Introduction
Acute pancreatitis (AP) is an acute inflammation of
the pancreas. The primary clinical presentation is acute abdominal
pain combined with an increase in serum amylase. For ~80% of
patients, the disease is self-limiting, whereas the remainder may
develop severe acute pancreatitis (SAP), the mortality rate of
which is ~30%. The pathogenic underlying mechanisms remain to be
fully elucidated. Previous theories of trypsin autodigestion
(1), pancreatic microcirculation
dysfunction (2), free radical
damage (3) and cell apoptosis
(4) do not fully account for the
disease. Rinderknecht (5) proposed
the ‘inflammatory mediator’ theory. According to this theory, a
large number of cytokines released by cells induces a complex
inflammatory cascade, which mediates damage in the pancreas and in
extrapancreatic organs, thus causing a systemic inflammatory
reaction.
Trypsin autodigestion is considered to be the
primary process involved in AP. Even in the early stages of AP,
pancreatic acinar cell damage occurs, and the type of acinar cell
death is important for the development and prognosis of AP. Cell
death type may influence the release of trypsin from acinar cells,
thus indirectly affecting the degree of pancreatitis. Therefore,
control of the activation and release of trypsin has become
critical for treating pancreatitis. The types of pancreatic cell
death include apoptosis and necrosis. If cells die from apoptosis,
the cell membrane remains intact; therefore, there is no
accompanying release of inflammatory factors and trypsin, and the
inflammatory reaction is milder. By contrast, necrosis is
accompanied by the release of intracellular contents and lysosomal
enzymes. Particularly for pancreatic acinar cells, necrosis is
associated with the release and activation of trypsin (6), which may induce the aggregation of a
variety of inflammatory cells and cause severe inflammatory
reactions (7).
The function of apoptosis and necrosis in AP is
complex. An understanding of the factors involved in these two
types of cell death may indicate a strategy for the promotion of
acinar cell death by apoptosis, rather than necrosis, which may be
important for the treatment of AP. The present study aimed to
investigate proteins differentially expressed in apoptosis and
necrosis in the AR42J rat pancreatic cell line.
Materials and methods
Establishment of the AP cell
model
The AR42J rat pancreatic acinar cell line was
purchased from the China Center for Type Culture Collection (Wuhan,
China). Cells were cultured in Ham's F12 K (Kaighn's) culture
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% foetal bovine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) in a 5% CO2 incubator at
37°C. Approximately 1×105/ml ARJ42 cells were seeded into 6-well
plates and cultured for 24 h. Caerulein (10–8 mol/l, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was added, and the cells were
incubated for a further 24 h. Finally, the ARJ42 cells were
collected, and the percentages of apoptosis and necrosis were
detected using a flow cytometer.
Labelling and flow cytometry sorting
of apoptotic and necrotic cells
For fluorescence staining, ARJ42 cells were detached
and collected in EDTA-free trypsin, washed twice with PBS and
centrifuged at 106 × g for 5 min at room temperature. A total of
5×105 cells were resuspended in 500 µl binding buffer (Beijing
Biosea Biotechnology Co., Ltd., Beijing, China). Following this, 5
µl Annexin V-fluorescein isothiocyanate (FITC) and 5 µl propidium
iodide (PI) (Beijing Biosea Biotechnology Co., Ltd.) were added and
mixed sequentially, and cells were incubated at room temperature in
the dark for 15 min.
For liquid flow monitoring, a 70-mm nozzle was
selected, and the amplitude was adjusted to make the liquid flow
break point between 1/2 and 1/3 of the window to ensure the regular
shape of all of the primary liquid drops. The vibration amplitude
of the liquid drops was adjusted to allow the Gap value to be as
stable as possible. The satellite points were <5 and were fused
into the liquid drops. The Amp1 value was 9.1, the Drop1 value was
252, and the Gap value was 7.
For adjustment of the sorting liquid path, the
voltage of the side liquid flow window was opened, and the Test
Sort test was activated. The amplitude of the primary liquid flow
window was gradually adjusted to clear the liquid flow separation.
The adjusted Drop1 value in the primary liquid flow window was
added to the default window. The Gap1 value, following liquid flow
adjustment, should be within the range of the default value ±3. The
Amp1 value was 9.1, the 2nd value was 14, and the 3rd value was
7.
For confirmation of liquid drop delay, the original
adjustment in precision was selected. The brightness in the left
frame was adjusted to 100%. The fine adjustment in precision was
selected, and the liquid drop delay value was adjusted to make the
brightness in the left frame ≥90%. Finally, during the adjustment
of the Drop Delay, following each single click, the adjustment was
conducted after the system reacted to the liquid drop delay time.
The Drop Delay value was 45.18.
Proteomics detection of apoptotic
cells and necrotic cells
Sorted Annexin V-positive cells (V group) or the
Annexin V/PI double-positive cells (VP group) at a density of 5×105
were mixed with 500 µl 2D lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Cells were sonicated (80 W, 5-sec
pulses; repeated three times). Protein concentrations were
determined using the Bradford method. Protein lysates were
aliquoted into tubes (100 µg/tube). Samples (20 µl) were loaded
into each well and separated by electrophoresis at 200 V for 45
min-1 h on a 12% SDS-PAGE gel. Gel samples were stained with silver
nitrate for 1–2 h and destained. The proteins in the gels were
enzymatically digested; gel samples were mixed with 5 µl Trypsin
(10 ng/µl, Gibco; Thermo Fisher Scientific, Inc.), buffer was added
at 37°C overnight and the protein solution was aspirated into EP
tube for the next step.
The Ettan™ Multidimensional Liquid
Chromatography system (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) was used for desalting and separation of tryptic peptides
mixtures. In this system, samples were desalted on Reversed-Phase
(RP) Trap Columns (Zorbax 300SB-C18; Agilent Technologies, Inc.,
Santa Clara, CA, USA), and subsequently separated on an RP column
(RP-C18; 150 µm ×100 mm; Column Technologies, Lombard, IL, USA).
Mobile phases A (0.1% formic acid in high performance liquid
chromatography-grade water) and B (0.1% formic acid in
acetonitrile) were selected. Tryptic peptide mixtures (20 µg) were
loaded onto the columns and separation was performed at a flow rate
of 2 µl/min using a linear gradient of 4–50% B for 120 min. A
Finnigan™ LTQ-VELOS™ Linear Ion Trap mass
spectrometer (Thermo Electron Corporation, Beverly, MA, USA)
equipped with an electrospray interface was connected to the liquid
chromatography setup for eluted peptide detection. Data-dependent
mass spectrometry (MS)/MS spectra were obtained simultaneously.
Each scan cycle consisted of one full MS scan in profile mode
followed by ten MS/MS scans in centroid mode with the following
Dynamic Exclusion™ (Thermo Electron Corporation)
settings: Repeat count, 2; repeat duration, 30 sec; and exclusion
duration, 90 sec.
Bioinformatics analysis
The Database for Annotation, Visualization and
Integrated Discovery (DAVID; https://david.abcc.ncifcrf.gov/) was used to screen
differentially expressed genes for Gene Ontology-Biological Process
(GO-BP) and pathway enrichment analysis based on the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database. The ‘Functional
Annotation Tool’ was used for analysis. Differentially expressed
genes previously identified were used for the GO-BP term and
pathway analysis. The results were arranged according to the
negative logarithms of the P-values. Cytoscape software version
2.6.3 (www.cytoscape.org/download.php) was used to plot the
network graphs of corresponding genes and the GO-BP term and KEGG
pathway.
The gene list of differentially expressed proteins
was uploaded onto DAVID. In addition, the corresponding gene
identifier was selected, and the rat whole genome was selected for
the background genes. The ‘Functional Annotation Tool’ was selected
as the analysis tool. The results of the functional enrichment
analysis were then obtained. P<0.05 was considered to indicate a
statistically significant difference.
Detection of type of cell death of
AR2J cells using Annexin V/PI double staining
AR42J cells (5×106 cells/ml) were seeded into
culture plates and incubated for 24 h, in a 5% CO2
incubator at 37°C. Caerulein (10–9, 10–8, 10-7, 10-6 or 10-5 mol/l)
was added to the AR42J cells. The control group did not receive
caerulein. According to the experimental results of our related
research (8), the number of
apoptotic cells decreased after the 8 h timepoint, so this was the
timepoint selected. All 6 groups of cells were incubated for 8 h.
Following this the cells were collected and apoptosis and necrosis
were detected using a flow cytometer.
Fluorescence staining was performed as
aforementioned. For flow cytometry, cells were evaluated at an
excitation wavelength of 488 nm, and an emission wavelength of 530
nm. The green fluorescence of Annexin V-FITC was detected using the
FITC channel (FL1), and the red fluorescence of PI was detected
using the PI channel (FL2). WinMDI version 2.8 (http://www.cyto.purdue.edu/flowcyt/software/Winmdi.htm)
was used for flow cytometric analysis.
Detection of high mobility group box-1
protein (HMGB1) expression
To validate the accuracy of the biological analyses,
HMBG1 protein expression levels were detected by western blot
analysis. Cells were treated with various concentrations of
caerulein as aforementioned. Following treatment, cells were lysed
with radioimmunoprecipitation assay buffer [0.15 M NaCl and 0.05 mM
Tris-HCl (pH 7.2) containing 1% NP-40, 1% sodium deoxycholate and
0.1% sodium dodecyl sulfate (SDS)] with 1 mM phenylmethylsulfonyl
fluoride, and subsequently centrifuged at 10,000 × g at 4°C for 10
min. Protein concentrations were quantified using a Bicinchoninic
Acid Protein assay kit (Beyotime Institute of Biotechnology,
Haimen, China). Proteins (40–50 µg) were separated by 12% SDS-PAGE
gel and subsequently transferred onto polyvinylidene difluoride
membranes. The membranes were incubated for 1 h at room temperature
with a protein blocker (5% skimmed milk in TBS containing Tween 20)
to occupy the spaces on the membranes that were not occupied by
protein. Then the membranes were incubated with the following
primary antibodies at 4°C overnight: Rabbit anti-HMBG1 (3935s;
1:200; Cell Signaling Technology, Inc., Danvers, MA, USA) and
rabbit anti-β-actin (WL0002; 1:200; Wanleibio, Co., Ltd., Shanghai,
China). Following this, membranes were incubated with a goat
anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibody (WLA023; 1:1,000; Wanleibio, Co., Ltd.). Proteins were
subsequently detected with an Enhanced Chemiluminescence Plus kit
(Beyotime Institute of Biotechnology) and the optical density
values of target bands were assessed using Gel-Pro Analyzer
software (version 6.3; Media Cybernetics, Inc., Rockville, MD,
USA).
Results
Proteomics analysis of apoptotic and
necrotic pancreatic cells
Annexin V-positive cells (V group) and Annexin V/PI
double-positive cells (VP group) were sorted by flow cytometry and
subjected to proteomics analysis. The results demonstrated that
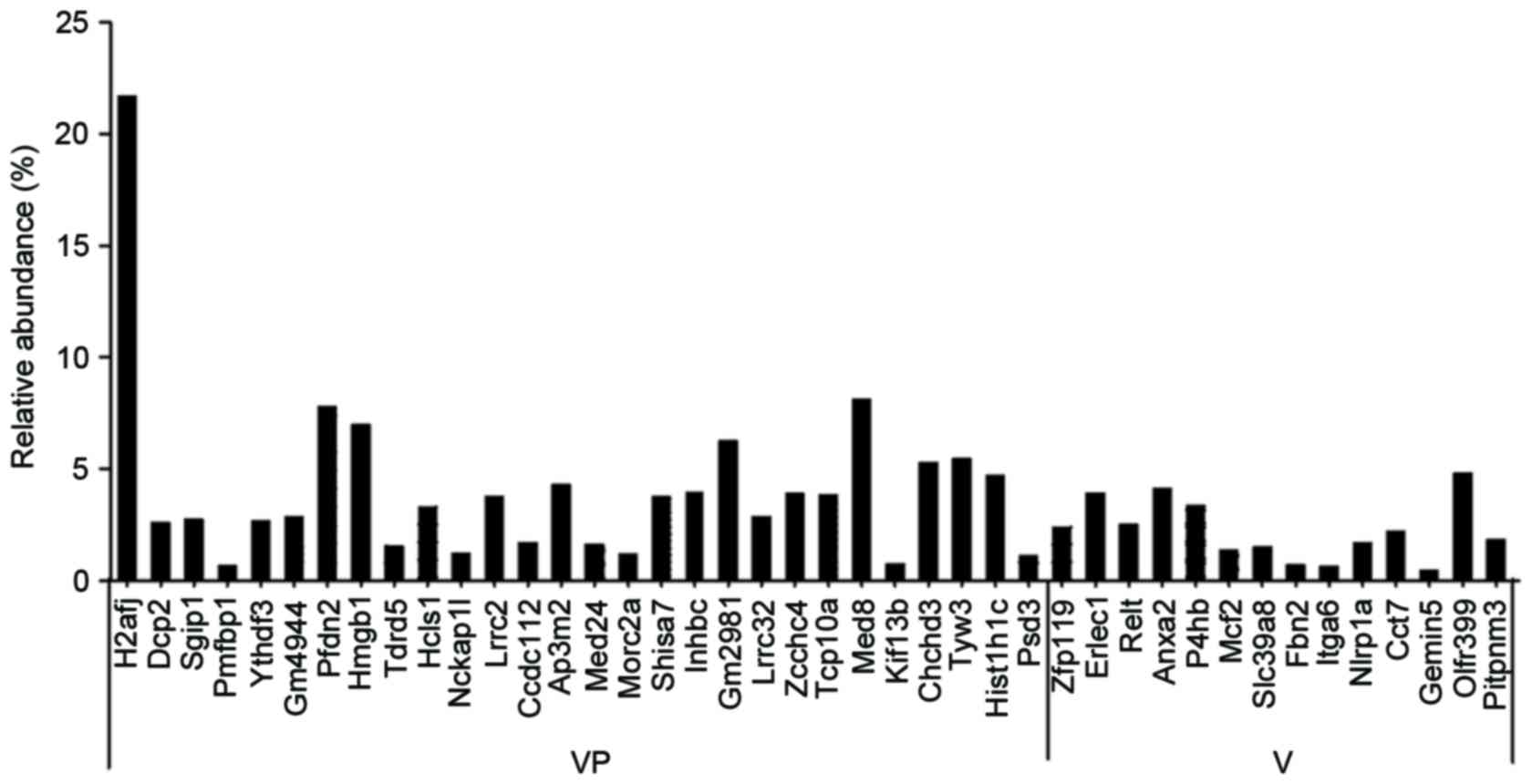
there were 28 different proteins specifically expressed in necrotic
cells (VP group), including H2A histone family member J (H2AFJ),
mRNA-decapping enzyme 2 (DCP2), SH3-domain GRB2-like (endophilin)
interacting protein 1 (SGIP1), polyamine modulated factor 1 binding
protein 1 (PMFBP1), YTHDF3, zinc finger protein 994 (GM4944),
prefoldin subunit 2 (PFDN2), HMGB1, tudor domain containing 5
(TDRD5) and hematopoietic linage cell-specific protein (HCLS1).
There were 14 different proteins specifically expressed in
apoptotic cells (V group), including endoplasmic reticulum lectin 1
(ERLEC1), tumour necrosis factor receptor superfamily member 19L
(RELT), Annexin A2 (ANXA2), protein disulphide-isomerase (P4HB),
proto-oncogene MCF-2 (MCF2) and solute carrier family 39 member 8
(SLC39A8; Fig. 1).
Functional analysis of genes
associated with differentially expressed proteins
The results revealed that HMGB1 was associated with
numerous biological processes, including the formation of the
chromosomal protein glycyl lysine isopeptide cross-link, and the
positive regulation of phosphorylation, protein acid
phosphorylation, the phosphate metabolic process and the phosphorus
metabolic process (Fig. 2).
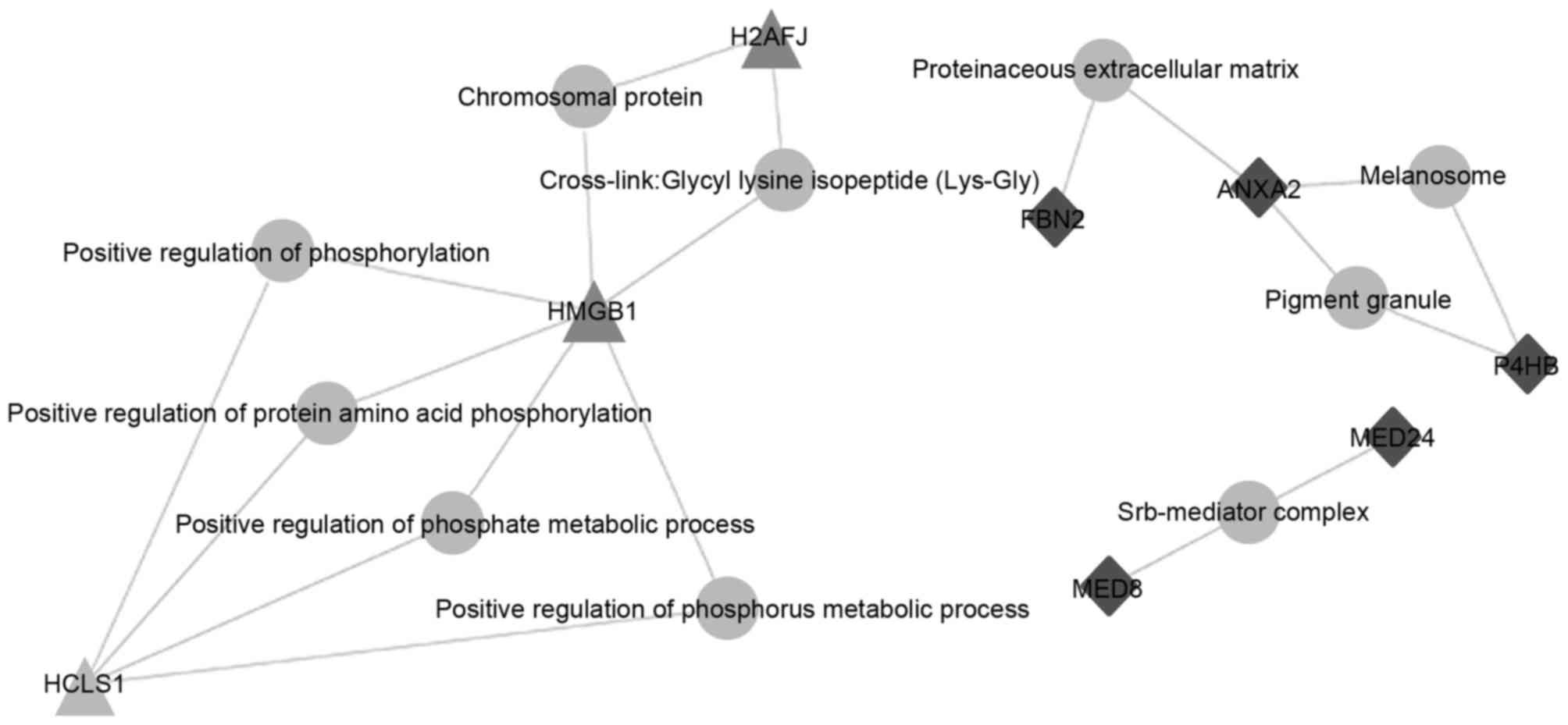 | Figure 2.Functional analysis of genes encoding
differentially expressed proteins. Schematic of genes and
associated signal transduction networks in apoptotic and necrotic
cells: grey circles, biological processes; grey triangles, proteins
specifically expressed in necrotic cells; black diamonds, proteins
specifically expressed in apoptotic cells. H2AFJ, H2A histone
family member J; HMGB1, high mobility group box-1 protein; HCLS1,
hematopoietic linage cell-specific protein 1. FBN2, fibrillin 2;
ANXA2, annexin A2; P4HB, protein disulphide-isomerase; MED24,
mediator complex subunit 24; MED8, mediator complex subunit 8. |
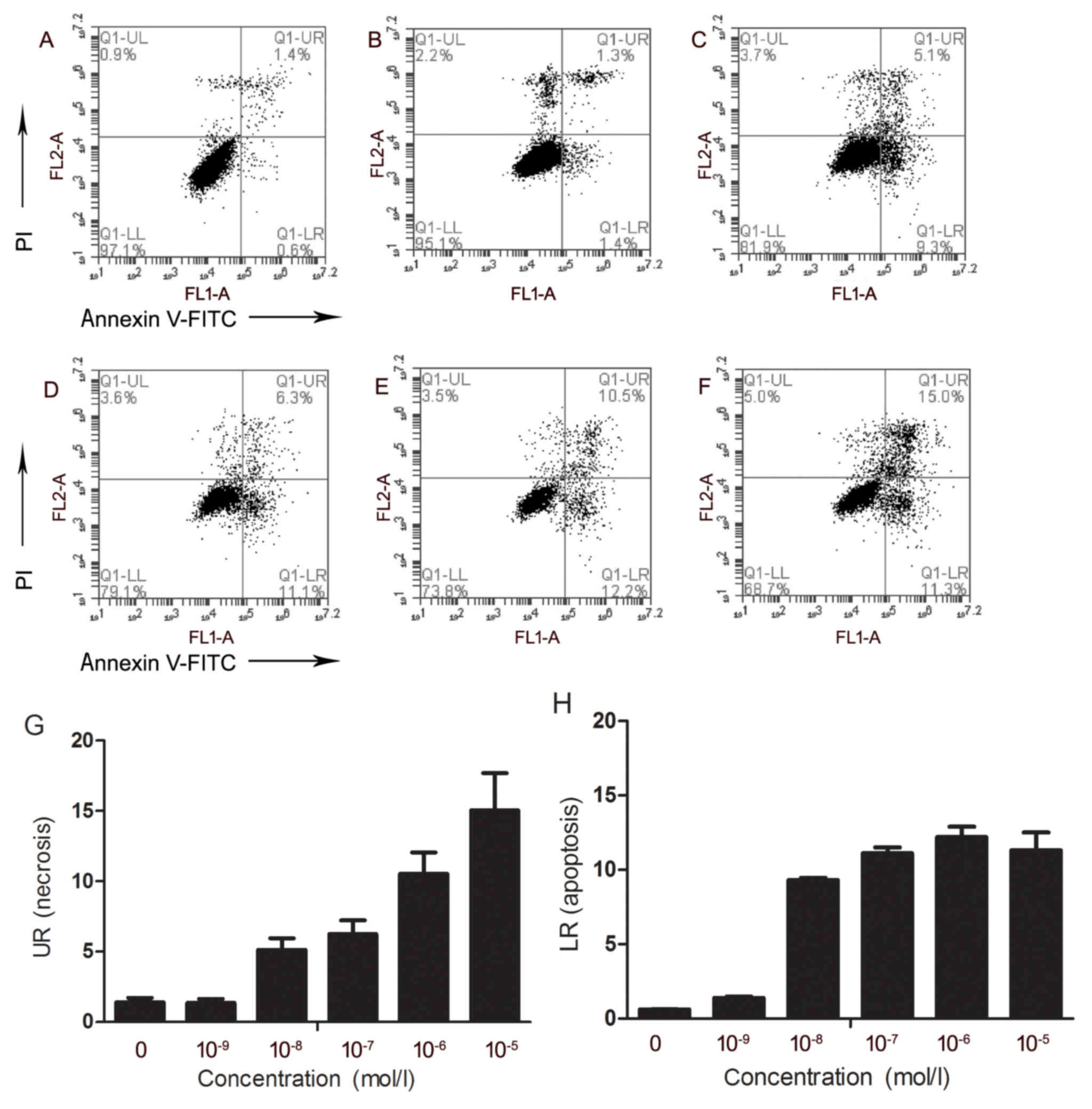
Flow cytometric analysis of AR42J
cells stimulated with various concentrations of caerulein
AR42J cells treated with 0 (Fig. 3A), 10-9 (Fig. 3B), 10-8 (Fig. 3C), 10-7 (Fig. 3D), 10-6 (Fig. 3F) or 10-5 mol/l (Fig. 3F) caerulein were assessed for
apoptosis and necrosis. As the concentration of caerulein increased
from 10-9 to 10-5 mol/l, the percentage of necrotic cells
increased, and cell necrosis frequency increased from 1.3 to 15.04%
(Fig. 3G). No obvious change was
observed in the percentage of apoptotic cells as the concentration
of caerulein increased from 10-8 to 10-5 mol/l (Fig. 3H).
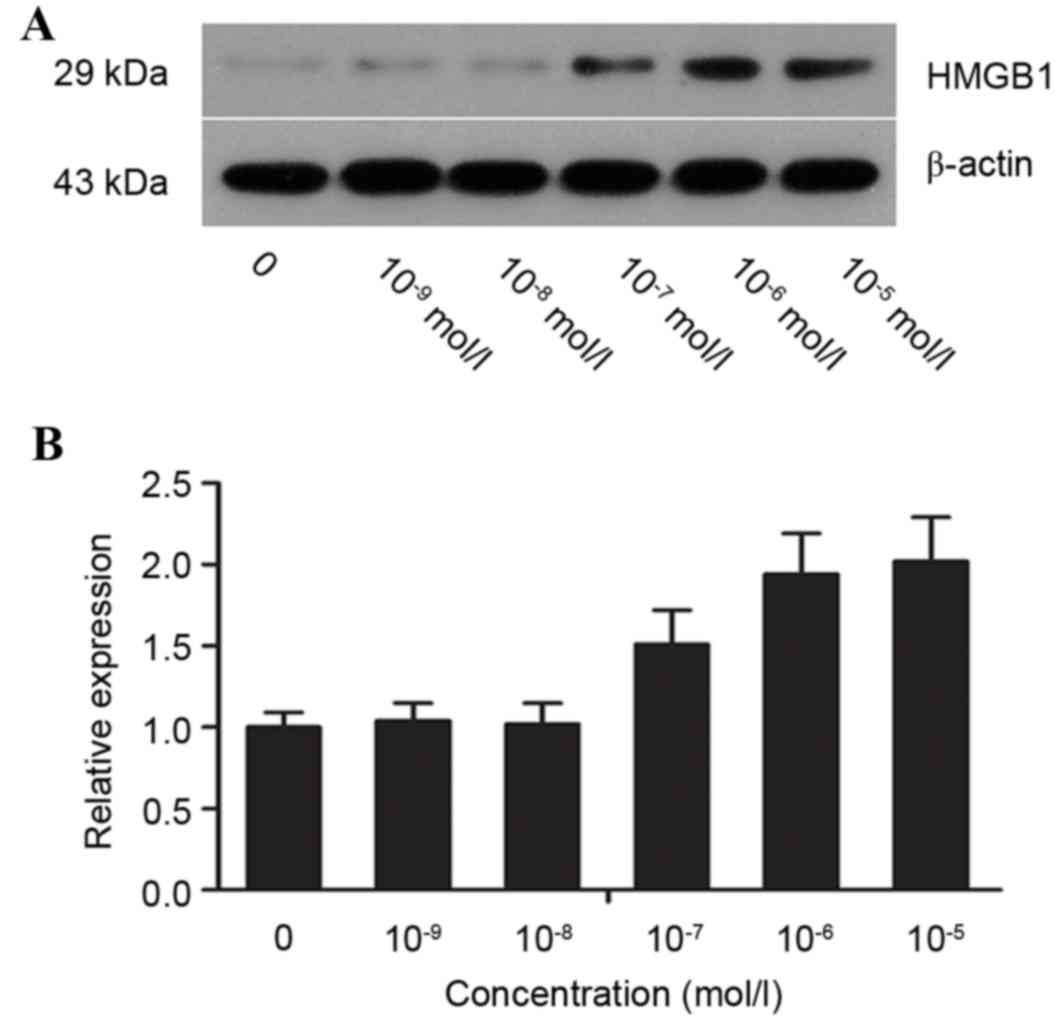
Detection of HMGB1 in AR42J cells
stimulated with various concentrations of caerulein
HGMB1 protein expression levels were detected by
western blot analysis (Fig. 4A).
The optical density value for the control group was set to 1, and
the values of HMGB1 and β-actin were calculated to obtain the
relative expression levels in each group. HMGB1 protein expression
levels increased in a dose-dependent manner. At 10-5 mol/l
caerulein, protein expression levels of HMGB1 were >2-fold those
of the control group (Fig.
4B).
Discussion
AP is a common acute abdominal syndrome, but SAP is
a disease with high morbidity and mortality. A variety of
experimental studies and clinical trials have been performed to
investigate the pathological process of AP. Notably, a previous
study demonstrated that the type of pancreatic cell death may
influence progression of the disease (9).
To examine the differences in protein expression
associated with different types of cell death in AP, flow cytometry
was performed following Annexin V/PI double staining to identify
and sort apoptotic and necrotic cells for proteomics analysis. The
results revealed that there were 28 different proteins specifically
expressed in necrotic cells (VP group), including H2AFJ, DCP2,
SGIP1, PMFBP1, YTHDF3, GM4944, PFDN2, HMGB1, TDRD5 and HCLS1. There
were 14 different proteins specifically expressed in apoptotic
cells (V group), including ERLEC1, RELT, ANXA2, P4HB, MCF2 and
SLC39A8. Functional enrichment analysis was performed using DAVID
to investigate which biological processes were associated with
these proteins. In the bioinformatics analysis of apoptotic and
necrotic cells, HMGB1 in the necrosis group was demonstrated to be
involved in numerous biological processes, including formation of
the chromosomal protein glycyl lysine isopeptide cross-link, and
the positive regulation of phosphorylation, protein acid
phosphorylation, the phosphate metabolic process and the phosphorus
metabolic process. Therefore, it was hypothesized that HMGB1 serves
important roles in necrosis to promote the occurrence and
development of pancreatitis.
HMGB1 is a non-histone chromosome binding protein in
eukaryotic cells. It is released from cells during necrosis and
injury to interact with the membrane receptors receptor for
advanced glycation end products, Toll-like receptor (TLR)2 and TLR4
(10–13). Previously, the function of HMGB1 in
the developmental process of inflammation has received increasing
attention (14,15). Studies have indicated that
extracellular HMGB1 is a novel pro-inflammatory cytokine in humans
(16–18). Previous studies have confirmed that
there is a clear association between extracellular HMGB1 and AP
(19,20). Yasuda et al (19) first reported that the serum level
of HMGB1 in AP patients significantly increased in the initial 72 h
of disease onset, and was associated with severity, infection and
organ dysfunction of AP. In addition, serum HMGB1 levels
demonstrated a positive correlation with serum lactate
dehydrogenase, C-reactive protein and total bilirubin. Therefore,
HMGB1 may be used to estimate the prognosis of SAP patients, with
high serum levels of HMGB1 being an indicator of poor
prognosis.
To confirm the above results, the present study
stimulated ARJ42 pancreatic cells with 10−9,
10−8, 10−7, 10−6 or
10−5 mol/l caerulein. The type of cell death was
detected by flow cytometry. The results indicated that with
increasing concentrations of caerulein, the percentage of necrotic
cells gradually increased from 1.3 to 15.04%. As the concentration
of caerulein increased from 10−8 to 10−5
mol/l, the percentage of apoptotic cells rose from 9.3 to 12.1%. A
previous study demonstrated that apoptotic cells do not release
HMGB1 (21) and do not induce an
inflammatory reaction, as HMGB1 in apoptotic cells is not
acetylated; therefore, HMGB1 is tightly bound to chromatin and will
not be released into the extracellular matrix. By contrast,
necrotic cells may passively release HMGB1 (21). HMGB1 loosely binds to the cell
nucleus at the interphase and division phases of cell division.
When cell necrosis occurs, cell membrane permeability increases and
cell membrane integrity declines; therefore, HMGB1 rapidly leaks
from the cells and is easily detected. To assess this, western blot
analyses were performed in the present study to detect protein
expression levels of HMGB1 in cells. The results demonstrated that
with increased concentrations of caerulein, HMGB1 protein
expression gradually increased, which was consistent with an
increase in necrotic cells. Therefore, at the early stage of
pancreatitis, HMGB1 may be involved in necrosis as a
pro-inflammatory cytokine.
The present study confirmed that there was an
association between HMGB1 and necrotic cells of AP. Yu et al
(22) used classic retrograde
pancreatic duct injection of 5% sodium taurocholate to induce a rat
AP model. Animals were sacrificed after 3, 6, 12 or 24 h, and
peripheral blood samples and pancreatic tissue samples were
collected to detect the levels of inflammatory factors. The results
demonstrated that early-stage inflammatory factors including
interleukin (IL)-1, tumour necrosis factor (TNF)-α and IL-6
increased rapidly, whereas the production, peak and regression time
of HMGB1 was slightly delayed. Therefore, HMGB1 may be involved in
the early-stage inflammatory reaction and serve important roles in
the prolongation and maintenance of inflammatory reactions
(22,23). In the present study, a significant
increase in HMGB1 levels were detected after 8 h of stimulation of
pancreatic acinar cells with caerulein; this result supported the
hypothesis that HMGB1 is involved in early-stage inflammatory
reactions. Considering the number of apoptotic cells, the 8 h
timepoint was selected (8).
However, spatial and temporal differences of HMGB1 expression also
exist in AP (22), detection at
multiple timepoints would aid the understanding of other
differentially expressed proteins. Future studies are required to
elucidate this. Yasuda et al (24) proposed the ‘HMGB1 circulation’
hypothesis. At the early stage of SAP, HMGB1 is primarily produced
by pancreatic and peritoneal macrophages. HMGB1 is subsequently
released into the blood and causes distant organ damage. The
injured organs may additionally release HMGB1, producing a cyclic
reaction. Therefore, HMGB1 inhibitors may have therapeutic effects
on AP (20). The inhibitors of
HMGB1, ethyl pyruvate and pyrrolidine dithiocarbamate, may inhibit
nuclear factor-κB activation (25), thus reducing HMGB1 levels and
blocking HMGB1 circulation; therefore, these inhibitors may protect
against the dysfunction of a variety of organs (26–30).
In conclusion, the present study used a pancreatic
acinar cell AP model to investigate the expression of HMGB1. The
results of the proteomics analysis revealed that HMGB1 protein
expression levels differed significantly between apoptotic and
necrotic cells. Therefore, as a pro-inflammatory cytokine, HMGB1
may be involved in inflammation and promote cell death by necrosis.
The biological processes involving HMGB1 were identified, and
provide an experimental basis for clinical intervention in AP.
However, the association between HMGB1 and other early-stage
inflammatory mediators, cellular processes and specific pathogenic
underlying mechanisms remain to be fully elucidated, and require
further study.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81370566).
References
|
1
|
Petersen OH, Tepikin AV, Gerasimenko JV,
Gerasimenko OV, Sutton R and Criddle DN: Fatty acids, alcohol and
fatty acid ethylesters: Toxic Ca2+ signal generation and
pancreatitis. Cell Calcium. 45:634–642. 2009. View Article : Google Scholar
|
|
2
|
Keck T, Friebe V, Warshaw AL, Antoniu BA,
Waneck G, Benz S, Hopt UT and Fernández-del-Castillo C: Pancreatic
proteases in serum Leukocyte-endothelial adhesion and pancreatic
microcirculatory failure. Pancreatology. 5:241–250. 2005.
View Article : Google Scholar
|
|
3
|
Leung PS and Chan YC: Role of oxidative
stress in pancreatic inflammation. Antioxid Redox Signal.
11:135–165. 2009. View Article : Google Scholar
|
|
4
|
Golstein P and Kroemer G: Cell death by
necrosis: Towards a molecular definition. Trends Biochem Sci.
32:37–43. 2007. View Article : Google Scholar
|
|
5
|
Rinderknecht H: Fatal pancreatitis, a
consequence of excessive leukocyte stimulation? Int J Pancreatol.
3:105–112. 1988.
|
|
6
|
Ziangirova GG and Antonova OV: The causes
of necrobiosis and apoptosis of corneal epithelial cells during
primary acquired keratoconus. Izv Akad Nauk Ser Biol. 5:517–521.
2002.(In Russian).
|
|
7
|
Andersson R and Wang XD: Patterns of
pancreatic cell death: Apoptosis versus oncosis. Pancreas.
17:281–288. 1998. View Article : Google Scholar
|
|
8
|
Chu J, Ji H, Lu M, Li Z, Qiao X, Sun B,
Zhang W and Xue D: Proteomic analysis of apoptotic and oncotic
pancreatic acinar AR42J cells treated with caerulein. Mol Cell
Biochem. 382:1–17. 2013. View Article : Google Scholar
|
|
9
|
Bhatia M: Apoptosis versus necrosis in
acute pancreatitis. Am J Physiol Gastrointest Liver Physiol.
286:G189–G196. 2004. View Article : Google Scholar
|
|
10
|
Hori O, Brett J, Slattery T, Cao R, Zhang
J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al: The
receptor for advanced glycation end products (RAGE) is a cellular
binding site for amphoterin. Mediation of neurite outgrowth and
co-expression of rage and amphoterin in the developing nervous
system. J Biol Chem. 270:25752–25761. 1995. View Article : Google Scholar
|
|
11
|
Park JS, Svetkauskaite D, He Q, Kim JY,
Strassheim D, Ishizaka A and Abraham E: Involvement of toll-like
receptors 2 and 4 in cellular activation by high mobility group box
1 protein. J Biol Chem. 279:7370–7307. 2004. View Article : Google Scholar
|
|
12
|
Urbonaviciute V, Fürnrohr BG, Meister S,
Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner
H, Manfredi AA, et al: Induction of inflammatory and immune
responses by HMGB1-nucleosome complexes: Implications for the
pathogenesis of SLE. J Exp Med. 205:3007–3018. 2008. View Article : Google Scholar :
|
|
13
|
Park JS, Gamboni-Robertson F, He Q,
Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama
I, Banerjee A, et al: High mobility group box 1 protein interacts
with multiple Toll-like receptors. Am J Physiol Cell Physiol.
290:C917–C924. 2006. View Article : Google Scholar
|
|
14
|
Goodwin GH, Sanders C and Johns EW: A new
group of chromatinassociated proteins with a high content of acidic
and basic amino acids. Eur J Biochem. 38:14–19. 1973. View Article : Google Scholar
|
|
15
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et
al: HMG-1 as a late mediator of endotoxin lethality in mice.
Science. 285:248–251. 1999. View Article : Google Scholar
|
|
16
|
Wang H, Yang H, Czura CJ, Sama AE and
Tracey KJ: HMGB1 as a late mediator of lethal systemic
inflammation. Am J Respir Crit Care Med. 164:1768–1773. 2001.
View Article : Google Scholar
|
|
17
|
Magna M and Pisetsky DS: The role of HMGB1
in the pathogenesis of inflammatory and autoimmune diseases. Mol
Med. 20:138–146. 2014. View Article : Google Scholar :
|
|
18
|
Singh A, Feng Y, Mahato N, Li J, Wu C and
Gong J: Role of high-mobility group box 1 in patients with acute
obstructive suppurative cholangitis-induced sepsis. J Inflamm Res.
8:71–77. 2015. View Article : Google Scholar :
|
|
19
|
Yasuda T, Ueda T, Takeyama Y, Shinzeki M,
Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y and Kuroda Y:
Significant increase of serum high-mobility group box chromosomal
protein 1 levels in patients with severe acute pancreatitis.
Pancreas. 33:359–363. 2006. View Article : Google Scholar
|
|
20
|
Sawa H, Ueda T, Takeyama Y, Yasuda T,
Shinzeki M, Nakajima T and Kuroda Y: Blockade of high mobility
group box-1 protein attenuates experimental severe acute
pancreatitis. World J Gastroenterol. 12:7666–7670. 2006. View Article : Google Scholar :
|
|
21
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar
|
|
22
|
Yu C, Huang L, Li X, Zhu H, Li Z and Yu X:
Spatial and temporal differences of HMGB1 expression in the
pancreas of rats with acute pancreatitis. Int J Clin Exp Pathol.
8:6928–6935. 2015.
|
|
23
|
Biscetti F, Ghirlanda G and Flex A:
Therapeutic potential of high mobility group box-1 in ischemic
injury and tissue regeneration. Curr Vasc Pharmacol. 9:677–681.
2011. View Article : Google Scholar
|
|
24
|
Yasuda T, Ueda T, Shinzeki M, Sawa H,
Nakajima T, Takeyama Y and Kuroda Y: Increase of high-mobility
group box chromosomal protein 1 in blood and injured organs in
experimental severe acute pancreatitis. Pancreas. 34:487–488. 2007.
View Article : Google Scholar
|
|
25
|
Sah RP and Saluja A: Molecular mechanisms
of pancreatic injury. Curr Opin Gastroenterol. 27:444–451. 2011.
View Article : Google Scholar :
|
|
26
|
Bünger R, Mallet RT and Hartman DA:
Pyruvate-enhanced phosphorylation potential and inotropism in
normoxic and postischemic isolated working heart. Near-complete
prevention of reperfusion contractile failure. Eur J Biochem.
180:221–233. 1989. View Article : Google Scholar
|
|
27
|
Cicalese L, Lee K, Schraut W, Watkins S,
Borle A and Stanko R: Pyruvate prevents ischemia-reperfusion
mucosal injury of rat small intestine. Am J Surg. 171:97–101. 1996.
View Article : Google Scholar
|
|
28
|
Sileri P, Schena S, Morini S, Rastellini
C, Pham S, Benedetti E and Cicalese L: Pyruvate inhibits hepatic
ischemia-reperfusion injury in rats. Transplantation. 72:27–30.
2001. View Article : Google Scholar
|
|
29
|
Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer
S, Hewitt SM and Star RA: Ethyl pyruvate decreases sepsis-induced
acute renal failure and multiple organ damage in aged mice. Kidney
Int. 64:1620–1631. 2003. View Article : Google Scholar
|
|
30
|
Satoh A, Shimosegawa T, Fujita M, Kimura
K, Masamune A, Koizumi M and Toyota T: Inhibition of nuclear
factor-kappaB activation improves the survival of rats with
taurocholate pancreatitis. Gut. 44:253–258. 1999. View Article : Google Scholar :
|