Introduction
Pelvic organ prolapse (POP) is a global health
problem that may seriously impact the quality of life of the
sufferer. It affects ~50% of women >50 years and surgery is
required in 20% of cases by the age of 80 (1). Vaginal childbirth, obesity and ageing
are important risk factors for developing POP (2,3) and
occur when a loss of healthy attachment and support results in
descent of the pelvic organs into the vaginal canal. The bladder,
uterus and rectum are all located around the vaginal canal. Owing
to the arrangement of these organs, bulging into the vaginal canal
due to weakness of the supportive tissues for these organs is
common. The descent of one or more out of the anterior vaginal
wall, posterior vaginal wall, the uterus (cervix) or the apex of
the vagina (vaginal vault or cuff scar following hysterectomy) is
defined as POP.
Female pelvic floor tissues are exposed to complex
biomechanical environments including pregnancy, childbirth and
other alterations in abdominal pressure. Coordination of ligaments,
fascia and muscle support pelvic organs. Previous studies have
demonstrated that POP is a disease based on the progressive decline
and abnormalities of the biomechanical properties of pelvic support
tissues (4,5).
The etiology of POP is complex and multifactorial,
and epidemiological studies have suggested that multiple
pathologies contribute to full anatomical loss, involving vaginal
parity and other obstetric risk factors (6,7), in
addition to advanced age, increased body mass index, smoking,
constipation and vaginal hysterectomy (8–10).
The mechanisms underlying pelvic floor support failure remain
poorly understood; however, studies in humans and animals implicate
defects in the extracellular matrix (ECM) or fibrous connective
tissue causing reduced tissue strength and defective repair
(11). It is hypothesized that
alterations in the connective tissue and ECM of the pelvic organs
may serve a role in the development of POP.
ECM composition, organization and compliance provide
architectural and chemical cues that regulate tissue homeostasis.
ECM produced by stromal fibroblasts serves a key role in POP. The
ECM of the pelvic floor, termed the endopelvic fascia, is
responsible for maintaining the position of organs adjacent to the
vagina. The primary fibrillar components of the ECM, collagen and
elastin, are hypothesized to contribute the most to its
biomechanical properties. Their alterations are potentially
involved in the physiopathology of POP (11). Collagen fibers are rigid and do not
easily distort, whereas elastin fibers provide elasticity and
recoil to the tissue. Elastic fibers are important for maintaining
vaginal structural integrity against mechanical strain (12).
The ECM is degraded by a family of enzymes called
the matrix metalloproteinases (MMPs). MMPs are Zn2+ dependent
endopeptidases responsible for the ECM degradation of connective
tissue matrix, including ligaments (13). Collectively, they are capable of
degrading all types of ECM proteins (14). The gelatinase sub-family is
composed of gelatinase A (MMP-2) and gelatinase B (MMP-9); the two
are capable of metabolizing native and denatured collagen, gelatin,
elastin, laminin, fibronectin and the basement membrane (14,15).
Transforming growth factor-β 1 (TGF-β1) has unique
and widespread actions in the remodeling of the ECM and is critical
for tissue integrity. TGF-β1 is a multifunctional cytokine and
dominant regulator of multiple ECM components and enzymes.
Sustained elevations of TGF-β1 have been associated with multiple
pathological conditions, including pulmonary fibrosis, keloid
formation, coronary artery restenosis and acute respiratory
distress syndrome (16). Pascual
et al (17) illustrated an
increase in expression of TGF-β1 in the fascia of inguinal hernias.
The pathogenesis of abdominal hernias and POP may be similar, as
the two conditions result in the loss of fascial support leading to
a protrusion or herniation of organs. Previous studies have
reported altered expression of TGF-β1 in women with POP (18,19).
Our previous studies (20,21) additionally demonstrated
downregulation of TGF-β1 in the pubocervical fascia tissue of
patients with POP.
The aim of the present study was to investigate the
expression of collagen and collagen metabolism-associated factors
in human parametrial ligament fibroblasts (hPLFs) under mechanical
strain and to examine potential alterations in collagen metabolism
induced by mechanical strain in the pathogenesis of POP.
Materials and methods
Patients and primary culture of
hPLFs
A total of 15 patients without POP or malignant
tumors who underwent vaginal hysterectomy surgery at the Department
of Obstetrics and Gynecology, Renmin Hospital of Wuhan University
(Wuhan, China) were selected. Ethical approval was obtained from
the Ethics Committee of Renmin Hospital of Wuhan University,
following which all patients signed informed consent. The patients
had no connective tissue diseases and oxidative stress-related
diseases, including coronary heart disease, diabetes and
hyperlipidemia. Patients with a history of estrogen application
within the past three months were excluded from the study.
Additionally, patients with endometriosis and ovarian, endocrine,
tumor and estrogen-associated diseases, as confirmed by
postoperative pathology, were removed from the study. Specimens
were extracted from part of the uterosacral and cardinal ligaments
in surgery. A collagenase digestion method was used for primary
culture of hPLFs as described in our previous study (22).
Mechanical strain loading on hPLFs in
vitro
Fibroblasts at generations 3–6 of exponential phase
were selected and a four-point bending device (SXG4201; Miracle
Technology Co., Ltd., Hsinchu, Taiwan) was used for mechanical
loading. The specific methods used are described in our previous
study (22). The fibroblasts were
cultured on a specially-made plate (Miracle Technology Co., Ltd.)
and stretched for mechanical strain loading. The parameters were
set to a frequency of 0.5 Hz for 4 h and cells were subject to
1,333 or 5,333 µ, using 0 µ strain as the control group.
Western blotting
Following mechanical strain loading at various
degrees, the cells were lysed on ice for 30 min in RIPA buffer
(Beyotime Institute of Biotechnology, Haimen, China) containing 1
mM PMSF (Sigma, USA) and then centrifuged at 12,000 × g at 4°C for
15 min. The supernatant was collected and quantified using a
Bincinchoninic Acid protein assay kit (Beyotime Institute of
Biotechnology). For each group, a total of 40 µg protein was
separated by SDS-PAGE with a 5% stacking and a 10% separating gel,
and subsequently transferred onto a polyvinylidene difluoride
membrane. The membrane was blocked in 5 g/l non-fat milk for 1 h
and washed with TBS, following which it was incubated with the
appropriate monoclonal antibodies at 4°C overnight. Following
washing with TBS with Tween-20 (TBST), the membrane was incubated
with a horseradish peroxidase-conjugated anti-IgG secondary
antibody at 37°C for 1 h. Subsequently, the membrane was washed
with TBST and target proteins were visualized using an Enhanced
Chemiluminescence kit (32106; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The following primary antibodies were used:
Collagen type I α 1 chain (COL1A1; 1:1,000; ab34710), collagen type
III α 1 chain (COL3A1; 1:5,000; ab7778), elastin (1:1,000;
ab23747), MMP-2 (1:200; ab7033), MMP-9 (1:1,000; ab38898) and
TGF-β1 (1:500; ab64715), all purchased from Abcam, Cambridge, MA,
USA. GAPDH (1:1,000; ab8245; Abcam) was used as an internal
reference control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression of COL1A1, COL3A1, MMP-2, MMP-9,
TGF-β1 and GAPDH were evaluated by RT-qPCR. The primers used for
amplification were purchased from Beijing SBS Genetech Co., Ltd.
(Beijing, China). Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA
was reverse transcribed to cDNA using a RevertAid First Strand cDNA
Synthesis kit (catalog no. k1622; Thermo Fisher Scientific, Inc.).
SYBR® Premix Ex Taq™ (Takara Bio, Inc., Otsu, Japan) was
used to detect gene expression in an ABI 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) for in
vitro qPCR which was performed as follows: 30 sec at 95°C, 40
cycles of 5 sec at 95°C, 34 sec at 60°C, 15 sec at 95°C, 1 min at
60°C, 15 sec at 95°C and 15 sec at 60°C. mRNA expression levels
were calculated and normalized to the expression levels of GAPDH.
The primers used in this study are presented in Table I. Normalized quantitation threshold
(Cq) values were used for comparison (23). Each sample was analyzed in
triplicate to ensure accuracy.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
GCACCGTCAAGGCTGAGAAC |
|
| R:
TGGTGAAGACGCCAGTGGA |
| COL1A1 | F:
CAAGACGAAGACATCCCACCAATC |
|
| R:
ACAGATCACGTCATCGCACAACA |
| COL3A1 | F:
TCGCTCTGCTTCATCCCACTAT |
|
| R:
CTTCCAGACATCTCTATCCGCAT |
| Elastin | F:
AAAGCAGCAGCAAAGTTCGG |
|
| R:
ACCTGGGACAACTGGAATCC |
| MMP-2 | F:
AGTTTCCATTCCGCTTCCAG |
|
| R:
CGGTCGTAGTCCTCAGTGGT |
| MMP-9 | F:
GTCCACCCTTGTGCTCTTCC |
|
| R:
GACTCTCCACGCATCTCTGC |
| TGF-β1 | F:
TATTGAGCACCTTGGGCACT |
|
| R:
ACCTCTCTGGGCTTGTTTCC |
Statistical analysis
Statistical analyses were performed with SPSS
version 19.0 (IBM SPSS, Armonk, NY, USA). All data are expressed as
the mean ± standard deviation. ANOVA was performed to evaluate the
differences between groups followed by Tukey post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of ECM components in
hPLFs following mechanical strain
hPLFs were isolated and cultured from human
uterosacral and cardinal ligaments and identified by positive
staining of vimentin and negative staining of cytokeratin (22).
To examine whether the ECM serves an important role
in the development of POP, western blotting and RT-qPCR were used
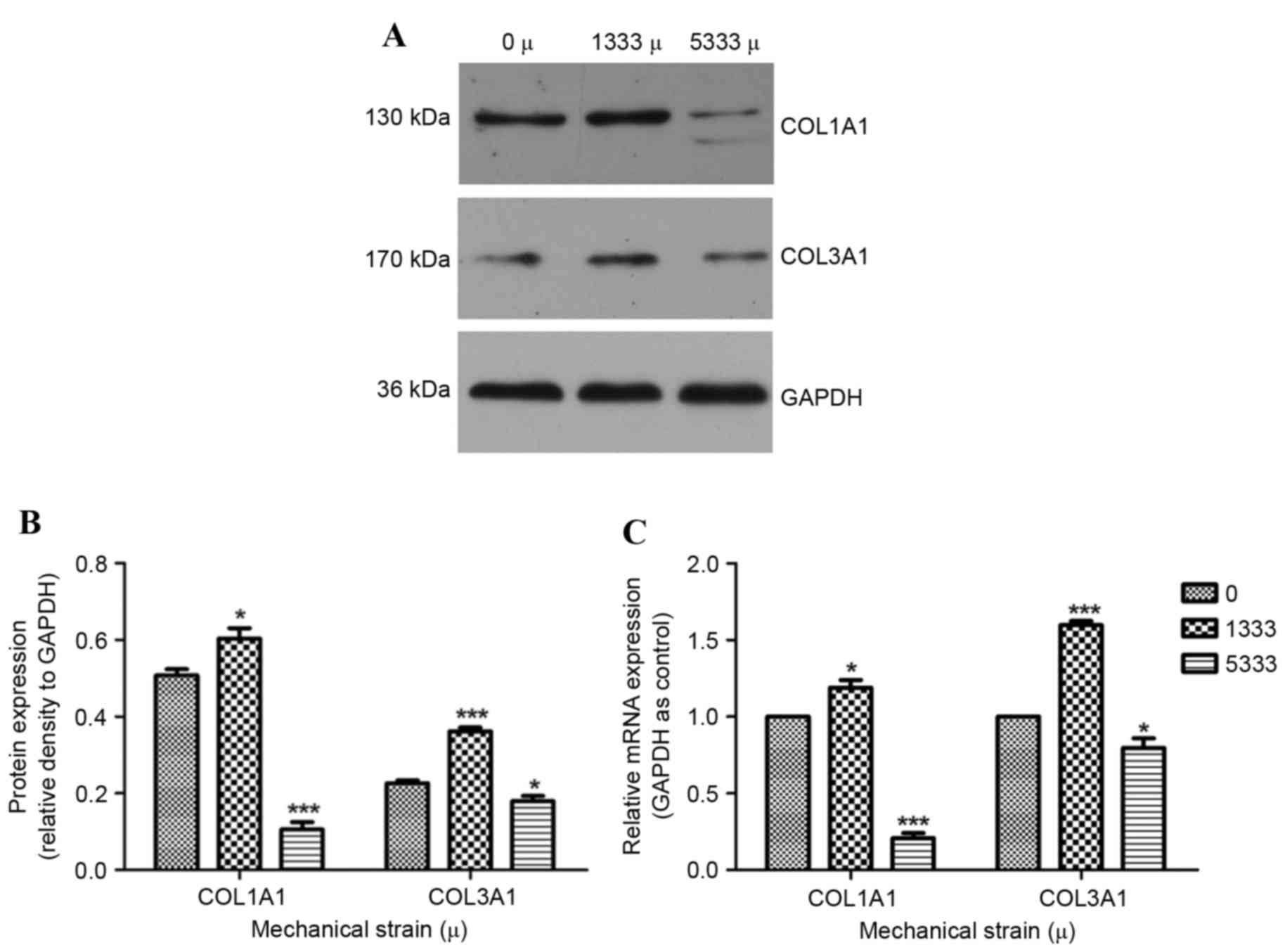
to detect protein (Fig. 1A and B)
and mRNA (Fig. 1C) expression
levels of its components under various mechanical strains (0, 1,333
or 5,333 µ). As collagen is a primary component of the ECM, and
COL1A1 and COL3A1 are the two principle components of pelvic
connective tissue, their expression levels were detected. Under
1,333 µ mechanical strain, COL3A1 protein and mRNA expression
levels were significantly increased compared with the control group
(P<0.001). This trend was additionally observed in COL1A1
protein and mRNA expression levels (P<0.05). In contrast,
decreased COL1A1 and COL3A1 protein and mRNA expression levels in
response to 5,333 µ mechanical strain were observed, compared with
the control group.
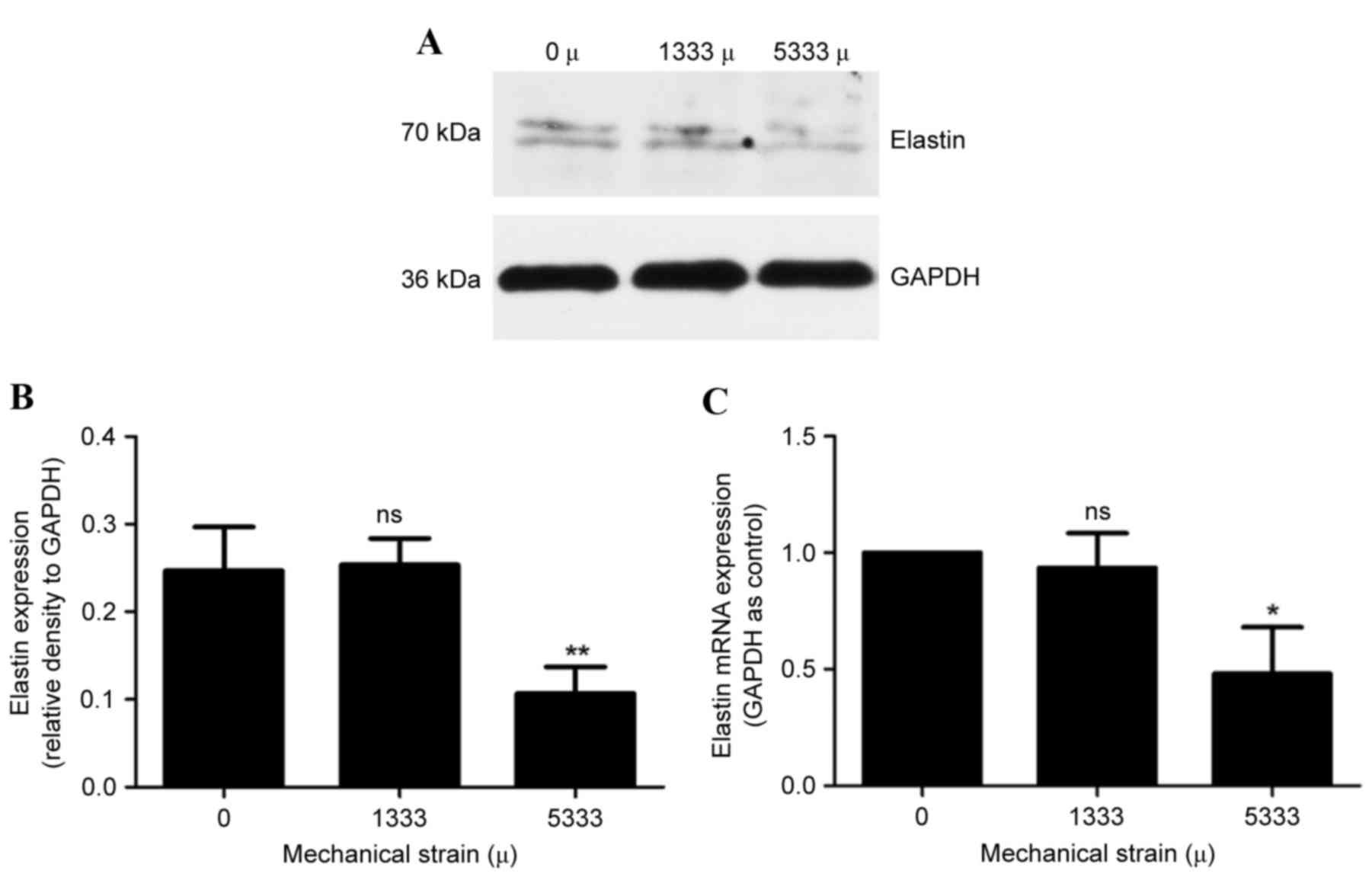
The effect of mechanical strain on elastin was
examined, which is another primary fibrillar component of the ECM
and provides elasticity and recoil to the ligament. As presented in
Fig. 2, no significant differences
in elastin protein (Fig. 2A and B)
and mRNA (Fig. 2C) expression
levels were observed between control cells and the 1,333 µ
mechanical strain group (P>0.05). On the other hand, the 5,333 µ
mechanical strain group exhibited reduced protein (Fig. 2A and B) and mRNA (Fig. 2C) expression levels (P<0.01 and
P<0.001, respectively). Taken together, these results suggested
that when hPLFs were subject to increased mechanical strain,
expression levels of ECM components reduced.
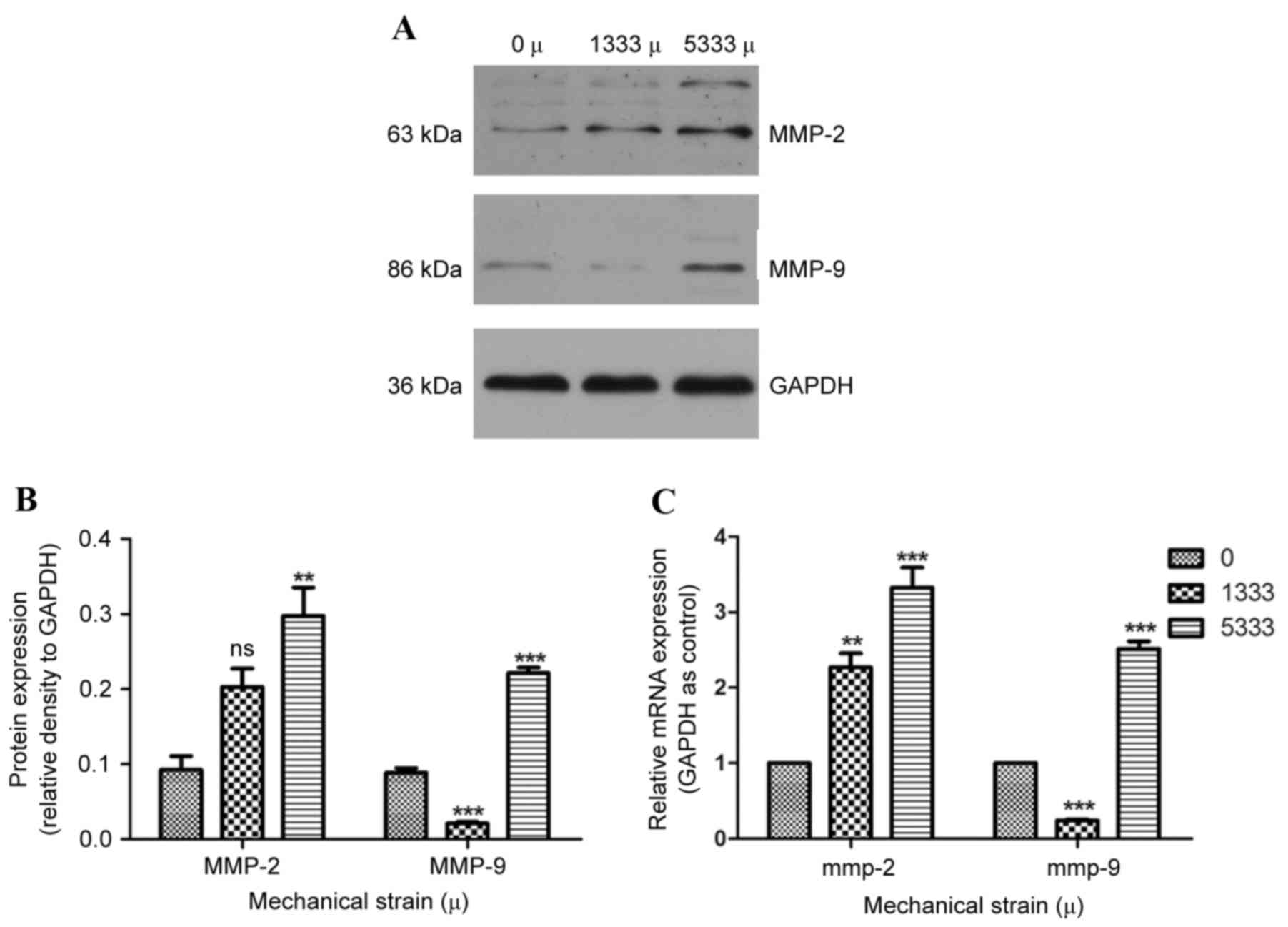
Expression levels of MMP-2 and −9 in
hPLFs following mechanical strain
MMP-2 and −9 are crucial enzymes for degrading the
ECM; the production of MMP is associated with pelvic fibrosis. To
demonstrate whether MMP is affected following the ECM alterations
observed above, the expression levels of MMP-2 and −9 were assessed
by western blotting and RT-qPCR. Compared with control cells, under
1,333 µ mechanical strain, no significant differences in MMP-2
protein expression levels were observed (Fig. 3A and B), whereas MMP-2 mRNA
expression levels were significantly increased (P<0.01, Fig. 3C). Protein (Fig. 3A and B) and mRNA (Fig. 3C) expression levels of MMP-9 were
reduced compared with the control group (P<0.001). When subject
to 5,333 µ mechanical strain, MMP-2 and MMP-9 protein (Fig. 3A and B) and mRNA (Fig. 3C) expression levels were
significantly increased compared with the control group
(P<0.001).
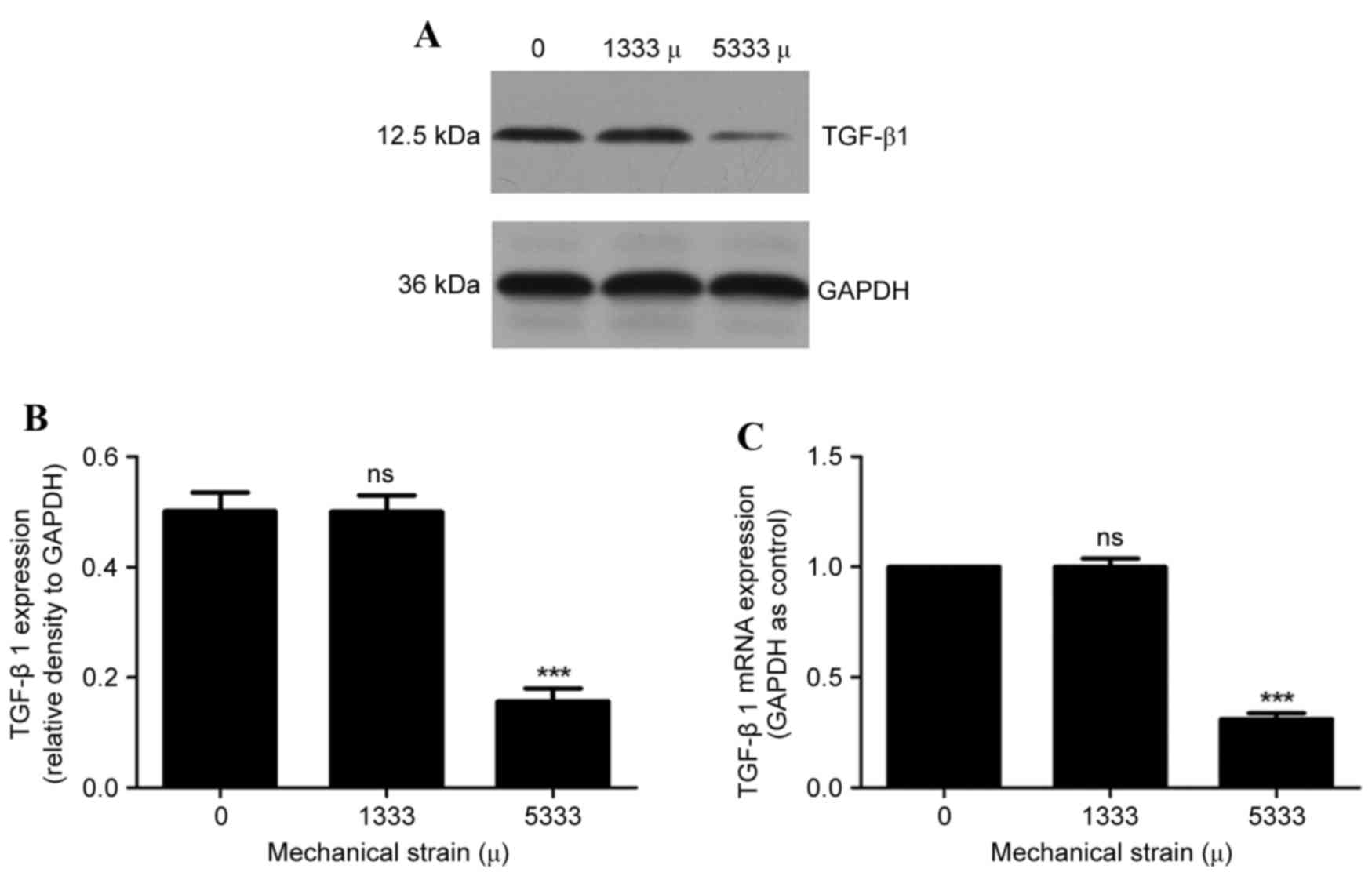
Expression levels of TGF-β1 in hPLFs
following mechanical strain
TGF-β1 is a multifunctional cytokine and dominant
regulator of multiple ECM components and enzymes. No significant
differences in TGF-β1 protein (Fig. 4A
and B) and mRNA (Fig. 4C)
expression levels were observed following 1,333 µ mechanical
strain, compared with compared with the control group (P>0.05).
However, significantly decreased protein (Fig. 4A and B) and mRNA (Fig. 4C) expression levels of TGF-β1 were
observed following 5,333 µ mechanical strain (P<0.001).
Discussion
To the best of our knowledge, this study is the
first evaluation of ECM metabolism using mechanical load in an hPLF
model to investigate the potential pathogenesis of POP. These
findings demonstrated that following mechanical strain, the
expression of components of the ECM altered, particularly MMP- and
−9, and TGF-β1, resulting in modified ECM metabolism. This
indicated that the TGF-β1 signaling pathway may be involved in the
ECM disorder caused by mechanical strain.
POP is a common pelvic floor disorder (3), occurring when a loss of healthy
attachment and support results in descent of the pelvic organs into
the vaginal canal. DeLancey (24)
reported three levels of connective tissue support of the vagina
when defining pelvic floor anatomy. Level I refers to the apical
portion of the posterior vaginal wall, which is suspended and
supported primarily by the cardinal-uterosacral ligaments. If level
I support is lost, apical POP follows. It has been reported that
dysfunction of level I, comprised of the uterosacral and cardinal
ligaments, is one of the key factors involved in POP (25). Therefore, the present study
selected hPLFs (derived from the uterosacral and cardinal
ligaments) to examine.
Previous studies in pelvic tissues have demonstrated
that alterations in the ECM lead to the development of POP.
Collagen is the primary component of pelvic connective tissue,
providing a scaffold for ECM assembly. Type I fibers are
well-organized and present in uterosacral ligaments that provide
DeLancey level I support of the cervix and vaginal apex (26). Type III fibers are more prominent
in the loose areolar tissue surrounding the vagina and pelvic
organs. Evaluation of the expression levels of COL1A1 and COL3A1 in
women with and without POP has yielded varying results, with some
studies demonstrating increased expression and others reporting
decreased expression (27,28). Numerous variables may contribute to
this, including different tissue types being studied (for example
uterosacral ligaments vs. the vaginal wall), harvesting and
extraction methods, patient characteristics and the molecular
makeup of the collagen. However, in the uterosacral and cardinal
ligaments of patients with POP, numerous studies have demonstrated
decreased COL1A1 and COL3A1 expression (29,30).
The present study on the expression levels of collagen in hPLFs
following greater mechanical strain was consistent with a previous
study in tissues (31), indicating
that decreased collagen expression may serve an important role in
the development of POP.
The results of the present study suggested that
elastin expression levels additionally decreased following
mechanical strain. Elastin fibers are key architectural elements of
connective tissues that are subject to mechanical strain, providing
extensibility and recoil ability to elastin tissues (32). Decrease in elastin content may
cause alterations in the properties of the ligament, resulting in
increased rigidity and decreased resistance to mechanical force.
These findings supported the importance of collagen and elastin
homeostasis in the development of POP.
MMP-2 and −9 expression levels were examined in
hPLFs following mechanical strain because of their important role
in remodeling of the ECM. MMP-2 contains three repeats of a type II
fibronectin domain in the catalytic domain, which binds to and may
degrade gelatin, collagens and laminin (33). MMP-9 is a gelatinase whose primary
function is the breakdown of basement membranes. Studies on the
role of MMPs have resulted in divergent findings (34,35).
Dviri et al (36)
demonstrated an increase in MMP-1 and −9 expression in uterosacral
ligaments in women with POP. Conversely, Phillips et al
(37) reported no difference in
expression of MMP-9 in uterosacral ligaments in women with POP
compared with controls. The present study demonstrated
significantly increased mRNA and protein expression levels of MMP-2
and MMP-9 in hPLFs following 5,333 µ mechanical strain, and
decreased expression levels of the ECM components elastin, COL1A1
and COL3A1, which indicated that increased expression of MMP-2 and
MMP-9 may lead to degradation of the ECM in POP development.
Studies examining the role of TGF-β1 in POP have
demonstrated contradictory results; Meijerink et al
(19) reported a positive
correlation between TGF-β1 expression and POP, whereas Qi et
al (20) observed a negative
correlation. The present study demonstrated that mRNA and protein
expression levels of TGF-β1 were reduced following stronger
mechanical strain (5,333 µ). The TGF-β1/mothers against
decapentaplegic homolog 3 (Smad3) signaling pathway is currently
viewed as an important regulator of fibrosis and degenerative
fibrotic diseases. Combined with these results, it was hypothesized
that the TGF-β1/Smad3 signaling pathway may serve a critical role
in the development of POP, which requires further confirmation in
future studies.
The present study additionally demonstrated that
hPLFs subject to weak mechanical strain exhibited slightly altered
ECM metabolism, resulting in an increase in synthesis of certain
components of the ECM and a decrease in degradation. It is
understood that clinical pelvic floor muscle training and other
methods may contribute to pelvic recovery postpartum and may be
used to treat pelvic floor dysfunction without symptoms (38). Therefore, moderate mechanical
strain may be conducive to the maintenance and restoration of
healthy pelvic tissue structure and functions. However, stronger
mechanical strain may damage the pelvic floor tissue and POP
develops. These results indicated that a weaker mechanical strain
may benefit ECM metabolism, whereas a stronger mechanical strain
may be harmful.
In conclusion, the present study demonstrated that
upregulation of MMP expression and downregulation of the TGF-β1
signaling pathway induced by stronger mechanical strain reduced ECM
synthesis and increased ECM degradation. These in vitro
results indicated that these factors may additionally be involved
in the process of POP in vivo. This may supply a new target
and strategy for the understanding of the etiology and treatment of
POP.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270684) and the
Foundation of Collaborative and Innovation Projects of Wuhan
University School of Medicine (grant no. 523-266078).
References
|
1
|
Wu JM, Matthews CA, Conover MM, Pate V and
Funk Jonsson M: Lifetime risk of stress urinary incontinence or
pelvic organ prolapse surgery. Obstet Gynecol. 123:1201–1206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buchsbaum GM, Duecy EE, Kerr LA, Huang LS,
Perevich M and Guzick DS: Pelvic organ prolapse in nulliparous
women and their parous sisters. Obstet Gynecol. 108:1388–1393.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelovsek JE, Maher C and Barber MD: Pelvic
organ prolapse. Lancet. 369:1027–1038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dviri M, Leron E, Dreiher J, Mazor M and
Shaco-Levy R: Increased matrix metalloproteinases-1, −9 in the
uterosacral ligaments and vaginal tissue from women with pelvic
organ prolapse. Eur J Obstet Gynecol Reprod Biol. 156:113–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ewies AA, Al-Azzawi F and Thompson J:
Changes in extracellular matrix proteins in the cardinal ligaments
of post-menopausal women with or without prolapse: A computerized
immunohistomorphometric analysis. Hum Reprod. 18:2189–2195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandberg LB, Weissman N and Gray WR:
Structural features of tropoelastin related to the sites of
cross-links in aortic elastin. Biochemistry. 10:52–56. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramirez F, Sakai LY, Dietz HC and Rifkin
DB: Fibrillin microfibrils: Multipurpose extracellular networks in
organismal physiology. Physiol Genomics. 19:151–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakai LY, Keene DR and Engvall E:
Fibrillin, a new 350-kD glycoprotein, is a component of
extracellular microfibrils. J Cell Biol. 103:2499–2509. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Apfelroth SD, Hu W, Davis EC,
Sanguineti C, Bonadio J, Mecham RP and Ramirez F: Structure and
expression of fibrillin-2, a novel microfibrillar component
preferentially located in elastic matrices. J Cell Biol.
124:855–863. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carley ME and Schaffer J: Urinary
incontinence and pelvic organ prolapse in women with Marfan or
Ehlers Danlos syndrome. Am J Obstet Gynecol. 182:1021–1023. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kerkhof MH, Hendriks L and Brölmann HA:
Changes in connective tissue in patients with pelvic organ
prolapse-a review of the current literature. Int Urogynecol J
Pelvic Floor Dysfunct. 20:461–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen B, Wen Y and Polan ML: Elastolytic
activity in women with stress urinary incontinence and pelvic organ
prolapse. Neurourol Urodyn. 23:119–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lisboa RA, Lisboa FA, de Castro Santos G,
Andrade MV and Cunha-Melo J: Matrix metalloproteinase 2 activity
decreases in human periodontal ligament fibroblast cultures
submitted to simulated orthodontic force. In Vitro Cell Dev Biol
Anim. 45:614–621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao PM, Buhler JM, d'Ortho MP, Lebargy F,
Delclaux C, Harf A and Lafuma C: Expression of matrix
metalloproteinase gelatinases A and B by cultured epithelial cells
from human bronchial explants. J Biol Chem. 271:15580–15589. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
Pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pascual G, Corrales C, Gómez-Gil V, Buján
J and Bellón JM: TGF-beta1 overexpression in the transversalis
fascia of patients with direct inguinal hernia. Eur J Clin Invest.
37:516–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen Y, Polan ML and Chen B: Do
extracellular matrix protein expressions change with cyclic
reproductive hormones in pelvic connective tissue from women with
stress urinary incontinence? Hum Reprod. 21:1266–1273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meijerink AM, van Rijssel RH and van der
Linden PJ: Tissue composition of the vaginal wall in women with
pelvic organ prolapse. Gynecol Obstet Invest. 75:21–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi XY, Hong L, Guo FQ, Fu Q, Chen L and Li
BS: Expression of transforming growth factor-beta 1 and connective
tissue growth factor in women with pelvic organ prolapse. Saudi Med
J. 32:474–478. 2011.PubMed/NCBI
|
|
21
|
Li BS, Hong L, Min J, Wu DB, Hu M and Guo
WJ: The expression of glutathione peroxidase-1 and the anabolism of
collagen regulation pathway transforming growth
factor-beta1-connective tissue growth factor in women with uterine
prolapse and the clinic significance. Clin Exp Obstet Gynecol.
40:586–690. 2013.PubMed/NCBI
|
|
22
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delancey JOL: Standing anatomy of the
pelvic floor. J Pelvic Surg. 2:1996.
|
|
25
|
Ramanah R, Berger MB, Chen L, Riethmuller
D and Delancey JO: See it in 3D!: Researchers examined structural
links between the cardinal and uterosacral ligaments. Am J Obstet
Gynecol. 207:437.e1–e7. 2012. View Article : Google Scholar
|
|
26
|
DeLancey JO: Anatomic aspects of vaginal
eversion after hysterectomy. Am J Obstet Gynecol. 166:1717–1728.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosier E, Lin VK and Zimmern P:
Extracellular matrix expression of human prolapsed vaginal wall.
Neurourol Urodyn. 29:582–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connell KA, Guess MK, Chen H, Andikyan V,
Bercik R and Taylor HS: HOXA11 is critical for development and
maintenance of uterosacral ligaments and deficient in pelvic
prolapse. J Clin Invest. 118:1050–1055. 2008.PubMed/NCBI
|
|
29
|
Sun ZJ, Zhu L, Lang JH, Wang Z and Liang
S: Proteomic analysis of the uterosacral ligament in postmenopausal
women with and without pelvic organ prolapse. Chin Med J (Engl).
128:3191–3196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gabriel B, Denschlag D, Göbel H, Fittkow
C, Werner M, Gitsch G and Watermann D: Uterosacral ligament in
postmenopausal women with or without pelvic organ prolapse. Int
Urogynecol J Pelvic Floor Dysfunct. 16:475–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu C, Yang Q, Fang G, Li BS, Wu DB, Guo
WJ, Hong SS and Hong L: Collagen metabolic disorder induced by
oxidative stress in human uterosacral ligament-derived fibroblasts:
A possible pathophysiological mechanism in pelvic organ prolapse.
Mol Med Rep. 13:2999–3008. 2016.PubMed/NCBI
|
|
32
|
Kielty CM, Sherratt MJ and Shuttleworth
CA: Elastic fibres. J Cell Sci. 115:2817–2828. 2002.PubMed/NCBI
|
|
33
|
Creemers LB, Jansen ID, Docherty AJ,
Reynolds JJ, Beertsen W and Everts V: Gelatinase A (MMP-2) and
cysteine proteinases are essential for the degradation of collagen
in soft connective tissue. Matrix Biol. 17:35–46. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gabriel B, Watermann D, Hancke K, Gitsch
G, Werner M, Tempfer C and zur Hausen A: Increased expression of
matrix metalloproteinase 2 in uterosacral ligaments is associated
with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct.
17:478–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang CC, Huang HY, Tseng LH, Chang SD, Lo
TS and Lee CL: Expression of matrix metalloproteinase-2 and tissue
inhibitors of metalloproteinase-1 (TIMP-1, TIMP-2 and TIMP-3) in
women with uterine prolapse but without urinary incontinence. Eur J
Obstet Gynecol Reprod Biol. 153:94–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dviri M, Leron E, Dreiher J, Mazor M and
Shaco-Levy R: Increased matrix metalloproteinases-1,-9 in the
uterosacral ligaments and vaginal tissue from women with pelvic
organ prolapse. Eur J Obstet Gynecol Reprod Biol. 156:113–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Phillips CH, Anthony F, Benyon C and Monga
AK: Collagen metabolism in the uterosacral ligaments and vaginal
skin of women with uterine prolapse. BJOG. 113:39–46. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trowbridge ER and Fenner DE: Conservative
management of pelvic organ prolapse. Clin Obstet Gynecol.
48:668–681. 2005. View Article : Google Scholar : PubMed/NCBI
|