Introduction
Systemic neoadjuvant chemotherapy can precede
conversion surgery, resulting in R0 resection, even in patients
with initially unresectable colorectal liver metastases (CRLM).
Although oxaliplatin-based chemotherapy plays a central role in the
treatment of patients with CRLM, oxaliplatin can induce sinusoidal
obstruction syndrome (SOS), which is characterized by hepatic
sinusoidal dilatation, hepatocyte atrophy, peri-sinusoidal
fibrosis, and nodular regenerative hyperplasia (1). These histological changes have been
reported in up to 40% of patients treated with oxaliplatin-based
regimens undergoing liver resection (2–6). SOS
prior to major hepatectomy has been associated with increased
peri-operative morbidity and prolonged hospital stay (7). Clinically, factors diagnostic of SOS
include portal hypertension, splenomegaly, thrombocytopenia, an
abnormal indocyanine green (ICG) retention rate and elevated liver
enzymes, bilirubin and hyaluronic acid (HA) (8–12).
On pathological examination, SOS is characterized by the disruption
of the sinusoidal endothelium, collagen deposition in
peri-sinusoidal spaces, fibrosis especially around the central vein
(in zone 3), dilatation of the sinusoidal space and congestion
(13). Platelets were shown to be
involved in the alterations in the sinusoidal endothelium observed
in patients with SOS (14).
Thrombocytopenia has been observed in patients with CRLM treated
with oxaliplatin based chemotherapy, and examination of their
resected livers showed platelet aggregation in zone 3 (15). Platelet aggregation in the
extra-sinusoidal (Disse's) space is called extravasated platelet
aggregation (EPA). This study assessed whether EPA was present in
the livers of rats with drug induced SOS.
Materials and methods
Reagents
Monocrotaline (MCT) was purchased from Wako Pure
Chemical Industries (Osaka, Japan). A 20 mg/ml solution of MCT was
prepared by dissolving 1,000 mg MCT in 1.0 N HCl, and adjusting the
pH to 7.4 with 0.5 N NaOH, followed by dilution in
phosphate-buffered saline (PBS), pH 7.4, to increase the total
volume to 50 ml (16,17).
Animals
Male Wistar rats, weighing 230–300 g, were purchased
from Charles River Inc. (Kanagawa, Japan), and allowed free access
to water and standard laboratory chow. This study complied with the
guidelines of the Division for Animal Research Resources,
University of Kanazawa. All experiments and procedures were
approved by the Animal Care and Use Committee of the University of
Kanazawa.
Experimental protocol
The animals were randomly divided into two groups of
10 rats each. Animals were fasted for 12 h, with one group
administered MCT (90 mg/kg) and the control group administered
water (Fig. 1). Rats were
thereafter allowed free access to water and standard laboratory
chow ad libitum. Because histopathological changes 48 h after MCT
administration are similar to those in patients with SOS, (16,18)
the rats were anesthetized by inhalation of diethyl ether and
sacrificed. Blood was collected from the inferior vena cava, and
liver tissues were obtained.
Macroscopic examination
The abdominal cavity of rats was accessed by middle
incision laparotomy, and the effect of MCT assessed macroscopically
by the accumulation of peritoneal fluid and the color of the liver
surface.
Biochemical analysis
Blood specimens were collected, and white blood cell
(WBC) and platelet counts, as well as hemoglobin (Hb)
concentrations, were measured using an automated blood cell counter
(Celltac α, MEK-6458, Nihon Kohden, Japan). Serum concentrations of
aspartate aminotransferase (AST), alanine aminotransferase (ALT),
total bilirubin (T-Bil), direct bilirubin (D-Bil), indirect
bilirubin (I-Bil) lactate dehydrogenase (LDH) and hyaluronic acid
(HA) were measured by SRL Inc. (Tokyo, Japan).
Histologic analysis
Liver tissue was fixed in 10% neutral buffered
formalin, embedded in paraffin, and cut serially into 4-µm
sections. Slides were stained with hematoxylin and eosin (H&E),
and ten randomly selected high-power fields (magnification, ×200)
were examined. The degree of SOS was assessed by examining
histological changes in sinusoidal dilatation, coagulative necrosis
of hepatocytes, endothelial damage to the central vein, and
sinusoidal hemorrhage (16,18,19).
Each of these four features was graded on a 4-point scale, with 0,
absent; 1, mild (1–30%); 2, moderate (31–60%); and 3, severe
(61–100%). The total SOS score for each rat was calculated as the
sum of the individual scores.
Immunohistochemistry
Tissue samples were fixed in 4% paraformaldehyde in
phosphate buffered saline for 3 days and embedded in a solution of
O.C.T. compound (Sakura Finetek, Tokyo, Japan) and 30% sucrose in
0.1 M phosphate buffer, pH 7.4, containing 0.05% NaN3. All tissue
samples were sectioned at 6 µm using cryostats (Thermo Fisher
Scientific, Waltham, MA, USA).
Immunohistochemical staining was performed with the
Dako Envision system (Dako, Carpinteria, CA, USA), which uses
dextran polymers conjugated with horseradish peroxidase, thus
avoiding contamination by endogenous biotin. Tissue samples were
dissected out, immersed in fixative for 4 h, dehydrated in a graded
alcohol series for 24 h, embedded in paraffin, and cut into
4-mm-thick sections. Endogenous peroxidases were blocked by
immersing the sections in 3% H2O2 in 100%
methanol for 20 min at room temperature. Antigen retrieval was
performed by micro-waving sections at 95°C for 10 min in 0.001 M
citrate buffer (pH 6.7). The sections were incubated with
serum-free protein block (Dako) at room temperature for 10 min to
block non-specific staining, followed by incubation for 2 h at room
temperature with primary monoclonal antibody. Peroxidase activity
was detected with the enzyme substrate 3-amino-9-ethyl carbazole.
As negative controls, tissue sections were incubated with
Tris-buffered saline without the primary antibodies. All samples
were counterstained with Meyer's hematoxylin.
Platelet aggregation was assessed by
immunohistochemical staining of liver tissues with antibodies to
platelet glycoprotein IIb (CD41) (1:100, orb4832; Biorbyt,
Cambridge, UK) and P-selectin (1:50, ERP1444(2)(B); Abcam, Tokyo, Japan), the latter
being a member of the selectin family of adhesion molecules
expressed on activated platelets and endothelium (20). Damage to sinusoidal endothelial
cells was determined by immunostaining rat liver tissues with
antibodies to rat endothelial cell antigen-1 (RECA-1) (#MCZ-970R;
Serotec, Oxford, UK) and CD34 (1:20, AF4117; R&D Systems, Inc.,
Minneapolis, MN, USA), the latter being a marker of sinusoidal
capillary formation in liver tissue, thereby differentiating
between normal and altered sinusoidal epithelium (21–24).
Hepatocyte apoptosis was determined by immunostaining with antibody
to cleaved caspase-3 (1:100, 9661; Cell Signaling Technology, Inc.,
Beverly, MA, USA). Dyeabilities of these factors were measured with
the BZ-Analyzer software (Keyence, Osaka, Japan). The total stained
areas were calculated from randomly selected images per high power
field (magnification, ×200) and compared between the two
groups.
Transmission electron microscopy
Rat liver specimens were fixed by immersion in 2%
paraformaldehyde plus 2.5% glutaraldehyde in phosphate buffer (pH
7.4) for 4 h at 4°C. After washing in phosphate buffer at 4°C, the
specimens were postfixed in 1% osmium tetraoxide for 2 h at 4°C,
washed repeatedly in distilled water, stained with 1% uranyl
acetate for 30 min, dehydrated through a graded ethanol series and
propylene oxide, and embedded in Glicidether (Selva Feinbiochemica,
Heidelberg, Germany). Ultrathin sections were cut and mounted onto
copper grids, stained with 1% uranyl acetate for 10 min and with
Reynolds lead citrate for 5 min, and evaluated using an H7650
electron microscope (Hitachi, Tokyo, Japan) (25).
Statistical analysis
All results were expressed as the mean ± standard
deviation (SD). Groups were compared by Student's t-tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
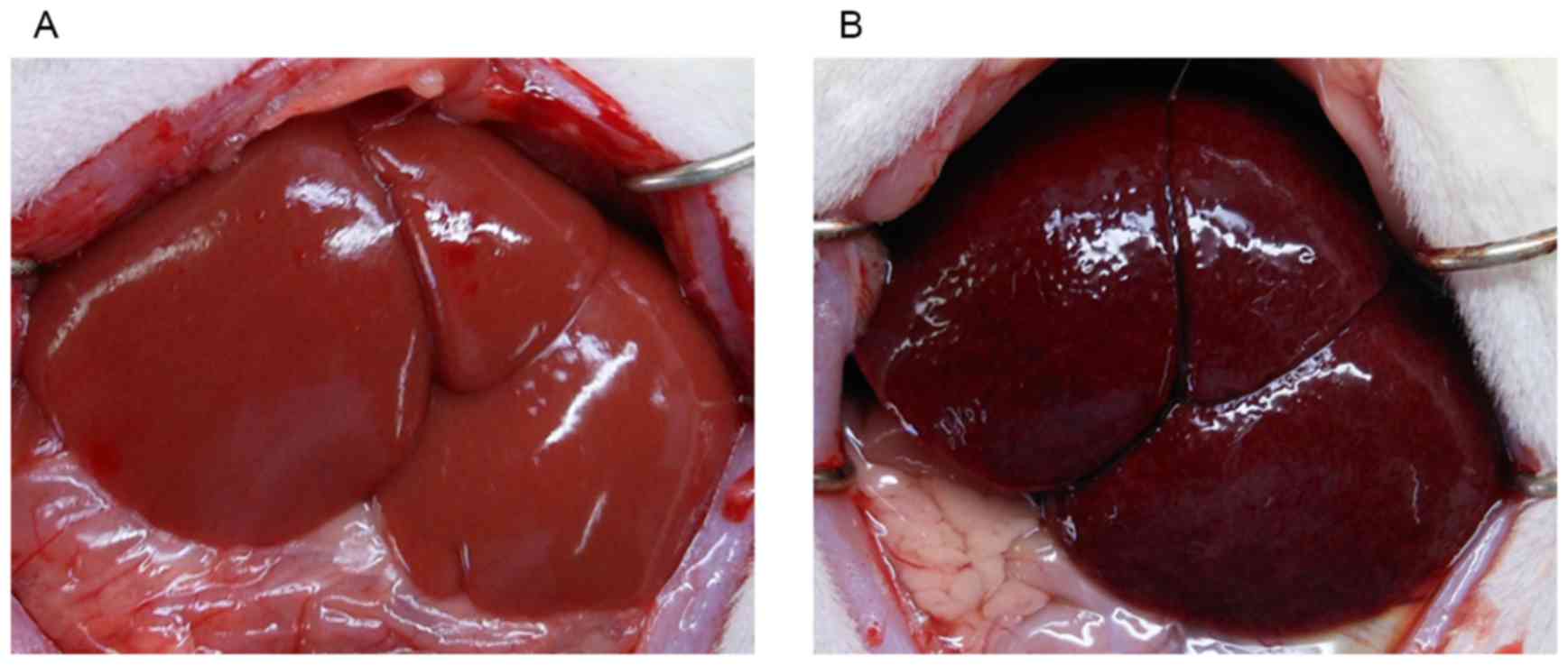
At sacrifice, macroscopic examination of the livers
in the MCT group showed accumulation of bloody ascites, with the
liver surface appearing congested and dark red in color (Fig. 1). These changes were not observed
in the control group. The difference of liver size and weight was
not observed between the two groups.
The results of complete blood counts and serum
biochemistry tests are shown in Table
I. There were no between group differences in WBC count and Hb
concentration, although platelet counts were significantly lower in
the MCT than in the control group (P=0.004). Serum concentrations
of AST (P=0.003), ALT (P=0.008), T-Bil (P=0.012), D-Bil (P=0.007),
I-Bil (P=0.003), LDH (P<0.001) and HA (P=0.0016) were all
significantly higher in the MCT than in the control group.
 | Table I.Blood counts and serum
biochemistry. |
Table I.
Blood counts and serum
biochemistry.
| Variables | MCT group
(n=10) | Control group
(n=10) | P-value |
|---|
| Hb (g/dl) | 14.73±1.43 | 14.34±1.04 | 0.281 |
| WBC
(x103/µl) | 48.6±17.8 | 73.6±11.1 | 0.232 |
| Plt
(x103/µl) | 5.87±2.7 | 75.78±8.3 | 0.004 |
| AST (IU/l) | 7,218.0±4071.3 | 82.9±9.1 | 0.003 |
| ALT (IU/l) | 1,539.2±837.7 | 33.4±5.4 | 0.008 |
| T-Bil (mg/dl) | 0.211±0.083 | 0.038±0.007 | 0.012 |
| D-Bil (mg/dl) | 0.126±0.053 | 0.032±0.004 | 0.007 |
| I-Bil (mg/dl) | 0.085±0.038 | 0.006±0.005 | 0.003 |
| LDH (mg/dl) |
5,664.9±3,700.9 | 312.9±158.7 | <0.001 |
| HA (ng/dl) | 1,188.3±729.0 | 36.8±3.9 | 0.016 |
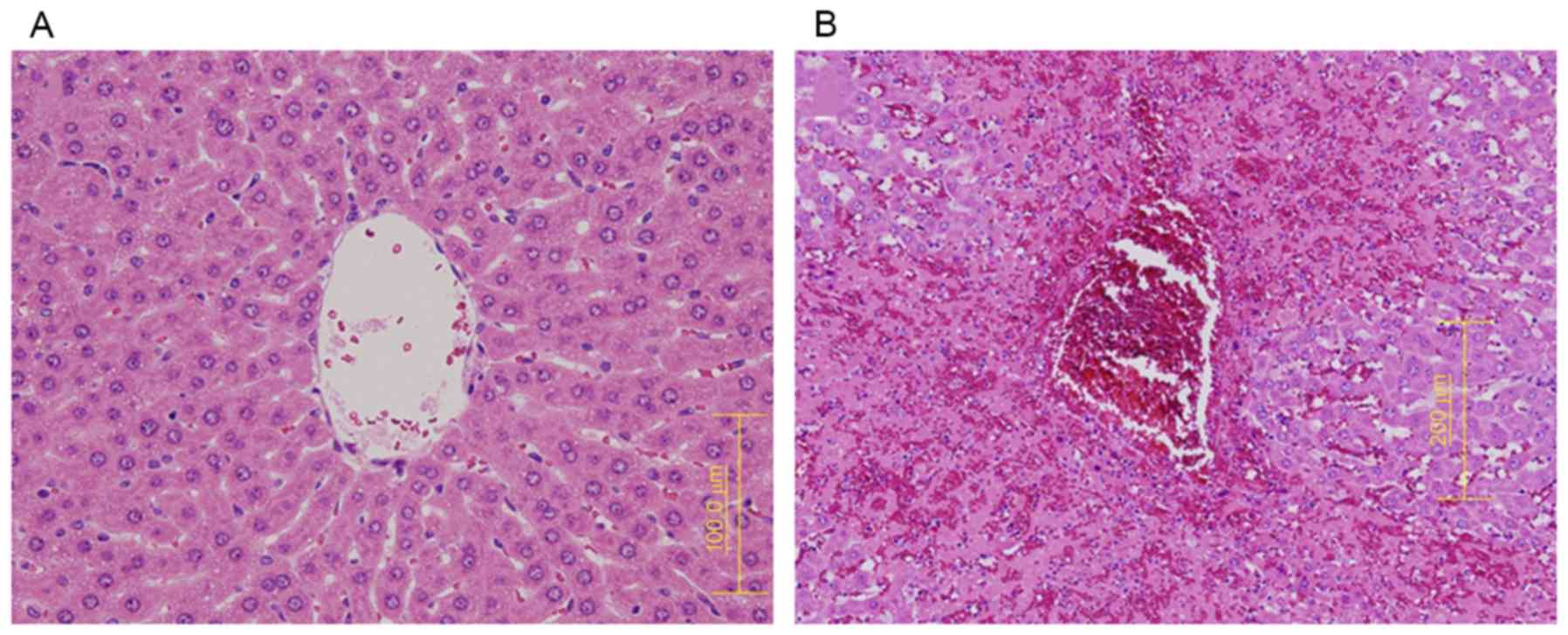
Histologically, H&E staining of liver tissue
showed higher SOS scores in the MCT than in the control group, with
the former group showing all four histological changes: sinusoidal
dilatation, coagulative necrosis of hepatocytes, endothelial damage
to the central vein, and sinusoidal hemorrhage (Fig. 2).
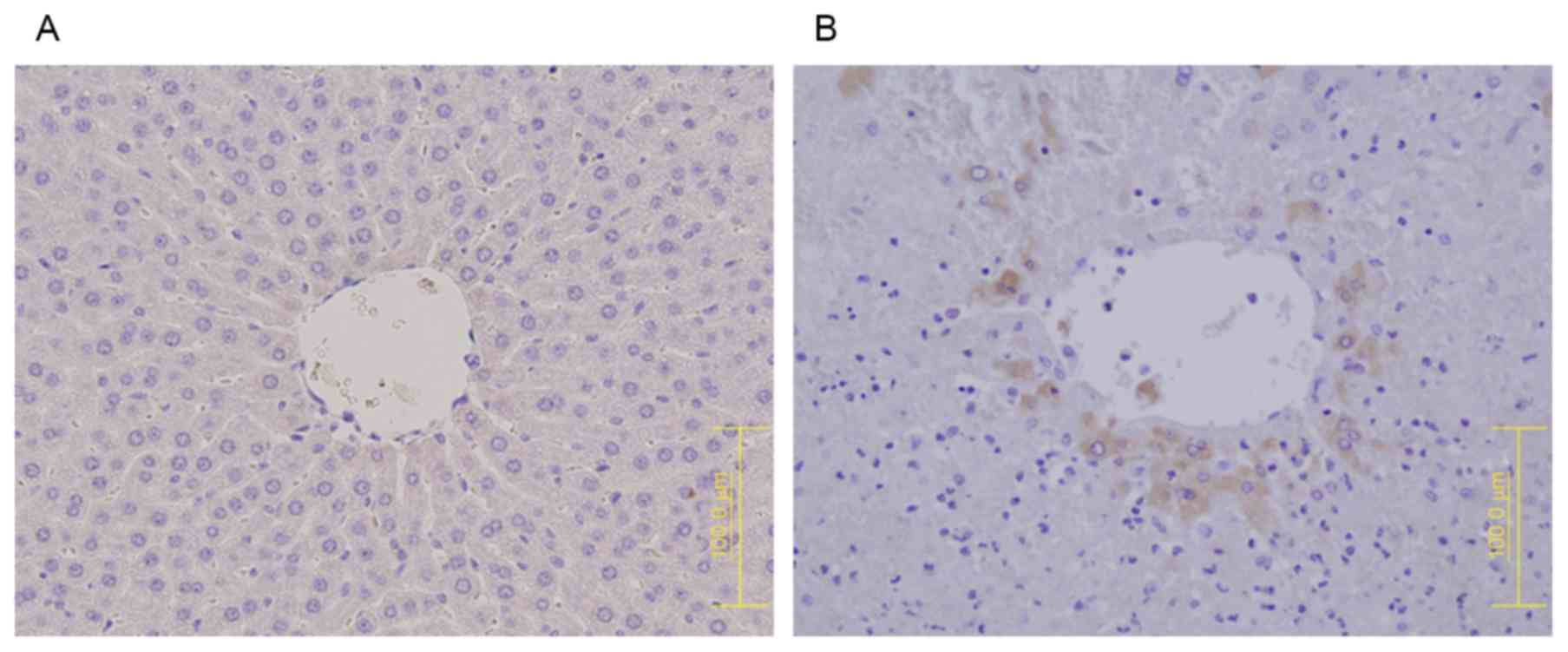
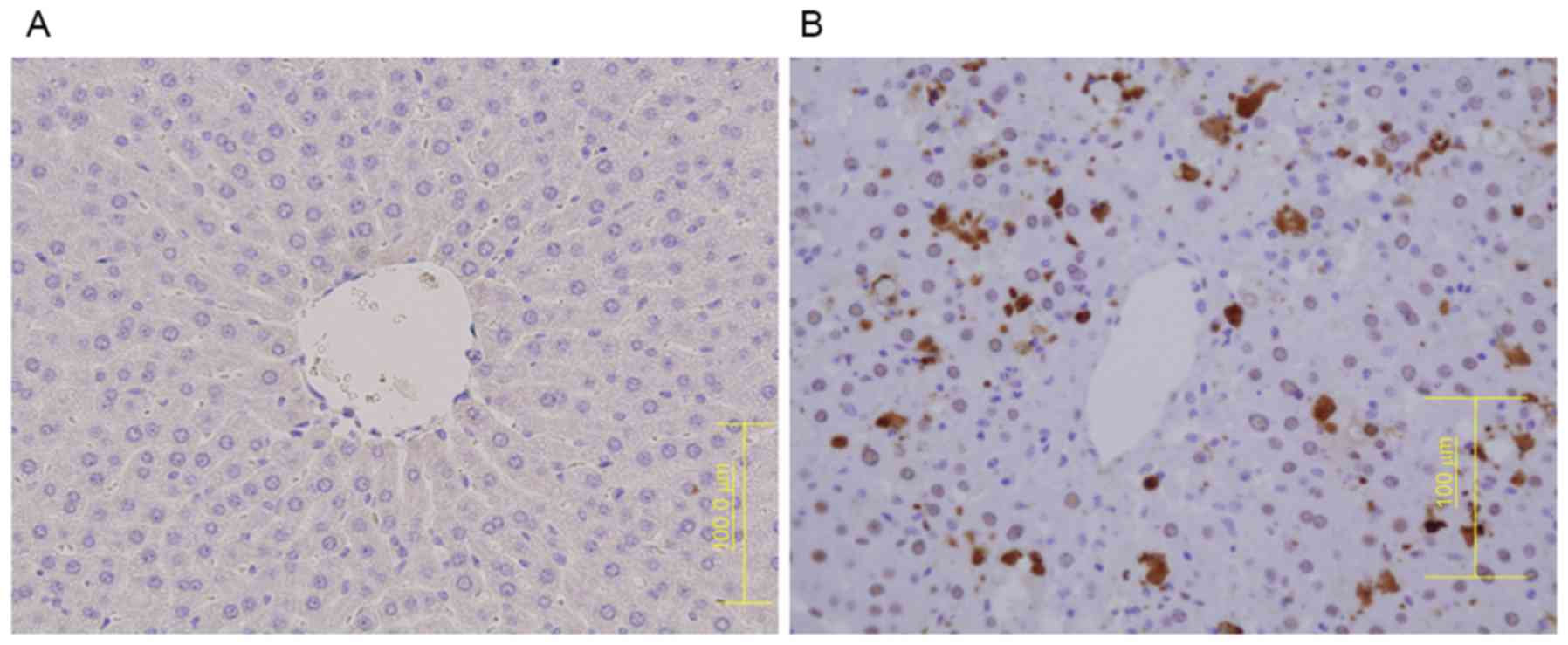
CD41 protein expression was observed in the MCT
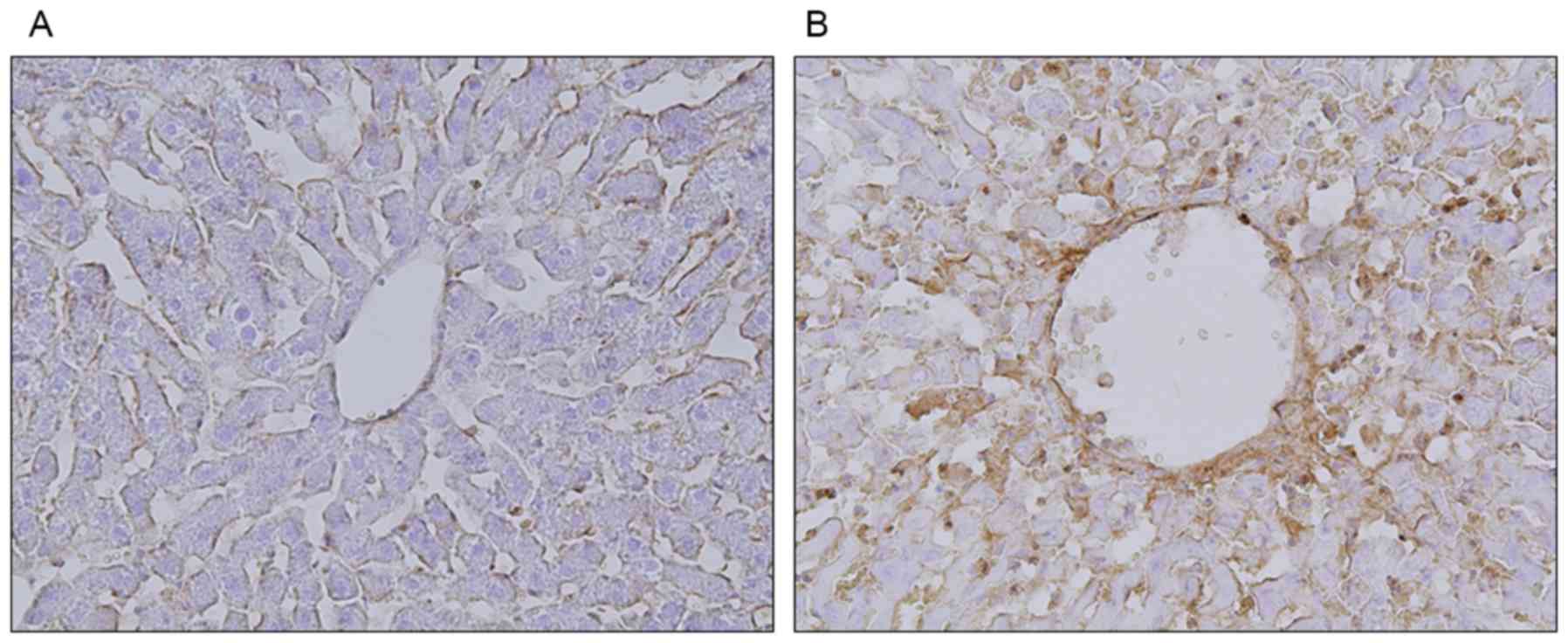
group, especially in zone 3, but not in the control group (Fig. 3). Assessment of P-selectin
expression showed that, in the control group, it was present only
on the endothelial cells of the sinusoids (Fig. 4A). In the MCT group, however,
P-selectin expression was observed on both sinusoidal endothelial
cells and granularly stained platelets (Fig. 4B).
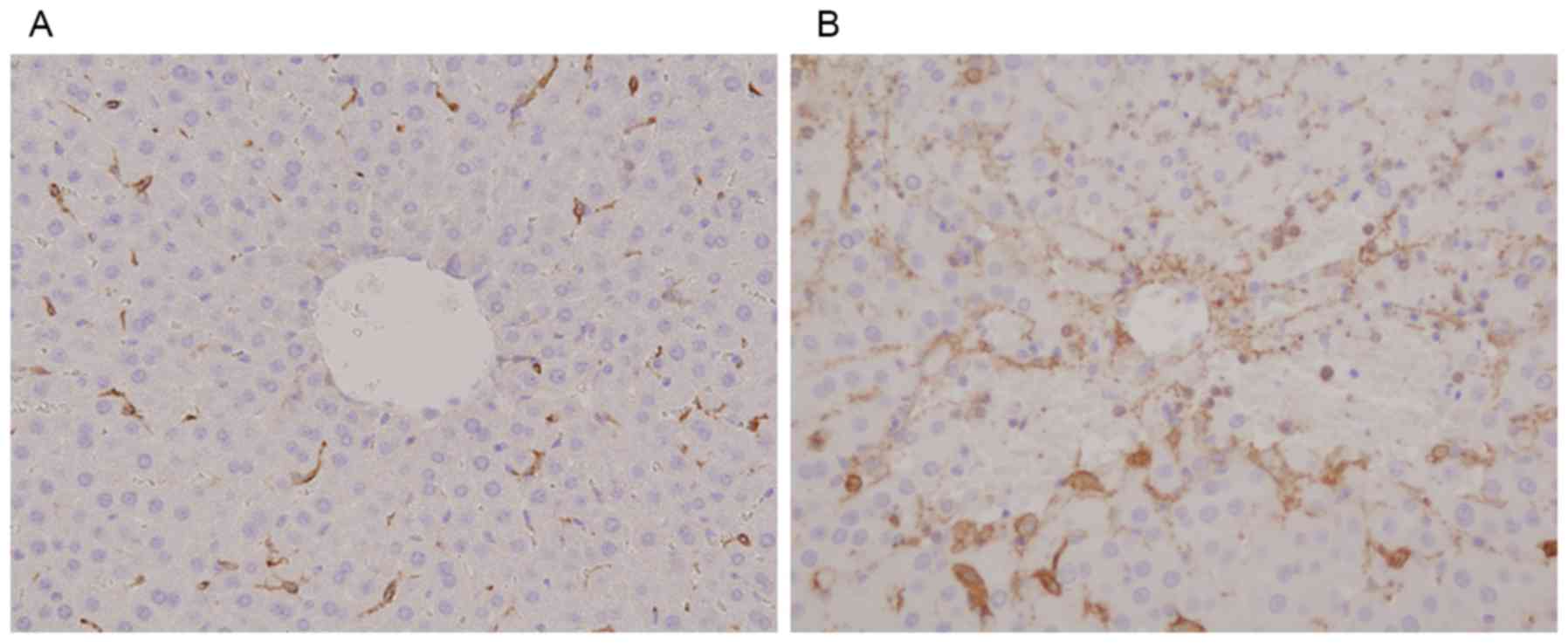
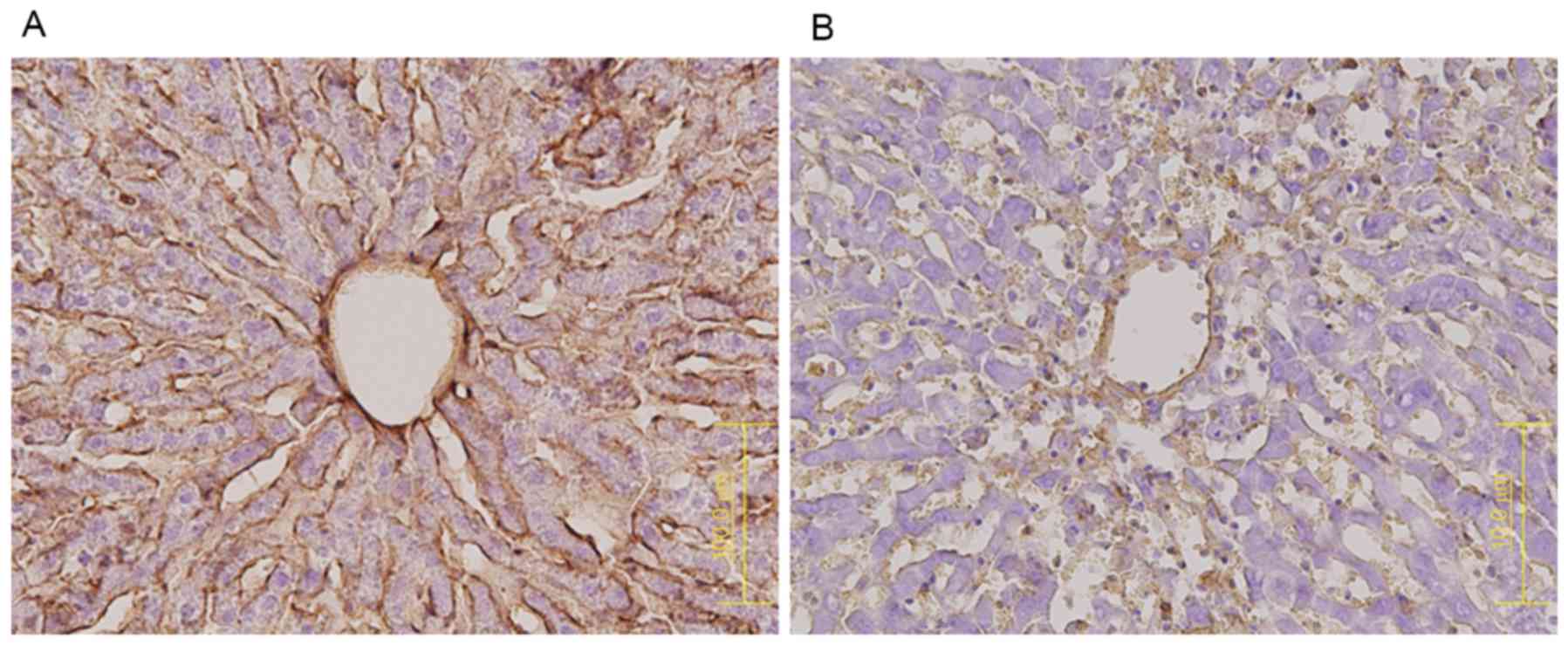
RECA-1 protein expression was markedly lower in the
MCT (Fig. 5B) than in the control
group (Fig. 5A), indicating that
the sinusoidal lining had largely disappeared from the former. In
contrast, CD34 expression was higher in the MCT group, especially
in zone 3 (Fig. 6B), than in the
control group (Fig. 6A).
Similarly, cells positive for cleaved caspase-3 were observed in
zone 3 in the MCT group 3 (Fig.
7B), but not in the control group. Measurements of the staining
areas of MCT and control group are shown in Table II. In the MCT group, significant
excessive staining was observed in all the factors in comparison
with the control group.
 | Table II.Expression areas of
immunohistochemical examination |
Table II.
Expression areas of
immunohistochemical examination
| Variables | MCT group
(µm2) | Control group
(µm2) | P-value |
|---|
| CD41 |
2,405.0±2,476.3 | 760.0±11.8 | 0.014 |
| P-selectin |
14,653.7±2,324.0 | 11,678.1±505.0 | 0.034 |
| RECA-1 | 51,503.0±174.0 |
246,664.0±64,171.0 | 0.001 |
| CD34 | 113,914±5,336 | 18,720±4,780 | <0.001 |
| Cleaved | 974±335 | 8.3±0.7 | 0.003 |
| Caspase-3 |
|
|
|
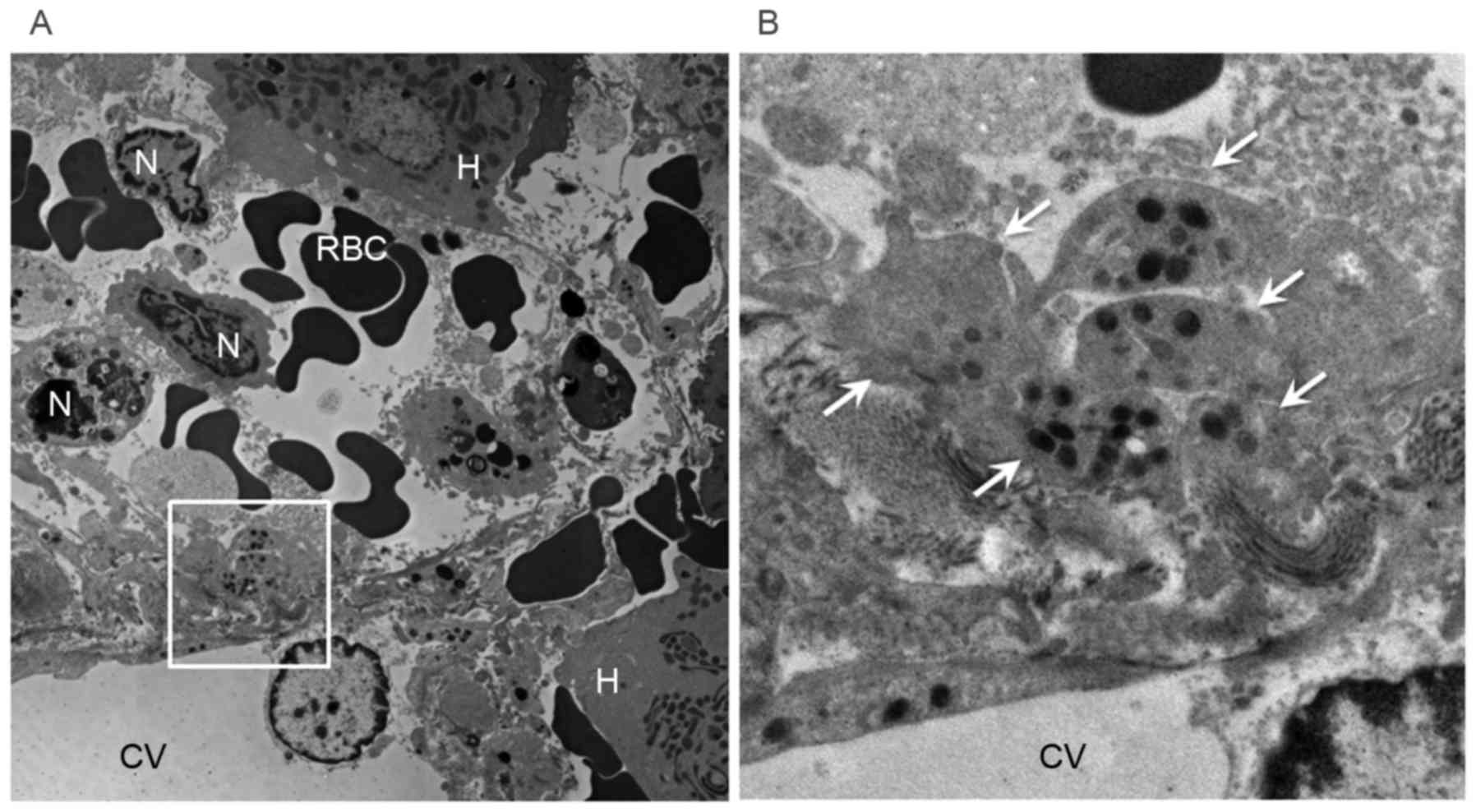
Electron microscopic examination of the MCT group
showed many platelets containing granules in the space of Disse
(Fig. 8).
Discussion
SOS is a veno-occlusive disease (VOD), characterized
by hepatic injury after exposure to a drug or toxin or after bone
marrow transplantation (26). SOS
can present in acute, subacute and chronic forms, usually as
abdominal pain and/or swelling, with evidence of portal
hypertension and variable degrees of serum enzyme elevation and
jaundice. Liver histology shows obstruction of the sinusoids in the
central areas, along with hepatocyte necrosis and hemorrhage.
Agents that cause SOS include cancer chemotherapeutic agents,
particularly alkylating agents such as cyclophosphamide and the
platinum coordination complexes cisplatin and oxaliplatin. The
diagnosis of SOS usually depends on a typical clinical
presentation, characterized by hepatomegaly, ascites, portal
hypertension, weight gain and jaundice (27), or by the exclusion of other causes
of liver injury. The usual differential diagnosis of SOS after bone
marrow transplantation includes graft vs. host disease, sepsis,
other forms of drug induced liver injury, and various forms of
viral hepatitis. SOS diagnosis is usually supported by imaging
modalities that demonstrate changes typical of sinusoidal
obstruction. Liver biopsy is diagnostic but is not usually needed;
moreover, concurrent coagulopathy or thrombocytopenia make it
difficult to acquire biopsy samples (28). Although thrombocytopenia in SOS
usually results from hypersplenism, decreased platelet counts have
been observed earlier than splenomegaly (15). Therefore, this research focused on
the role of platelets in the pathogenesis of SOS.
Histologic examination of the liver in patients with
SOS shows obstruction of the sinusoids around the central vein
(zone 3), along with hepatocyte necrosis and congestion. SOS may be
caused by cancer chemotherapeutic and immunosuppressing agents. SOS
was shown to be induced by oxaliplatin based chemotherapy in
patients with CRLM, appearing as blue liver. Administration of MCT
to rats induced SOS, with the macroscopic appearance of blue liver
and SOS detected histologically. Blood analysis showed that
treatment with MCT significantly increased serum concentrations of
AST, ALT, T-Bil, D-Bil and HA, with the latter reflecting injury to
liver sinusoidal endothelial cells (LSECs) (13).
Histopathologically, administration of MCT resulted
in sinusoidal dilatation, coagulative necrosis of hepatocytes,
endothelial damage to the central vein, and sinusoidal hemorrhage.
Immunohistochemical assays showed that MCT administration resulted
in reduced RECA-1 and increased CD34 expression. Reduced RECA-1
expression indicated that the sinusoidal lining had largely
disappeared. CD34 has been used to detect sinusoidal capillary
formation in liver tissue and to differentiate between normal and
altered sinusoidal epithelium (20–23).
Neutrophils and platelets participate in the
pathogenesis of severe sepsis, as well as forming neutrophil
extracellular trapping systems (NETs) to kill pathogens
extracellularly (29). NETs,
however, have been reported to contribute to organ damage in
patients with infectious diseases (30) Furthermore, sivelestat sodium, a
neutrophil elastase inhibitor, was found to inhibit neutrophil
adhesion and migration to vascular endothelium during hepatic
ischemia reperfusion and prevent liver injury (31). Platelets are not stained by
H&E. because they lack nuclei. Platelets can be visualized in
rats by immunostaining with antibody to the surface marker, CD41.
Clinically, we observed EPA in SOS patients after liver
transplantation (32,33) and after oxaliplatin chemotherapy
(15). Platelets can be indirectly
localized by detection of their surface markers. However, it was
possible to directly verify the presence of platelets in the space
of Disse around zone 3 by electron microscopy in the MCT group.
SOS has also been referred to as VOD in
transplantation. Autopsy reports have shown that the pathology of
human VOD includes reticulin deposition within sinusoids, central
vein occlusion, hepatocyte atrophy/necrosis, sinusoidal hemorrhage,
and sparing of portal tracts (34). Earlier histopathological findings
of VOD can be detected by examining liver biopsy specimens. The
affected venules show a lumen narrowed by a wide, edematous
subendothelial zone containing a fibrous material and fragmented
red cells. The sinusoids draining into these veins are congested,
and the surrounding hepatocytes are pale and necrotic. Hemorrhage
is also observed in the space of Disse.
Although sinusoidal fibrosis in zone 3 is regarded
as an equivalent of early VOD, this diagnosis is not usually
accepted unless there are significant changes in the central vein
(35). Despite venous reflux
disorder, with portal blood flowing back, being observed in
patients with severe SOS, but venous obstruction or thrombosis has
not been detected pathologically. Platelets not only form a white
thrombus, but may cause spasms in the central vein by secreting
various growth factors, including thromboxane A2 (TXA2), vascular
endothelial growth factor (VEGF)-A, transforming growth factor
(TGF)-β and plasminogen activator inhibitor (PAI)-1 (36). These growth factors can explain the
liver injury in SOS. For example, TXA2 can cause central vein
occlusion and portal hypertension, (37) and thrombospondin-activated TGF-β
can cause collagen deposition in the peri-sinusoidal space and
inhibit substance exchange in the space of Disse (28). TGF-β can also increase serum
bilirubin concentration and may be responsible for an abnormal ICG
retention rate. PAI-1 and TGF-β interfere with liver regeneration
by suppressing hepatocyte growth factor (38). Although VEGF-A usually acts as a
vasodilator, it can act as a vasoconstrictor under conditions of
endothelial failure and hepatic sinusoidal injury (39). Platelet aggregation in the space of
Disse may explain many of the symptoms, venous perfusion
abnormalities, and portal hypertension detected in SOS, findings
that cannot be confirmed pathologically. Thus, antiplatelet agents,
including vasodilators such as prostaglandin E1 (40) and low dose heparin, (41) may prevent or reduce SOS.
In conclusion, EPA in the space of Disse has been
confirmed in this rat model of MCT-induced liver SOS. Platelets are
heavily involved in the development of SOS.
References
|
1
|
Rubbia-Barandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hubert C, Fevaille C, Sempoux C, Horsmans
Y, Humblet Y, Machiels JP, Zech F, Ceratti A and Gigot JF:
Prevalence and clinical relevance of pathological hepatic changes
occurring after neoadjuvant chemotherapy for colorectal liver
metastases. Surgery. 147:185–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vauthey JN, Pawlik TM, Ribero D, Wu TT,
Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et
al: Chemotherapy regimen predicts steatohepatitis and an increase
in 90-day mortality after surgery for hepatic colorectal
metastases. J Clin Oncol. 24:2065–2072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamandl D, Klinger M, Eipeldauer S,
Herberger B, Kaczirek K, Gruenberger B and Gruenberger T: Sinusoid
obstruction syndrome impairs long-term outcome of colorectal liver
metastases treated with resection after neoadjuvant chemotherapy.
Ann Surg Oncol. 18:421–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson SM, Wilson CH, Burt AD, Manas DM
and White SA: Chemotherapy associated liver injury in patients with
colorectal liver metastases: A systemic review and meta-analysis.
Ann Surg Oncol. 19:4287–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aloysius MM, Zaitoun AM, Beckingham IJ,
Neal KR, Aithal GP, Bessell EM and Lobo DN: The pathological
response to neoadjuvant chemotherapy with FOLFOX-4 for colorectal
liver metastases: A comparative study. Virchows Arch. 451:943–948.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakano H, Oussoultzoqlou E, Rosso E,
Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P and Jaeck D:
Sinusoidal injury increases morbidity after major hepatectomy in
patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jardim DL, Rodorigues CA, Novis YA, Rocha
VG and Hoff PM: Oxaliplatin-related thrombocytopenia. Ann Oncol.
23:1937–1942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miura K, Nakano H, Sakurai J, Kobayashi S,
Koizumi S, Arai T, Shimamura T, Makizumi R, Yamada K, Miyajima N,
et al: Splenomegaly in FOLFOX-naïve stage IV or recurrent
colorectal cancer patients due to chemotherapy- associated
hepatotoxicity can be predicted by the aspartate aminotransferase
to platelet ratio before chemotherapy. Int J Clin Oncol.
16:257–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klieger PM, Tamandl D, Herberger B, Faybik
P, Fleichmann E, Maresch J and Gruengerger T: Evaluation of
chemotherapy-associated liver injury in patients with colorectal
cancer liver metastases using indocyanine green clearance testing.
Ann Surg Oncol. 18:1644–1650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato S, Nakano H, Ishida Y and Otsubo T:
The aspartate aminotransferase to platelet ratio before
chemotherapy predicts adverse events for FOLFOX and XELOX regimens
including bevacizumab as the first-line therapy for stage IV,
recurrent and metastatic colorectal cancer. J Gastrointest Oncol.
4:203–209. 2013.PubMed/NCBI
|
|
12
|
Narita M, Oussoultzoglou E, Chenard MP,
Fuchshuber P, Rather M, Rosso E, Addeo P, Jaeck D and Bachellier P:
Liver injury due to chemotherapy-induced sinusoidal obstruction
syndrome is associated with sinusoidal capillarization. Ann Surg
Oncol. 19:2230–2237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morine Y, Shimada M and Utsunomiya T:
Evaluation and management of hepatic injury induced by
oxaliplatin-based chemotherapy in patients with hepatic resection
for colorectal liver metastasis. Hepatol Res. 44:59–69. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lalor PF, Herbert J, Bicknell R and Adams
DH: Hepatic sinusoidal endothelium avidly binds platelets in an
integrin-dependent manner, leading to platelet and endothelial
activation and leucocyte recruitment. Am J Physiol Gastrointest
Liver Physiol. 304:G469–G478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tajima H, Ohta T, Miyashita T, Nakanuma S,
Matoba M, Miyata T, Sakai S, Okamoto K, Makino I, Kinoshita J, et
al: Oxaliplatin-based chemotherapy induces extravasated platelet
aggregation in the liver. Mol Clinic Oncol. 3:555–558. 2015.
|
|
16
|
Narita M, Hatano E, Ikai I,
Miyagawa-Hayashino A, Yanagida A, Nagata H, Asechi H, Taura K and
Uemoto S: A phosphodiesterase III inhibitor protects rat liver from
sinusoidal obstruction syndrome through heme oxygenase-1 induction.
Ann Surg. 249:806–813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prié S, Stewart DJ and Dupuis J:
EndothelinA receptor blockade improves nitric oxide-mediated
vasodilation in monocrotaline-induced pulmonary hypertension.
Circulation. 97:2169–2174. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura K, Hatano E, Narita M,
Miyagawa-Hayashino A, Koyama Y, Nagata H, Iwaisako K, Taura K and
Uemoto S: Sorafenib attenuates monocrotaline-induced sinusoidal
obstruction syndrome in rats through suppression of JNK and MMP-9.
Hepatology. 57:1037–1043. 2012. View Article : Google Scholar
|
|
19
|
DeLeve LD, McCuskey RS, Wang X, Hu L,
McCuskey MK, Epstein RB and Kanel GC: Characterization of a
reproducible rat model of hepatic veno-occlusive disease.
Hepatology. 29:1779–1791. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki M, Bachelet-Violette L, Rouzet F,
Beilvert A, Autret G, Maire M, Menager C, Louedec L, Choqueux C,
Saboural P, et al: Ultrasmall superparamagnetic iron oxide
nanoparticles coated with fucoidan for molecular MRI of
intraluminal thrombus. Nanomedicine (Lond). 10:73–87. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wisse E, De Zanger RB, Charels K, Van Der
Smissen P and McCuskey RS: The liver sieve: Considerations
concerning the structure and function of endothelial fenestrae, the
sinusoidal wall and the space of Disse. Hepatology. 5:683–692.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fina L, Molgaard HV, Robertson D, Bradley
NJ, Monaghan P, Delia D, Sutherland DR, Baker MA and Greaves MF:
Expression of the CD34 gene in vascular endothelial cells. Blood.
75:2417–2426. 1990.PubMed/NCBI
|
|
23
|
Couvelard A, Scoazec JY, Dauge MC,
Bringuier AF, Potet F and Feldmann G: Structural and functional
differentiation of sinusoidal endothelial cells during liver
organogenesis in humans. Blood. 87:4568–4580. 1996.PubMed/NCBI
|
|
24
|
Kuntz E and Kuntz HD: Textbook and Atlas:
History, Morphology, Biochemistry, Diagnostics, Clinic,
TherapyHepatology. 3rd. Springer Medizin Verlag; Heidelberg: 2008,
View Article : Google Scholar
|
|
25
|
Wakayama T, Nakata H, Kurobo M, Sai Y and
Iseki S: Expression, localization, and binding activity of the
ezrin/radixin/moesin proteins in the mouse testis. J Histochem
Cytochem. 57:351–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jelliffe DB, Bras G and Stuart KL:
Veno-occlusive disease of the liver. Pediatrics. 14:334–339.
1954.PubMed/NCBI
|
|
27
|
Campos-Varela I, Castells L, Dopazo C,
Pérez-Lafuente M, Allende H, Len O, Llopart L, Vargas V and Charco
R: Transjuglar intrahepatic portsystemic shunt for the treatment of
sinusoidal obstruction syndrome in a liver transplant recipient and
review of the literature. Liver Transpl. 18:201–205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao L, Yao ZM and Yu T: Influence of BOL
on hyaluronic acid, laminin and hyperplasia in hepatofibrotic rats.
World J Gastroenterol. 7:872–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2001. View Article : Google Scholar
|
|
30
|
Merza M, Hartman H, Rahman M, Hwaiz R,
Zhang E, Renström E, Luo L, Mörgelin M, Regner S and Thorlacius H:
Neutrophil extracellular traps induce trypsin activation,
inflammation and tissue damage in mice with severe acute
pancreatitis. Gastroenterology. 149:1920–1931, e8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakai S, Tajima H, Miyashita T, Nakanuma
S, Makino I, Hayashi H, Nakagawara H, Kitagawa H, Fushida S,
Fujimura T, et al: Sivelestat sodium hydrate inhibits neutrophil
migration to the vessel wall and suppresses hepatic ischemia
reperfusion injury. Dig Dig Sci. 59:787–794. 2014. View Article : Google Scholar
|
|
32
|
Nakanuma S, Miyashita T, Hayashi H, Tajima
H, Takamura H, Tsukada T, Okamoto K, Sakai S, Makino I, Kinoshita
J, et al: Extravasated platelet aggregation in liver zone 3 may
correlate with the progression of sinusoidal obstruction syndrome
following living donor liver transplantation: A case report. Exp
Therap Med. 9:1119–1124. 2015.
|
|
33
|
Takamura H, Nakanuma S, Hayashi H, Tajima
H, Kakinoki K, Kitahara M, Sakai S, Makino I, Nakagawara H,
Miyashita T, et al: Severe veno-occlusive disease/sinusoidal
obstruction syndrome after deceased-donor and living-donor liver
transplantation. Transplant Proc. 46:3523–3535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pai RK, van Besien K, Hart J, Artz AS and
O'Donnell PH: Clinicopathologic features of late onset
veno-occlusive disease/sinusoidal obstructive syndrome after high
dose busulfan and hematopoietic cell transplantation. Leuk
Lymphoma. 53:1552–1557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carreras E, Grañena A and Rozman C:
Hepatic veno-occlusive disease after bone marrow transplant. Blood
Rev. 7:43–51. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Battinelli EM, Markens BA and Italiano JE
Jr: Release of angiogenesis regulatory proteins from platelet alpha
granules: Modulation of physiologic and pathologic angiogenesis.
Blood. 118:1359–1368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui S, Shibamoto T, Liu W, Takano H and
Kurata Y: Effects of platelet- activating factor, thromboxane A2
and leukotriene D4 on isolated perfused rat liver. Prostaglandins
Other Lipid Mediat. 80:35–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Narmada BC, Chia SM, Tucker-Kellogg L and
Yu H: HGF regulates the activation on TGF-β1 in rat hepatocytes and
hepatic stellate cells. J Cell Physiol. 228:393–401. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parenti A, Brogelli L, Filippi S, Donnini
S and Ledda F: Effect of hypoxia and endothelial loss on vascular
smooth muscle cell responsiveness to VEGF-A: Role of
fit-1/VEGF-receptor-1. Cardiovasc Res. 55:201–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oates JA, FitzGerald GA, Branch RA,
Jackson EK, Knapp HR and Roberts LJ II: Clinical implications of
prostaglandin and thromboxane A2 formation (1). N Eng J Med.
319:689–698. 1988. View Article : Google Scholar
|
|
41
|
Attal M, Huguet F, Rubie H, Huynh A,
Charlet JP, Payen JL, Voigt JJ, Brousset P, Selves J and Muller C:
Prevention of hepatic veno-occlusive disease after bone marrow
transplantation by continuous infusion of low-dose heparin: A
prospective, randomized trial. Blood. 79:2834–2840. 1992.PubMed/NCBI
|