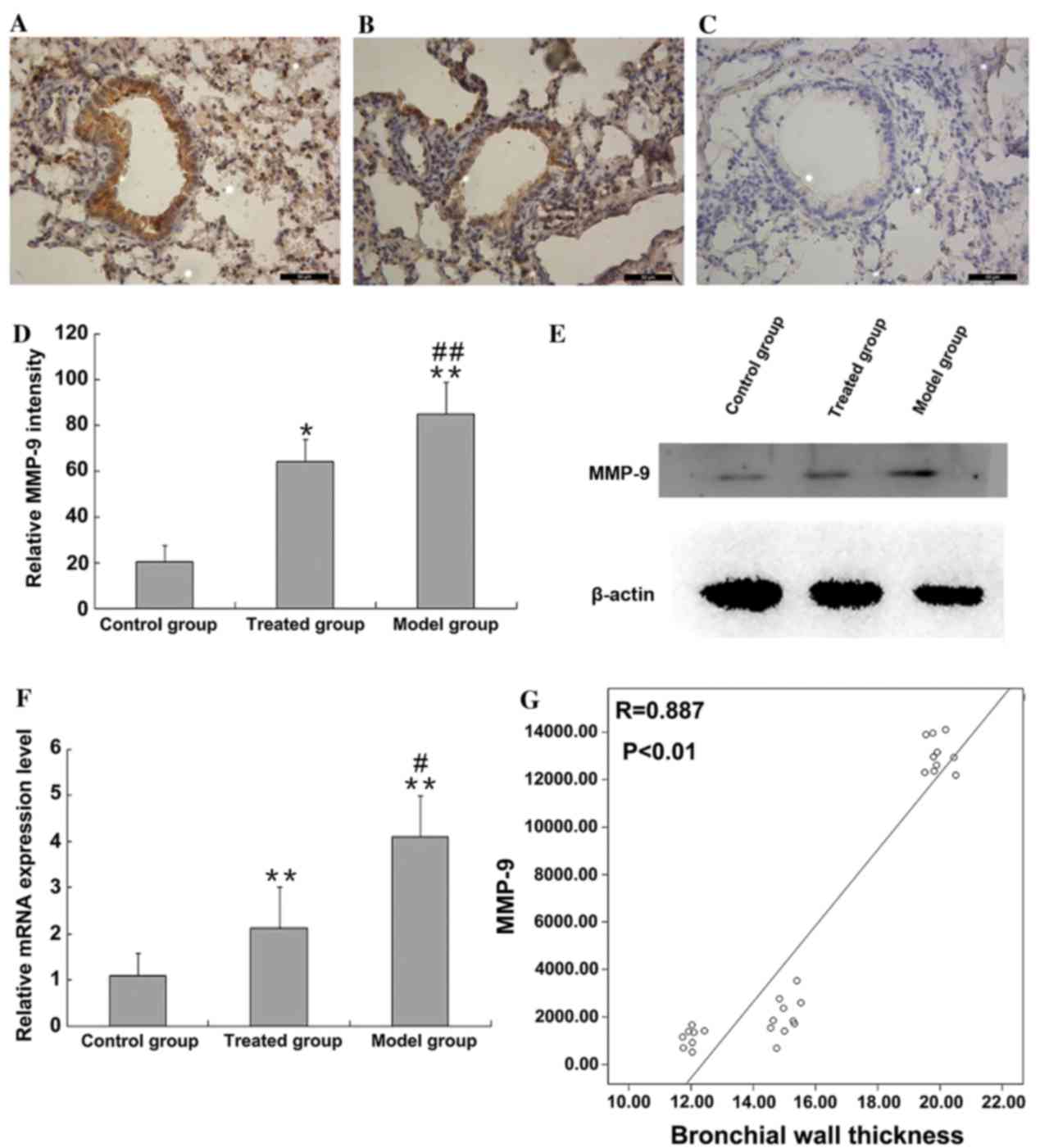
MMP-9 expression level
MMP-9 expression level of lung tissues was detected
by immunohistochemical staining. MMP-9 was primarily located in the
nucleus and cytoplasm of epithelial cells, as indicated in Fig. 4A-C. The level of MMP-9
immunohistochemical staining was quantified using ImageJ software.
Low levels of MMP-9 expression were detected in the control group;
however, MMP-9 expression was greater in asthmatic mice (85±13.57
and 20.28±7.33%, P<0.01; Fig.
4D). Administration of budesonide significantly reduced MMP-9
expression level (85±13.57 and 64.07±9.53%, P<0.01; Fig. 4D); however, did not reduce the
level of MMP-9 expression to healthy control levels (64.07±9.53 and
20.28±7.33%, P<0.05; P=0.013 Fig
4D). Western blotting confirmed these observations (Fig. 4E). Additionally, the mRNA MMP-9
level in lung tissues was assessed by RT-qPCR (Fig. 4F). MMP-9 mRNA level in the lung
tissues of asthmatic model mice was significantly increased
compared with healthy control mice (P<0.01; Fig. 4F) and treatment with budesonide of
asthmatic mice significantly reduced the mRNA expression of MMP-9
(P<0.05; P=0.011); however, not to healthy control levels
(P<0.01). Upregulation of MMP-9 protein expression was
significantly positively correlated with bronchial wall thickness
(r=0.887, P<0.01; Fig. 4G).
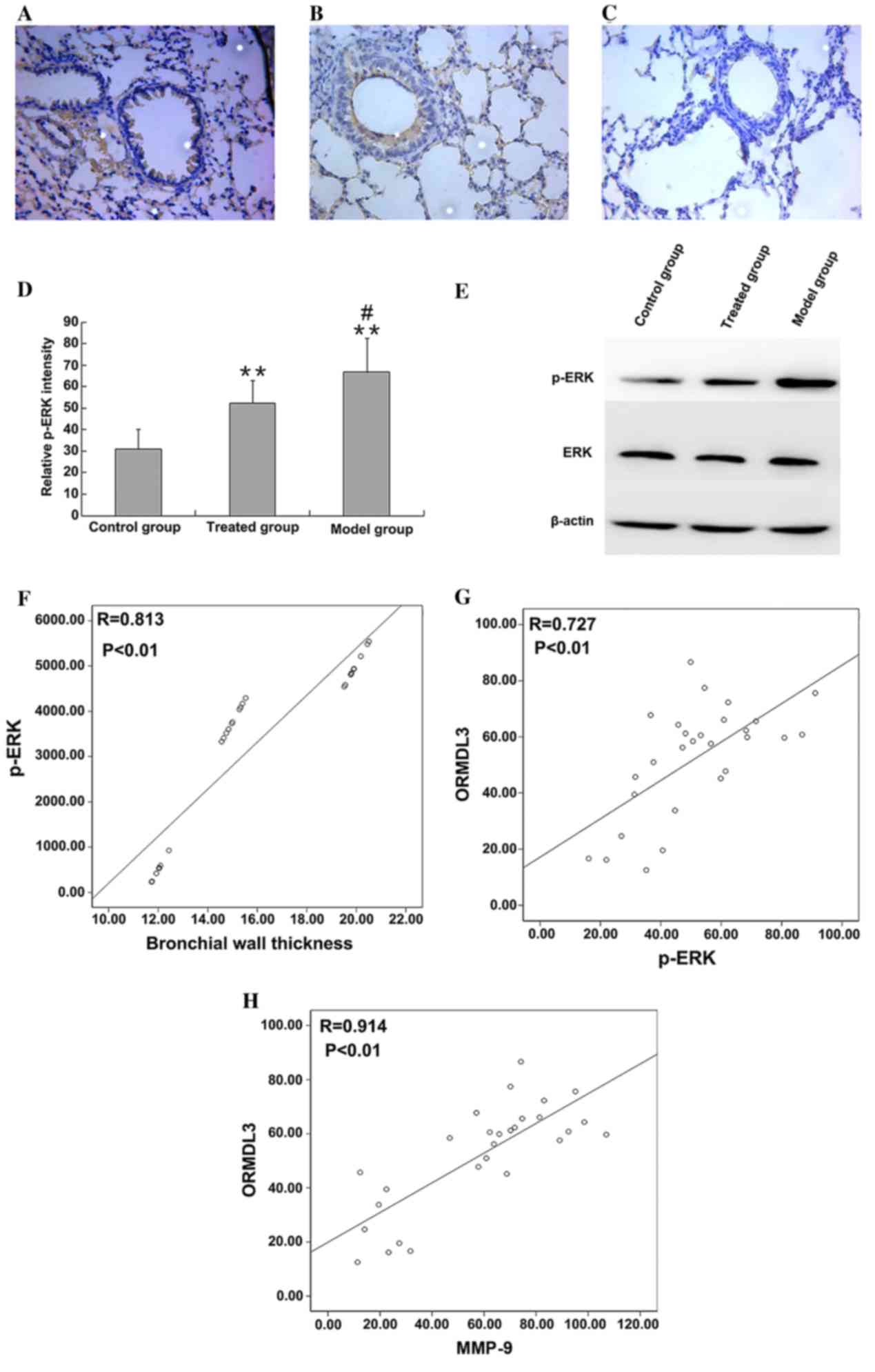
ERK and p-ERK expression
p-ERK level in lung tissues was detected by
immunohistochemical staining. p-ERK was primarily located in the
nucleus and cytoplasm of inflammatory and epithelial cells, as
indicated in Fig. 5A-C. p-ERK
immunohistochemical staining was quantified using ImageJ. The p-ERK
level was low in the control group; however, p-ERK was
significantly increased in asthmatic mice (66.79±15.7 and
31.04±9.39%, respectively; P<0.01). Administration of budesonide
significantly reduced the p-ERK level (52.38±10.42%) compared with
the model group (P<0.05; P=0.015), but did not entirely reduce
the level of p-ERK to healthy control levels (52.38±10.42 and
31.04±9.39%, respectively; P<0.01; Fig. 5D). Western blotting confirmed that
while the expression level of ERK did not differ between the
groups, the level of p-ERK protein was higher in asthmatic mice
(P<0.01), and significantly reduced by administration of
budesonide (P<0.01; Fig. 5E).
p-ERK levels, measured using western blotting, were also
significantly positively correlated with bronchial wall thickness
(r=0.813, P<0.01; Fig. 5F).
Correlation between ORMDL3, MMP-9 and
p-ERK levels
Correlational analysis was performed according to
the results of western blotting. The present study determined that
protein levels of ORMDL3 were significantly positively associated
with p-ERK (r=0.727; P<0.01; Fig.
5G) and MMP-9 (r=0.914, P<0.01; Fig. 5H).
Discussion
ORMDL3 was previously defined as a novel oncogene,
that may contribute to airway remodeling (16). The current study determined that
ORMDL3 was upregulated in the lung tissues of an asthma mouse
model. ORMDL3 was primarily located in the nucleus and cytoplasm of
inflammatory and epithelial cells. Expression of ORMDL3 occurred
along with markers of airway remodeling and pathological signs of
airway remodeling, including epithelial cell shedding, tube wall
edema, smooth muscle hyperplasia, collagen deposition and
inflammatory cell infiltration were observed.
Airway remodeling may be a secondary process
occurring in response to a chronic inflammatory environment and
involves increased proliferation of inflammatory cells, epithelial
cells and cytokine release. These factors affect the airway
structures and pulmonary functions (11,17).
The present study identified exacerbated airway modeling in the
OVA-induced asthma model, which was reduced by the administration
of budesonide.
In the asthma mouse model established in the present
study the expression of ORMDL3, MMP-9 and p-ERK was positively
associated bronchial wall thickness, an indicator of airway
remodeling severity. ORMDL3 was previously reported to be
associated with asthma (18,19).
Allergen challenge was reported to induce a 127-fold increase in
ORMDL3 mRNA expression in the bronchial epithelium of wild-type
mice (20–24). Previous studies have also revealed
that ORMDL3 increased expression of proteases, including MMP-9,
which is also associated with asthma pathogenesis (25–30).
MMP-9 is produced by epithelial and inflammatory cells and
upregulates release of matrix-associated growth factors, including
transforming growth factor-β1, leading to the degradation of
collagen and thus induces infiltration of inflammatory cells
through the basement membrane and ECM, accelerating collagen
deposition. Therefore, these processes induce airway remodeling
(31,32). Thus, our observation that ORMDL3,
MMP-9 and p-ERK expression were positively associated with
bronchial wall thickness was consistent with previous studies.
In the present study ORMDL3 expression was also
positively associated with levels of MMP-9 and p-ERK; therefore,
ORMDL3 may induce the activation of MMP-9, and ORMDL3 may promote
airway remodeling through the ERK/MMP-9 pathway. Previous studies
of ORMDL3 expression in HEK 293 cells suggested that the primary
impact of ORMDL3 expression is on the p-ERK/ERK pathway (14,33).
The ERK1/2 signaling pathway may stimulate extracellular signal
transduction from the cell to the nucleus, through phosphorylation
of transcription factors that regulate the activation of nuclear
factor-κB, ETS protooncogene 1 and other transcription factors,
thereby regulating the transcription of target genes, such as MMP-9
(34). Activation of the
ROS-ERK-MMP-9 pathway may induce metastasis (35).
Administration of budesonide ameliorated airway
remodeling in the OVA-induced asthma model and reduced the
expression levels of ORMDL3, MMP-9 and p-ERK, indicating that
budesonide may reduce airway remodeling by inhibiting the
ORMDL3/ERK/MMP-9 pathway.
In conclusion, the findings of the present study
suggest that ORMDL3 may induce MMP-9 expression in asthmatic mice
via the ERK pathway, by inducing airway remodeling and that
budesonide may alleviate bronchial hyperplasia and collagen
deposition through the ORMDL3/ERK/MMP-9 pathway. However, the
association between ORMDL3 and the ERK/MMP-9 pathway remains to be
determined. Future investigations should identify the effects of
ORMDL3 on the ROS-ERK/MMP-9 pathway using in vitro
experiments and ORMDL3-knockout mice. Further understanding of the
mechanisms responsible for pathological remodeling in asthma may
highlight novel therapeutic strategies.
Acknowledgements
The authors would like to thank Dr Ningjiao at
Traditional Chinese Hospital (Zibo, China) for proofreading of the
manuscript. The present study was supported primarily by the
Shandong Provincial Natural Science Foundation (grant no.
ZR2013HQ034) and partly by the National Natural Science Foundation
of China (grant no. 81500020).
References
|
1
|
Liu YN, Zha WJ, Ma Y, Chen FF, Zhu W, Ge
A, Zeng XN and Huang M: Galangin attenuates airway remodelling by
inhibiting TGF-β1-mediated ROS generation and MAPK/Akt
phosphorylation in asthma. Sci Rep. 5:117582015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller M, Rosenthal P, Beppu A, Mueller
JL, Hoffman HM, Tam AB, Doherty TA, McGeough MD, Pena CA, Suzukawa
M, et al: ORMDL3 transgenic mice have increased airway remodeling
and airway responsiveness characteristic of asthma. J Immunol.
192:3475–3487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y, Wang J, Li H, Sun L, Wang Y and Han
X: The effects of budesonide on angiogenesis in a murine asthma
model. Arch Med Sci. 9:361–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin R, Xu HG, Yuan WX, Zhuang LL, Liu LF,
Jiang L, Zhu LH, Liu JY and Zhou GP: Mechanisms elevating ORMDL3
expression in recurrent wheeze patients: Role of Ets-1, p300 and
CREB. Int J Biochem Cell Biol. 44:1174–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hrdlickova B and Holla LI: Relationship
between the 17q21 locus and adult asthma in a Czech population. Hum
Immunol. 72:921–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moffatt MF, Kabesch M, Liang L, Dixon AL,
Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, et
al: Genetic variants regulating ORMDL3 expression contribute to the
risk of childhood asthma. Nature. 448:470–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirota T, Harada M, Sakashita M, Doi S,
Miyatake A, Fujita K, Enomoto T, Ebisawa M, Yoshihara S, Noguchi E,
et al: Genetic polymorphism regulating ORM1-like 3
(Saccharomyces cerevisiae) expression is associated with
childhood atopic asthma in a Japanese population. J Allergy Clin
Immunol. 121:769–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller M, Tam AB, Cho JY, Doherty TA, Pham
A, Khorram N, Rosenthal P, Mueller JL, Hoffman HM, Suzukawa M, et
al: ORMDL3 is an inducible lung epithelial gene regulating
metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci
USA. 109:16648–16653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Kim CH, Ahn JH, Kim MS, Kim SC,
Lee SY, Kwon SS, Kim YK, Kim KH, Moon HS, et al: Time sequence of
airway remodeling in a mouse model of chronic asthma: The relation
with airway hyperresponsiveness. J Korean Med Sci. 22:183–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schönbeck U, Mach F and Libby P:
Generation of biologically active IL-1 beta by matrix
metalloproteinases: A novel caspase-1-independent pathway of IL-1
beta processing. J Immunol. 161:3340–3346. 1998.PubMed/NCBI
|
|
11
|
Felsen CN, Savariar EN, Whitney M and
Tsien RY: Detection and monitoring of localized matrix
metalloproteinase upregulation in a murine model of asthma. Am J
Physiol Lung Cell Mol Physiol. 306:L764–L774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan YY, Hsiao JR, Chang KC, Chang JS, Chen
CW, Lai HC, Wu SY, Yeh TH, Chang FH, Lin WH, et al: Epstein-Barr
virus latent membrane protein 2A promotes invasion of
nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction
of matrix metalloproteinase 9. J Virol. 86:6656–6667. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma HP, Li W and Liu XM: Matrix
metalloproteinase 9 is involved in airway inflammation in cough
variant asthma. Exp Ther Med. 8:1197–1200. 2014.PubMed/NCBI
|
|
14
|
Sands MF: Localization of matrix
metalloproteinase (MMP)-9 in lung tissue of a murine model of
allergic asthma. Immunol Invest. 41:87–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gueders MM, Foidart JM, Noel A and Cataldo
DD: Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs
in the respiratory tract: Potential implications in asthma and
other lung diseases. Eur J Pharmacol. 533:133–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van den Steen PE, Proost P, Wuyts A, Van
Damme J and Opdenakker G: Neutrophil gelatinase B potentiates
interleukin-8 tenfold by aminoterminal processing, whereas it
degrades CTAP-III, PF-4 and GRO-alpha and leaves RANTES and MCP-2
intact. Blood. 96:2673–2681. 2000.PubMed/NCBI
|
|
17
|
Hsu CH, Hu CM, Lu KH, Yang SF, Tsai CH, Ko
CL, Sun HL and Lue KH: Effect of selective cysteinyl leukotriene
receptor antagonists on airway inflammation and matrix
metalloproteinase expression in a mouse asthma model. Pediatr
Neonatol. 53:235–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferreira MA, McRae AF, Medland SE, Nyholt
DR, Gordon SD, Wright MJ, Henders AK, Madden PA, Visscher PM, Wray
NR, et al: Association between ORMDL3, IL1RL1 and a deletion on
chromosome 17q21 with asthma risk in Australia. Eur J Hum Genet.
19:458–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang FF, Huang Y, Li QB, Dai JH and Fu Z:
Single nucleotide polymorphisms in the ORM1-like 3 gene associated
with childhood asthma in a Chinese population. Genet Mol Res.
11:4646–4653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galanter J, Choudhry S, Eng C, Nazario S,
Rodríguez-Santana JR, Casal J, Torres-Palacios A, Salas J, Chapela
R, Watson HG, et al: ORMDL3 gene is associated with asthma in three
ethnically diverse populations. Am J Respir Crit Care Med.
177:1194–1200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murray LA, Argentieri RL, Farrell FX,
Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff
HL, et al: Hyper-responsiveness of IPF/UIP fibroblasts: Interplay
between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol.
40:2174–2182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao CN, Fan Y, Huang JJ, Zhang HX, Gao T,
Wang C, Wang T and Hou LF: The association of GSDMB and ORMDL3 gene
polymorphisms with asthma: A meta-analysis. Allergy Asthma Immunol
Res. 7:175–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura Y, Esnault S, Maeda T, Kelly EA,
Malter JS and Jarjour NN: Ets-1 regulates TNF-alpha-induced matrix
metalloproteinase-9 and tenascin expression in primary bronchial
fibroblasts. J Immunol. 172:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong KK: Recent developments in
anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent
Pat Anticancer Drug Discov. 4:28–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ha SG, Ge XN, Bahaie NS, Kang BN, Rao A,
Rao SP and Sriramarao P: ORMDL3 promotes eosinophil trafficking and
activation via regulation of integrins and CD48. Nat Commun.
4:24792013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cataldo D, Munaut C, Noël A, Frankenne F,
Bartsch P, Foidart JM and Louis R: MMP-2- and MMP-9-linked
gelatinolytic activity in the sputum from patients with asthma and
chronic obstructive pulmonary disease. Int Arch Allergy Immunol.
123:259–267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kavalar MS, Balantic M, Silar M, Kosnik M,
Korosec P and Rijavec M: Association of ORMDL3, STAT6 and TBXA2R
gene polymorphisms with asthma. Int J Immunogenet. 39:20–25. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian Y, Guan Y, Jia Y, Meng Q and Yang J:
Chloride intracellular channel 1 regulates prostate cancer cell
proliferation and migration through the MAPK/ERK pathway. Cancer
Biother Radiopharm. 29:339–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelley J, Kovacs EJ, Nicholson K and
Fabisiak JP: Transforming growth factor-beta production by lung
macrophages and fibroblasts. Chest. 99:(3 Suppl). 85S–86S. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shifren A, Witt C, Christie C and Castro
M: Mechanisms of remodeling in asthmatic airways. J Allergy
(Cairo). 2012:3160492012.PubMed/NCBI
|
|
31
|
Ameredes BT, Zamora R, Gibson KF, Billiar
TR, Dixon-McCarthy B, Watkins S and Calhoun WJ: Increased nitric
oxide production by airway cells of sensitized and challenged IL-10
knockout mice. J Leukoc Biol. 70:730–736. 2001.PubMed/NCBI
|
|
32
|
Liu P and Wilson MJ: miR-520c and miR-373
upregulate MMP9 expression by targeting mTOR and SIRT1 and activate
the Ras/Raf/MEK/Erk signaling pathway and NF-κB factor in human
fibrosarcoma cells. J Cell Physiol. 227:867–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cantero-Recasens G, Fandos C,
Rubio-Moscardo F, Valverde MA and Vicente R: The asthma-associated
ORMDL3 gene product regulates endoplasmic reticulum-mediated
calcium signaling and cellular stress. Hum Mol Genet. 19:111–121.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bergeron C, Tulic MK and Hamid Q: Airway
remodelling in asthma: From benchside to clinical practice. Can
Respir J. 17:e85–e93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Montagut C and Settleman J: Targeting the
RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 283:125–134.
2009. View Article : Google Scholar : PubMed/NCBI
|