Introduction
Bioaccumulation and biomagnification of pollutants
are considered the major threats to human and ecosystem health
(1–4). In particular, heavy metals can be
readily assimilated and bioaccumulated in organisms inducing a
potential risk to human health by consuming contaminated food
(5).
Since 2002, food safety is a major concern of the
European Union (EU) member states and protecting consumers' health
with independent scientific advice on the food chain is the main
objective of the EFSA strategy 2020 (6). For this reason, studies dealing with
risks and benefits of seafood consumption related to the presence
of toxic chemicals in fish, are growing (7–11).
Due to their place in the food chain, the large
predatory fish, such as swordfish and tuna, are considered the main
source of human exposure to metals. A large part of the scientific
literature, dealing with health risk linked to the consumption of
fish in the world, endorses this statement focusing, for example,
on levels of toxic metals in fresh and canned tuna (12–15).
In Europe, limits for contaminants in foodstuffs are established by
the Commission Regulation (EC) no. 1881/2006 of December 19, 2006,
setting maximum levels for lead (Pb), cadmium (Cd), and mercury
(Hg) (Pb in muscle meat of fish must not exceed 0.30 mg/kg wet
weight (w.w.); Cd and Hg must not exceed 0.10 and 1.0 mg/kg w.w.,
respectively, in Thunnus species, Euthynnus species
and Katsuwonus pelamis).
Interspecific differences in metal concentrations
exist due to the trophic level occupied by the fish, the region it
inhabits, the size and the age of the fish. For example Thunnus
albacares (Yellowfin tuna) from Taiwan contains level of heavy
metals in the muscle highest than those detected in the same
species from New Jersey; T. albacares from Reunion island,
in the Western Indian Ocean, contains level of heavy metals highest
than those detected in Katsuwonis pelamis from the same
region (16). For these reasons,
the EFSA Scientific Committee recommended to each country to
consider its own pattern of fish consumption, especially the
species of fish consumed, and carefully assess the risk of
exceeding the tolerable daily intake (TWI), in particular, of
methylmercury (17). Because of
the expansion of the fish global market and the citizen awareness
of the risks, a high and growing interest in the origin of seafood
products has been triggered in consumers, who demand for food
quality and safety assurance. In this context and because of the
alarming levels of seafood mislabeling worldwide detected (18–24),
the accurate identification of fish species in transformed products
is essential to verify food quality.
The reliability and sensitivity of species
authentication through molecular biology techniques is widely
acknowledged, especially when species lose the diagnostic
morphological characters useful to recognize them, due to the
industrial processing of food. The DNA barcoding methodology is
currently used to identify species and a partial sequence of the
mitochondrial gene cytochrome oxidase I (COI) referred to as a
barcode sequence, has been widely used for fish species
identification in transformed fishery products (25–39).
Of note, Lowenstein et al (40) used COI DNA barcode to identify tuna
sushi samples analyzed for mercury to more accurately measure the
health risk to consumers. Based on consideration above, the aims of
the present work are: i) to authenticate tuna species via COI DNA
barcode; ii) to evaluate the levels of heavy metals in samples of
canned tuna of the most popular brands sold in Italy; iii) to
assess which brands could pose a health risk to consumers.
Materials and methods
Sampling
A total of 5 brands of canned tuna in olive oil
(coded from X1 to X5 in the tables) and 5 brands of canned tuna in
brine (coded from X6 to X10 in the tables) packaged in metal cans,
representing the most popular brands sold in Italy, were purchased
from local markets during 2015 and 2016. Sampling was repeated
three times to analyze three different batches of the same brand. A
total of 30 canned tuna were processed, stored in laboratory at
−80°C until analysis.
DNA analysis
For each brand three samples were chosen randomly
and processed to investigate the presence of multiple species in
the product. The canned tissue samples were washed, at least three
times, in Milli-Q water (Q-Gard® 1, Merck Millipore,
Darmstadt, Germany) and then preserved in 95% ethanol (J.T. Baker,
Deventer, Netherlands). Total genomic DNA was extracted using
DNeasy tissue extraction kit (Qiagen) following the manufacturer's
protocol. All PCR amplifications were carried out using the primers
FishF1 and FishR1 described in Ward et al (35) and following the methodology
detailed in Pappalardo and Ferrito (33). Sequences were carefully checked and
deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The
chromatograms obtained were edited using BioEdit v7.0.8 (Ibis
Biosciences, Carlsbad, CA, USA) (41) to generate a consensus sequence for
each specimen. The DNA sequences were aligned using the ClustalX
(42) tool in the MEGA v5.0
software (Society for Molecular Biology and Evolution, Oxford, UK)
(43). A COI reference library of
six tuna species sequences from GenBank (Thunnus albacares,
KT211348; Katsuwonus pelamis, KJ968132; Thunnus
thynnus, KT352985; Thunnus obesus, KP975908; Thunnus
alalunga, KC501691; Euthynnus alletteratus, KJ709729)
and 27 sequences from processed samples were used to build a
Maximum Likelihood tree in MEGA v 5.0 using Tamura Nei model.
Evaluation of statistical confidence in nodes was based on 1,000
non-parametric bootstrap replicates (44). Ambiguous extremities of the
sequences were trimmed after alignment.
Heavy metals analysis
Three aliquots of 0.5 g of each sample were weighted
for metals extraction and quantification. As previously described
in detail (10), the samples were
acid digested in an Ethos TC microwave system (Milestone S.r.l,
Bergamo, Italy). A digestion solution was prepared with 6 ml of 65%
nitric acid (HNO3) (Carlo Erba, Milan, Italy) and 2 ml
of 30% peroxide hydrogen (H2O2-Carlo Erba)
over a 50 min, operation cycle at 200°C. After the cycle, at a
temperature <25°C the vessels were opened and ultra-pure water
(Merck Millipore, Bedford, MA, USA) was added to the samples up to
30 ml; an ICP-MS Elan-DRC-e (Perkin-Elmer, Norwalk, CT, USA) was
used for metal quantification. Standards for the instrument
calibration were prepared with mono element certified reference
solution ICP Standard (Merck Millipore). Standard reference
material Lake Superior fish 1946 NIST (NIST, Gaithersburg, MD, USA)
was used to validate analysis. We obtained recoveries of 96.2, 94.1
and 89.4%, respectively, for Cd, Pb and Hg certified values. Pb
reference value was not given in the certificate of analysis, thus
we spiked 10 real samples in duplicate with 5 µg/l of each element
to validate analysis, and the percentage of recovery ranged was of
110.6%. The method detection limits (MDL) estimated with 3σ of the
procedure blanks were (mg/kg w.w.): Cd 0.003, Pb 0.001 and Hg
0.002.
Statistical analysis
Statistical analysis was performed with IBM SPSS
20.0 software (IBM SPSS, Armonk, NY, USA). Mann-Whitney U test for
medians comparison between groups, canned tuna in olive oil and in
brine, and between declared species, was applied.
Risk assessment for consumers
The daily intake and the risk assessment were
calculated according to the equation reported in previous studies
(45–47).
EDI = MS×C/BW
THQ = (EF×ED×MS×C) / (RfDo×BW×AT)
Where EDI is the estimated daily intake and THQ is
the target hazard quotient estimated per meal size (MS=120 g),
corresponding to a whole packaged canned tuna. When THQ risk is
above 1, systemic effects may occur, and it means that THQ is
higher than the maximum permitted reference dose. The Environmental
Protection Agency (EPA) for consumption limits calculation in adult
population provides most of the variables as default values of the
equations. In particular they are the following: BW is the body
weight (adults, 70 kg); EF is the exposure frequency, or number of
exposure events per year of exposure (365 days/year for people who
eat fish seven times a week; 208 days/year for people who eat fish
four times a week; 52 days/year for people who eat fish once a
week); ED is the exposure duration (adults, 70 years); RfDo is the
oral reference dose (µg/g/day); AT is the averaging time (it is
equal to EF х ED), C is the metal concentration found in this study
(expressed as µg/kg w.w. in EDI and as µg/g in THQ).
Values of the RfDo are provided by EPA's Integrated
Risk Information System online database. If it was not available,
we used the respective metal provisional tolerable daily intake
(PTDI) provided by World Health Organization (WHO) for THQ
calculation.
Results
COI DNA barcode
Unambiguously aligned sequences were obtained for
655–659 bp of the COI gene from 30 samples of tuna canned products.
The three samples randomly chosen for each brand, yielded sequences
belonging to the same species. No insertions, deletions or stop
codons were observed. The lack of stop codons is consistent with
all amplified sequences being functional mitochondrial COI
sequences, along with the fact that all amplified sequences were of
the same length. This suggests that NUMTs (nuclear DNA sequences
originating from mitochondrial DNA sequences) were not sequenced
[vertebrate NUMTS are generally smaller than 600 bp (48)].
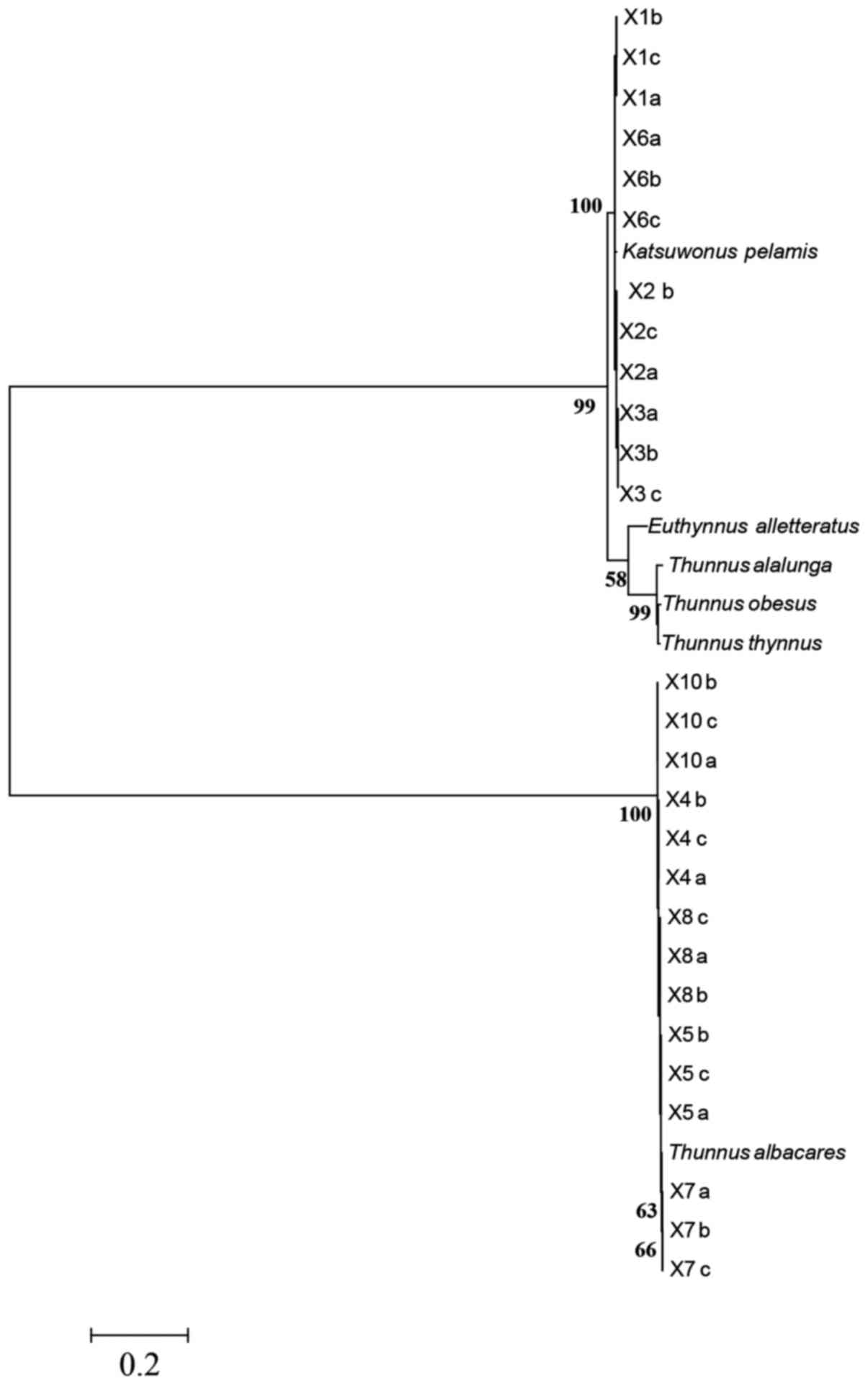
The ML tree built using the 6 reference sequences
and 27 COI sequences of tuna canned samples (Fig. 1), revealed that all sequences from
brands labeled as yellowfin tuna clustered with the Thunnus
albacares reference sequence; only four COI sequences out of
five products labeled as skipjack tuna clustered with the
Katsuwonis pelamis reference sequence. One sample out of ten
products labeled as tuna yielded no amplification (Table I).
 | Table I.Samples of canned tuna from Italian
supermarkets included in this study. |
Table I.
Samples of canned tuna from Italian
supermarkets included in this study.
| Code | Canned product | Declared
species | Identified
species | GenBank acc.
no. |
|---|
| X1 | Tuna in olive
oil | Katsuwonus
pelamis | Katsuwonus
pelamis | KY652762 |
| X2 | Tuna in olive
oil | Katsuwonus
pelamis | Katsuwonus
pelamis | KY652763 |
| X3 | Tuna in olive
oil | Katsuwonus
pelamis | Katsuwonus
pelamis | KY652764 |
| X4 | Tuna in olive
oil | Thunnus
albacares | Thunnus
albacares | KY652765 |
| X5 | Tuna in olive
oil | Thunnus
albacares | Thunnus
albacares | KY652766 |
| X6 | Tuna in brine | Katsuwonus
pelamis | Katsuwonus
pelamis | KY652767 |
| X7 | Tuna in brine | Thunnus
albacares | Thunnus
albacares | KY652768 |
| X8 | Tuna in brine | Thunnus
albacares | Thunnus
albacares | KY652769 |
| X9 | Tuna in brine | Tuna spp. | Not identified |
|
| X10 | Tuna in brine | Thunnus
albacares | Thunnus
albacares | KY652770 |
Heavy metals
Ten brands of canned tuna were analyzed to detect
concentrations of metals regulated by the EC no. 1881/2006
(Table II). We found mean
concentration of Cd of all brands below the law limit of 0.10 mg/kg
w.w., although one batch of the brand 1 in olive oil was over (0.13
mg/kg w.w.). Mean concentration of Pb was always found below the
law limit (0.30 mg/kg w.w.), moreover, in 5 brands (X1, X2, X5, X6
and X9) Pb was found below the method detection limit. Regarding
Hg, all the analyzed brands had Hg concentrations below the law
limit (1.0 mg/kg w.w.), with a large range of concentrations, from
0.02 to 0.71 mg/kg w.w.), and highest values in canned tuna in
brine X6 and X9 (Table II).
 | Table II.Mean concentrations and standard
deviations (SD) of Cd, Pb and Hg (mg/Kg wet weight) in canned tuna
of different brands. |
Table II.
Mean concentrations and standard
deviations (SD) of Cd, Pb and Hg (mg/Kg wet weight) in canned tuna
of different brands.
|
| Cd (EU limit: 0.10
mg/Kg w.w.) | Pb (EU limit: 0.30
mg/Kg w.w.) | Hg (EU limit: 1
mg/Kg w.w.) |
|---|
|
|
|
|
|
|---|
| Canned tuna | Mean | SD | Mean | SD | Mean | SD |
|---|
| Olive oil |
|
|
|
|
|
|
| X1 | 0.060 | 0.062 | <0.001 | / | 0.240 | 0.320 |
| X2 | 0.020 | 0.006 | <0.001 | / | 0.050 | 0.050 |
| X3 | 0.030 | 0.010 | 0.010 | 0.006 | 0.050 | 0.053 |
| X4 | 0.010 | 0.015 | 0.010 | 0.006 | 0.170 | 0.170 |
| X5 | 0.010 | 0.006 | <0.001 | / | 0.260 | 0.263 |
| Brine |
|
|
|
|
|
|
| X6 | 0.030 | 0.029 | <0.001 | / | 0.290 | 0.290 |
| X7 | 0.010 | 0.006 | 0.010 | 0.006 | 0.060 | 0.060 |
| X8 | <0.003 | 0.006 | 0.010 | 0.006 | 0.060 | 0.057 |
| X9 | 0.010 | 0.002 | 0.020 | 0.010 | 0.480 | 0.480 |
|
X10 | <0.003 | 0.006 | <0.001 | / | 0.150 | 0.150 |
Mann-Whitney U test did not highlight any
significant differences in metal concentrations between canned tuna
in olive oil and canned tuna in brine. Conversely, the comparison
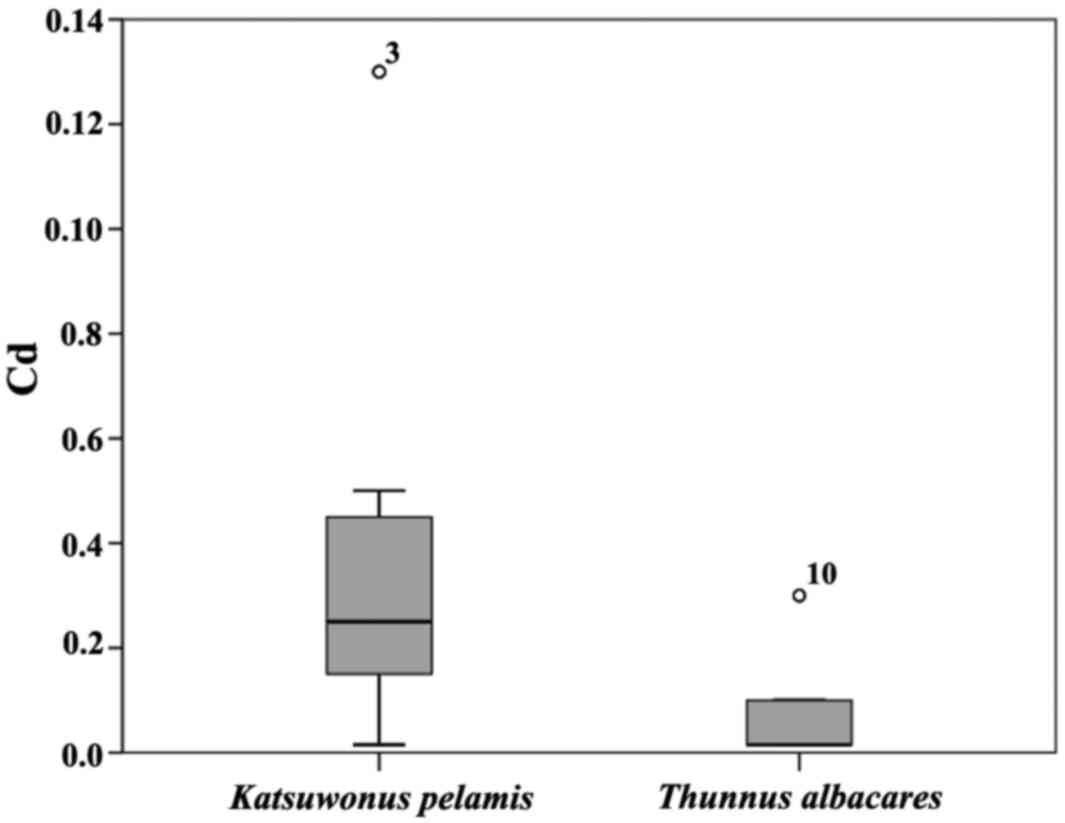
between species revealed significantly higher concentrations of Cd
(p=0.001) in Katsuwonus pelamis than in Thunnus
albacares (Fig. 2). The brand
X9 to which belongs an undeclared species as well as an
unidentified one was not included in this statistical test, but, as
it can be seen in Table II, it
has the highest value of Hg.
Exposure to daily intake, calculated supposing a
meal size corresponding to a full package of canned tuna (120 g)
revealed intake values for Pb and Cd significantly below the levels
of risk for human consumption (Table
III). The consumption of most of the brands analyzed, revealed
high levels of Hg daily intake if compared with the RfD suggested
by EPA for CH3Hg, conversely, Hg-EDI are very close to
the threshold suggested by WHO for tHg.
 | Table III.Exposure daily intake (EDI). |
Table III.
Exposure daily intake (EDI).
| Canned tuna | Pb EDI | Cd EDI | Hg EDI |
|---|
| Olive oil |
|
|
|
| X1 | – | 0.103 | 0.411 |
| X2 | – | 0.034 | 0.086 |
| X3 | 0.002 | 0.051 | 0.086 |
| X4 | 0.002 | 0.017 | 0.291 |
| X5 | – | 0.005 | 0.446 |
| Brine |
|
|
|
| X6 | – | 0.051 | 0.497 |
| X7 | 0.002 | 0.017 | 0.103 |
| X8 | 0.002 | 0.005 | 0.103 |
| X9 | 0.017 | 0.017 | 0.823 |
|
X10 | – | 0.005 | 0.257 |
| EPA-RDo
µg/kg-day | / | 1 | 0.1a |
| WHO-PTDI | 3.57 | 0.833 | 0.571 |
Target Hazard Quotient (THQ) was calculated to
evaluate the risk of chronic systemic effects due to the
consumption of the chosen brands of canned tuna. Furthermore, we
evaluated this health risk factor supposing a different exposure
scenario. For each brand, we have calculated the THQ with three
levels of exposure frequency. In detail, for seven, four and one
meals per week (Table IV).
 | Table IV.Target hazard quotient (THQ)
calculated for Cd, Pb and Hg with different levels of exposure
frequency (EF) of sampled brands. |
Table IV.
Target hazard quotient (THQ)
calculated for Cd, Pb and Hg with different levels of exposure
frequency (EF) of sampled brands.
| Brands | EF day/week | THQ Cd | THQ Pb | THQ Hg | THQ Hg |
|---|
| Olive oil |
|
|
|
|
|
| X1 | 7 | 0.102 | / | 4.114 | 0.721 |
|
| 4 | 0.058 | / | 2.344 | 0.411 |
|
| 1 | 0.014 | / | 0.586 | 0.103 |
| X2 | 7 | 0.034 | / | 0.857 | 0.150 |
|
| 4 | 0.019 | / | 0.488 | 0.086 |
|
| 1 | 0.004 | / | 0.122 | 0.021 |
| X3 | 7 | 0.051 | 0.0004 | 0.857 | 0.150 |
|
| 4 | 0.029 | 0.0002 | 0.488 | 0.086 |
|
| 1 | 0.007 | 0.0001 | 0.122 | 0.021 |
| X4 | 7 | 0.017 | 0.0004 | 2.914 | 0.510 |
|
| 4 | 0.009 | 0.0002 | 1.660 | 0.291 |
|
| 1 | 0.002 | 0.0001 | 0.415 | 0.073 |
| X5 | 7 | 0.005 | / | 4.457 | 0.781 |
|
| 4 | 0.002 | / | 2.539 | 0.445 |
|
| 1 | 0.001 | / | 0.634 | 0.111 |
| Brine |
|
|
|
|
|
| X6 | 7 | 0.051 | / | 4.971 | 0.871 |
|
| 4 | 0.029 | / | 2.833 | 0.496 |
|
| 1 | 0.007 | / | 0.708 | 0.124 |
| X7 | 7 | 0.017 | 0.0004 | 1.028 | 0.180 |
|
| 4 | 0.009 | 0.0002 | 0.586 | 0.103 |
|
| 1 | 0.002 | 0.0001 | 0.146 | 0.026 |
| X8 | 7 | 0.005 | 0.0004 | 1.028 | 0.180 |
|
| 4 | 0.002 | 0.0002 | 0.586 | 0.103 |
|
| 1 | 0.001 | 0.0001 | 0.146 | 0.026 |
| X9 | 7 | 0.017 | 0.0043 | 8.228 | 1.441 |
|
| 4 | 0.009 | 0.0024 | 4.689 | 0.821 |
|
| 1 | 0.002 | 0.0006 | 0.172 | 0.205 |
|
X10 | 7 | 0.005 | / | 2.571 | 0.450 |
|
| 4 | 0.002 | / | 1.465 | 0.257 |
|
| 1 | 0.007 | / | 0.366 | 0.064 |
|
| RfD or PTDI | RfD for Cd | PTDI for Pb and
compounds | RfD for
CH3Hg | PTDI for tHg |
|
| µg/kg-day | 1 | 3.57 | 0.1 | 0.571 |
We found the THQ always below the level of risk (THQ
<1) both for Cd and Pb in all analyzed brands. The oral exposure
to Hg derived by the ingestion of canned tuna is high for most of
the selected brands, even if, as said before, the concentrations
found are not over the law limit. Supposing that Hg found is 100%
CH3Hg, a level of exposure of one meal per week could be
protective against the toxic effect of CH3Hg for all the
brands. Higher exposure than one meal per week could be at risk
with the consumptions of brands X1, X4, X5, X6, X9 and X10. Only
the brands X2 and X3 have Hg concentrations so low that they could
be consumed even 7 times a week. Conversely, performing the THQ
calculation by using the PTDI given by WHO for tHg, we found value
higher than 1 in the brand X9 for a level of exposure of seven
meals per week. The other brands, with the exception of the X2, X3,
X7 and X8, for the same level of exposure, have values close to
1.
Discussion
The results obtained in this work deal with two main
issues of food safety: the species authentication in transformed
products, to unveil commercial frauds due to the substitutions of
high value species with species of low commercial value, and the
assessment of health risk to consumers related to the level of
heavy metal contents in canned tuna in olive oil or in brine. The
COI DNA barcode analysis revealed that nine out of ten of most
popular commercial brands of tuna sold in Italy contain the species
declared in the label, Thunnus albacares and Katsuwonis
pelamis. Although appropriate species traceability and labeling
is nowadays requested by laws, mislabeling is often difficult to
demonstrate because more than one species is marketed under the
same name.
The current list of trade names of fish species of
commercial interest marketed in Italy (Italian Ministerial Decree -
MD - of January 31, 2008 as further supplemented and amended by MD
August 12, 2011 and December 23, 2010) includes ten tuna species.
Three species (Euthynnus affinis, Euthynnus lineatus and
Thunnus tonggol) are marketed as Indopacific tuna, while
each of the remaining seven species are marketed under specific
names and in particular these were yellowfin tuna for T.
albacares and skipjack tuna for K. pelamis. These two
species are the more common varieties used for preparation of
canned tuna and the products studied by us were properly labeled.
However, the canned tuna has high potential to be the target of
intentional or unintentional mislabeling: for example striped
bonito (Sarda orientalis) was found in products labeled as
tongol tuna (Thunnus tonggol), a case of evident economic
fraud (49). Studies by Lowenstein
et al (50) uncovered
pieces of tuna sushi to contain endangered species (protected under
the Convention on International Trade in Endangered Species of Wild
Fauna and Flora - CITES) such as the northern bluefin tuna (T.
thynnus) and the southern bluefin tuna (T. maccoyii);
they also found the escolar (Lepidocybium flavorunneum),
banned for sale in Italy and Japan because it contains gempylotoxin
causing digestive symptoms, sold as white tuna (T.
albacore). The authentication of tuna species by DNA barcoding
was also of paramount importance to relate the health risk to
consumers to the content of heavy metals in tuna species (40).
Fish that is almost of the higher trophic level,
such as tuna, would be considered unsafe for human consumption
because to the bioaccumulation of contaminants up to the food
chain. Heavy metals are known for their toxicity and because they
can cause health risks in consumers through ingestion of
contaminated foods (51). Although
the outcomes of more recent monitoring studies have been different
in that they stressed that the maximum levels set by regulations of
the respective countries had been frequently exceeded, a food that
exceeds the maximum food standard is not necessarily unfit for
human consumption. Conversely, a food that does not exceed the
maximum food standard could be unfit for human consumption: these
limits are conservatively set for regulatory purpose and assume a
worst-case scenario. For this reason it is important to evaluate
the metal daily intake and the health risk during the lifetime,
building different scenarios of food habits.
In the brands analyzed in this study, we found
concentrations of Cd, Pb and Hg, almost within the limits set by
the European Regulation, with the exception of one batch belonging
to the brand X3, identified as K. pelamis. Overall, we did
not find any significant differences in concentrations of metals
between canned tuna processed in olive oil or in brine. K.
pelamis was identified as the species with the highest Cd
bioaccumulation values (p<0.01), with a mean value of 0.035 if
compared with T. albacore where a mean value of 0.007 mg/kg
was found. From a literature review, the concentrations of Cd we
found, have comparable values to that of canned tuna distributed in
other national and international markets (52–56).
Some markets in Iran, Nigeria and Kingdom of Saudi
Arabia revealed Cd concentrations of canned tuna significantly
higher than the regulation followed in the European countries
(57,58). In literature it is known that Cd is
a very dangerous toxicant with adverse health effects after
long-term oral exposure including kidney dysfunction, bone damage
via oxidative stress (osteomalacia, osteoporosis, fractures) and
nephrotoxicity (59). Moreover, it
was classified as a human carcinogen, group I, by the International
Agency for Research on Cancer (IARC) (60). Observed alterations of DNA, as
consequences of Cd applications in experimental models of mammalian
cell cultures, higher plants, and intact animals include decreased
fidelity of DNA synthesis and DNA repair, gene mutations, and
chromosomal abnormalities. However, Cd does not appear to possess
significant genotoxic potential via the oral route (61). The Environmental Protection Agency
(EPA) and the World Health Organization (WHO) suggest similar value
of maximum daily intake (RfD=0.1 and PTDI=0.833 µg/kg-day,
respectively) unlikely to cause adverse health effects. Our results
related to the Cd-EDI and Cd-THQ revealed daily intake values
largely below the recommended doses for each brands as well as THQ
below the level of risk, supposing a consumption of canned tuna
from one to seven portions of 120 g per week.
Regarding to Pb, concentrations found are
unexpectedly lower than literature finding related to canned tuna
(52,54–58).
Pollution of Pb is an environmental and public health hazard for
its persistence and toxicity. To date, the introduction of unleaded
petrol has progressively reduced its spread. Children are more
susceptible to Pb than adults due to higher gastrointestinal uptake
and the permeable blood-brain barrier (62). This leads to behavioral
disturbances, learning and concentration difficulties, and
diminished intellectual capacity. The pathogenic effect of Pb is
multifactorial because it directly interrupts the activity of
enzymes, competitively inhibits absorption of important trace
minerals, and deactivates antioxidant sulfhydryl pools (63). Besides its ability to induce brain
disorders, Pb may also cause hypertension, kidney damage, anemia,
and negative effects on fertility (64). Pb compounds also cause genetic
damage by several indirect mechanisms, including inhibition of DNA
synthesis and repair, oxidative damage, and interaction with
DNA-binding proteins and tumor-suppressor proteins and increase in
frequency of chromosomal aberrations (65). Inorganic Pb compounds are
classified as probably carcinogenic to humans (group 2A) by IARC
(66).
WHO suggests for Pb a maximum provisional daily
intake of 3.57 µg/kg-day, conversely EPA is reviewing the
information, and RfD was not yet estimated. On the basis of
concentrations found in the brands chosen for this study, surely,
both the Pb-EDI and the Pb-THQ estimated are widely lower the level
of risk.
Concentrations of Hg detected in our samples are of
concern for human consumption, although below the threshold set by
European regulation, as well as most of literature data on
processed canned tuna around the world (52–54,67–70).
It is recognized as the most deleterious pollutant with regard to
both its effects on marine organisms and its potential hazard to
humans. The most toxic chemical species of mercury is
methylmercury, formed by bacterial methylation of inorganic mercury
in aquatic sediment; it may cause permanent harm to the central
nervous system, such as affecting normal neuronal development,
behavioral disorders, and deficiencies in the immune systems
(71). Prenatal life is more
sensitive than adult life to the toxic effects of methylmercury. In
the case of prenatal exposure, the effects of methylmercury seem to
be quite different and of a much more general nature. It affects
normal neuronal development, with altered brain architecture and
decreased brain size.
Methylmercury may also exert an effect on cell
division during critical stages in the formation of the central
nervous system. Exposure to Hg in utero during pregnancy or in
early childhood, in fact, is related to neurodevelopmental
disorders such as dyslexia, attention deficit hyperactivity
disorder, intellectual retardation, and autism (72). In adults, methylmercury has the
potential to induce delayed neurotoxicity years after cessation of
exposure or as a result of low-level exposure over a large portion
of the life span (73). Its
neurotoxicity includes symptoms such as excessive tremor, insomnia,
fatigue, and various psychological disorders (64). The average daily intake of Hg from
food is in the range of 2–20 µg (73), and seafood is recognized as the
main source of methylmercury in the general population (74,75).
This is also an important point for the food chain, as mercury
increases in the upper level, ending up in the diet of humans.
Maximum provisional daily intake suggested by EPA and WHO and
considered for this study are different because EPA provides only
the reference dose for methylmercury (CH3Hg) (0.1
µg/kg-day), instead WHO provides also the PTDI of total mercury
(0.571 µg/kg-day). Our results highlighted lower value of EDI if
compared with PTDI, but higher values if compared with the RfD,
assuming the total amount we found as methylmercury. THQ is also of
great concern, and overall, we would suggest a consumption of
canned tuna with no more than two meals per week.
Overall, in all tested samples commercial fraud
related to the identification of declared species were not
recognized. Furthermore, none of the products surpassed the
European regulatory limits no. 1881/2006 fixed for Hg and Pb,
whereas one batch of canned tuna in olive oil exceeded standard for
Cd. However, the data obtained clearly indicated that mercury
exposure to human could be minimized if the consumption of tuna is
limited to one meal per week. In addition, risk reduction should
include a better risk communication to allow the understanding of
the relationship among Hg and fish size, fish species, and trophic
levels, to reduce the consumption of the number of higher predatory
meals.
References
|
1
|
Tomasello B, Copat C, Pulvirenti V,
Ferrito V, Ferrante M, Renis M, Sciacca S and Tigano C: Biochemical
and bioaccumulation approaches for investigating marine pollution
using Mediterranean rainbow wrasse, Coris julis (Linneaus
1798). Ecotoxicol Environ Saf. 86:168–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrante M, Copat C, Mauceri C, Grasso A,
Schilirò T and Gilli G: The importance of indicators in monitoring
water quality according to European directives. Epidemiol Prev.
39:(Suppl 1). 71–75. 2015.PubMed/NCBI
|
|
3
|
D'Agata A, Fasulo S, Dallas LJ, Fisher AS,
Maisano M, Readman JW and Jha AN: Enhanced toxicity of ‘bulk’
titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine
mussels (Mytilus galloprovincialis). Nanotoxicology.
8:549–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longo G, Trovato M, Mazzei V, Ferrante M
and Conti GO: Ligia italica (Isopoda, Oniscidea) as bioindicator of
mercury pollution of marine rocky coasts. PLoS One. 8:e585482013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Copat C, Arena G, Fiore M, Ledda C,
Fallico R, Sciacca S and Ferrante M: Heavy metals concentrations in
fish and shellfish from eastern Mediterranean Sea: Consumption
advisories. Food Chem Toxicol. 53:33–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
European Food Safety Authority: EFSA
Strategy 2020: Trusted Science for Safe Food. EFSA; Parma: 2015,
https://www.efsa.europa.eu/sites/default/files/151008.pdf
|
|
7
|
Conti Oliveri G, Copat C, Wang Z, D'Agati
P, Cristaldi A and Ferrante M: Determination of illegal
antimicrobials in aquaculture feed and fish: An ELISA study. Food
Control. 50:937–941. 2015. View Article : Google Scholar
|
|
8
|
Dadar M, Adel M, Ferrante M, Saravi N,
Copat C and Conti Oliveri G: Potential risk assessment of trace
metals accumulation in food, water and edible tissue of rainbow
trout (Oncorhynchus mykiss) farmed in Haraz River, northern
Iran. Toxin Rev. 35:141–146. 2016. View Article : Google Scholar
|
|
9
|
Copat C, Brundo MV, Arena G, Grasso A,
Conti Oliveri G, Ledda C, Fallico R, Sciacca S and Ferrante M:
Seasonal variation of bioaccumulation in Engraulis
encrasicolus (Linneaus, 1758) and related biomarkers of
exposure. Ecotoxicol Environ Saf. 86:31–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conte F, Copat C, Longo S, Conti GO,
Grasso A, Arena G, Brundo MV and Ferrante M: First data on trace
elements in Haliotis tuberculata (Linnaeus, 1758) from
southern Italy: Safety issues. Food Chem Toxicol. 81:143–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conte F, Copat C, Longo S, Conti GO,
Grasso A, Arena G, Dimartino A, Brundo MV and Ferrante M:
Polycyclic aromatic hydrocarbons in Haliotis tuberculata
(Linnaeus, 1758) (Mollusca, Gastropoda): Considerations on food
safety and source investigation. Food Chem Toxicol. 94:57–63. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ababneh F and Al-Momani I: Levels of
mercury, cadmium, lead and other selected elements in canned tuna
fish commercialised in Jordan. Int J Environ Anal Chem. 93:755–766.
2013. View Article : Google Scholar
|
|
13
|
Burger J and Gochfeld M: Mercury and
selenium levels in 19 species of saltwater fish from New Jersey as
a function of species, size, and season. Sci Total Environ.
409:1418–1429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Storelli MM and Marcotrigiano GO: Content
of mercury and cadmium in fish (Thunnus alalunga) and
cephalopods (Eledone moschata) from the south-eastern
Mediterranean Sea. Food Addit Contam. 21:1051–1056. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teffer AK, Staudinger MD, Taylor DL and
Juanes F: Trophic influences on mercury accumulation in top pelagic
predators from offshore New England waters of the northwest
Atlantic Ocean. Mar Environ Res. 101:124–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jakimska A, Konieczka P, Skora K and
Namiesnik J: Bioaccumulation of metals in tissue of marine animals,
part II: Metal concentrations in animal tissues. Pol J Environ
Stud. 5:1127–1146. 2011.
|
|
17
|
EFSA: Statement on the benefits of
fish/seafood consumption compared to the risks of methylmercury in
fish/seafood. EFSA J. 13:39822015. View Article : Google Scholar
|
|
18
|
Garcia-Vazquez E, Perez J, Martinez JL,
Pardiñas AF, Lopez B, Karaiskou N, Casa MF, Machado-Schiaffino G
and Triantafyllidis A: High level of mislabeling in Spanish and
Greek hake markets suggests the fraudulent introduction of African
species. J Agric Food Chem. 59:475–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Changizi R, Farahmand H, Soltani M, Asareh
R and Ghiasvand Z: Species identification reveals mislabeling of
important fish products in Iran by DNA barcoding. Iran J Fish Sci.
12:783–791. 2013.http://jifro.ir/article-1-1338-en.pdf
|
|
20
|
Helyar SJ, Lloyd HA, de Bruyn M, Leake J,
Bennett N and Carvalho GR: Fish product mislabelling: Failings of
traceability in the production chain and implications for illegal,
unreported and unregulated (IUU) fishing. PLoS One. 9:e986912014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang YR, Yin MC, Hsieh YL, Yeh YH, Yang
YC, Chung YL and Hsieh CH: Authentication of consumer fraud in
Taiwanese fish products by molecular trace evidence and
forensically informative nucleotide sequencing. Food Res Int.
55:294–302. 2014. View Article : Google Scholar
|
|
22
|
Armani A, Guardone L, Castigliego L,
D'Amico P, Messina A, Malandra R, Gianfaldoni D and Guidi A: DNA
and Mini-DNA barcoding for the identification of Porgies species
(family Sparidae) of commercial interest on the international
market. Food Control. 50:589–596. 2015. View Article : Google Scholar
|
|
23
|
Bénard-Capelle J, Guillonneau V, Nouvian
C, Fournier N, Le Loët K and Dettai A: Fish mislabelling in France:
Substitution rates and retail types. PeerJ. 2:e7142015. View Article : Google Scholar
|
|
24
|
Lamendin R, Millen K and Ward R: Labelling
accuracy in Tasmanian seafood: An investigation using of DNA
barcoding. Food Control. 47:436–443. 2015. View Article : Google Scholar
|
|
25
|
Galimberti A, De Mattia F, Losa A, Bruni
I, Federici S, Casiraghi M, Martellos S and Labra M: DNA barcoding
as a new tool for food traceability. Food Res Int. 50:55–63. 2013.
View Article : Google Scholar
|
|
26
|
Hebert PD, Cywinska A, Ball SL and deWaard
JR: Biological identifications through DNA barcodes. Proc Biol Sci.
270:313–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hebert PD, Ratnasingham S and deWaard JR:
Barcoding animal life: Cytochrome c oxidase subunit 1 divergences
among closely related species. Proc Biol Sci. 270:(Suppl 1).
S96–S99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hebert PD, Stoeckle MY, Zemlak TS and
Francis CM: Identification of birds through DNA barcodes. PLoS
Biol. 2:e3122004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hajibabaei M, Smith MA, Janzen DH,
Rodriguez JJ, Whitfield JB and Hebert PD: A minimalist barcode can
identify a specimen whose DNA is degraded. Mol Ecol Notes.
6:959–964. 2006. View Article : Google Scholar
|
|
30
|
Pappalardo A and Ferrito V: A COIBar-RFLP
strategy for the rapid detection of Engraulis encrasicolus
in processed anchovy products. Food Control. 57:385–392. 2015.
View Article : Google Scholar
|
|
31
|
Pappalardo AM, Guarino F, Reina S, Messina
A and De Pinto V: Geographically widespread swordfish barcode stock
identification: A case study of its application. PLoS One.
6:e255162011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pappalardo AM, Federico C, Sabella G,
Saccone S and Ferrito V: A COI nonsynonymous mutation as diagnostic
tool for intraspecific discrimination in the European anchovy
Engraulis encrasicolus (Linnaeus). PLoS One.
10:e01432972015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pappalardo A and Ferrito V: DNA barcoding
species identification unveils mislabeling of processed flatfish
products in southern Italy markets. Fish Res. 164:153–158. 2015.
View Article : Google Scholar
|
|
34
|
Ferrito V, Bertolino V and Pappalardo A:
White fish authentication by COIBar-RFLP: Toward a common strategy
for the rapid identification of species in convenience seafood.
Food Control. 70:130–137. 2016. View Article : Google Scholar
|
|
35
|
Ward RD, Zemlak TS, Innes BH, Last PR and
Hebert PD: DNA barcoding Australia's fish species. Philos Trans R
Soc Lond B Biol Sci. 360:1847–1857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lefébure T, Douady CJ, Gouy M and Gibert
J: Relationship between morphological taxonomy and molecular
divergence within Crustacea: Proposal of a molecular threshold to
help species delimitation. Mol Phylogenet Evol. 40:435–447. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogden R: Fisheries forensics: The use of
DNA tools for improving compliance, traceability and enforcement in
the fishing industry. Fish Fish. 9:462–472. 2008. View Article : Google Scholar
|
|
38
|
Dawnay N, Ogden R, McEwing R, Carvalho GR
and Thorpe RS: Validation of the barcoding gene COI for use in
forensic genetic species identification. Forensic Sci Int. 173:1–6.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pappalardo A, Cuttitta A, Sardella A,
Musco M, Maggio T, Patti B, Mazzola S and Ferrito V: DNA barcoding
and COI sequence variation in Mediterranean lanternfishes larvae.
Hydrobiologia. 749:155–167. 2015. View Article : Google Scholar
|
|
40
|
Lowenstein JH, Burger J, Jeitner CW, Amato
G, Kolokotronis SO and Gochfeld M: DNA barcodes reveal
species-specific mercury levels in tuna sushi that pose a health
risk to consumers. Biol Lett. 6:692–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hall TA: BioEdit: A user-friendly
biological sequence editor and analysis program for Windows
95/08/NT. Nucleic Avids Symp Ser. 41:95–98. 1999.
|
|
42
|
Thompson JD, Gibson TJ, Plewniak F,
Jeanmougin F and Higgins DG: The CLUSTAL_X windows interface:
Flexible strategies for multiple sequence alignment aided by
quality analysis tools. Nucleic Acids Res. 25:4876–4882. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tamura K, Peterson D, Peterson N, Stecher
G, Nei M and Kumar S: MEGA5: Molecular evolutionary genetics
analysis using maximum likelihood, evolutionary distance, and
maximum parsimony methods. Mol Biol Evol. 28:2731–2739. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Felsenstein J: Confidence limits on
phylogenies: An approach using the bootstrap. Evolution.
39:783–791. 1985. View Article : Google Scholar
|
|
45
|
Adel M, Conti Oliveri G, Dadar M, Mahjoub
M, Copat C and Ferrante M: Heavy metal concentrations in edible
muscle of whitecheek shark, Carcharhinus dussumieri
(elasmobranchii, chondrichthyes) from the Persian Gulf: A food
safety issue. Food Chem Toxicol. 97:135–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Copat C, Bella F, Castaing M, Fallico R,
Sciacca S and Ferrante M: Heavy metals concentrations in fish from
Sicily (Mediterranean Sea) and evaluation of possible health risks
to consumers. Bull Environ Contam Toxicol. 88:78–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Conti GO, Copat C, Ledda C, Fiore M,
Fallico R, Sciacca S and Ferrante M: Evaluation of heavy metals and
polycyclic aromatic hydrocarbons (PAHs) in Mullus barbatus
from Sicily Channel and risk-based consumption limits. Bull Environ
Contam Toxicol. 88:946–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang DX and Hewitt GM: Nuclear
integrations: Challenges for mitochondrial DNA markers. Trends Ecol
Evol. 11:247–251. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mitchell JK and Hellberg RS: Use of the
mitochondrial control region as a potential DNA mini-barcoding
target for the identification of canned tuna species. Food Anal
Methods. 9:2711–2720. 2016. View Article : Google Scholar
|
|
50
|
Lowenstein JH, Amato G and Kolokotronis
SO: The real maccoyii: Identifying tuna sushi with DNA barcodes -
contrasting characteristic attributes and genetic distances. PLoS
One. 4:e78662009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bosch AC, O'Neill B, Sigge GO, Kerwath SE
and Hoffman LC: Heavy metals in marine fish meat and consumer
health: A review. J Sci Food Agric. 96:32–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Okyere H, Voegborlo RB and Agorku SE:
Human exposure to mercury, lead and cadmium through consumption of
canned mackerel, tuna, pilchard and sardine. Food Chem.
179:331–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Storelli MM, Barone G, Cuttone G, Giungato
D and Garofalo R: Occurrence of toxic metals (Hg, Cd and Pb) in
fresh and canned tuna: Public health implications. Food Chem
Toxicol. 48:3167–3170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Russo R, Lo Voi A, De Simone A, Serpe FP,
Anastasio A, Pepe T, Cacace D and Severino L: Heavy metals in
canned tuna from Italian markets. J Food Prot. 76:355–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Andayesh S, Hadiani MR, Mousavi Z and
Shoeibi S: Lead, cadmium, arsenic and mercury in canned tuna fish
marketed in Tehran, Iran. Food Addit Contam Part B Surveill.
8:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Morshdy AE, Hafez AE, Darwish WS, Hussein
MA and Tharwat AE: Heavy metal residues in canned fishes in Egypt.
Jpn J Vet Res. 61:(Suppl). S54–S57. 2013.PubMed/NCBI
|
|
57
|
Hosseini SV, Sobhanardakani S, Miandare
HK, Harsij M and Regenstein JM: Determination of toxic (Pb, Cd) and
essential (Zn, Mn) metals in canned tuna fish produced in Iran. J
Environ Health Sci Eng. 13:592015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Iwegbue CM: Metal concentrations in
selected brands of canned fish in Nigeria: Estimation of dietary
intakes and target hazard quotients. Environ Monit Assess.
187:852015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sabath E and Robles-Osorio ML: Renal
health and the environment: Heavy metal nephrotoxicity. Nefrologia.
32:279–286. 2012.PubMed/NCBI
|
|
60
|
IARC: Beryllium, cadmium, mercury and
exposure in the glass manufacturing industryCadmium and Cadmium
Compounds. 58. WHO Press; Lyon: pp. 119–237. 1993
|
|
61
|
World Health Organization: Background
document for development of WHO guidelines for drinking-water
qualityCadmium in Drinking Water. WHO Press; Geneva: 2011
|
|
62
|
Järup L: Hazards of heavy metal
contamination. Br Med Bull. 68:167–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kakkar P and Jaffery FN: Biological
markers for metal toxicity. Environ Toxicol Pharmacol. 19:335–349.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
National Toxicology Program. U.S.
Department of Health and Human Services: Report on Carcinogens
(RoC) (12th). 2011.http://ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf
|
|
66
|
International Agency for Research on
Cancer (IARC): IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans: Inorganic and Organic Lead Compounds. 87. WHO
Press; Lyon: 2006
|
|
67
|
Burger J and Gochfeld M: Mercury in fish
available in supermarkets in Illinois: Are there regional
differences. Sci Total Environ. 367:1010–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ruelas-Inzunza J, Patiño-Mejía C,
Soto-Jiménez M, Barba-Quintero G and Spanopoulos-Hernández M: Total
mercury in canned yellowfin tuna Thunnus albacares marketed
in northwest Mexico. Food Chem Toxicol. 49:3070–3073. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dabeka RW, Mckenzie AD and Forsyth DS:
Total mercury in canned tuna sold in Canada in 2006. Food Addit
Contam Part B Surveill. 7:110–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
García MÁ, Núñez R, Alonso J and Melgar
MJ: Total mercury in fresh and processed tuna marketed in Galicia
(NW Spain) in relation to dietary exposure. Environ Sci Pollut Res
Int. 23:24960–24969. 2016.PubMed/NCBI
|
|
71
|
United Nations Environment Programme:
Global Mercury Assessment. UNEP Chemicals; Geneva: 2002
|
|
72
|
Weiss B: Vulnerability of children and the
developing brain to neurotoxic hazards. Environ Health Perspect.
108:(Suppl 3). 375–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
World Health Organization: Background
document for development of WHO guidelines for drinking-water
qualityMercury in Drinking-Water. WHO Press; Geneva: 2005
|
|
74
|
Storelli MM, Giacominelli-Stuffler R and
Marcotrigiano GO: Relationship between total mercury concentration
and fish size in two pelagic fish species: Implications for
consumer health. J Food Prot. 69:1402–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Copat C, Vinceti M, D'Agati MG, Arena G,
Mauceri V, Grasso A, Fallico R, Sciacca S and Ferrante M: Mercury
and selenium intake by seafood from the Ionian Sea: A risk
evaluation. Ecotoxicol Environ Saf. 100:87–92. 2014. View Article : Google Scholar : PubMed/NCBI
|