Introduction
Breast cancer is the most common type of invasive
cancer in women and accounts for 16% of all female cancers, with
the second highest mortality rate among women worldwide (1), while in USA and Iran it comprises 26
and 23% of all female cancers (2–4),
respectively. In addition, breast cancer is an important public
health issue; with ~8,000 women being diagnosed annually in Iran,
of which 97.2% are women. However, due to the relatively early
diagnosis, it is the fifth most common cause of cancer death in
Iran (3). The two major
susceptibility genes, BRCA1 and BRCA2, account for a
maximum of 20% of familial breast cancer cases worldwide. The
remaining cases may be explained by mutations in other cancer
susceptibility genes together with environmental factors (5).
The Fanconi anemia complementation group A
(FANCA) gene is located at 16q24.3, and is primarily
recognized as a gene involved in Fanconi anemia (FA), which is a
rare autosomal recessive disorder with a prevalence of ~1–5 per
million in the western world (6).
FA genes are assigned into the following 8 distinct complementation
FA groups: FANCA, FANCB, FANCC, FANCD1,
FANCD2, FANCE, FANCF and FANCG.
FANCA is the most common FA subtype in the majority of
populations and is defective in >65% of FA cases; FANCC,
FANCG and FANCD2 each account for ~5–15% of cases,
with the remaining subtypes being rare (6–8).
Furthermore, FANCA is a potential tumor suppressor gene due
to its role in the repair of DNA damage, and it remains an
attractive candidate as either a cancer predisposition gene or a
target of genetic sporadic cancer (9). The proteins encoded by FA genes are
closely associated to each other in molecular pathways involved in
DNA repair, and interact directly to form a multi-subunit nuclear
complex (10) that is required to
respond to DNA damage (11,12).
Notably, the 16q24.3 genomic region, where FANCA resides, is
a common target for loss-of-heterozygosity in breast tumors
(13), and in addition, an
intronic FANCA single nucleotide polymorphism has been
previously associated with an 8% increase in breast cancer risk
(14). Only 1 potential
FANCA missense mutation has been identified in UK families
with breast cancer (15), and 4
large FANCA deletions have been previously reported in
sporadic acute myeloid leukemia (16). As polymorphisms in promoters alter
the transcription or regulation of a gene, it was hypothesized that
the FANCA gene duplication in the promoter region may alter
the risk of developing breast cancer in an Iranian population,
similar to previous reports in other populations (17), and may be associated with an
altered risk of developing breast cancer. The present study
therefore performed a case control study to determine whether
duplication allele may be responsible for an increase in the risk
of breast cancer among a population of Iranian women.
Materials and methods
Subjects
A total of 304 breast cancer cases were
systematically ascertained through Imam Khomeini Hospital Complex
(Tehran University of Medical Sciences, Tehran, Iran). Of these
cases, 50% (152) were selected on the basis of a family history of
breast cancer (defined as ≥2 cases of breast cancer in a first- or
second-degree female relative). Blood was taken from all recruits
who consented to molecular analysis for breast cancer
predisposition genes at the Central Laboratory of Imam Khomeini
Hospital Complex hospital. The age range of the all breast cancer
participants was 28–74 with a mean age of 49.41±10.44 years
(familial and non-familial breast cancer). The controls were
selected from the same population from which the cases arose and
consisted of 295 healthy female volunteers who were attending the
Cancer Institute of Imam Khomeini Hospital Complex for a checkup.
The age range of the controls was 28–74 with a mean age of
49.28±10.48 years. None of the control individuals had any history
of breast cancer or any other neoplastic diseases, and had no
family history of breast cancer diagnosed at the same clinics.
Women with hysterectomy and artificial menopause, or who had been
exposed to any kind of radiation, including X-rays, or chemotherapy
in their life time were excluded from the study. Control and cancer
groups were drawn from the same geographical area. Demographical
and epidemiological risk factor data was collected from a short,
structured questionnaire, which included information on age at
menarche, age at menopause, marriage status, race, age at breast
cancer onset, number of pregnancies and children, age at first
child birth and average lactation term. An ongoing protocol to
collect and store blood samples for future genomic tests was
approved by the institutional review board and appropriate ethics
committee. Peripheral blood was collected and genotyping analysis
was performed for selected regions in the FANCA gene.
Ethical approval and consent to
participate
The study was approved by the Research Ethics
Committee for Tehran University of Medical Sciences
(IR.TUMS.REC.1395.2500). Informed consent for testing and
publication was obtained from all participants prior to
participation in the present study (or their parents/legal
guardians).
Molecular genetic analysis
DNA for genotyping was prepared from the peripheral
blood sample of patients and controls. The DNA promoter region
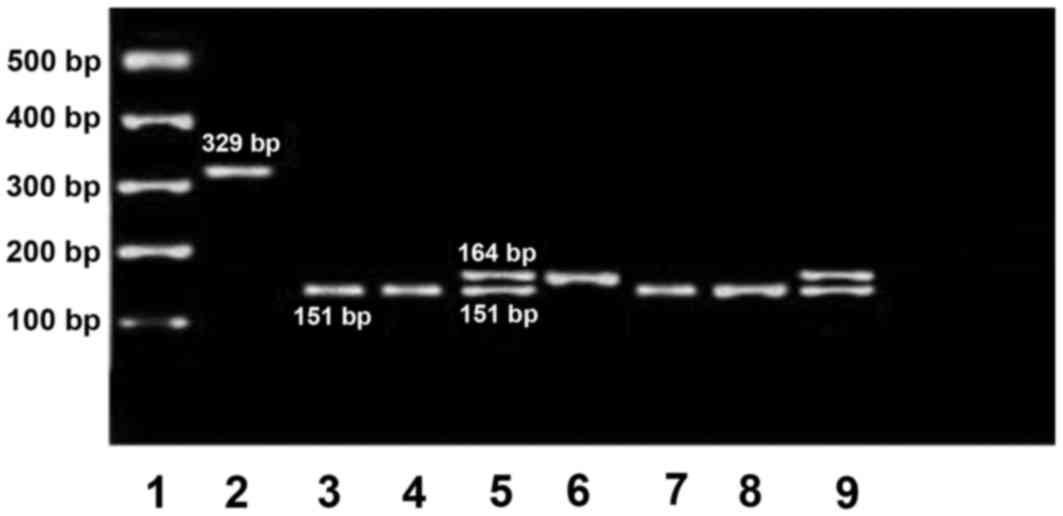
containing the duplication (164 bp) was amplified using primers
designed by Primer3 (version 0.4.0) software and positive control
primers were used to amplify estrogen receptor 1 gene exon 4 (329
bp), as published elsewhere (18).
Primer sequences are presented in Table I.
 | Table I.Polymerase chain reaction primers. |
Table I.
Polymerase chain reaction primers.
| Primer | Sequence 5′→3′ | Base pairs |
|---|
| Duplicated region
FANCA primers | F:
CCAAACGCAAAAACTACCTCACCG | 164 |
|
| R:
CGCTGCCTTCCTATTGGCTGC |
|
| ESR1 exon 4
primers (positive control) | F:
ACCTGTGTTTTCAGGGATACGA | 329 |
|
| R:
GCTGCGCTTCGCATTCTTAC |
|
Phenol-chloroform DNA extraction
DNA was isolated using AccuPrep® (high
pure phenol-chloroform) Genomic DNA extraction kit (Bioneer
Corporation; Takapouzist Co., Tehran, Iran). Lysis buffer (200 µl)
was added to 200 µl whole blood and incubated at 60°C for 10 min,
500 µl phenol was then added and centrifuged at 12,000 × g
at 4°C for 5 min. Chloroform (500 µl) was added to the upper phase
and centrifuged for 5 min at 12,000 × g at 4°C. Sodium
Acetate (1:10) plus cold 100% ethanol (2:1) was added to the upper
phase, frozen for 20 min at −20°C and centrifuged for 10 min at
12,000 × g and 4°C. To precipitate DNA, 70% ethanol was
added and centrifugation was performed for 15 min at 12,000 ×
g and 4°C. The DNA was measured using a spectrophotometer;
the amount of DNA was calculated in µg/ml (85 µg/ml) by absorbance
at 260 nm and the purity was tested by determining the 260/280 nm
ratio (a ratio of 1.7 was detected).
Polymerase chain reaction (PCR)
conditions
The following was added to each 50 µl PCR reaction
tube: 43.5 µl master mix (5X HOT FIREPol® Blend; TAG
Copenhagen A/S, Frederiksberg, Denmark); 2 µl (200 nM) primers
synthesized by TAG Copenhagen A/S; 2 µl (50 ng) DNA template
(extracted genomic DNA); and 2.52 µl Taq DNA polymerase (0.5 U
Super Taq enzymes; Cambridge Bioscience, Ltd., Cambridge, UK) and
PCR was performed in an Eppendorf thermo-cycler following the
protocol in Table II. The first
set of PCR primers amplified a 151-base pair product for allele 0
(normal) and a 164 base pair product for allele 1 (duplication).
The DNA ladder (Biotium) was also purchased from Cambridge
Bioscience, Ltd. The products were separated by electrophoresis on
3% agarose gel and stained by 1% ethidium bromide (Cambridge
Bioscience, Ltd.). PCR products from the three genotypes were
sequenced on the 96-capillary ABI 3730xl (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) by the Sanger
sequencing technique.
 | Table II.Polymerase chain reaction cycling
conditions. |
Table II.
Polymerase chain reaction cycling
conditions.
| Step number | Reaction | Temperature (°C) | Duration (min) | Number of cycles |
|---|
| 1 | Primary
denaturation | 94 | 4 | 1 |
| 2 | Denaturation | 94 | 1 | 35 |
| 3 | Annealing | 67 | 1 | 35 |
| 4 | Extension | 72 | 1 | 35 |
| 5 | Final
extension | 72 | 5 | 2 |
Statistical analysis
The Hardy-Weinberg equilibrium was assessed by the
standard methods (19). The data
were considered using normal (00; women without a copy of the
duplication allele), heterozygotes (01; women with 1 copy of the
duplication allele) and homozygotes (11; women with 2 duplication
alleles). Data was analyzed using SPSS, version 17.0 (SPSS, Inc.,
Chicago, IL, USA). Stratification with respect to demographics and
risk factors was performed and post-stratification Pearson's
χ2 analysis was used to calculate the significance and
odds ratio (OR) with a 95% confidence interval. All tests were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of the FANCA promoter
duplication
Variation in the FANCA gene promoter region
(NCBI reference sequence, NM_000135.2) was screened using PCR
analysis. Variant bands were purified and sequenced directly with
forward and reverse primers. The comparison of band sequences and
the sequence of the FANCA promoter demonstrated that a
13-base pair sequence (5′-GGCCACGACGCAA-3′) located from −98 to
−110 bases upstream of the transcription start point, which was
present either as a single or double copy, causes variation in
patterns observed on the agarose gel. In the present study, allele
0 is defined as having has a single copy of the sequence
(5′-GGCCACGACGCAA-3′), whilst allele 1 has 2 tandem copies of this
sequence. Notably, the sequence directly adjacent to (in the
downstream direction) the 13-base pair sequence shares homology
with the duplicate sequence.
Case-control study of FANCA
variations
A single set of primers was used to amplify both
alleles by PCR in the promoter region (Fig. 1). The frequency of the polymorphism
was determined in patients with breast cancer and controls to
assess if the presence of either allele was associated with a
predisposition to breast cancer. Table III presents the genotypic
frequency distribution in patients with breast cancer and controls.
The distribution of the genotypes within overall breast cancer and
familial breast cancer groups deviated significantly from those
expected under Hardy-Weinberg equilibrium with a P-value of 0.001.
In patients with familial breast cancer, the estimated risk was 1.3
fold higher for individuals who were 01 heterozygote duplication
(OR, 1.27; 95% CI, 0.825–1.955) or 1.5 fold higher for those who
were 11 homozygote duplication (OR, 1.511; 95% CI, 0.73–3.131)
compared with 00 homozygotes. Therefore, the results indicated that
a higher frequency of allele 1 may be associated with an increased
risk of developing familial breast cancer. However, the genotypic
frequency did not show significant (P=0.365) elevation among
the-non family history group. Furthermore, the frequency of the
duplication allele (allele 1) in all breast cancer patients and
familial breast cancer patients only was significantly higher
compared with controls (P=0.001), with exception of non-familiar
breast cancer patients (P=0.131; Table IV). Promoter duplication genotypes
were compared with selected clinical breast cancer features,
including age at menarche, age at breast cancer onset, age at
menopause and lymph node metastasis in patients with breast cancer.
The only significant association was for age at menarche, as
indicated by the ORs presented in Table V. Genotype frequencies exhibited
significantly different distributions in age at menarche (<12
years old vs. ≥12; P=0.001). The estimated risk was higher for
individuals who were 11 homozygotes for the duplication (OR 0.148,
95% CI: 0.069–0.316) compared with the corresponding 01
heterozygotes (OR, 0.775; 95% CI, 0.428–1.405). Furthermore, the
estimated risk of developing breast cancer (familiar breast cancer
vs. non-familiar breast cancer) was 4-fold higher for those who
were 11 homozygotes (OR 4.093, 95%; CI, 1.957–8.561) or 3-fold
higher for those who were 01 heterozygotes (OR, 3.315; 95% CI,
1.996–5.506) compared with the corresponding 00 homozygotes. The
results suggest that the higher the frequency of allele 1, the
greater the risk of developing breast cancer.
 | Table III.The distribution of Fanconi anemia
complementation group A gene promoter polymorphism genotypes and
estimated risk in breast cancer cases and controls. |
Table III.
The distribution of Fanconi anemia
complementation group A gene promoter polymorphism genotypes and
estimated risk in breast cancer cases and controls.
|
| Study group |
|
|---|
|
|
|
|
|---|
| Group | 00 | 01 | 11 | P-value |
|---|
| Control (%) | 190 (64.4) | 85 (28.8) | 20 (6.8) |
|
| All breast cancer
cases |
|
|
| 0.001 |
| Number
(%) | 131 (43.1) | 131 (43.1) | 42 (13.8) |
|
| OR (95%
CI) | 1.0 | 2.235
(1.57–3.17) | 3.046
(1.71–5.424) |
|
| Familial breast
cancer cases |
|
|
| 0.001 |
| Number
(%) | 43 (28.3) | 81 (53.3) | 28 (18.4) |
|
| OR (95%
CI) | 1.0 | 1.27
(0.825–1.955) | 1.511
(0.73–3.131) |
|
| Non-familial breast
cancer cases |
|
|
| 0.365 |
| Number
(%) | 88 (57.9) | 50 (32.9) | 14 (9.2) |
|
| OR (95%
CI) | 1.0 | 4.211
(2.686–6.601) | 6.186
(3.189–11.998) |
|
 | Table IV.The distribution of Fanconi anemia
complementation group A promoter polymorphism allelic frequencies
in familial and non-familial breast cases compared with
controls. |
Table IV.
The distribution of Fanconi anemia
complementation group A promoter polymorphism allelic frequencies
in familial and non-familial breast cases compared with
controls.
| Study group | Allele 0(%) | Allele 1(%) | P-value | χ2 |
|---|
| All breast cancer
cases | 393 (46.6) | 215 (53.4) | 0.001 | 29.6 |
| Control | 465 (78.8) | 125 (21.2) |
|
|
| Familial breast
cancer | 167 (54.9) | 137 (45.1) | 0.001 | 55.21 |
| Control | 465 (78.8) | 125 (21.2) |
|
|
| Non-familial breast
cancer | 226 (74.3) | 78 (25.7) | 0.131 | 2.286 |
| Control | 465 (78.8) | 125 (21.2) |
|
|
 | Table V.Estimated risk of breast cancer for
major risk factors in different genotypes. |
Table V.
Estimated risk of breast cancer for
major risk factors in different genotypes.
|
| Genotype |
|
|---|
|
|
|
|
|---|
| Category | All | 01 | 11 | 00 | P-value |
|---|
| Age at
menarche |
|
|
|
| 0.001 |
| ≤12
years (%) | 220 | 100 (76.3) | 16 (38.1) 26
(61.9) | 104 (80.6) |
|
| >12
years (%) | 82 | 31 (23.7) | 26 (61.9) | 25 (19.4) |
|
| OR (95%
CI) |
| 0.775
(0.428–1.405) | 0.148
(0.069–0.316) | 1 |
|
| Age at breast
cancer onset |
|
|
|
| 0.335 |
| ≤40
years (%) | 118 | 57 (44.2) | 14 (34.1) | 47 (36.4) |
|
| >40
years (%) | 181 | 72 (55.8) | 27 (65.9) | 82 (63.6) |
|
| OR (95%
CI) |
| 1.381 (0.838-
2.276) | 0.905
(0.432–1.893) | 1 |
|
| Age at
menopause |
|
|
|
| 0.693 |
| ≤50
years (%) | 39 | 20 (27.8) | 5 (19.2) | 14 (25.5) |
|
| >50
years (%) | 114 | 52 (72.2) | 21 (80.8) | 41 (74.5) |
|
| OR (95%
CI) |
| 1.126
(0.508–2.497) | 0.697
(0.221–2.199) | 1 |
|
| Metastasis
status |
|
|
|
| 0.348 |
| Yes
(%) | 37 | 14 (10.8) | 8 (19) | 15 (11.6) |
|
| No
(%) | 264 | 116 (89.2) | 34 (81) | 114 (88.4) |
|
| OR (95%
CI) |
| 0.917
(0.423–1.987) | 1.788
(0.699–4.576) | 1 |
|
| Cancer status |
|
|
|
| 0.001 |
|
Familial (%) | 152 | 81 (61.8) | 28 (66.7) | 43 (32.8) |
|
|
Non-familial (%) | 152 | 50 (38.2) | 14 (33.3) | 88 (67.2) |
|
| OR (95%
CI) |
| 3.315
(1.996–5.506) | 4.093
(1.957–8.561) | 1 |
|
Discussion
To the best of our knowledge, the present study was
the first to evaluate the association between variations in the
FANCA gene, in 304 breast cancer cases, and breast cancer
risk in Iranian women. As this duplication was relatively common in
healthy individuals, it may be regarded as a polymorphism. It is
already established that alterations in the FANC group of genes are
associated with several types of cancer (20–23).
Following the identification of BRCA2 as a member of the
FANC family (FANCD1), researchers hypothesize that
variations in other FANC genes, such as FANCA, may increase
breast cancer susceptibility (24). For example, the absence of
FANCF by aberrant promoter methylation (25,26)
and BACH1/FANCJ mutations (27) have been previously identified in
breast cancer cases. Furthermore, deletions (28) and duplications (17) in the FANCA gene are
pathogenic and have been previously detected in patients with
breast cancer.
As even single base changes in gene sequences may
change gene expression regulation and lead to tumorigenesis,
particularly if this affects a transcription factor binding site
(17,29,30),
the 13-base pair duplication alleles in the promoter region
identified in the present study are important. Considering the
common occurrence of both FANCA polymorphism alleles,
neither allele is expected to be a high penetrance predisposition
allele. The current study evaluated whether duplication in the
promoter region of the FANCA gene may increase breast cancer
susceptibility. The results of the present study were consistent
with previous epidemiological studies, which indicated the role of
FANCA gene variations in increasing the risk of breast
cancer (31). According to the
results of the present study, FANCA gene mutations generally
increase the risk of breast cancer and contribute to the
development of familial breast cancer. Previous research has
highlighted the role of various genes involved in DNA repair, other
than BRCA1 or BRCA2, in increasing breast cancer
susceptibility. For example, a study on the Finnish population
reported that FANCM mutations caused a strong predisposition
to breast cancer (31–35). Johnson et al (31) reported missense variants in the
BRCA1, BRCA2, ATM, CHEK2 and ATM
genes to be significantly associated with breast cancer risk among
cases with bilateral disease (P=0.005), particularly for less
common alleles (P=0.00004). However, despite the evidence to
support the potential role of the FANCA family in increasing
susceptibility to breast cancer and other types of cancer (15), the investigation of the role of
these genes in cancer susceptibility in a monoallelic context has
been limited. However, heterozygous FANCA deletions have
been reported as potential low penetrance alleles for acute myeloid
leukemia (16). Recently, Virts
et al (36) in the US
demonstrated the FANCT gene to be a rare cancer
susceptibility gene. In a study on a Spanish population, Blanco
et al (37) introduced
RAD51 paralog C, a novel FA gene necessary for homologous
recombination repair, as a rare susceptibility gene for hereditary
breast and ovarian cancer. By contrast, Cleton-Jansen et al
(38) reported the absence of
mutations in 19 breast cancer patients with 16q24.3.
Genotyping of the promoter polymorphism indicated a
significant difference in the allele or genotype distribution
between patients with breast cancer and normal controls. The
present study had 80% power to detect an OR ≥1.27 for heterozygous
carriers of the duplication and an OR ≥1.511 for homozygous
carriers of the duplication in hereditary breast cancer.
Furthermore, the frequency of the allele 1 was significantly higher
in patients with a family history of breast cancer compared with
the control group (45 and 21%, respectively; P=0.001), indicating
that allele 1 may increase the risk of breast cancer development.
Nevertheless, larger studies are required to compare the frequency
of FANCA gene sequence variants between breast cancer
patients and healthy controls with different ethnicities. In
conclusion, the current study was the first to report a promoter
region variation in the FANCA gene among women with breast
cancer in Iran. The results of the present study confirmed the
allelic variants in the FANCA promoter region as a tumor
suppressor gene. This gene affects cell activities, such as the
basal rate of transcription or the regulation of transcription,
which increase the risk of breast cancer. However, a more extensive
evaluation of the role of other FA pathway genes in hereditary
susceptibility to cancer is required. Further studies are required
to clarify the full implication of FANCA gene variation in
breast cancer susceptibility and predisposition to other types of
cancer. Such genetic markers may be useful as an early breast
cancer diagnosis tool in developing countries such as Iran.
Acknowledgements
The current study was supported by Tehran University
of Medical Sciences and Health Services (grant no.
91-01-31-14816).
References
|
1
|
WHO, . Global Burden of Disease. Geneva,
Switzerland: 2004
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
3
|
Akbari ME, Abachizade K, Tabatabaei M,
Motlagh A Ghanbari, Jabari Z Majd and Khayamzadeh M: Cancer in
Iran. Darolfekr Publications; Ghom: 2008
|
|
4
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
5
|
Stratton MR and Rahman N: The emerging
landscape of breast cancer susceptibility. Nat Genet. 40:17–22.
2008. View Article : Google Scholar
|
|
6
|
Joenje H and Patel KJ: The emerging
genetic and molecular basis of Fanconi anaemia. Nat Rev Genet.
2:446–457. 2001. View
Article : Google Scholar
|
|
7
|
Tischkowitz MD and Hodgson SV: Fanconi
anaemia. J Med Genet. 40:1–10. 2003. View Article : Google Scholar :
|
|
8
|
Pronk JC, Gibson RA, Savoia A, Wijker M,
Morgan NV, Melchionda S, Ford D, Temtamy S, Ortega JJ, Jansen S, et
al: Localisation of the Fanconi anaemia complementation group A
gene to chromosome 16q24.3. Nat Genet. 11:338–340. 1995. View Article : Google Scholar
|
|
9
|
Han SS, Tompkins VS, Son DJ, Han S, Yun H,
Kamberos NL, Dehoedt CL, Gu C, Holman C, Tricot G, et al: CDKN1A
and FANCD2 are potential oncotargets in Burkitt lymphoma and
multiple myeloma. Exp Hematol Oncol. 4:92015. View Article : Google Scholar :
|
|
10
|
Medhurst AL, Huber PA, Waisfisz Q, de
Winter JP and Mathew CG: Direct interactions of the five known
Fanconi anaemia proteins suggest a common functional pathway. Hum
Mol Genet. 10:423–429. 2001. View Article : Google Scholar
|
|
11
|
Hussain S, Witt E, Huber PA, Medhurst AL,
Ashworth A and Mathew CG: Direct interaction of the Fanconi anaemia
protein FANCG with BRCA2/FANCD1. Hum Mol Genet. 12:2503–2510. 2003.
View Article : Google Scholar
|
|
12
|
Howlett NG, Taniguchi T, Olson S, Cox B,
Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals
G, et al: Biallelic inactivation of BRCA2 in Fanconi anemia.
Science. 297:606–609. 2002. View Article : Google Scholar
|
|
13
|
Cleton-Jansen AM, Callen DF, Seshadri R,
Goldup S, Mccallum B, Crawford J, Powell JA, Settasatian C, Van
Beerendonk H, Moerland EW, et al: Loss of heterozygosity mapping at
chromosome arm 16q in 712 breast tumors reveals factors that
influence delineation of candidate regions. Cancer Res.
61:1171–1177. 2001.
|
|
14
|
Haiman CA, Hsu C, de Bakker PI, Frasco M,
Sheng X, Van Den Berg D, Casagrande JT, Kolonel LN, Le Marchand L,
Hankinson SE, et al: Comprehensive association testing of common
genetic variation in DNA repair pathway genes in relationship with
breast cancer risk in multiple populations. Hum Mol Genet.
17:825–834. 2008. View Article : Google Scholar
|
|
15
|
Seal S, Barfoot R, Jayatilake H, Smith P,
Renwick A, Bascombe L, McGuffog L, Evans DG, Eccles D, Easton DF,
et al: Evaluation of Fanconi anemia genes in familial breast cancer
predisposition. Cancer Res. 63:8596–8599. 2003.
|
|
16
|
Tischkowitz MD, Morgan NV, Grimwade D,
Eddy C, Ball S, Vorechovsky I, Langabeer S, Stöger R, Hodgson SV
and Mathew CG: Deletion and reduced expression of the Fanconi
anemia FANCA gene in sporadic acute myeloid leukemia. Leukemia.
18:420–425. 2004. View Article : Google Scholar
|
|
17
|
Thompson E, Dragovic RL, Stephenson SA,
Eccles DM, Campbell IG and Dobrovic A: A novel duplication
polymorphism in the FANCA promoter and its association with breast
and ovarian cancer. BMC Cancer. 5:432005. View Article : Google Scholar :
|
|
18
|
Abbasi S, Rasouli M, Nouri M and Kalbasi
S: Association of estrogen receptor-α A908G (K303R) mutation with
breast cancer risk. Int J Clin Exp Med. 6:39–49. 2013.
|
|
19
|
Beck A, Luedtke A, Liu K and Tintle N: A
powerful method for including genotype uncertainty in tests of
hardy-weinberg equilibrium. Pac Symp Biocomput. 22:368–379.
2016.
|
|
20
|
Colleu-Durel S, Guitton N, Nourgalieva K,
Lévêque J, Danic B and Chenal C: Genomic instability and breast
cancer. Oncol Rep. 8:1001–1005. 2001.
|
|
21
|
Shen Y, Lee YH, Panneerselvam J, Zhang J,
Loo LW and Fei P: Mutated Fanconi anemia pathway in non-Fanconi
anemia cancers. Oncotarget. 6:20396–20403. 2015. View Article : Google Scholar :
|
|
22
|
Bakker JL, Thirthagiri E, Van Mil SE,
Adank MA, Ikeda H, Verheul HM, Meijers-Heijboer H, de Winter JP,
Sharan SK and Waisfisz Q: A novel splice site mutation in the
noncoding region of BRCA2: Implications for Fanconi anemia and
familial breast cancer diagnostics. Hum Mutat. 35:442–446. 2014.
View Article : Google Scholar :
|
|
23
|
Sawyer SL, Tian L, Kähkönen M,
Schwartzentruber J, Kircher M; University of Washington Centre for
Mendelian Genomics, ; FORGE Canada Consortium, ; Majewski J, Dyment
DA, Innes AM, et al: Biallelic mutations in BRCA1 cause a new
Fanconi anemia subtype. Cancer Discov. 5:135–142. 2015. View Article : Google Scholar
|
|
24
|
Venkitaraman AR: Connecting Fanconi's
anaemia to breast cancer predisposition. Lancet. 360:1344–1345.
2002. View Article : Google Scholar
|
|
25
|
Van Der Heijden MS, Brody JR and Kern SE:
Functional screen of the fanconi anemia pathway in cancer cells by
Fancd2 immunoblot. Cancer Biol Ther. 3:534–537. 2004. View Article : Google Scholar
|
|
26
|
Turner N, Tutt A and Ashworth A: Hallmarks
of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 4:814–819. 2004.
View Article : Google Scholar
|
|
27
|
Lewis AG, Flanagan J, Marsh A, Pupo GM,
Mann G, Spurdle AB, Lindeman GJ, Visvader JE, Brown MA and
Chenevix-Trench G: Kathleen Cuningham Foundation Consortium for
Research into Familial Breast Cancer: Mutation analysis of FANCD2,
BRIP1/BACH1, LMO4 and SFN in familial breast cancer. Breast Cancer
Res. 7:R1005–R1016. 2005. View
Article : Google Scholar :
|
|
28
|
Solyom S, Winqvist R, Nikkilä J, Rapakko
K, Hirvikoski P, Kokkonen H and Pylkäs K: Screening for large
genomic rearrangements in the FANCA gene reveals extensive deletion
in a Finnish breast cancer family. Cancer Lett. 302:113–118. 2011.
View Article : Google Scholar
|
|
29
|
Zhu Y, Spitz MR, Lei L, Mills GB and Wu X:
A single nucleotide polymorphism in the matrix metalloproteinase-1
promoter enhances lung cancer susceptibility. Cancer Res.
61:7825–7829. 2001.
|
|
30
|
Bond GL, Hu W, Bond EE, Robins H, Lutzker
SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al: A
single nucleotide polymorphism in the MDM2 promoter attenuates the
p53 tumor suppressor pathway and accelerates tumor formation in
humans. Cell. 119:591–602. 2004. View Article : Google Scholar
|
|
31
|
Johnson N, Fletcher O, Palles C, Rudd M,
Webb E, Sellick G, dos Santos Silva I, McCormack V, Gibson L,
Fraser A, et al: Counting potentially functional variants in BRCA1,
BRCA2 and ATM predicts breast cancer susceptibility. Hum Mol Genet.
16:1051–1057. 2007. View Article : Google Scholar
|
|
32
|
Rodríguez-López R, Osorio A,
Sánchez-Pulido L, De La Hoya M, Barroso A, Caldés T and Benítez J:
No mutations in the XRCC2 gene in BRCA1/2-negative high-risk breast
cancer families. Int J Cancer. 103:136–137. 2003. View Article : Google Scholar
|
|
33
|
Smith TR, Miller MS, Lohman K, Lange EM,
Case LD, Mohrenweiser HW and Hu JJ: Polymorphisms of XRCC1 and
XRCC3 genes and susceptibility to breast cancer. Cancer Lett.
190:183–190. 2003. View Article : Google Scholar
|
|
34
|
Kiiski JI, Pelttari LM, Khan S,
Freysteinsdottir ES, Reynisdottir I, Hart SN, Shimelis H, Vilske S,
Kallioniemi A, Schleutker J, et al: Exome sequencing identifies
FANCM as a susceptibility gene for triple-negative breast cancer.
Proc Natl Acad Sci USA. 111:pp. 15172–15177. 2014; View Article : Google Scholar :
|
|
35
|
Fearnhead NS, Wilding JL, Winney B, Tonks
S, Bartlett S, Bicknell DC, Tomlinson IP, Mortensen NJ and Bodmer
WF: Multiple rare variants in different genes account for
multifactorial inherited susceptibility to colorectal adenomas.
Proc Natl Acad Sci USA. 101:pp. 15992–15997. 2004; View Article : Google Scholar :
|
|
36
|
Virts EL, Jankowska A, Mackay C, Glaas MF,
Wiek C, Kelich SL, Lottmann N, Kennedy FM, Marchal C, Lehnert E, et
al: AluY-mediated germline deletion, duplication and somatic stem
cell reversion in UBE2T defines a new subtype of Fanconi anemia.
Hum Mol Genet. 24:5093–5108. 2015. View Article : Google Scholar :
|
|
37
|
Blanco A, Gutiérrez-Enríquez S,
Santamariña M, Montalban G, Bonache S, Balmaña J, Carracedo A, Diez
O and Vega A: RAD51C germline mutations found in Spanish
site-specific breast cancer and breast-ovarian cancer families.
Breast Cancer Res Treat. 147:133–143. 2014. View Article : Google Scholar
|
|
38
|
Cleton-Jansen AM, Moerland EW, Pronk JC,
Van Berkel CG, Apostolou S, Crawford J, Savoia A, Auerbach AD,
Mathew CG, Callen DF and Cornelisse CJ: Mutation analysis of the
Fanconi anaemia A gene in breast tumours with loss of
heterozygosity at 16q24.3. Br J Cancer. 79:1049–1052. 1999.
View Article : Google Scholar :
|