Introduction
Endothelial dysfunction associated with type 2
diabetes mellitus and insulin resistance (1) is involved in multiple complications
of diabetes. Numerous studies have suggested that endothelial cell
dysfunction increases macromolecule permeability (2,3),
causes abnormal vasodilation/vasoconstriction (4–6) and
results in procoagulant activation (7).
Endothelial migration contributes to vascular repair
and is inhibited under conditions of high glucose (8). Furthermore, previous data have shown
that cluster of differentiation (CD)97, a surface molecule
abundantly expressed in endothelial cells, can stimulate migration,
invasion and angiogenesis (9).
Therefore, the present study hypothesized that CD97 may act to
promote endothelial cell migration under high glucose treatment
conditions.
CD97 is encoded by the adhesion G protein-coupled
receptor 5 (ADGRE5) gene (10) and
is a member of the epidermal growth factor (EGF)-seven
transmembrane family, which belongs to the adhesion family of G
protein-coupled receptors (GPCRs) (11–13).
CD97 is widely expressed on the surface of lymphoid cells,
macrophages, smooth muscle cells and several types of tumor cell
(14–19). A previous study also found that
CD97 enhances cell invasion via Ras homolog (RHO) and extracellular
signal-regulated kinase activation by associating with
lysophosphatidic acid receptor 1 in prostate cancer cells (19).
In the present study, a lentivirus-mediated
endothelial cell line overexpressing CD97 was constructed and the
effects of CD97 on cell migration were investigated. It was found
that CD97 ameliorated the inhibition of high glucose-induced
endothelial cell migration. In addition, the molecular mechanism
whereby high levels of glucose regulate the expression of CD97 was
also characterized in detail.
Materials and methods
Cell culture
The human umbilical vein endothelial cell (HUVEC)
line was purchased from American Type Culture Collection (Manassas,
VA, USA). The cells were cultured in an incubator at 37°C, 5%
CO2in complete medium containing10% FBS (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) and Dulbecco's modified
Eagle's medium (DMEM; 5.5 mM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Prior to exposure to glucose at
concentrations of 5.5, 10 or 33 mM for 24 h, 1×105cells
were starved of FBS for 12 h at 37°C in the incubator. A 5.5 mM
concentration of glucose was used as a control.
Small interfering (si)RNA and plasmid
transfection
Transfection of the cells was performed using
Polyplus transfection reagent (jetPPRIME; Polyplus Transfection,
Illkirch, France). In brief, 1 µl of siRNA (50 µM; Ruibo
Biotechnology Co., Ltd., Guangzhou, China) or 2.5 µg plasmid cDNA
(ViGene Biosciences, Inc., Shandong, China) was added to 200 µl of
jetPRIME buffer. Following mixing with 4 µl of jetPRIME, the
solution was vortexed for 10 sec. Following incubation for 10 min
at room temperature, the mixture was added into one well of a
6-well plate with 1×106 cells cultured in 1 ml complete
medium. Following culture for an additional 24 h, the cells were
harvested and used in subsequent assays.
Western blot analysis
The cells were lysed on ice in RIPA lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China), which
included a cocktail of protease inhibitors (Cell Signaling
Technology, Inc., Danvers, MA, USA) and protein levels was
determined by bicinchoninic acid assay method. Subsequently, 40 µg
of total protein was loaded onto 10% SDS-PAGE gels, electrophoresed
and transferred onto PVDF membranes (EMD Millipore, Billerica, MA,
USA). Following blocking using 5% non-fat milk (Nestlé, Vevey,
Switzerland), antibody incubation and immunoblotting using ECL
(Kangwei, Beijing China) were used to detect fluorescence. The
following primary antibodies were used: anti-human CD97 (cat. no.
ab108368; 1:1,000; Abcam, Cambridge, MA, USA), anti-human rhodopsin
(RHO; cat. no. ab5417; 1:1,000; Abcam), anti-human Ras-related C3
botulinum toxin substrate 1 (RAC; cat. no. ab33186; 1:1,000;
Abcam), anti-human cell division cycle 42 (CDC42; cat. no.
ab155940; 1:1,000; Abcam), anti-human actin-related protein 2
(ARP2; cat. no. ab47654; 1:1,000; Abcam), anti-human signal
transducer and activator of transcription 1 (STAT1; cat. no.
ab3987; 1:1,000; Abcam) and anti-human GAPDH (cat. no. CW0101M;
1:2,000; Nuoyang, Hangzhou, China). All primary antibodies were
incubated at 4°C overnight. The relevant secondary antibody was
goat anti-rabbit antibody (cat. no. A0208; 1:5,000; Beyotime
Institute of Biotechnology) incubated at room temperature for 1
h.
Flow cytometry
Following treatment, the cells were washed twice
with PBS and then incubated with FITC-conjugated anti-CD97 (BD
Biosciences, Franklin Lakes, NJ, USA). The subsequent analysis of
the expression of CD97 was performed using a flow cytometer (BD
Biosciences).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from the endothelial cells
using an RNAsimple total RNA kit (Tiangen Biotech Co., Ltd.,
Beijing, China). First-strand cDNA was synthesized using a
Primescript RT reagent kit (Takara Bio, Inc., Otsu, Japan). A total
of 5 ng cDNA of each sample was subjected to PCR reactions
consisting of 40 cycles of 95°C for 10 sec, 68°C for 30 sec and
72°C for 30 sec using SYBR-Green Premix Ex Taq (Takara Bio, Inc.,
Otsu, Japan) and detected by ABI PRISM 7500 Sequence Detection
System (Thermo Fisher Scientific, Inc.). The relative expression
level results were analyzed using the 2−ΔΔCq method
(20). Finally, the PCR products
were examined using DNA agarose gel electrophoresis. The following
primers were used for RT-PCR analysis: CD97, forward
5′-ACTCTGCCGGGAGCTGAAAC-3′ and reverse 5′-TGGATGGTGACCTCGGCTGA-3′;
18S, forward 5′-CCGCACTTGATACGGTTCCT-3′ and reverse
5′-CCAGGCTGATCTATCCCACTG-3′.
Wound healing assay
Endothelial cells were seeded at density
1×105 and cultured in 6-well plates to 80–90% confluence
and were serum-starved for 24 h. Two scratches were then introduced
to the cell layer in each well using a 100–1,000 µl tip. Following
washing twice with PBS, the cells were incubated in DMEM with
glucose (33 mM), and 5.5 mM glucose DMEM treatment was used as a
control. Images of the same regions were captured at 0 and 24 h
following stimulation with light microscope (Stemi 2000; Zeiss
GmbH, Jena, Germany); the paired images were analyzed.
Chromatin immunoprecipitation
(ChIP)
ChIP assays were performed according to the
manufacturer's protocol using a kit from Cell Signaling Technology,
Inc. In brief, for each group, 1×107 cells were fixed
with 1% formaldehyde (Aladdin Industrial, Inc., Nashville, TN,
USA). Subsequently, chromatin DNA was sheared using micrococcal
nuclease (Cell Signaling Technology, Inc.) to yield DNA fragments
ranging between 300 and 900 bp. Following preclearance with 10 µl
protein A/G agarose beads, the samples were incubated with rabbit
anti-STAT1 monoclonal antibody (2 µg; cat. no. ab3987; 1:100;
Abcam) or control rabbit IgG antibody (2 µg; cat. no. 2729; 1:100;
Cell Signaling Technology, Inc.). The samples were then
immunoprecipitated by incubation with 30 µl protein A/G agarose
beads, and complexes were reverse cross-linked by protease K and
NaCl (5 M) treatment. Finally, DNA was purified using a DNA
purification kit (Cell Signaling Technology, Inc.). The content of
the purified DNA was assessed using RT-quantitative PCR analysis as
aforementioned. The following primers were used to amplify the
STAT1 binding element in the promoter of the CD97 gene: Forward
5′-TAGCGCTAAGACACAGTTGGACC-3′ and reverse
5′-ACTCGCCAGTTGCAACAGTTC-3′.
Generation of the CD97-Cas9
endothelial cell line
To knockout CD97 in an endothelial cell line,
experiments were performed according to a previously published
protocol (21). In brief, a custom
designed gRNA for CD97 was cloned into the Pep-ko (Pep-330x)
plasmid. Then the plasmid was transfected into endothelial cells at
a density 3×105 using Polyplus transfection reagent
(JetPRIME; Polyplus Transfection) at 37°C for 24 h, the endothelial
cells were filtered using puromycin (2 µg/ml; Sigma-Aldrich; Merck
Millipore). The surviving cells were seeded into a 96-well plate
and cultured into monoclonal cell lines for further assessment of
the expression of CD97. The CD97 gRNA sequences were as follows:
Forward 5′-accgTCCGGTGGACGAGGCGGCGG-3′ and reverse
5′-accgCGGCCGACCACCACCGCTTC-3′.
Construction of stable CD97-expressing
endothelial cells by lentivirus transfection
A customized CD97-overexpression lentivirus vector
was obtained from ViGene Biosciences, Inc. Endothelial cells were
transfected with the CD97 lentivirus and screened/selected using
puromycin (2 µg/ml; Sigma-Aldrich; Merck Millipore). The surviving
cells were cultured into multiple monoclonal cell lines and were
assessed for the expression of CD97 using western blot
analysis.
Immunofluorescence staining
Endothelial cells were seeded at a density of
1×105 in a cell culture dish (Nest Scientific, Rahway,
NJ, USA). Following treatment with glucose (control, 5.5 mM; high,
33 mM) for 24 h, the cells were fixed using 1% formaldehyde and
incubated with FITC-phalloidin (Thermo Fisher Scientific, Inc.).
Finally, the cells were observed using a Zeiss Confocal Imaging
system (Zeiss GmbH).
Animal model
A total of 20 male C57BL/6 J mice (age, 4 weeks;
weight, 20±4 g, maintained at 20°C, normal lighting) were purchased
from the Shanghai Institute for Biological Sciences (Shanghai,
China). Diabetes was induced in these mice by intraperitoneal
injection of STZ (70 mg/kg; Sigma-Aldrich; Merck Millipore), and
the mice were continually fed a high-fat diet for 3 months. Mice
with glucose levels >16.4 mM were considered a successful
diabetic mouse model. Three months later, mice were sacrificed by
cervical dislocation. The aortic endothelium was harvested and
subjected to analysis of the expression of CD97 using
immunohistochemistry (IHC).
IHC
Following deparaffinization, hydration and blocking,
the paraffin-embedded tissue transverse sections (thickness, 5 µm)
were incubated with primary antibodies (CD97; cat. no. ab108368,
1:200; Abcam) for 2 h at 37°C. Following incubation, the tissue
sections were washed with PBS for 15 min, followed by incubation
with anti-digoxigenin-conjugated secondary antibodies (1:200; cat.
no. ZX300, Nuoyang, Beijing, China) for 1 h at room temperature.
Subsequently, the sections were washed again with PBS and incubated
with DAB reagent (ViGene Biosciences, Inc., China). Finally, images
of the stained samples were captured using an optical microscope
(Olympus, Tokyo, Japan).
Dual luciferase reporter assay
Genomic DNA was extracted from the endothelial cells
using a Genome DNA Extract kit (Kangwei, China). The 2,000, 1,500,
1,000 and 500 bp upstream regions from the transcription initiation
site of the ADGRE5 promoter were amplified, gel-purified and
sub-cloned into a pGL3-basic luciferase reporter vector (Promega
Corporation, USA) between the HindIII and SacI sites. The primer
sequences used were as follows: 2 k-promoter, forward
5′-CAAGTCACGCCGAATCCAATA-3′ and reverse
5′-CGGTCCTGAACTTTCCGAGATG-3′; 1.5k-promoter, forward
5′-GACGGCTCAGGACCTACATAA-3′ and reverse
5′-CGGTCCTGAACTTTCCGAGATG-3′; 1k-promoter, forward
5′-GAATCCCAATACGTCAAGCCA-3′ and reverse
5′-CGGTCCTGAACTTTCCGAGATG-3′; 0.5k-promoter, forward
5′-CTAAGCAACCGTGTCGAACAC-3′ and reverse
5′-CGGTCCTGAACTTTCCGAGATG-3′. Each vector was transfected into the
endothelial cells with glucose treatment (control, 5.5 mM; high, 33
mM) for 24 h. The activity of each was determined using a dual
luciferase reporter assay system.
Transcription factor (TF) filter plate
assay
TF filter plate (Signosis, Santa Clara, CA, USA)
assays were performed according to the manufacturer's protocol. In
brief, endothelial cells were collected to extract nuclear
proteins. TF DNA complexes were created by mixing TF probes with a
500 bp sequence of the ADGRE5 promoter. Subsequently, complexes
were separated from free probes and the bound probes were eluted.
Following hybridization of the eluted probes on a hybridization
plate, which included nuclear proteins, the relative activity was
detected using a luminometer (BioTek Instruments, Inc., Winooski,
VT, USA).
Statistical analysis
All significant differences between the mean were
analyzed using GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). Data are presented as the mean ± standard
deviation. Comparisons were performed using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of CD97/ADGRE5 in high
glucose-induced HUVECs and diabetic mice
The expression of CD97 in endothelial cells
subjected to glucose treatment was assessed using western blot
analysis and flow cytometry; a basal concentration (5.5 mM) of
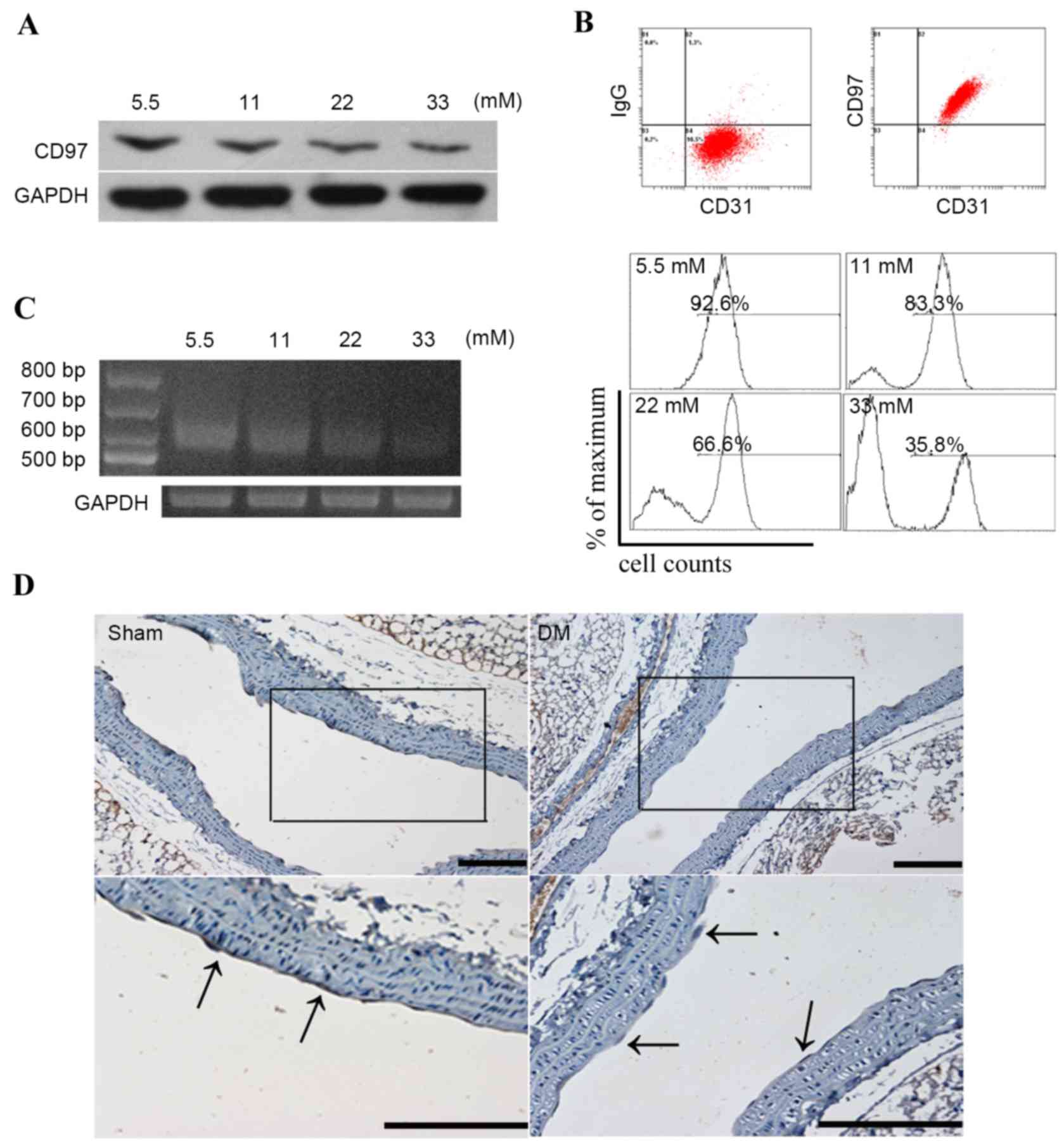
glucose was used as a control. As shown in Fig. 1A and B, the expression of CD97 was
reduced when exposed to an increasing glucose concentration
gradient. Alterations in the three CD97 isoforms, CD97 (EGF1,2,5),
CD97 (EGF1,2,3,5) and CD97 (EGF1,2,3,4,5), were also analyzed in
high glucose-induced endothelial cells. As shown in Fig. 1C, the endothelial cells
predominantly expressed CD97 (EGF1,2,5), which was ~600 bp in
length, and its pattern was altered in response to high glucose
treatment. Furthermore, staining of the aortic endothelial tissues
from the diabetic mice using anti-CD97 antibody showed lower
expression of CD97, compared with the physiological saline-treated
group (Fig. 1D).
Overexpression of CD97 (EGF1,2,5) in
HUVECs attenuates high glucose-induced dysregulation of
migration
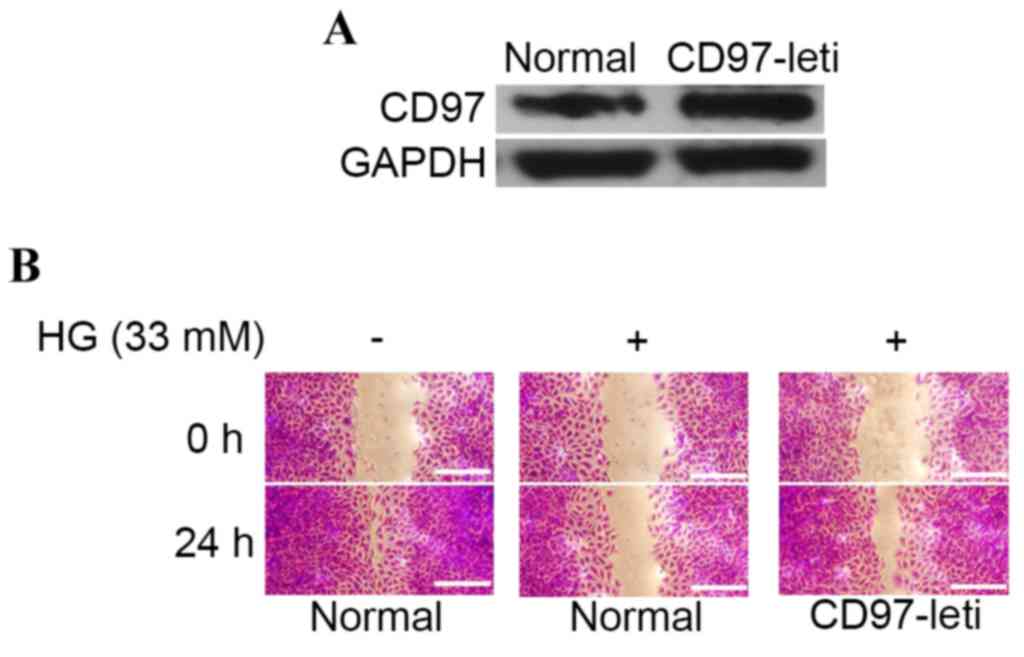
To determine whether CD97 (EGF1,2,5) enhanced the
dysregulation of endothelial cell migration induced by high
glucose, the present study initially constructed a CD97
(EGF1,2,5)-overexpression endothelial cell line via lentivirus
transfection (Fig. 2A). As shown
in Fig. 2B, high glucose
stimulation reduced the mobility ratio of the endothelial cells,
whereas the overexpression of CD97 partially attenuated this.
CD97 promotes actin enrichment and
reorganization in a CDC42-ARP2 dependent manner
To analyze the effects of CD97 in the cytoskeleton
of endothelial cells, a CD97-knockout endothelial cell line
(CD97-Cas9) was constructed using clustered regularly interspaced
short palindromic repeats (CRISPR)/CRISPR-associated protein 9
(Cas9) technology (Fig. 3A).
Cytoskeletal staining revealed that the expression level of CD97
was associated with membrane ruffling and lamellipodia formation
(Fig. 3B). In the previous study
by Wojciak-Stothard et al (22), RHO, RAC and CDC42 were found to
control reorganization of the actin cytoskeleton and to promote
migration in endothelial cells. The present study hypothesized that
these GTP-binding proteins are also activated in the
CD97-overexpressing cell line. As shown in Fig. 3C, the expression of CDC42 was
positively regulated by CD97. Furthermore, to elucidate the
mechanism underlying the effects of CD97 on cytoskeletal
alterations, an evolutionarily conserved actin nucleation factor,
the ARP2/3 complex, which is necessary for lamellipodia extension
and cell migration in fibroblasts, was examined (23). As shown in Fig. 3D, the upregulation of CD97
increased the levels of ARP2, whereas the downregulation of CDC42,
induced by using siRNA to target the mRNA transcripts encoding
CDC42, abrogated the increase levels of ARP2. This suggested that
CD97 promoted lamellipodia formation, which was dependent upon the
activation of CDC42 and ARP2.
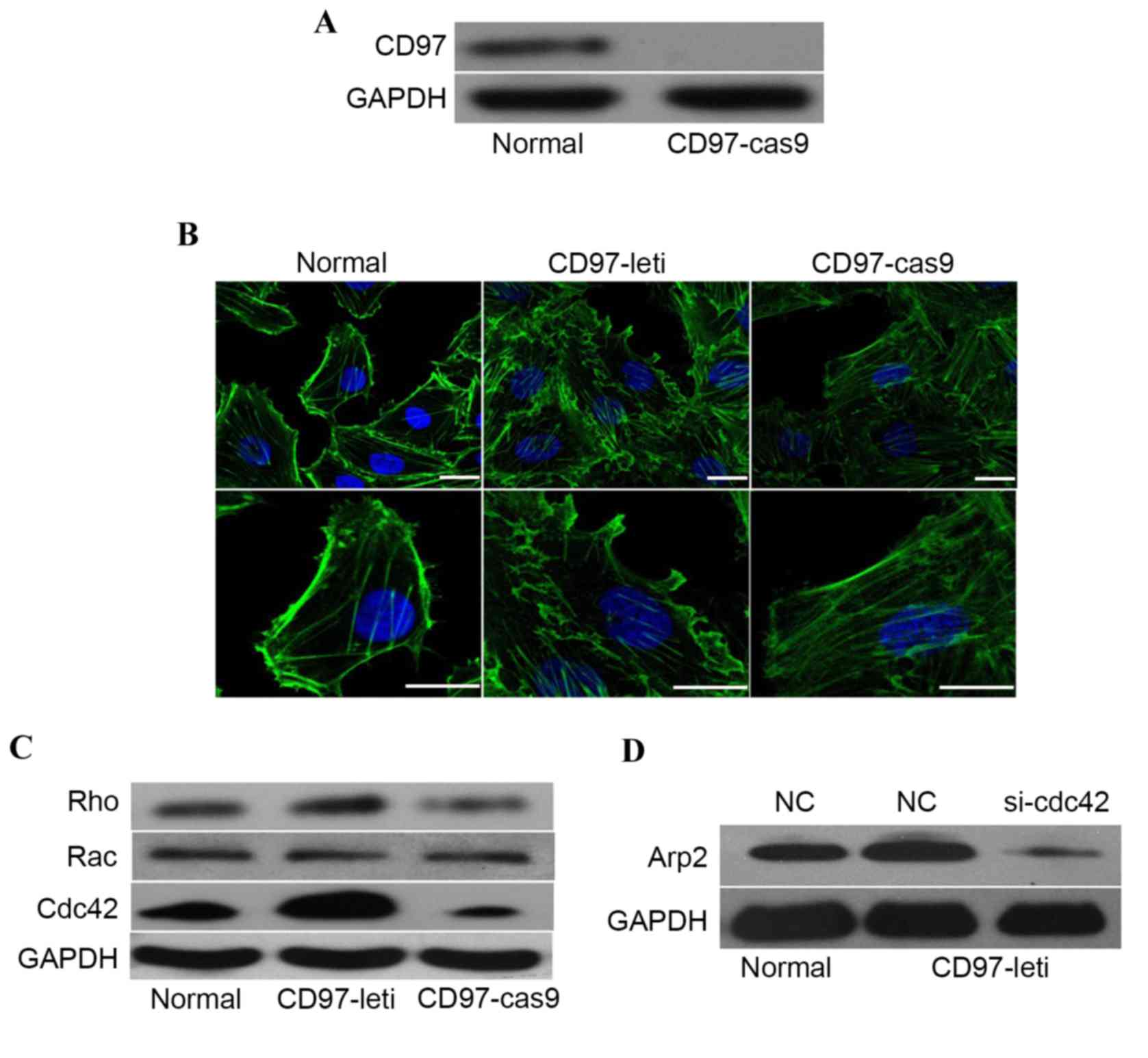 | Figure 3.CD97 promotes membrane ruffling and
lamellipodia formation in endothelial cells. (A) Validation of the
CD97 knockout status of endothelial cells generated by clustered
regularly interspaced short palindromic repeats/Cas9 using western
blotting. (B) Alterations in the distribution of F-actin in
endothelial cells, CD97-Cas9 endothelial cells or CD97-lentivirus
endothelial cells. Stress fibers or lamellipodia are indicated by
white arrows. Scale bar, 3 µm. (C) Protein levels of RHO, RAC and
CDC42 in endothelial cells, CD97-Cas9 endothelial cells or
CD97-lentivirus endothelial cells. (D) Analysis of protein
expression levels of CD97 in endothelial cells, CD97-lentivirus
endothelial cells or CD97-lentivirus endothelial cells transfected
with siRNA to knockdown the expression of CDC42. CD97, cluster of
differentiation 97; Cas9, clustered regularly interspaced short
palindromic repeats-associated protein9; Rho, Ras homolog; Rac,
Ras-related C3 botulinum toxin substrate; Cdc42, cell division
cycle 42; Arp2, actin-related protein 2; NC, negative control; si,
small interfering RNA; leti, lentivirus. |
High glucose inhibits CD97
transcription via the regulation of STAT1
The mechanism underlying the regulatory effect of
high glucose on the expression of CD97 was also examined in detail.
To characterize the promoter region of CD97, a series of luciferase
reporter plasmids, including the 500, 1,000, 1,500 and 2,000 bp
sequences upstream of the transcription start point (24), were constructed (Fig. 4A). Dual luciferase reporter assays
revealed that the promoters, which included a 500 bp sequence,
represented the minimal length required to suppress CD97
transcription. Subsequently, TF filter plate assays were performed
using this 500 bp sequence of the CD97 promoter. As shown in
Fig. 4B, STAT1 was the most
prominent factor to be activated by high glucose concentrations;
this factor also bound to the 500 bp promoter region upstream of
CD97. Therefore, the present study aimed to characterize the role
of STAT1 in the regulation of CD97 transcription. Nuclear extracts
from high glucose-induced endothelial cells showed higher
expression levels of STAT1 (Fig.
4C). Additionally, transfection with siRNA targeting STAT1
under high glucose conditions revealed that high glucose resulted
in reduced expression levels of CD97 via the upregulation of STAT1
(Fig. 4D). The effect of high
glucose on the binding activity of STAT1 to the CD97 promoter was
also examined using ChIP assays. As shown in Fig. 4E, high levels of glucose increased
the binding activity of STAT1 at the CD97 promoter.
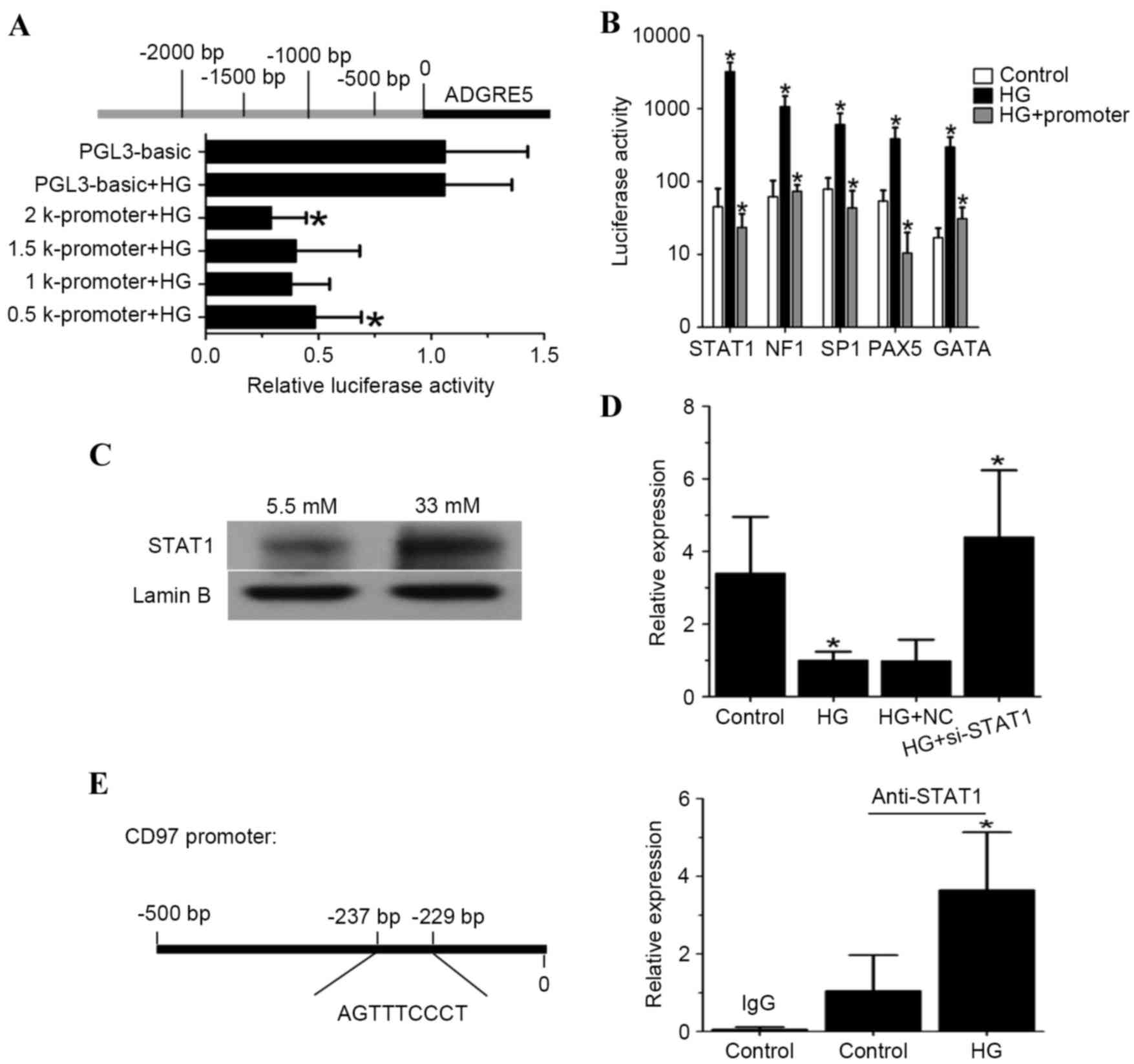 | Figure 4.High glucose concentrations inhibit
CD97 transcription via the upregulation of STAT1. (A) Schematic
representation of the promoter regions (upper), which were
sub-cloned into the pGL3-basic luciferase reporter. Activation of
the promoter-luciferase reporters in response to high glucose
concentrations in endothelial cells is shown below. (B) Top five
most robust binding transcription factors in the CD97 promoter
region, determined using TF filter plate assays. (C) High glucose
(33 mM) stimulation promoted STAT1 nuclear translocation; 5.5 mM
glucose stimulation was used as a control. (D) Analysis of the mRNA
transcript expression of CD97 in endothelial cells transfected with
siRNA targeting STAT1 prior to high glucose (33 mM) treatment (5.5
mM glucose served as a control). (E) Schematic illustration of
potential binding sites in the CD97 promoter for STAT1. High
glucose (33 mM) treatment increased STAT1 binding to its binding
site in the CD97 promoter (5.5 mM glucose served as a control).
*P<0.05. CD97, cluster of differentiation 97; ADGRE5, adhesion G
protein-coupled receptor 5; STAT1, signal transducer and activator
of transcription 1; NF1, neurofibromatosis type 1; SP1, specificity
protein 1; PAX5, paired box 5; GATA, GATA binding protein; HG, high
glucose; si, small interfering RNA. |
Discussion
CD97/ADGRE5 belongs to the GPCR family (11–13)
and has been found to regulate migration in multiple cell types,
including granulocytes (25),
prostate cancer cells (19) and
endothelial cells (9). In the
present study, the role of CD97 in high glucose-induced
dysregulation of endothelial cell migration was reported. By
activating CDC42 and ARP2, CD97 promotes the formation and
extension of lamellipodia by endothelial cells. Unlike prostate
cancer cells, for which CD97-mediated invasion is primarily
RHO-dependent (19), CD97
primarily regulates the expression of CDC42 rather than RHO in
endothelial cells. This difference may be attributed to differences
in cell type or treatment. In addition, the present study found
that specificity protein 1 (SP1) controlled the transcription of
CD97 in smooth muscle cells (26)
and regulated the transcription of CD97 under conditions of high
glucose treatment. However, compared with the STAT1 transcription
factor, SP1 exhibits lower activity in high glucose-stimulated
endothelial cell assays. However, in endothelial cells stimulated
by high glucose concentrations, whether these two factors can
promote the transcription of CD97 in a cooperative manner remains
to be elucidated. Additionally, whether CD97 acts in high
glucose-induced apoptosis or other modes of dysfunction in
endothelial cells remains to be elucidated. The present study is
the first, to the best of our knowledge, to describe a link between
CD97 and the dysregulation of high glucose-induced endothelial
migration, which may provide insights in the identification of
novel therapeutic targets for the treatment of diabetic
complications.
In conclusion, the overexpression of CD97 reversed
the dysregulation of high glucose-induced endothelial cell
migration by activating CDC42, which acts via its downstream
signaling adaptor ARP2.
References
|
1
|
Creager MA, Lüscher TF, Cosentino F and
Beckman JA: Diabetes and vascular disease: Pathophysiology,
clinical consequences, and medical therapy: Part I. Circulation.
108:1527–1532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antonetti DA, Barber AJ, Khin S, Lieth E,
Tarbell JM and Gardner TW: Vascular permeability in experimental
diabetes is associated with reduced endothelial occludin content:
Vascular endothelial growth factor decreases occludin in retinal
endothelial cells. Penn State Retina Research Group, Diabetes.
47:1953–1959. 1998.
|
|
3
|
Campanini M, Airoldi G, Cusinato S,
Ballarè M and Monteverde A: Arterial blood pressure as a factor in
endothelial permeability. J Hypertens Suppl. 9:S200–S201. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bassenge E: Clinical relevance of
endothelium-derived relaxing factor (EDRF). Br J Clin Pharmacol.
34:(Suppl 1). S37–S42. 1992. View Article : Google Scholar
|
|
5
|
Cohen RA: The role of nitric oxide and
other endothelium-derived vasoactive substances in vascular
disease. Prog Cardiovasc Dis. 38:105–128. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Meyer GR and Herman AG: Vascular
endothelial dysfunction. Prog Cardiovasc Dis. 39:325–342. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kario K, Matsuo T, Kobayashi H, Matsuo M,
Sakata T and Miyata T: Activation of tissue factor-induced
coagulation and endothelial cell dysfunction in
non-insulin-dependent diabetic patients with microalbuminuria.
Arterioscler Thromb Vasc Biol. 15:1114–1120. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamuro M, Polan J, Natarajan M and Mohan
S: High glucose induced nuclear factor kappa B mediated inhibition
of endothelial cell migration. Atherosclerosis. 162:277–287. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang T, Ward Y, Tian L, Lake R, Guedez L,
Stetler-Stevenson WG and Kelly K: CD97, an adhesion receptor on
inflammatory cells, stimulates angiogenesis through binding
integrin counterreceptors on endothelial cells. Blood.
105:2836–2844. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamann J, Hartmann E and van Lier RA:
Structure of the human CD97 gene: Exon shuffling has generated a
new type of seven-span transmembrane molecule related to the
secretin receptor superfamily. Genomics. 32:144–147. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwakkenbos MJ, Kop EN, Stacey M, Matmati
M, Gordon S, Lin HH and Hamann J: The EGF-TM7 family: A postgenomic
view. Immunogenetics. 55:655–666. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leemans JC, te Velde AA, Florquin S,
Bennink RJ, de Bruin K, van Lier RA, van der Poll T and Hamann J:
The epidermal growth factor-seven transmembrane (EGF-TM7) receptor
CD97 is required for neutrophil migration and host defense. J
Immunol. 172:1125–1131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McKnight AJ and Gordon S: EGF-TM7: A novel
subfamily of seven-transmembrane-region leukocyte cell-surface
molecules. Immunol Today. 17:283–287. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aust G, Eichler W, Laue S, Lehmann I,
Heldin NE, Lotz O, Scherbaum WA, Dralle H and Hoang-Vu C: CD97: A
dedifferentiation marker in human thyroid carcinomas. Cancer Res.
57:1798–1806. 1997.PubMed/NCBI
|
|
15
|
Eichler W, Aust G and Hamann D:
Characterization of an early activation-dependent antigen on
lymphocytes defined by the monoclonal antibody BL-Ac (F2). Scand J
Immunol. 39:111–115. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamann J, Eichler W, Hamann D, Kerstens
HM, Poddighe PJ, Hoovers JM, Hartmann E, Strauss M and van Lier RA:
Expression cloning and chromosomal mapping of the leukocyte
activation antigen CD97, a new seven-span transmembrane molecule of
the secretion receptor superfamily with an unusual extracellular
domain. J Immunol. 155:1942–1950. 1995.PubMed/NCBI
|
|
17
|
Jaspars LH, Vos W, Aust G, Van Lier RA and
Hamann J: Tissue distribution of the human CD97 EGF-TM7 receptor.
Tissue Antigens. 57:325–331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steinert M, Wobus M, Boltze C, Schütz A,
Wahlbuhl M, Hamann J and Aust G: Expression and regulation of CD97
in colorectal carcinoma cell lines and tumor tissues. Am J Pathol.
161:1657–1667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ward Y, Lake R, Yin JJ, Heger CD, Raffeld
M, Goldsmith PK, Merino M and Kelly K: LPA receptor heterodimerizes
with CD97 to amplify LPA-initiated RHO-dependent signaling and
invasion in prostate cancer cells. Cancer Res. 71:7301–7311. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ran FA, Hsu PD, Wright J, Agarwala V,
Scott DA and Zhang F: Genome engineering using the CRISPR-Cas9
system. Nat Protoc. 8:2281–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wójciak-Stothard B, Entwistle A, Garg R
and Ridley AJ: Regulation of TNF-alpha induced reorganization of
the actin cytoskeleton and cell-cell junctions by Rho, Rac, and
Cdc42 in human endothelial cells. J Cell Physiol. 176:150–165.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suraneni P, Rubinstein B, Unruh JR, Durnin
M, Hanein D and Li R: The Arp2/3 complex is required for
lamellipodia extension and directional fibroblast cell migration. J
Cell Biol. 197:239–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li P, Grgurevic S, Liu Z, Harris D,
Rozovski U, Calin GA, Keating MJ and Estrov Z: Signal transducer
and activator of transcription-3 induces microRNA-155 expression in
chronic lymphocytic leukemia. PLoS One. 8:e646782013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Veninga H, Becker S, Hoek RM, Wobus M,
Wandel E, van der Kaa J, van der Valk M, de Vos AF, Haase H, Owens
B, et al: Analysis of CD97 expression and manipulation: Antibody
treatment but not gene targeting curtails granulocyte migration. J
Immunol. 181:6574–6583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wobus M, Wandel E, Prohaska S, Findeiss S,
Tschöp K and Aust G: Transcriptional regulation of the human CD97
promoter by Sp1/Sp3 in smooth muscle cells. Gene. 413:67–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|