Introduction
Although surgical intervention is effective for the
majority of early-stage non-small cell lung cancer (NSCLC), ~20–30%
of patients experience relapses (1). Therefore, the identification of
prognostic markers and novel therapeutic targets is of crucial
importance. The serine/threonine protein kinase tumor progression
locus 2 [TPL2/MAP3 kinase 8 (MAP3K8)] acts as an essential signal
for the expression of pro-inflammatory mediators in several cell
systems, including peripheral macrophages (2), adipocytes (3), hepatic stellate cells (4), airway epithelial cells (5) and glial cells (6). Inflammatory molecules have emerged as
significant in the development of cancer, with roles in events
leading to the formation, growth and metastasis of tumors. TPL2 has
been suggested to enhance tumor growth and metastatic progression
in distinct types of human cancer (7–9).
Overexpression of TPL2 has been reported in human gastric/colon
adenocarcinomas (10), large
granular T-cell neoplasias (11)
and breast cancer (12,13). However, a detailed evaluation of
TPL2 expression and function in human malignancies and normal
tissues remains to be performed, and the role of TPL2 in
carcinogenesis remains unclear. TPL2 may stimulate oncogenic
events, including the cellular response to therapy and immune
control of cancer growth (14).
However, in certain cases, TPL2 may function as a tumor suppressor
(15). Little information
regarding the prognostic role of TPL2 in the oncogenesis of NSCLC
exists to assist with identifying those at high-risk of relapse
following surgical resection.
Several previous studies have identified micro (mi)
RNAs as critical regulators of immune responses, including
inflammation (16,17). Mechanistically, miRNAs are small,
non-coding RNA molecules that pair with partially complementary
sequences in their target mRNAs and regulate their stability and/or
translation. Extracellular signals, differentiation and oncogenic
transformation bring about alterations in the expression of miRNAs,
allowing miRNAs to regulate these processes. Previous studies have
suggested that miR-21 overexpression may be an independent
negative prognostic factor in the overall survival of patients with
NSCLC (18), and that
miR-21 targets numerous genes involved in cancer cell
phenotypes (19). Using three
miRNA target identification programs (miRNAorg, MicroCosm Targets
and PITA), TPL2 was identified among the potential targets
of miR-21. Therefore, the present study was based on the
analysis of the potential association between TPL2 and
miR-21 in cases of early-stage NSCLC.
Materials and methods
Patients
A total of 101 patients with NSCLC at peripheral
stage I (T1N0M0) underwent surgical resection in the Unit of
Thoracic Surgery in the Department of Surgical, Medical, Molecular
Pathology and Critical Care at Pisa University (Pisa, Italy), and
were retrospectively selected. Histological diagnoses were
formulated by two pathologists independently (G.F. and A.S.), in
accordance with the World Health Organization classification
(20,21). Clinicopathological characteristics
were noted in all cases. Written informed consent was obtained from
each patient for tissue collection and molecular analysis.
Target prediction
Alignment of miRNAs with the TPL2 target gene
was predicted using the microRNA target prediction programs
microRNA.org (Harvard Medical School, Boston, MA,
USA), MicroCosm Targets 5.0 (The European Bioinformatics Institute)
and PITA (Weizmann Institute of Science).
RNA isolation
Following standard deparaffinization and manual
tumor macrodissection of the areas with prevalent adenocarcinomas,
the miRNeasy formalin-fixed and paraffin-embedded (FFPE) kit
(Qiagen GmbH, Hilden, Germany) was used to isolate total RNA,
including miRNAs, from 5 µm sections of FFPE tissues, according to
the manufacturer's protocol. A total of 600 ng of total RNA was
used to synthesize cDNA using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
a reaction volume of 20 µl.
Expression of TPL2 mRNA and miR-21
using the reverse transcription quantitative-polymerase chain
reaction (RT-qPCR)
Quantification of the mRNA expression of TPL2
was performed in triplicate using the Rotor Gene SYBR Green PCR kit
(Qiagen GmbH) on a Rotor Gene 6000 (Qiagen GmbH). The following
primers used for RT-qPCR are as follows: TPL2, forward:
5′-TAATCCACAAGCAAGCACCTC-3′ and reverse:
5′-TGATTGGGGTTTCTCTCCAG-3′); β-actin, forward:
5′-CCAACCGCGAGAAGATGA-3′ and reverse: 5′-CCAGAGGCGTACAGGGATAG-3′.
Specific TaqMan® MicroRNA assays (Applied Biosystems;
Thermo Fisher Scientific, Inc.) were used to amplify miR-21
and RNU6B, according to the manufacturer's protocol. In
addition, the threshold cycle and baselines were determined in an
identical manner. The expression was calculated by relative
quantification using β-actin and RNU6B as reference
controls for TPL2 and miR-21, respectively. Fold
expression changes were determined by the 2−∆∆Cq method
(22) using a pool of 12
non-cancerous tissues as a calibrator group. The analysis was
performed using the DataAssist™ software (Applied Biosystems;
Thermo Fisher Scientific, Inc.).
Protein expression of TPL2
The protein expression of TPL2 was assessed in FFPE
tissue samples using immunohistochemistry (IHC). The Cot (M-20)
anti-TPL2 rabbit polyclonal antibody (dilution, 1:50; cat. no.
sc-720, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was
incubated at 37°C for 36 min to detect the protein. The
avidin-biotin peroxidase method was used by developing the
immunoreaction with diaminobenzidine. Simultaneous staining of a
known TPL2-positive case was used as a positive control. Incubation
of parallel slides omitting the first antibody was performed as a
negative control. The number of TPL2 immunoreactive cells was
determined by scoring a minimum of five high-power fields
(magnification, ×40) and counting the number of immunoreactive
cells out of the total epithelial cells analyzed in each field of
view.
A proportion score (PS) was assigned by representing
the estimated proportion of positively stained cells, and was
divided into three groups: negative (score 0), 1–60% (score 1) and
>60% (score 2). An intensity score (IS) was also assigned and
divided into four classes: None (score 0), weak (score 1),
intermediate (score 2) and strong (score 3). A total score was
obtained from the sum of the PS and IS, and was graded into two
classes: High (4–5, with a higher expression of TPL2) and low (0–3,
with a lower or negative TPL2 expression).
Statistical analysis
One-way analysis of variance and chi-squared tests
were used to determine the association between the expression of
TPL2, miR-21 levels and the clinicopathological
parameters. Survival analyses were performed using the Kaplan-Meier
method, along with the log-rank test and the Cox proportional
hazard model. Statistical analyses were performed using the JMP10
software (SAS Institute, Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The present study was performed using 101 patients
with early-stage NSCLC (71 males and 30 females), including 54
patients with adenocarcinoma (ADC) and 47 patients with squamous
cell carcinoma (SCC). Regarding their histological classification,
different histological subtypes of ADC were identified: Acinar
(19/54, 35.2%), lepidic (18/54, 33.3%), mucinous (9/54, 16.7%),
papillary (6/54, 11.1%) and solid (2/54, 3.7%). The median age at
diagnosis was 67 years (range, 49–81; mean, 67.03 years), and the
median follow-up was 63 months (range, 6–161 months). In terms of
tumor grade, five tumors were G1 phase, 71 tumors were G2 phase and
25 were G3 phase. Disease progression and mortality from lung
cancer were observed in 27 patients with ADC (26.7%) and 20 with
SCC (19.8%) of the 101 patients with NSCLC, respectively. The
median progression-free survival and the overall survival were 54
months (95% CI: 6–161) and 68 months (95% CI: 11–161),
respectively. Regarding smoking habits of the entire patient group,
there were 15 non-smokers, 44 former smokers and 42 current smokers
(Table I).
 | Table I.Correlation between the mRNA
expression of tumor progression locus 2 and the predominant
clinicopathological characteristics of patients with early-stage
non-small cell lung cancer. |
Table I.
Correlation between the mRNA
expression of tumor progression locus 2 and the predominant
clinicopathological characteristics of patients with early-stage
non-small cell lung cancer.
|
| TPL2
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | Low % | High % | P-value |
|---|
| Age, years |
|
| 0.368 |
|
≤67 | 24 (47.1) | 27 (52.9) |
|
|
>67 | 28 (56.0) | 22 (44.0) |
|
| Gender |
|
| 0.809 |
|
Male | 36 (50.7) | 35 (49.3) |
|
|
Female | 16 (53.3) | 14 (46.7) |
|
| Histology |
|
| 0.471 |
|
Adenocarcinoma | 26 (48.1) | 28 (51.9) |
|
|
Squamous cell carcinoma | 26 (55.3) | 21 (44.7) |
|
| Smoking
history |
|
| 0.512 |
|
Never | 6 (40.0) | 9 (60.0) |
|
|
Former | 25 (56.8) | 19 (43.2) |
|
|
Current | 21 (50.0) | 21 (50.0) |
|
| Tumor grade |
|
| 0.661 |
| G1 | 3 (60.0) | 2 (54.5) |
|
| G2 | 38 (53.5) | 33 (46.5) |
|
| G3 | 11 (44.0) | 14 (56.0) |
|
TPL2 expression and
clinicopathological characteristics
TPL2 mRNA expression was quantified in 101
NSCLC tissues and in 12 non-cancerous tissues, and was normalized
against the β-actin housekeeping gene, using RT-qPCR. The
samples were divided into high and low expression groups based on
the median fold-change value (0.815 for TPL2). TPL2
mRNA expression was low in 52/101 (51%) cases and high in 49/101
(49%) cases (data not shown). The present study determined whether
TPL2 expression was correlated with the predominant
clinicopathological characteristics. No statistically significant
associations were observed between TPL2 mRNA expression and
any of the predominant clinicopathological characteristics of the
patients with NSCLC (Table I).
However, focusing on the difference in TPL2 levels among the
different subtypes of the 54 adenocarcinomas, it was identified
that TPL2 mRNA expression was high in 6/19 (31.6%) acinar,
13/18 (72.3%) lepidic, 5/9 (55.6%) mucinous, 3/6 (50%) papillary
and 1/2 (50%) solid subtypes, with a significantly higher level in
lepidic when compared with all other subtypes (Chi-squared test,
P=0.012; data not shown).
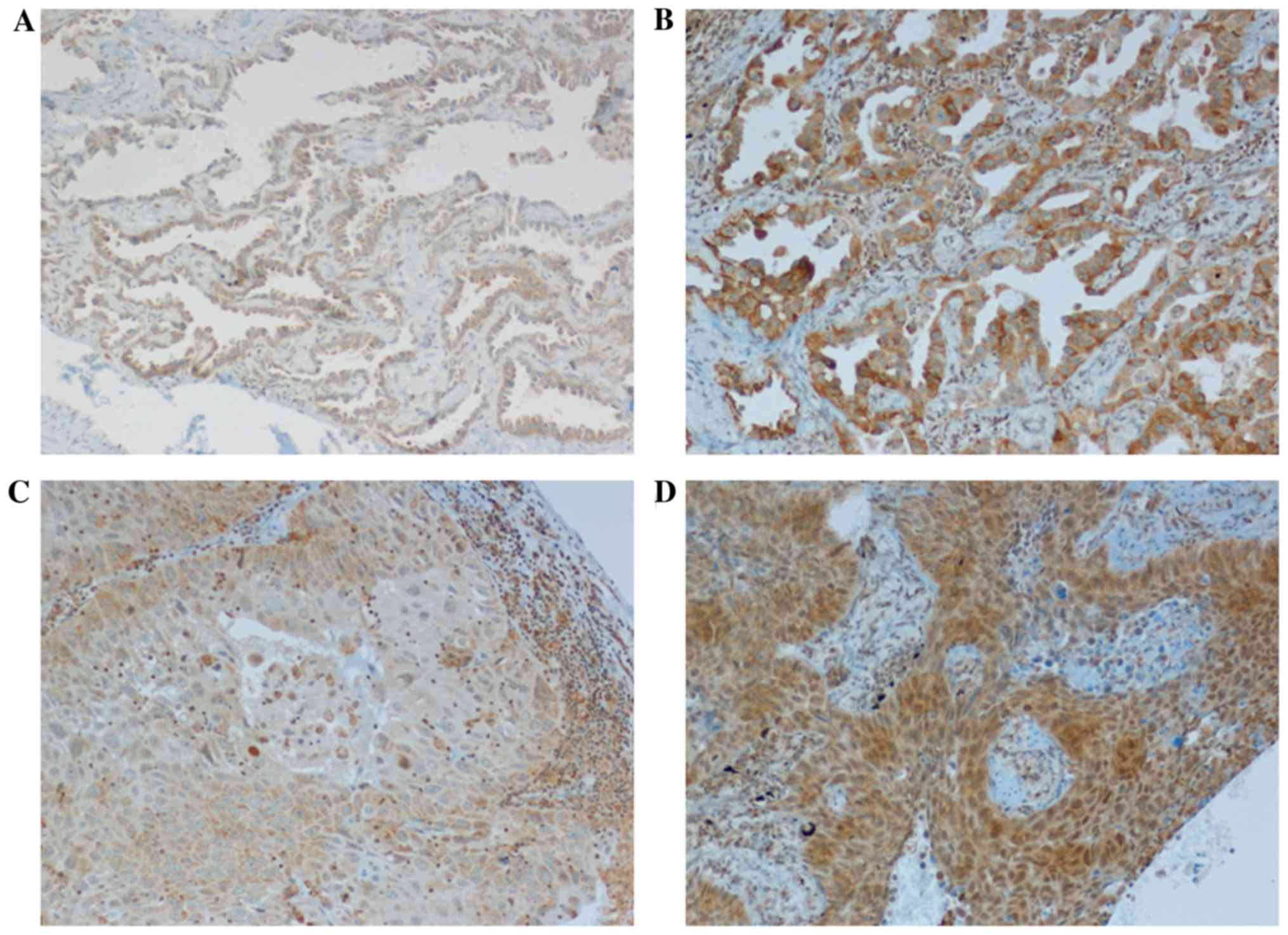
Using the 2-level (high/low) immunohistochemical
scoring system, TPL2 protein expression was low in 45/101 (44%) and
high in 56/101 (56%) cases (Fig.
1). Even if the correlation between the level of TPL2
mRNA and the score of its protein was not significant (chi-squared
test, P=0.36), samples with a low TPL2 protein score expression
typically exhibited lower mRNA levels (2.33-fold change value
±1.55) when compared with samples with high TPL2 score (4.0±1.2).
In addition, a high score for TPL2 protein was identified in the
lepidic adenocarcinoma subtype (P=0.003), as aforementioned for
TPL2 mRNA expression.
Target prediction and correlation
between TPL2 and miR-21 expression
Alignment of miRNAs with the TPL2 target mRNA
was predicted by the miRNA target prediction programs miRBase,
MicroCosm Targets and PITA. The present study identified the
TPL2 gene as a potential target of miR-21 (Fig. 2). Firstly, the patients with NSCLC
were divided into miR-21 high and low expression groups
based on the median fold change values, which resulted as 1.7.
Following this, the present study determined whether TPL2
expression was correlated with miR-21 levels, but no
statistically significant associations were observed between groups
(Chi-squared test, P=0.66).
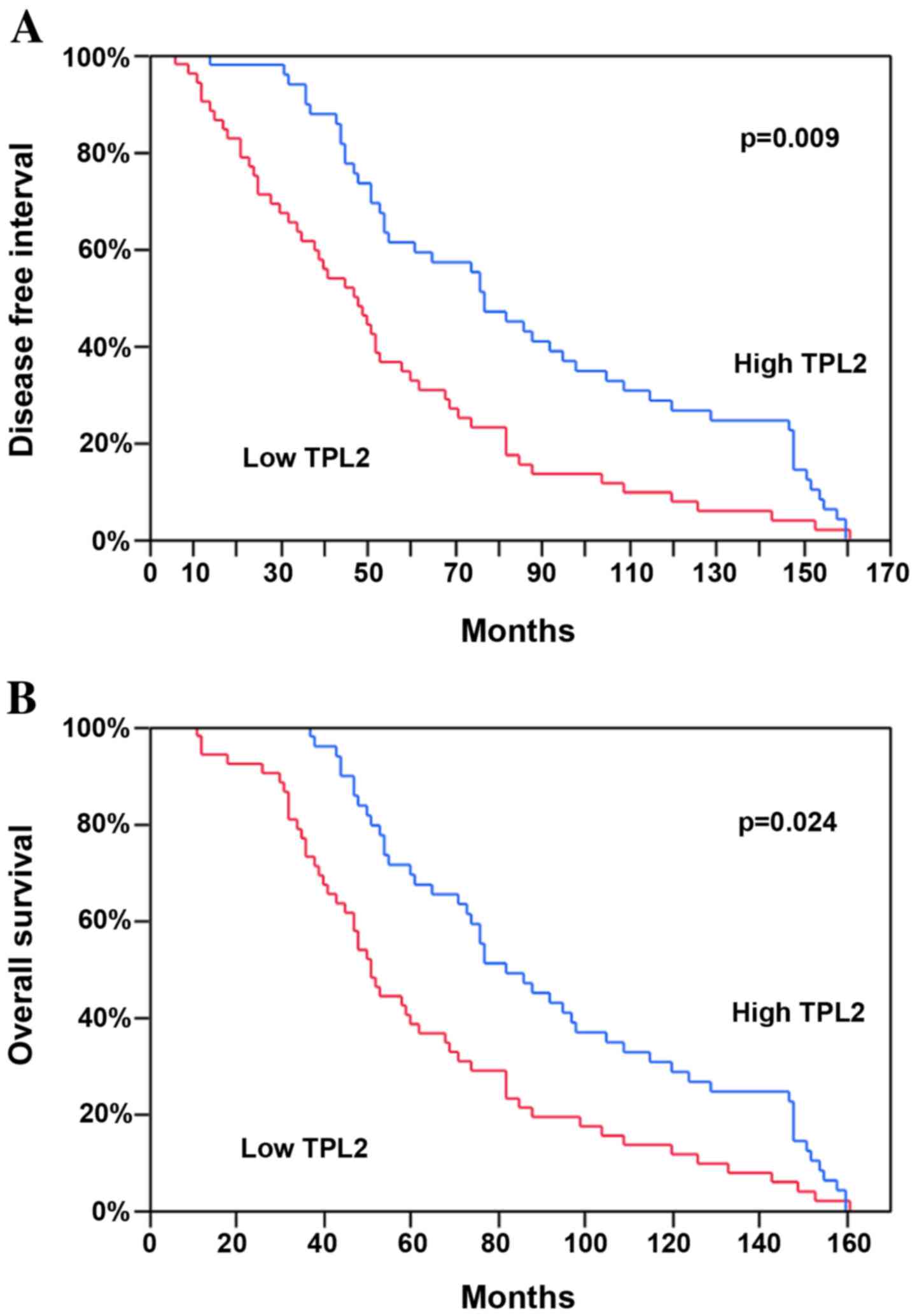
Survival analysis
To evaluate the potential association between
TPL2 expression and the prognosis of the patients with
NSCLC, a survival analysis by the Kaplan-Meier method using the
disease-free interval (DFI) and the overall post-operative survival
(OS) as endpoints was performed. It was noted that the tumors with
a high TPL2 mRNA expression demonstrated a significantly
longer mean DFI and OS compared with the patients with low
expression of this mRNA (P=0.009 and P=0.024, respectively;
Fig. 3 and Table II). In the multivariate analysis,
TPL2 mRNA expression continued to be a good prognostic
factor for DFI and OS (P=0.0005 and P=0.005; Table II). Tumor grading, a classical
prognostic factor, was also associated with the DFI in the
univariate (P=0.0019) and multivariate analyses (P=0.0008; Table II), as well as with the OS
(P=0.0009 and P=0.0002, respectively). No significant differences
were identified for other demographics or clinical characteristics
(Table II). Furthermore, the
median DFI and OS were similar in examined patients with either low
or high miR-21 expression (data not shown).
 | Table II.Factors associated with disease-free
survival in patients with early-stage non-small cell lung
cancer. |
Table II.
Factors associated with disease-free
survival in patients with early-stage non-small cell lung
cancer.
| Characteristic | Univariate analyses
(P-value) | Multivariate
analyses (P-value) |
|---|
| Age (≤67 vs. >67
years) | 0.7107 | 0.7141 |
| Gender (male vs.
female) | 0.0866 | 0.4067 |
| Histology (ADC vs.
SCC) | 0.1030 | 0.9908 |
| Smoking
(current/former vs. never) | 0.5915 | 0.5477 |
| Tumor grading (G1
vs. G2/G3) | 0.0019 | 0.0008 |
| TPL2 mRNA level
(low vs. high) | 0.0009 | 0.0005 |
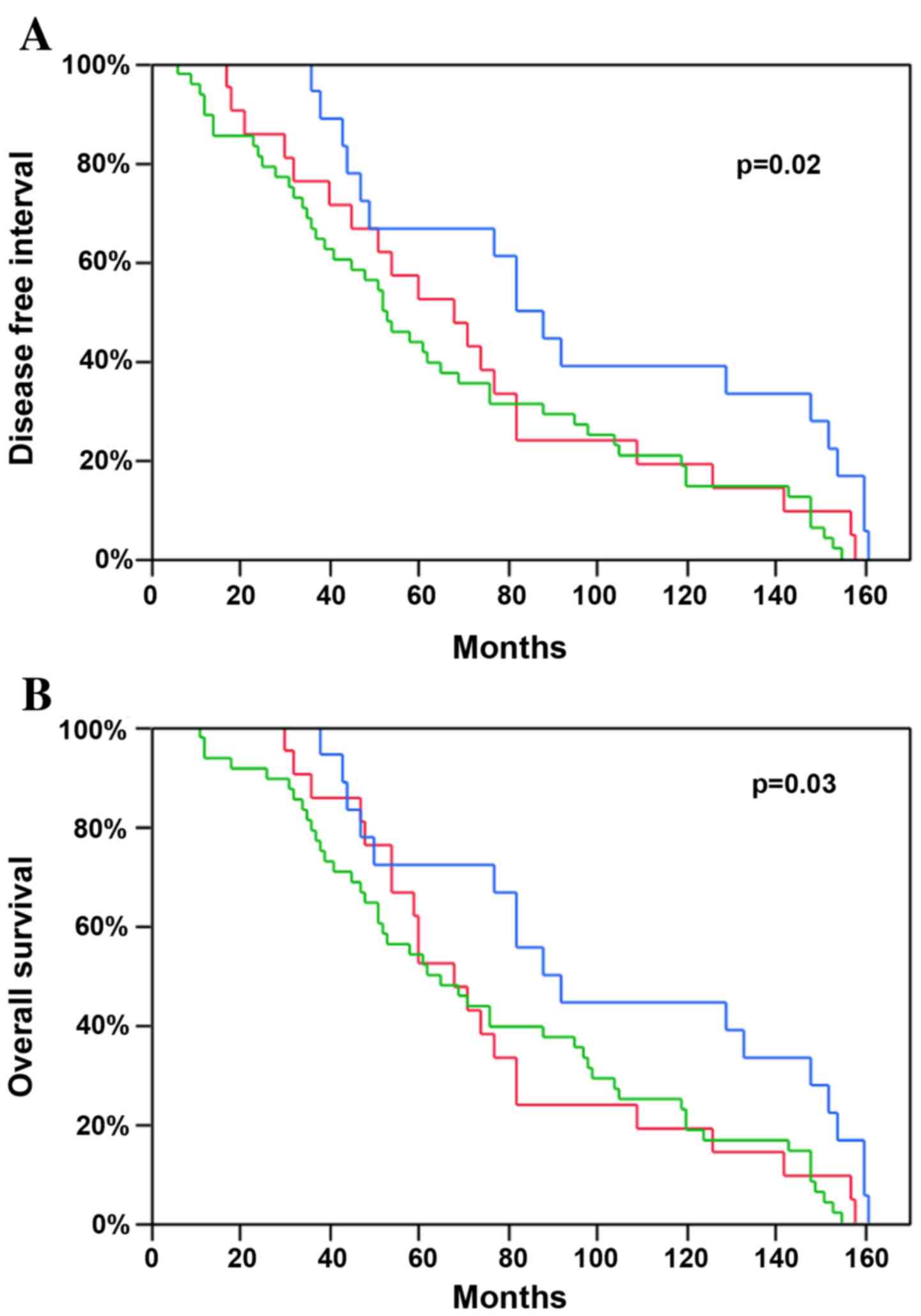
Following this analysis, the survival difference
between the histological cell types was investigated. No
significant difference was observed between SCC and ADC (Table II). However, based on the
difference of the TPL2 levels reported above, the NSCLCs
were divided into three prognostic groups: Squamous cell carcinoma
and the two predominant adenocarcinoma growth patterns (acinar and
lepidic subtypes). The group with the most favorable prognosis was
the lepidic predominant subtype, which was demonstrated earlier in
the study to possess a higher level of TPL2, with a median
DFI of 85 months and a median OS of 90 months. SCC and acinar ADC,
both NSCLC tumors with lower TPL2 levels, presented a worse
prognosis with a mean DFI of 53 and 68 months and with a mean OS of
65 and 68 months, respectively (P=0.02 and P=0.03; Fig. 4).
Discussion
TPL2 is involved in numerous intracellular signaling
pathways, with a prominent role in the regulation of inflammatory
signal transduction (2–6). TPL2 may be able to engage a plethora
of signaling pathways and to act in concert with other kinases and
signaling molecules. A major role of TPL2 is in the activation of
the ERK/MAPK pathway through the direct phosphorylation of MEK, an
extracellular signal-regulated kinase, which is a pro-inflammatory
mediator. Conversely, in certain disease models, TPL2 deficiency
exacerbates the inflammatory response (23). The diverse and often contradictory
effects of TPL2 may be due to the utilization of different kinases
and signal transduction pathways in different cell types. Similar
contradictory data concerning the role of TPL2 in oncogenic signal
transduction exist in the literature. Gkirtzimanaki et al
(15) demonstrated a tumor
suppressor function for TPL2 in lung cancer. However, in contrast,
elevated levels of TPL2 have been reported in several tumor types
(10–13). TPL2 inhibitors are currently in
advanced pharmaceutical development for the treatment of myeloma
(24). The effects of inhibiting
different MAP kinases may have different consequences in a
cell-type-dependent manner, and therefore, any therapeutic use of
kinase inhibitors must be carefully considered.
Considering these discrepancies, the aim of the
present study was to elucidate the role of TPL2 in lung cancer,
specifically in early-stage NSCLC. NSCLC accounts for ~70% of all
lung cancer-associated mortalities worldwide (25). The current standard of care for
stage I NSCLC is surgery; however, 20–30% of these early-stage
patients will experience recurrence (1), reflecting the urgent requirement to
develop adjunctive, specific markers that can aid in the prognosis
of lung cancer. In the present report, it was demonstrated that low
TPL2 mRNA levels correlated with reduced survival in TNM
stage I lung cancer patients, suggesting loss of its expression was
an early event in the development of the disease.
Immunohistochemical analysis of TPL2 protein expression confirmed
the corresponding mRNA results, even if not fully. There are
presumably several reasons for a weak correlation between mRNA and
protein levels, including mRNA post-transcriptional modifications,
translational efficiency, miRNA actions and errors in both mRNA and
protein measuring techniques. In addition, due to subcellular
localization, TPL2 protein abundance may not represent its
biological activity.
It was later hypothesized that TPL2 may be
correlated with miRNAs, which are small, non-coding RNAs that
regulate target gene expression (26,27).
Several miRNAs are aberrantly expressed in the majority of human
cancer types (19). Oncogenes and
tumor suppressor genes exert their activity, in part, by regulating
the expression of specific miRNAs (28). miR-21 has proven to be a
useful prognostic indicator in numerous cancer types, including
NSCLC (29,30). Using the miRNA target prediction
programs miRBase, MicroCosm Targets and PITA, the present study
identified TPL2 as a potential target of miR-21. As a
result, the study evaluated miR-21 expression in early-stage
NSCLC and its association with TPL2 levels by combining
miR-21 expression with TPL2 levels. The present study
is the first, to the best of our knowledge, to test the hypothesis
that miR-21 may cooperate with TPL2. It was revealed
that TPL2 levels were not correlated with miR-21,
suggesting no association between miR-21 and TPL2 in
lung carcinogenesis. In addition, no difference in the disease
prognosis was observed for miR-21 in the present cases, as
also previously reported by the authors (31). The prognostic role of this miRNA is
controversial, whilst certain studies have demonstrated that
miR-21 overexpression is associated with poor NSCLC survival
(18,32,33),
other studies have indicated a converse or insignificant
association (34,35). In view of the present results, the
prognostic role of TPL2 is independent from the expression of
miR-21.
Furthermore, histological subtyping has been
proposed as a potential screening tool to identify the patients
with lung cancer at a high risk for recurrent disease and an
unfavorable prognosis (36). In
the comparisons between the patients with adenocarcinoma and
squamous cell carcinoma, ADCs with a lepidic pattern exhibited
significantly improved survival. Although this prognosis of lepidic
adenocarcinoma (37–39), and the more aggressive course of
the disease and poor prognosis in the SCC histotype (40) have been well-documented, the
finding of the lepidic ADC pattern correlation with high
TPL2 expression has been not previously reported. Squamous
cell lung carcinoma, even if clearly biologically different from
the adenocarcinoma histotype, may behave as an adenocarcinoma
arising from a non-terminal respiratory unit that is centrally
originated, solid in morphology, poorly differentiated and often
necrotic (41).
TPL2 appears to have divergent roles in different
cells and tissues, serving as an oncogene as well as a tumor
suppressor gene. In addition, how TPL2 acts in carcinogenesis
remains to be elucidated. Gkirtzimanaki et al (15) hypothesized that TPL2 may lead to
increased accumulation and activation of p53, resulting in an
accelerated cell death and reduced malignant transformation. This
hypothesis is corroborated by the good prognosis of lung cancer
patients bearing tumors with elevated levels of TPL2, as it
was identified in early-stage NSCLCs. Downregulation of TPL2, as a
result of a genetic or epigenetic alteration, including LOH,
overexpression of miRNAs, or oncogenic RAS signaling, depending
upon the histological subtype, may have an impact on the early
stages of human lung disease similar to the effects of TPL2
ablation on lung cancer initiation in the mouse (15).
In conclusion, the present study is the first
examination, to the best of our knowledge, of the association
between TPL2 and early-stage lung carcinogenesis,
underscoring its tumor suppressor gene role. In the present
analysis, miR-21 expression did not impact on TPL2
expression. Future studies must involve an analysis of TPL2 in
advanced lung cancer stages and its downstream signaling. In
addition, studies must correlate findings with other miRNA
expression profiles to clarify the mechanism by which TPL2 is
involved in lung carcinogenesis, taking into consideration all
histological subtypes, and any important translational
implications.
References
|
1
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dumitru CD, Ceci JD, Tsatsanis C,
Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA,
Copeland NG, Kollias G and Tsichlis PN: TNF-alpha induction by LPS
is regulated posttranscriptionally via a TPL2/ERK-dependent
pathway. Cell. 103:1071–1083. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jager J, Gremeaux T, Gonzalez T, Bonnafous
S, Debard C, Laville M, Vidal H, Tran A, Gual P, Le
Marchand-Brustel Y, et al: TPL2 kinase is upregulated in adipose
tissue in obesity and may mediate interleukin-1beta and tumor
necrosis factor-{alpha} effects on extracellular signal-regulated
kinase activation and lipolysis. Diabetes. 59:61–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perugorria MJ, Murphy LB, Fullard N,
Chakraborty JB, Vyrla D, Wilson CL, Oakley F, Mann J and Mann DA:
Tumor progression locus 2/Cot is required for activation of
extracellular regulated kinase in liver injury and toll-like
receptor-induced TIMP-1 gene transcription in hepatic stellate
cells in mice. Hepatology. 57:1238–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martel G, Bérubé J and Rousseau S: The
protein kinase TPL2 is essential for ERK1/ERK2 activation and
cytokine gene expressionin airway epithelial cells exposed to
pathogen-associated molecular patterns (PAMPs). PLoS One.
8:e591162013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirschhorn J, Mohanty S and Bhat NR: The
role of tumor progression locus 2 protein kinase in glial
inflammatory response. J Neurochem. 128:919–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu YP, Landsittel D, Jing L, Nelson J, Ren
B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, et al: Gene
expression alterations in prostate cancer predicting tumor
aggression and preceding development of malignancy. J Clin Oncol.
22:2790–2799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hatziapostolou M, Polytarchou C,
Panutsopulos D, Covic L and Tsichlis PN: Proteinase-activated
receptor-1-triggered activation of tumor progression locus-2
promotes actin cytoskeleton reorganization and cell migration.
Cancer Res. 68:1851–1861. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Wang X, Hawkins CA, Chen K,
Vaynberg J, Mao X, Tu Y, Zuo X, Wang J, Wang YX, et al: Structural
basis of focal adhesion localization of LIM-only adaptor PINCH by
integrin-linked kinase. J Biol Chem. 284:5836–5844. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohara R, Hirota S, Onoue H, Nomura S,
Kitamura Y and Toyoshima K: Identification of the cells expressing
cot proto-oncogene mRNA. J Cell Sci. 108:97–103. 1995.PubMed/NCBI
|
|
11
|
Christoforidou AV, Papadaki HA, Margioris
AN, Eliopoulos GD and Tsatsanis C: Expression of the TPL2/Cot
oncogene in human T-cell neoplasias. Mol Cancer. 3:342004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sourvinos G, Tsatsanis C and Spandidos DA:
Overexpression of the Tpl-2/Cot oncogene in human breast cancer.
Oncogene. 18:4968–4973. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krcova Z, Ehrmann J, Krejci V, Eliopoulos
A and Kolar Z: Tpl-2/Cot and COX-2 in breast cancer. Biomed Pap Med
Fac Univ Palacky Olomouc Czech Repub. 152:21–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HW, Joo KM, Lim JE, Cho HJ, Cho HJ,
Park MC, Seol HJ, Seo SI, Lee JI, Kim S, et al: TPL2 kinase impacts
tumor growth and metastasis of clear cell renal cell carcinoma. Mol
Cancer Res. 11:1375–1386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gkirtzimanaki K, Gkouskou KK, Oleksiewicz
U, Nikolaidis G, Vyrla D, Liontos M, Pelekanou V, Kanellis DC,
Evangelou K, Stathopoulos EN, et al: TPL2 kinase is a suppressor of
lung carcinogenesis. Proc Natl Acad Sci USA. 110:pp. E1470–E1479.
2013; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baltimore D, Boldin MP, O'Connell RM, Rao
DS and Taganov KD: MicroRNAs: New regulators of immune cell
development and function. Nat Immunol. 9:839–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao C and Rajewsky K: MicroRNA control in
the immune system: Basic principles. Cell. 136:26–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao W, Yu Y, Cao H, Shen H, Li X, Pan S
and Shu Y: Deregulated expression of miR-21, miR-143 and miR-181a
in non small cell lung cancer is related to clinicopathologic
characteristics or patient prognosis. Biomed Pharmacother.
64:399–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:pp. 2257–2261.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/American thoracic society/European respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Travis WD, Brambilla E, Noguchi M,
Nicholson A, Geisinger K, Yatabe Y, Ishikawa Y, Wistuba I, Flieder
DB, Franklin W, et al: Diagnosis of lung cancer in small biopsies
and cytology: Implications of the 2011 international association
for the study of lung cancer/American thoracic society/European
respiratory society classification. Arch Pathol Lab Med.
137:668–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Decicco-Skinner KL, Trovato EL, Simmons
JK, Lepage PK and Wiest JS: Loss of tumor progression locus 2
(tpl2) enhances tumorigenesis and inflammation in two-stage skin
carcinogenesis. Oncogene. 30:389–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asimakopoulos F, Kim J, Denu RA, Hope C,
Jensen JL, Ollar SJ, Hebron E, Flanagan C, Callander N and Hematti
P: Macrophages in multiple myeloma: Emerging concepts and
therapeutic implications. Leuk Lymphoma. 54:2112–2121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
27
|
Schetter AJ, Heegaard NH and Harris CC:
Inflammation and cancer: Interweaving microRNA, free radical,
cytokine and p53 pathways. Carcinogenesis. 31:37–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujita K, Mondal AM, Horikawa I, Nguyn GH,
Kumamoto K, Sohn JJ, Bowman ED, Mathe EA, Schetter AJ, Pine SR, et
al: p53isoforms Delta133p53 and p53beta are endogenous regulators
of replicative cellular senescence. Nat Cell Biol. 11:1135–1142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito M, Schetter AJ, Mollerup S, Kohno T,
Skaug V, Bowman ED, Mathé EA, Takenoshita S, Yokota J, Haugen A and
Harris CC: The association of microRNA expression with prognosis
and progression in early-stage, non small cell lung adenocarcinoma:
A retrospective analysis of three cohorts. Clin Cancer Res.
17:1875–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Capodanno A, Boldrini L, Proietti A, Alì
G, Pelliccioni S, Niccoli C, D'Incecco A, Cappuzzo F, Chella A,
Lucchi M, et al: Let-7g and miR-21 expression in non-small cell
lung cancer: Correlation with clinicopathological and molecular
features. Int J Oncol. 43:765–774. 2013.PubMed/NCBI
|
|
32
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao W, Shen H, Lui L, Xu J, Xu J and Shu
Y: MiR-21 overexpression in human primary squamous cell lung
carcinoma is associated with poor patient prognosis. J Cancer Res
Clin Oncol. 137:557–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Markou A, Tsaroucha EG, Kaklamanis L,
Fotinou M, Georgoulias V and Lianidou ES: Prognostic value of
mature microRNA-21 and microRNA-205 overexpression in non-small
cell lung cancer by quantitative real-time RT-PCR. Clin Chem.
54:1696–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Voortman J, Goto A, Mendiboure J, Sohn JJ,
Schetter AJ, Saito M, Dunant A, Pham TC, Petrini I, Lee A, et al:
MicroRNA expression and clinical outcomes in patients treated with
adjuvant chemotherapy after complete resection of non-small cell
lung carcinoma. Cancer Res. 70:8288–8298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu J, Lu C, Guo J, Chen L, Chu Y, Ji Y and
Ge D: Prognostic significance of the IASLC/ATS/ERS classification
in Chinese patients-A single institution retrospective study of 292
lung adenocarcinoma. J Surg Oncol. 107:474–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Russell PA, Wainer Z, Wright GM, Daniels
M, Conron M and Williams RA: Does lung adenocarcinoma subtype
predict patient survival?: A clinic-pathologic study based on the
new international association for the study of lung cancer/American
thoracic society/European respiratory society international
multidisciplinary lung adenocarcinoma classification. J Thorac
Oncol. 6:1496–1504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Solis LM, Behrens C, Raso MG, Lin HY,
Kadara H, Yuan P, Galindo H, Tang X, Lee JJ, Kalhor N, et al:
Histological patterns and molecular characteristics of lung
adenocarcinoma associated with clinical outcome. Cancer.
118:2889–2899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Araki K, Kidokoro Y, Hosoya K, Wakahara M,
Matsuoka Y, Takagi Y, Haruki T, Miwa K, Taniguchi Y, Horie S and
Nakamura H: Excellent prognosis of lepidic-predominant lung
adenocarcinoma: Low incidence of lymphatic vessel invasion as a key
factor. Anticancer Res. 34:3153–3156. 2014.PubMed/NCBI
|
|
40
|
Okamoto T, Maruyama R, Suemitsu R, Aoki Y,
Wataya H, Kojo M and Ichinose Y: Prognostic value of the
histological subtype in completely resected non-small cell lung
cancer. Interact Cardiovasc Thorac Surg. 5:362–366. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yatabe Y, Mitsudomi T and Takahashi T:
TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol.
26:767–773. 2002. View Article : Google Scholar : PubMed/NCBI
|